Fabrication of Ni–Co–BN (h) Nanocomposite Coatings with Jet Electrodeposition in Different Pulse Parameters
Abstract
1. Introduction
2. Experimental
2.1. Experimental Materials and Pretreatment
2.2. Preparation of Ni–Co–BN (h) Nanocomposite Coating
2.3. Sample Characterization
3. Results and Discussion
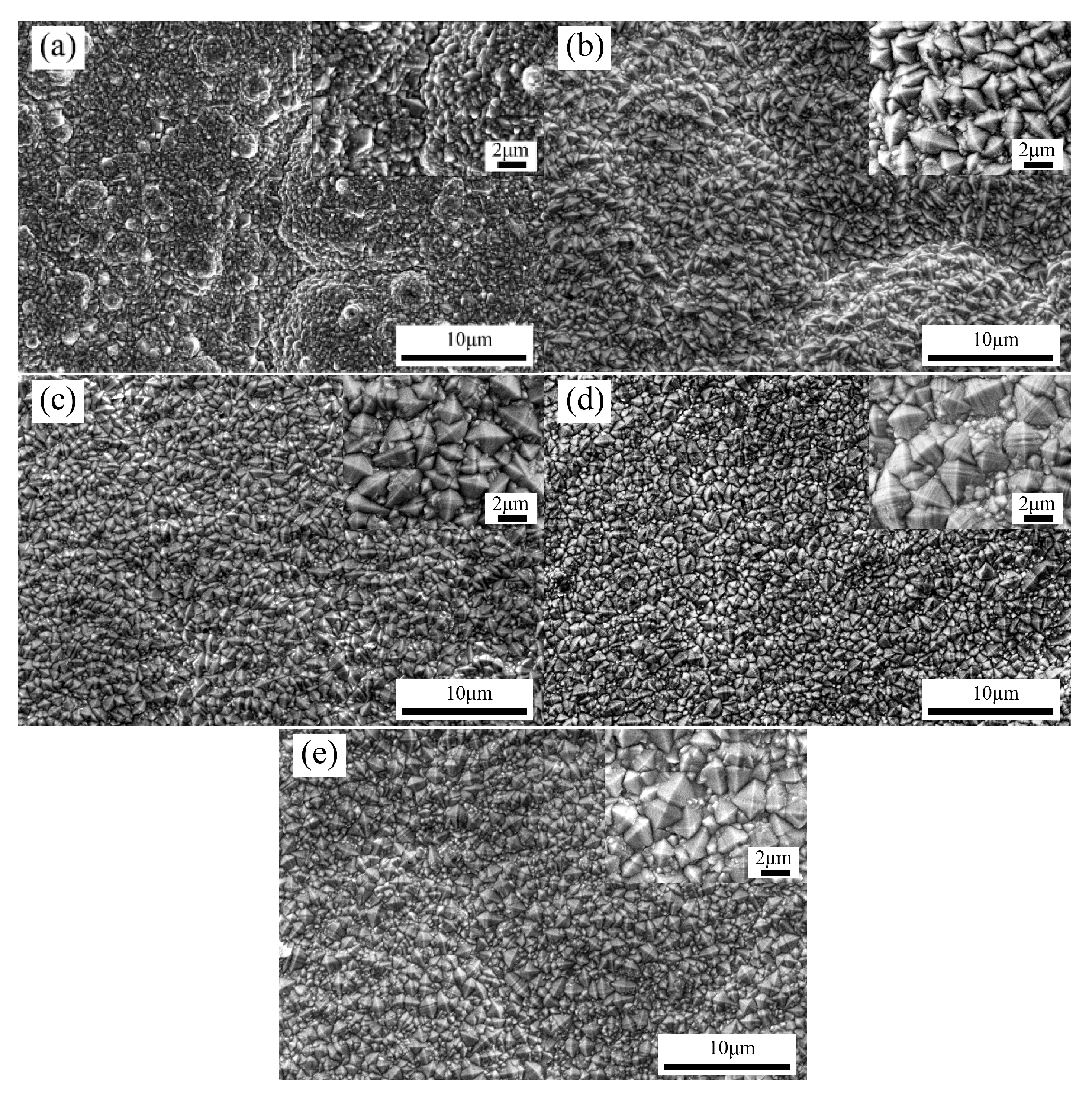
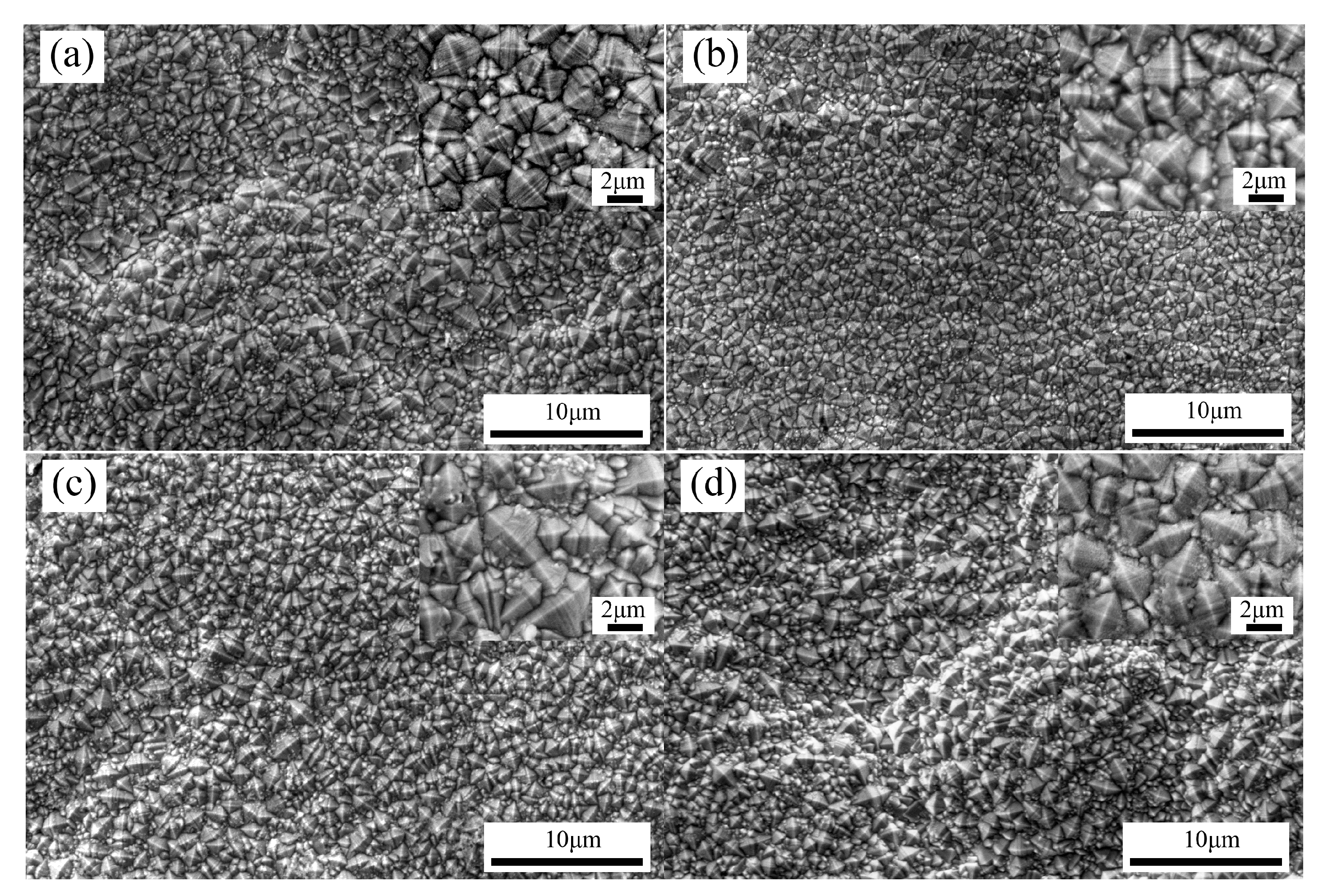
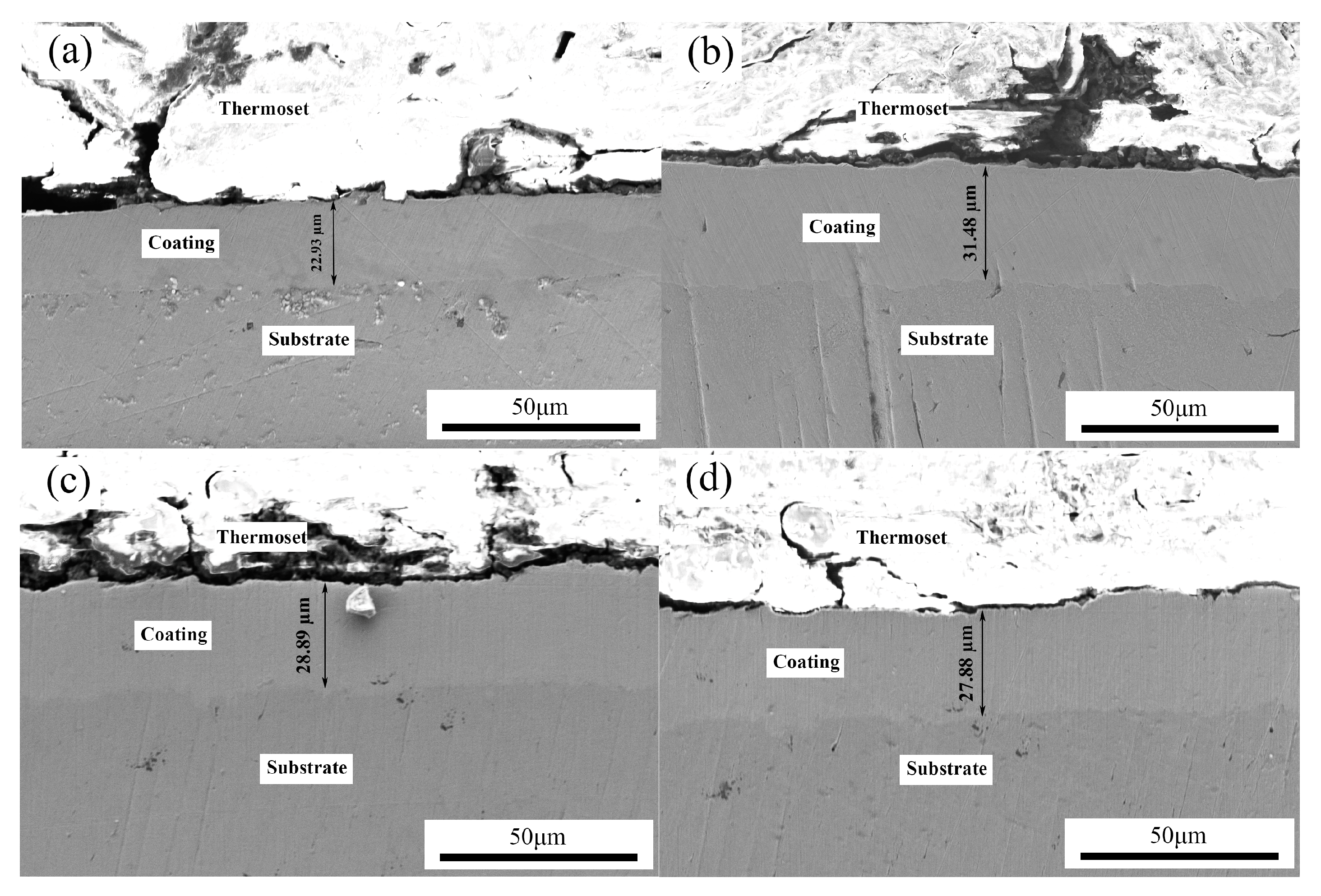
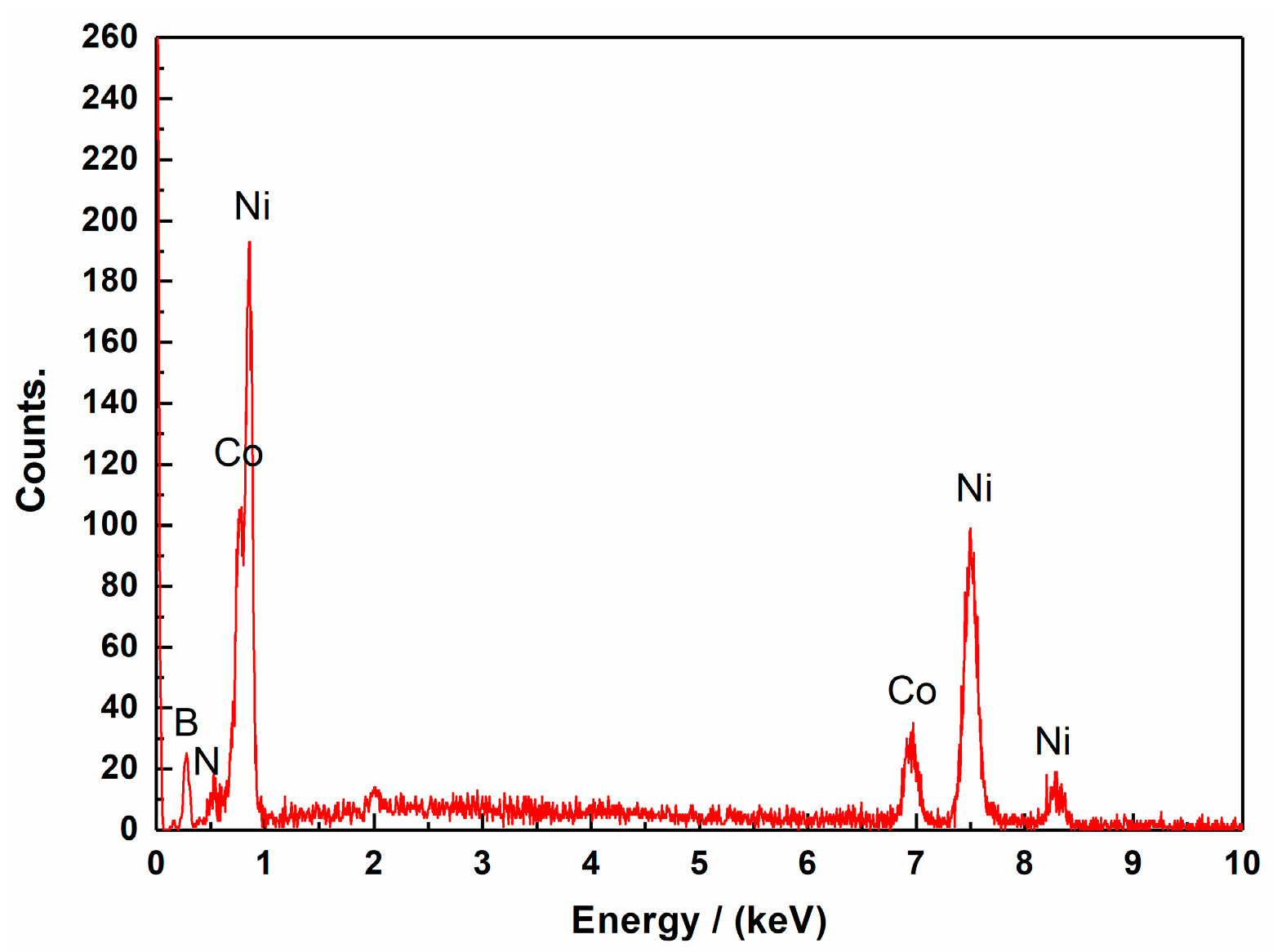
3.1. Effects of Pulse Parameters on the Surface Morphology and Element Content
