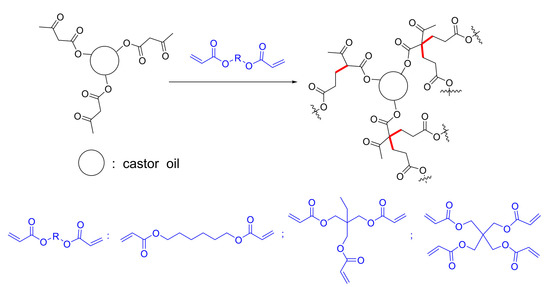
An Ambient Curable Coating Material Based on the Michael Addition Reaction of Acetoacetylated Castor Oil and Multifunctional Acrylate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
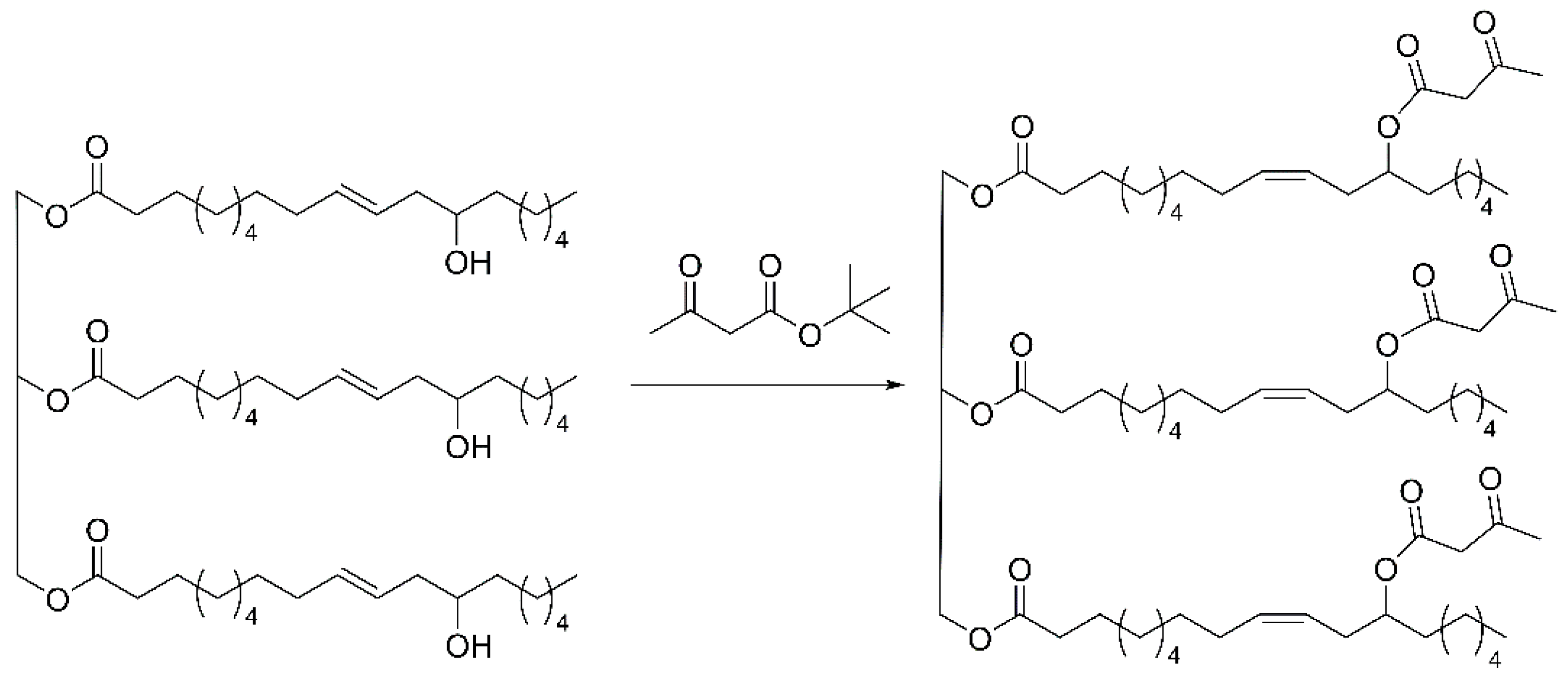
2.2. The Synthesis of Acetoacetylated Castor Oil
2.3. Preparation of Films
2.4. Characterization