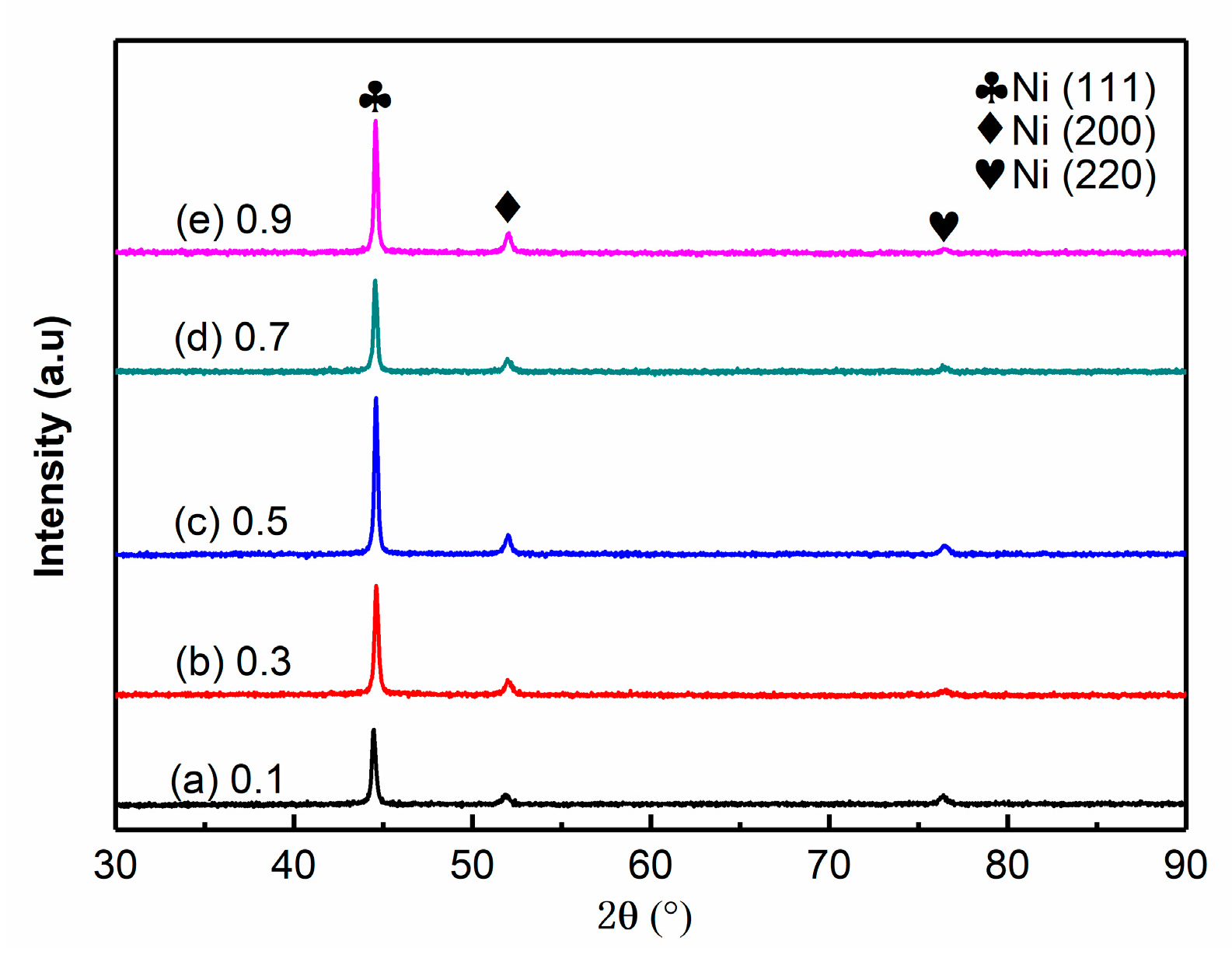
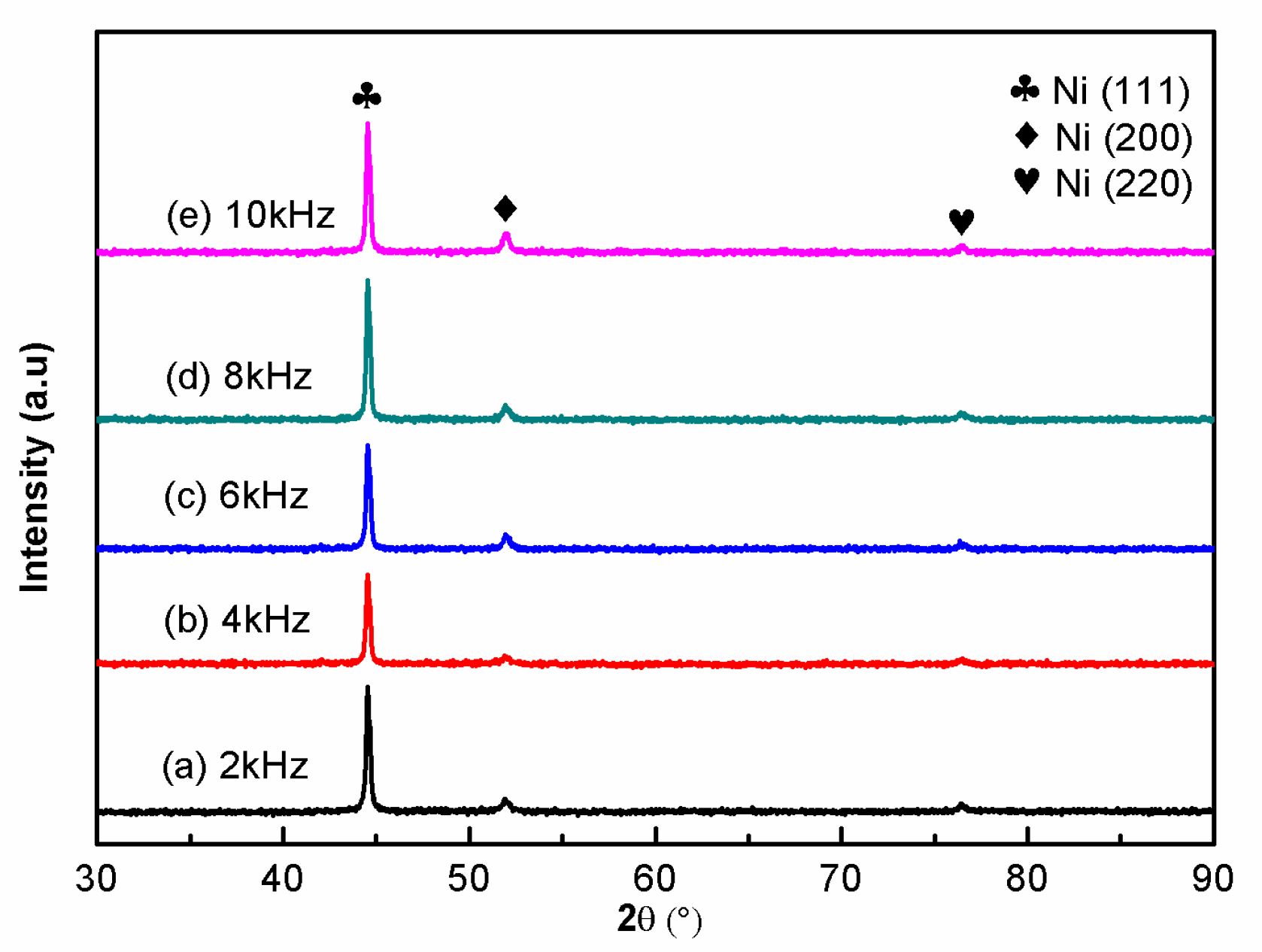
3.2. Effects of Pulse Parameters on the Phase Structure
3.3. Effects of Pulse Parameters on the Surface Roughness
3.4. Effects of Pulse Parameters on the Microhardness
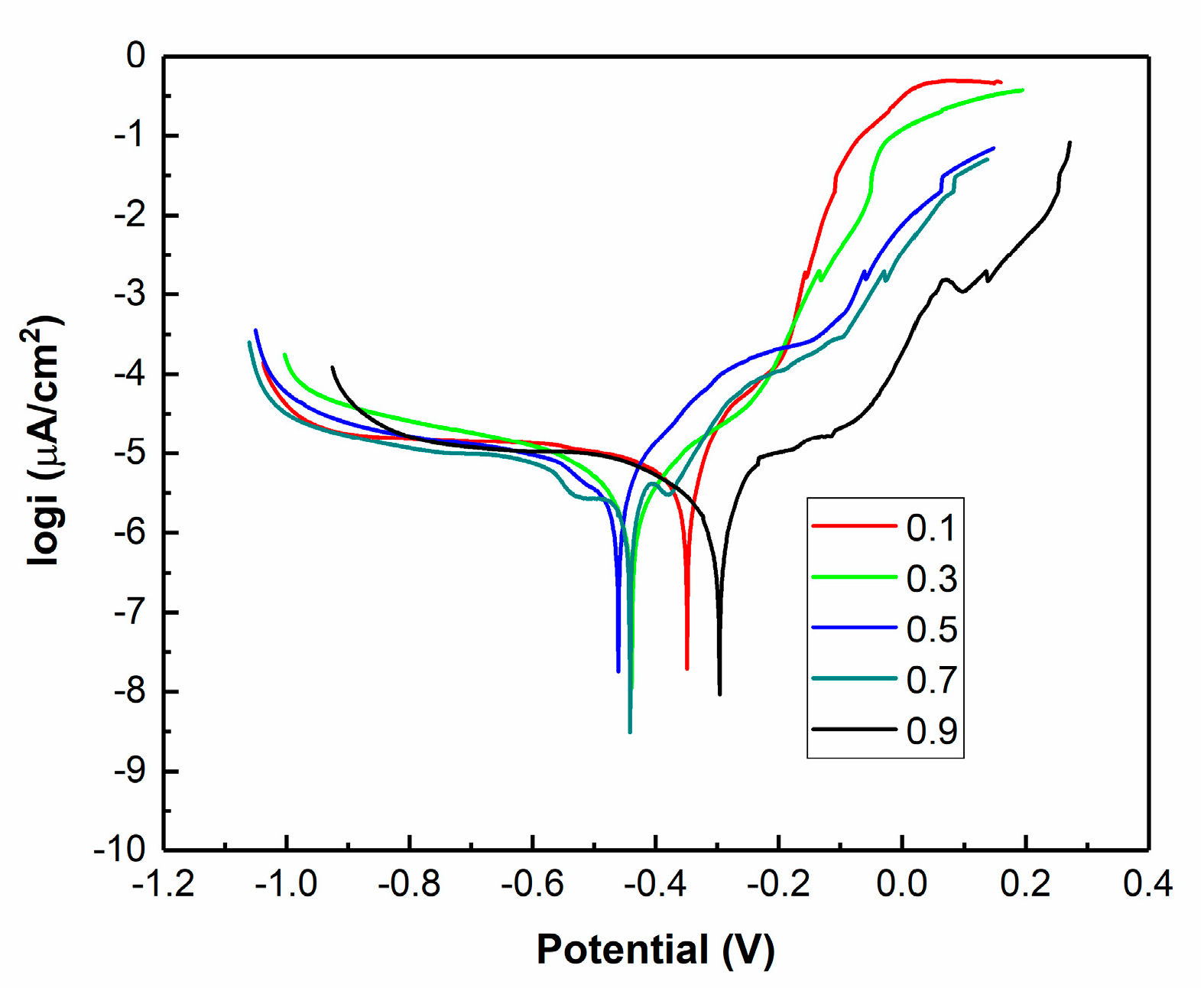
3.5. Effects of Pulse Parameters on the Corrosion Resistance
4. Conclusions
- The change of pulse parameters affected the surface morphology, thickness, composition, and surface roughness of the coatings. Reasonable pulse parameters were not only conducive to the growth of the coatings, but also increased the contents of Co and BN (h) nanoparticles. When the pulse frequency was 4 kHz and the duty cycle was 0.7, the surface roughness Sa was 0.664 µm, the contents of Co and the BN (h) nanoparticles were 27.34 wt % and 3.82 wt %, respectively.
- The Ni–Co–BN (h) nanocomposite coatings prepared by pulse jet electrodeposition had a face-centered cubic structure, and the Ni atoms and Co atoms in the coatings formed a single α-phase solid solution. In the process of coatings growth, the grains had obvious preferred orientation in the (111) plane. The grain size of the coatings decreased with the increase in duty cycle, and decreased first and then increased with the increase in pulse frequency.
- In the process of pulse jet electrodeposition, the variation of duty cycle and pulse frequency had similar effects on the microhardness of the coatings. With the increase in pulse parameters, the microhardness first increased and then decreased.
- Polarization curves of the Ni–Co–BN (h) nanocomposite coatings showed that the pulse parameters had great effects on the corrosion resistance. The change in corrosion current density and polarization resistance indicated that too high or too low pulse parameters were not conducive to the improvement of corrosion resistance of the coatings. The sample with pulse frequency of 4 kHz and duty cycle of 0.7 exhibited good performance in corrosion current density and polarization resistance.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiang, W.; Shen, L.D.; Qiu, M.B. Microhardness, wear, and corrosion resistance of Ni-SiC composite coating with magnetic-field-assisted jet electrodeposition. Mater. Res. Express 2018, 5, 096407. [Google Scholar] [CrossRef]
- Dheeraj, P.R.; Patra, A.; Sengupta, S. Synergistic effect of peak current density and nature of surfactant on microstructure, mechanical and electrochemical properties of pulsed electrodeposited Ni-Co-SiC nanocomposites. J. Alloy. Compd. 2017, 729, 1093–1107. [Google Scholar] [CrossRef]
- Ratajski, T.; Kalemba-Rec, I.; Indyka, P. Microstructural characterization of SiO2/Ni nanocomposites electrodeposited from a sulphate bath modified by PEI. Mater. Charact. 2018, 142, 478–491. [Google Scholar] [CrossRef]
- Alizadeh, M.; Safaei, H. Characterization of Ni-Cu matrix, Al2O3 reinforced nano-composite coatings prepared by electrodeposition. Appl. Surf. Sci. 2018, 456, 195–203. [Google Scholar] [CrossRef]
- Li, B.S.; Li, X.; Huan, Y.X. Influence of alumina nanoparticles on microstructure and properties of Ni-B composite coating. J. Alloy. Compd. 2018, 762, 133–142. [Google Scholar] [CrossRef]
- Ansari, M.I.; Julka, S.; Thakur, D.G. Enhancement of surface properties with influence of bath pH on electroless Ni-P-ZnO/Al2O3 nano-composite deposits for defence applications. J. Mol. Liq. 2017, 247, 22–33. [Google Scholar] [CrossRef]
- Lopes, N.I.A.; Freire, N.H.J.; Resende, P.D. Electrochemical deposition and characterization of ZrO2 ceramic nanocoatings on superelastic NiTi alloy. Appl. Surf. Sci. 2018, 450, 21–30. [Google Scholar] [CrossRef]
- Nazir, M.H.; Khan, Z.A.; Saeed, A. Analyzing and Modelling the Corrosion Behavior of Ni/Al2O3, Ni/SiC, Ni/ZrO2 and Ni/Graphene Nanocomposite Coatings. Materials 2017, 10, 1225. [Google Scholar] [CrossRef]
- Jiang, Y.C.; Xu, Y.H.; Wang, M. Effects of pulse plating parameters on the microstructure and properties of high frequency pulse electrodeposited Ni-Co-ZrO2 nano-composite coatings. J. Mater. Sci. Mater. Electron. 2017, 28, 610–616. [Google Scholar] [CrossRef]
- Adachi, T.; Latthe, S.S.; Gosavi, S.W. Photocatalytic, superhydrophilic, self-cleaning TiO2 coating on cheap, light-weight, flexible polycarbonate substrates. Appl. Surf. Sci. 2018, 458, 917–923. [Google Scholar] [CrossRef]
- Wang, Y.X.; Tay, S.L.; Wei, S.H. Microstructure and properties of sol-enhanced Ni-Co-TiO2, nanocomposite coatings on mild steel. J. Alloy. Compd. 2015, 649, 222–228. [Google Scholar] [CrossRef]