3. Results
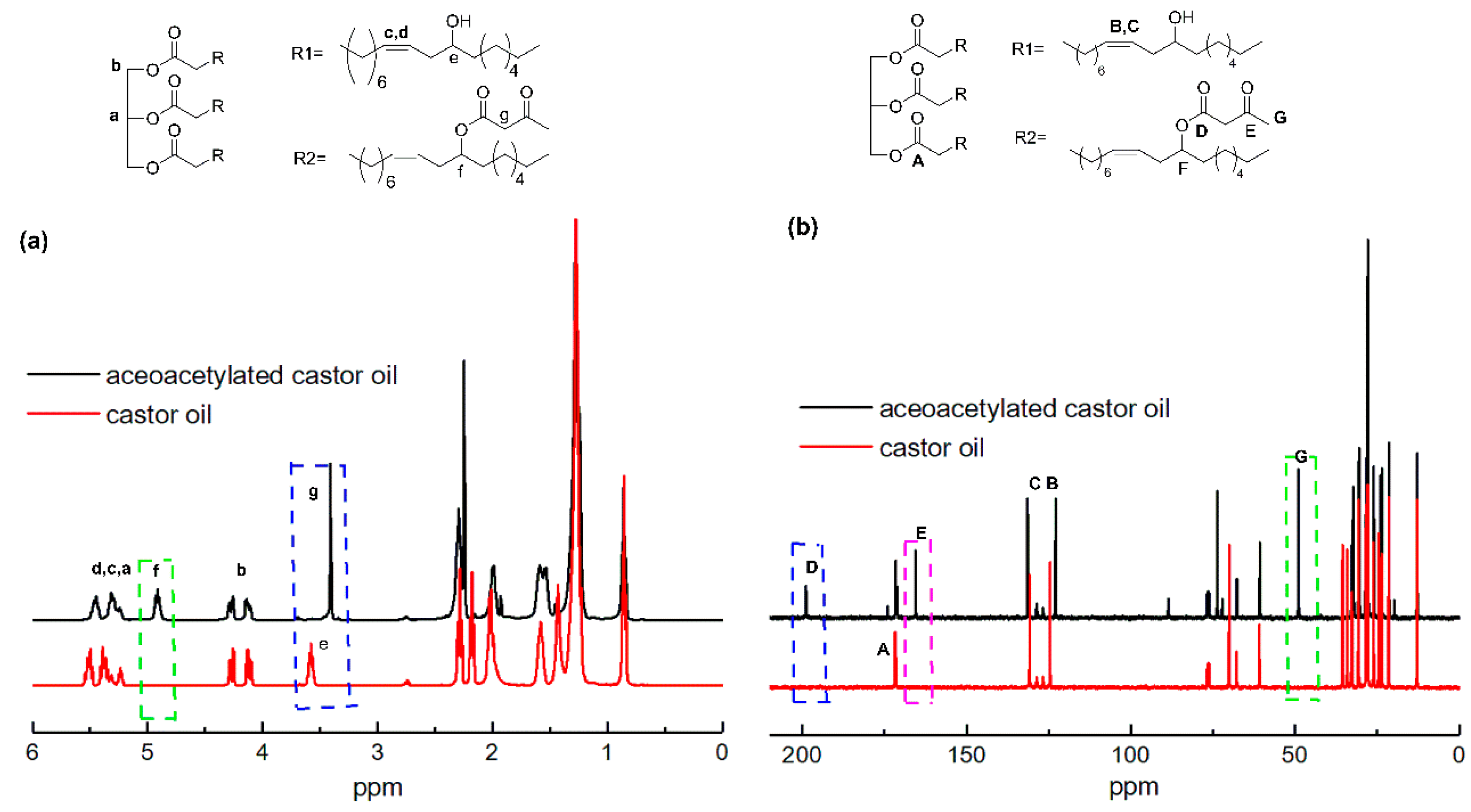
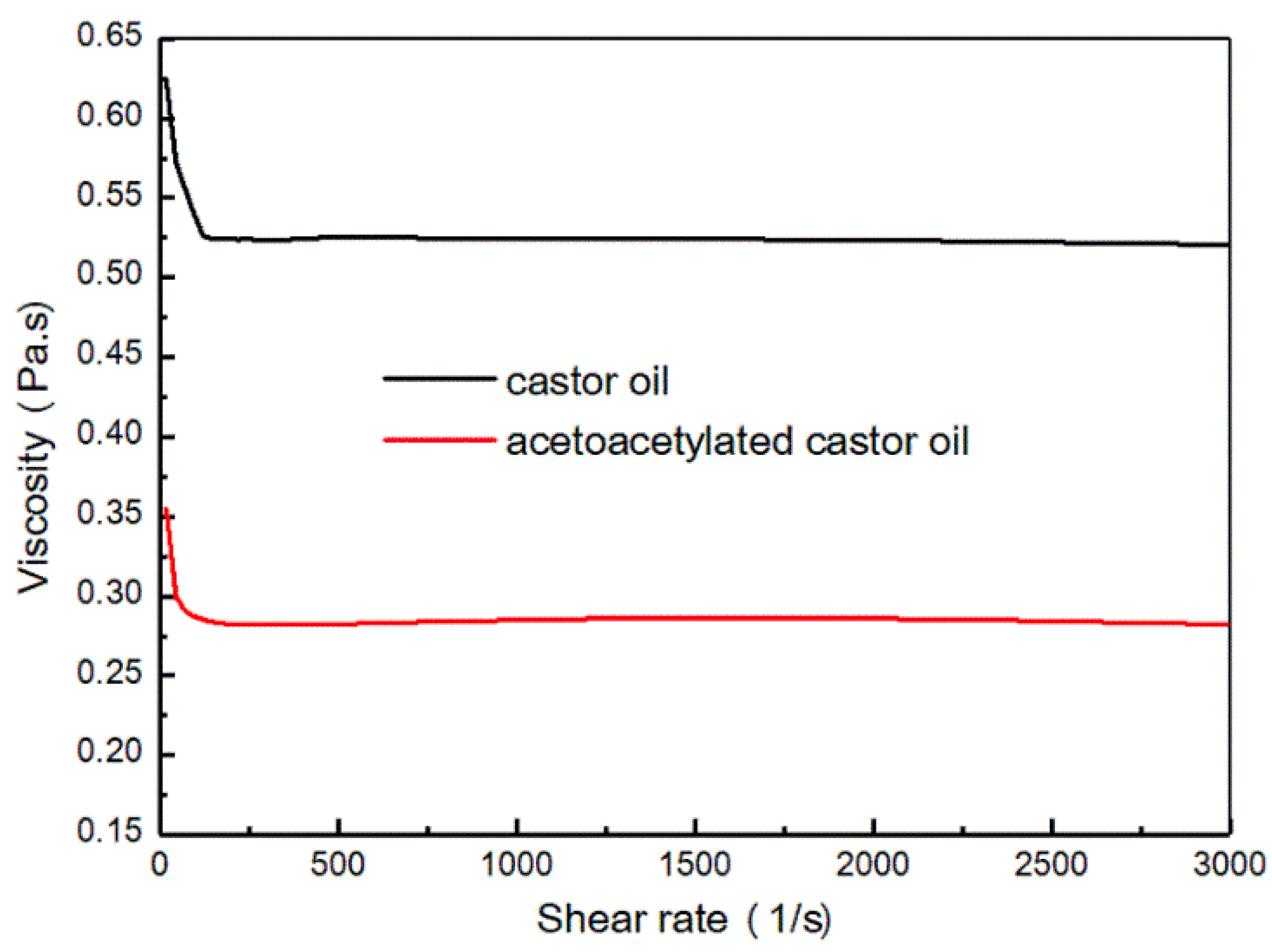
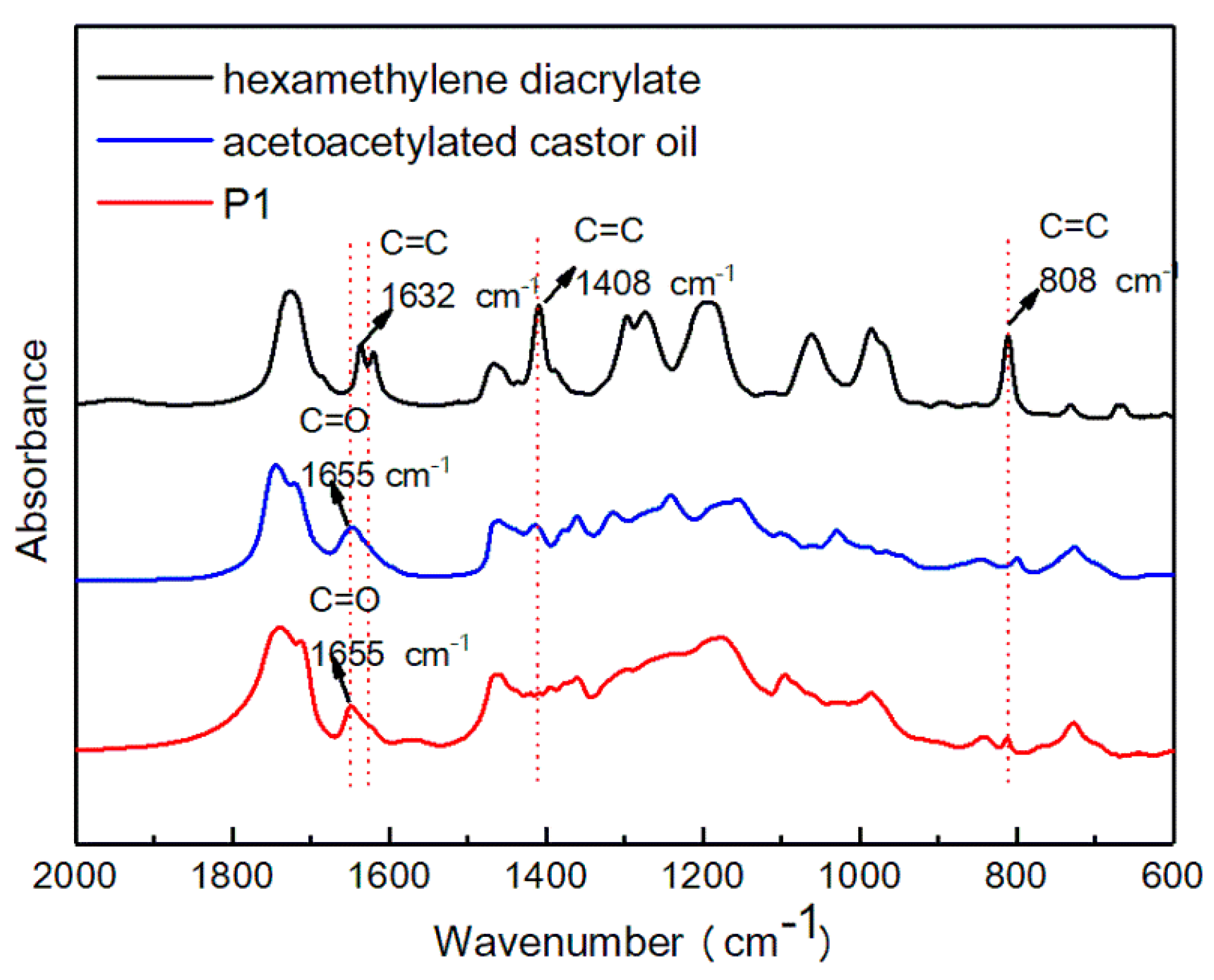