- Karslioglu, R.; Akbulut, H. Comparison microstructure and sliding wear properties of nickel-cobalt-CNT composite coatings by DC, PC and PRC current electrodeposition. Appl. Surf. Sci. 2015, 353, 615–627. [Google Scholar] [CrossRef]
- Liu, Z.; Tabakman, S.; Welsher, K. Carbon Nanotubes in Biology and Medicine: In vitro and in vivo Detection, Imaging and Drug Delivery. Nano Res. 2009, 2, 85–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.M.; Xia, J.G.; Zhao, Q.H. Functional Graphene Oxide as a Nanocarrier for Controlled Loading and Targeted Delivery of Mixed Anticancer Drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Wu, Z.F.; Xu, S.G. Probing the electron states and metal-insulator transition mechanisms in molybdenum disulphide vertical heterostructures. Nat. Commun. 2015, 6, 6088. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.D.; Vurgaftman, I.; Tischler, J.G. Atomic-scale photonic hybrids for mid-infrared and terahertz nanophotonics. Nat. Nanotechnol. 2016, 11, 9–15. [Google Scholar] [CrossRef]
- Tajaddod, N.; Song, K.; Green, E.C. Exfoliation of Boron Nitride Platelets by Enhanced Interfacial Interaction with Polyethylene. Macromol. Mater. Eng. 2016, 301, 315–327. [Google Scholar] [CrossRef]
- Jin, H.L.; Huang, H.H.; He, Y.H. Graphene Quantum Dots Supported by Graphene Nanoribbons with Ultrahigh Electrocatalytic Performance for Oxygen Reduction. J. Am. Chem. Soc. 2015, 137, 7588–7591. [Google Scholar] [CrossRef]
- Hu, S.; Lozada-Hidalgo, M.; Wang, F.C. Proton transport through one-atom-thick crystals. Nature 2014, 516, 227–230. [Google Scholar] [CrossRef]
- Weng, W.H.; Wang, X.B.; Wang, X. Functionalized hexagonal boron nitride nanomaterials: Emerging properties and applications. Chem. Soc. Rev. 2016, 45, 3989–4012. [Google Scholar] [CrossRef]
- Ghazanlou, S.I.; Farhood, A.H.S.; Hosouli, S. Pulse frequency and duty cycle effects on the electrodeposited Ni-Co reinforced with micro and nano-sized ZnO. J. Mater. Sci. Mater. Electron. 2017, 28, 15537–15551. [Google Scholar] [CrossRef]
- Yi, D.G.; Shen, L.D.; Zhu, J. Electrochemical corrosion behavior of nano-crytalline nickle prepared by pulsed friction aided jet electrodeposition. Mater. Sci. Technol. 2015, 23, 96–101. (In Chinese) [Google Scholar]
- Wang, X.; Shen, L.D.; Qiu, M.B. Effect of Friction on Preparation of NdFeB Nickel Coating by Jet Electrodepostion. J. Electrochem. Sci. 2018, 13, 7706–7717. [Google Scholar] [CrossRef]
- Liu, Z.D. Non-Conventional Machining; Peking University Press: Beijing, China, 2017; pp. 221–225. (In Chinese) [Google Scholar]
- Bakhit, B. The influence of electrolyte composition on the properties of Ni-Co alloy coatings reinforced by SiC nano-particles. Surf. Coat. Technol. 2015, 275, 324–331. [Google Scholar] [CrossRef]
- Bakhit, B.; Akbari, A.; Nasirpouri, F. Corrosion resistance of Ni-Co alloy and Ni-Co/SiC nanocomposite coatings electrodeposited by sediment codeposition technique. Appl. Surf. Sci. 2014, 307, 351–359. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, H.; Wand, D. Effects of saccharin and cobalt concentration in electrolytic solution on microhardness of nanocrystalline Ni-Co alloys. Surf. Coat. Technol. 2008, 202, 4952–4956. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Liu, H. Effects of bivalent Co ion on the co-deposition of nickel and nanodiamond particles. Surf. Coat. Technol. 2005, 191, 1–6. [Google Scholar] [CrossRef]
- Hou, J. Pulse electroplating and its power supply. Electroplat. Pollut. Control 2011, 31, 4–9. (In Chinese) [Google Scholar]
- Xiang, G.P. The Theory and Application of Pulse Plating; Tianjin science and Technology Press: Tianjin, China, 1989; pp. 122–124. (In Chinese) [Google Scholar]
- Seifzadeh, D.; Hollagh, A.R. Corrosion Resistance Enhancement of AZ91D Magnesium Alloy by Electroless Ni-Co-P Coating and Ni-Co-P-SiO2 Nanocomposite. J. Mater. Eng. Perform. 2014, 23, 4109–4121. [Google Scholar] [CrossRef]
- Wang, Z.C.; Jia, F.; Yu, L. Direct electroless nickel-boron plating on AZ91D magnesium alloy. Surf. Coat. Technol. 2012, 206, 3676–3685. [Google Scholar] [CrossRef]
- Zamani, M.; Amadeh, A.; Baghal, S.M.L. Effect of Co content on electrodeposition mechanism and mechanical properties of electrodeposited Ni-Co alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 484–491. [Google Scholar] [CrossRef]
- Bakhit, B.; Akbari, A. Effect of particle size and co-deposition technique on hardness and corrosion properties of Ni-Co/SiC composite coatings. Surf. Coat. Technol. 2012, 206, 4964–4975. [Google Scholar] [CrossRef]



| Solution | Composition |
|---|---|
| Plating Solution/(g L−1) | 200.0 NiSO4·6H2O + 5.0 CoSO4·7H2O + 50.0 NiCl2·6H2O + 30.0 H3BO3 + 0.05 Sodium dodecyl sulfate + 0.002 Thiourea + 5.0 BN (h) nanoparticles |
| Electric Cleaning Solution/(g L−1) | 25.0 NaOH + 21.7 Na2CO3 + 50.0 Na3PO4 + 2.4 NaCl |
| Strong Activation Solution/(g L−1) | 25.0 Hydrochloric acid + 140.1 NaCl |
| Weak Activation Solution/(g L−1) | 141.2 Na3C6H5O7·2H2O + 94.2 H3C6H5O7·H2O + 3.0 NiCl2·6H2O |
| Pulse Parameters | Co (wt %) | N (wt %) | Ni (wt %) | Grain Size (nm) | Sa (µm) | Microhardness (HV0.05) | |
|---|---|---|---|---|---|---|---|
| Frequency (kHz) | Duty Cycle | ||||||
| 4 | 0.1 | 12.60 | 1.58 | 85.82 | 20.3 | 1.466 | 411.7 |
| 4 | 0.3 | 13.71 | 1.72 | 84.57 | 21.6 | 1.305 | 447.8 |
| 4 | 0.5 | 19.90 | 3.04 | 78.38 | 25.3 | 0.832 | 640.7 |
| 4 | 0.7 | 27.34 | 3.82 | 68.84 | 27.2 | 0.664 | 719.2 |
| 4 | 0.9 | 21.68 | 2.89 | 75.43 | 28.4 | 0.936 | 631.3 |
| 2 | 0.7 | 24.77 | 3.48 | 71.75 | 28.1 | 0.870 | 685.2 |
| 6 | 0.7 | 22.87 | 3.33 | 73.80 | 28.9 | 0.764 | 673.6 |
| 8 | 0.7 | 19.93 | 3.16 | 76.91 | 30.4 | 0.890 | 655.4 |
| 10 | 0.7 | 17.62 | 2.97 | 79.41 | 31.6 | 0.995 | 641.6 |
| Pulse Parameters | Eocp (mV) | βa (mV/dec) | βc (mV/dec) | Icorr (µA/cm2) | Ecorr (mV) | Rp (kΩ cm2) | |
|---|---|---|---|---|---|---|---|
| Frequency (kHz) | Duty Cycle | ||||||
| 4 | 0.1 | −435 | 138.69 | 361.46 | 5.90 | −389 | 7.37 |
| 4 | 0.3 | −425 | 111.80 | 195.03 | 2.81 | −476 | 10.97 |
| 4 | 0.5 | −431 | 136.69 | 215.85 | 2.62 | −433 | 13.87 |
| 4 | 0.7 | −462 | 77.59 | 174.35 | 0.77 | −422 | 30.11 |
| 4 | 0.9 | −327 | 361.82 | 200.09 | 3.57 | −365 | 15.69 |
| 2 | 0.7 | −475 | 77.35 | 261.35 | 1.62 | −425 | 15.97 |
| 6 | 0.7 | −269 | 72.95 | 176.26 | 1.76 | −313 | 12.74 |
| 8 | 0.7 | −477 | 70.48 | 411.13 | 2.76 | −380 | 9.44 |
| 10 | 0.7 | −487 | 129.67 | 520.75 | 4.74 | −438 | 9.52 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Kang, M.; Zhang, Y.; Liu, Y.; Jin, M.; Mbugua, N.S.; Zhu, G.; Liu, C. Fabrication of Ni–Co–BN (h) Nanocomposite Coatings with Jet Electrodeposition in Different Pulse Parameters. Coatings 2019, 9, 50. https://doi.org/10.3390/coatings9010050
Li H, Kang M, Zhang Y, Liu Y, Jin M, Mbugua NS, Zhu G, Liu C. Fabrication of Ni–Co–BN (h) Nanocomposite Coatings with Jet Electrodeposition in Different Pulse Parameters. Coatings. 2019; 9(1):50. https://doi.org/10.3390/coatings9010050
Chicago/Turabian StyleLi, Hengzheng, Min Kang, Yin Zhang, Yuntong Liu, Meifu Jin, Nyambura Samuel Mbugua, Guang Zhu, and Conghu Liu. 2019. "Fabrication of Ni–Co–BN (h) Nanocomposite Coatings with Jet Electrodeposition in Different Pulse Parameters" Coatings 9, no. 1: 50. https://doi.org/10.3390/coatings9010050
APA StyleLi, H., Kang, M., Zhang, Y., Liu, Y., Jin, M., Mbugua, N. S., Zhu, G., & Liu, C. (2019). Fabrication of Ni–Co–BN (h) Nanocomposite Coatings with Jet Electrodeposition in Different Pulse Parameters. Coatings, 9(1), 50. https://doi.org/10.3390/coatings9010050