3.1. Characterization of Acetoacetylated Castor Oil
3.2. Optimization of the Curing Reaction Conditions
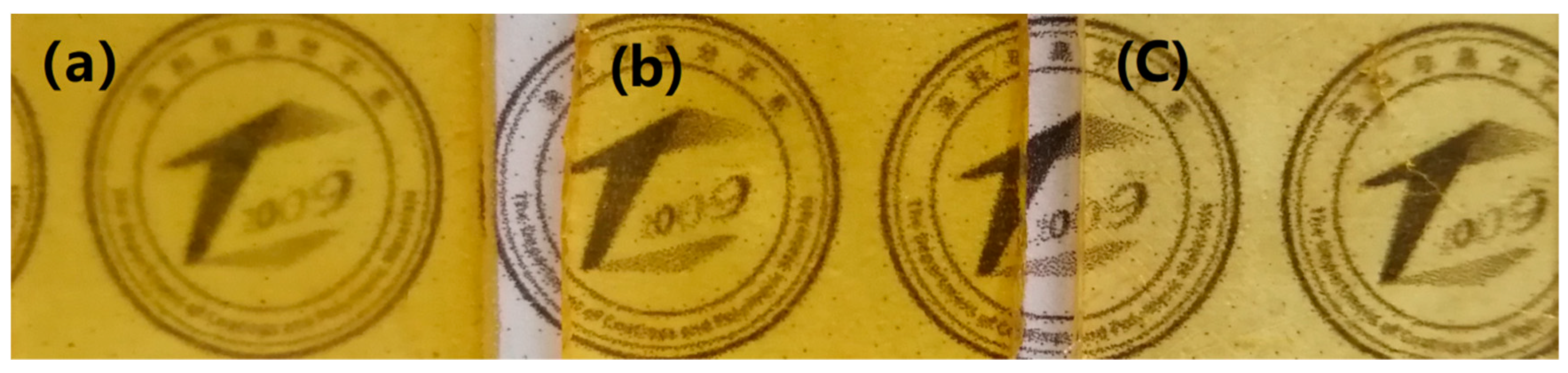
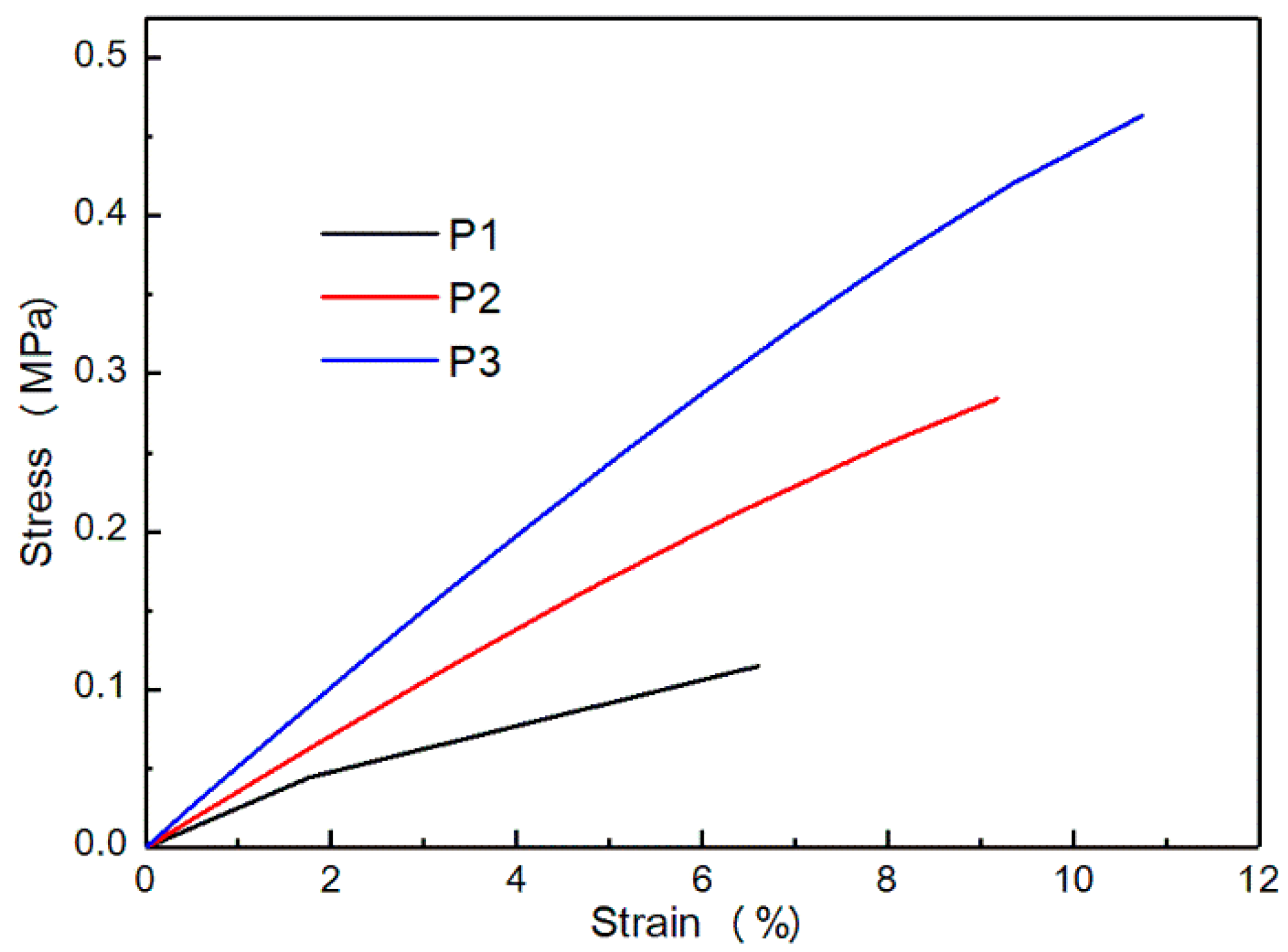
3.3. Characterization of the Films
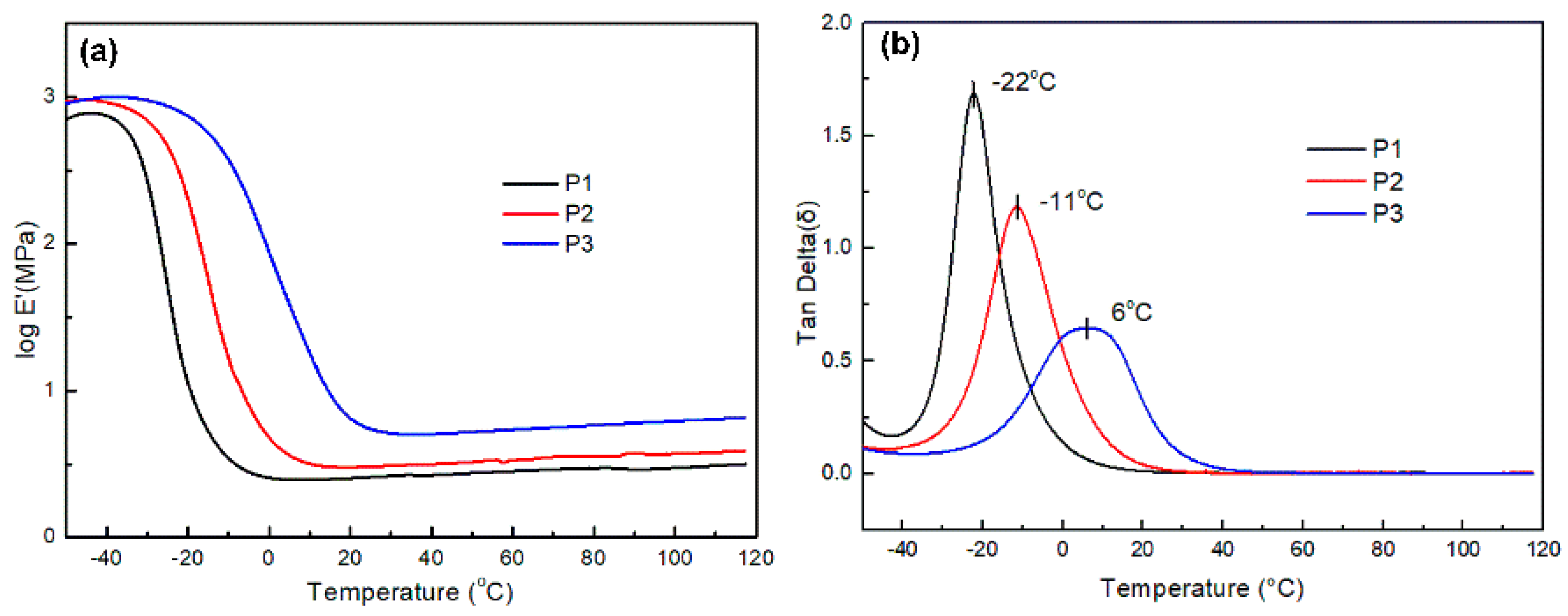
3.3.1. DMA Analysis
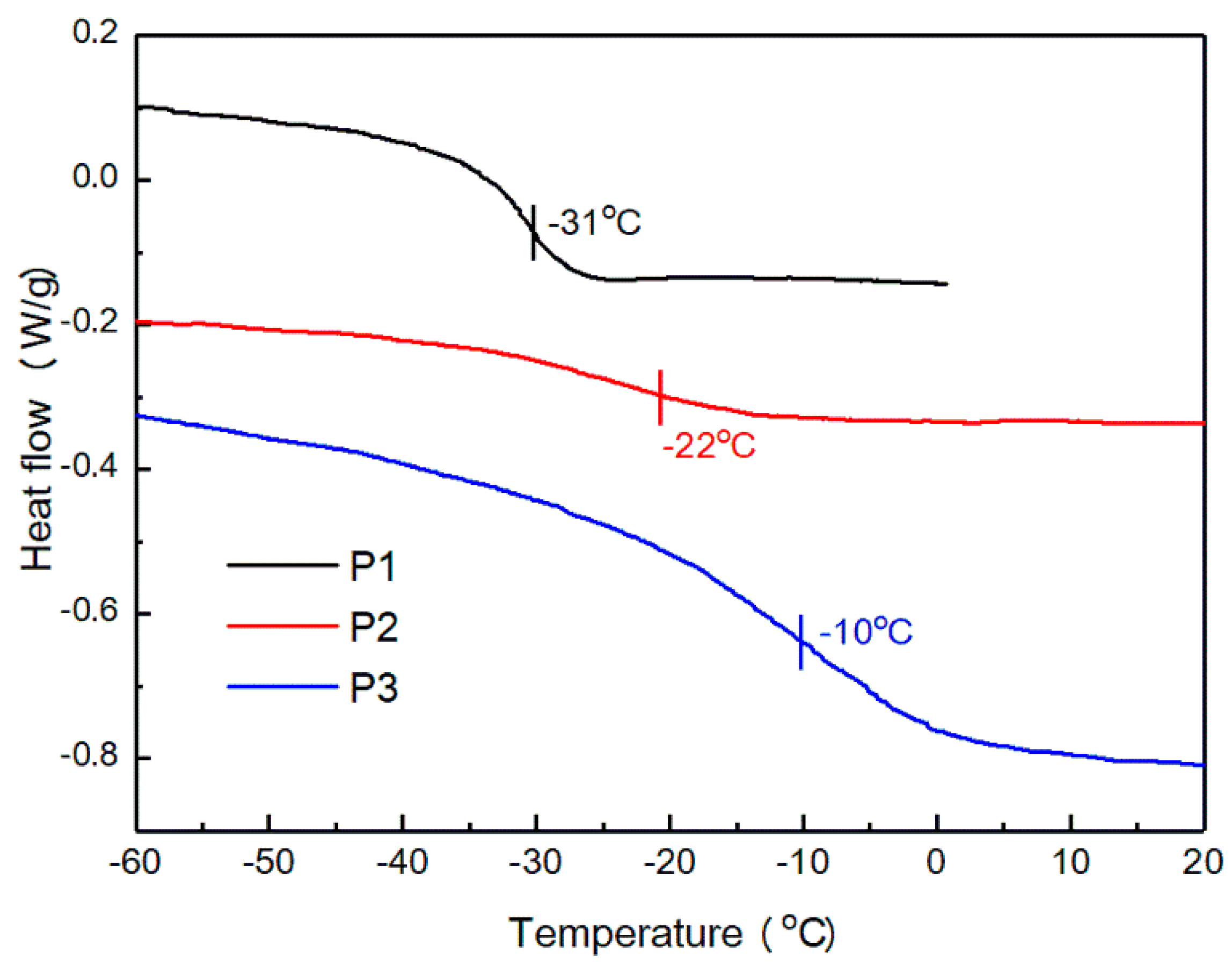
3.3.2. DSC Analysis
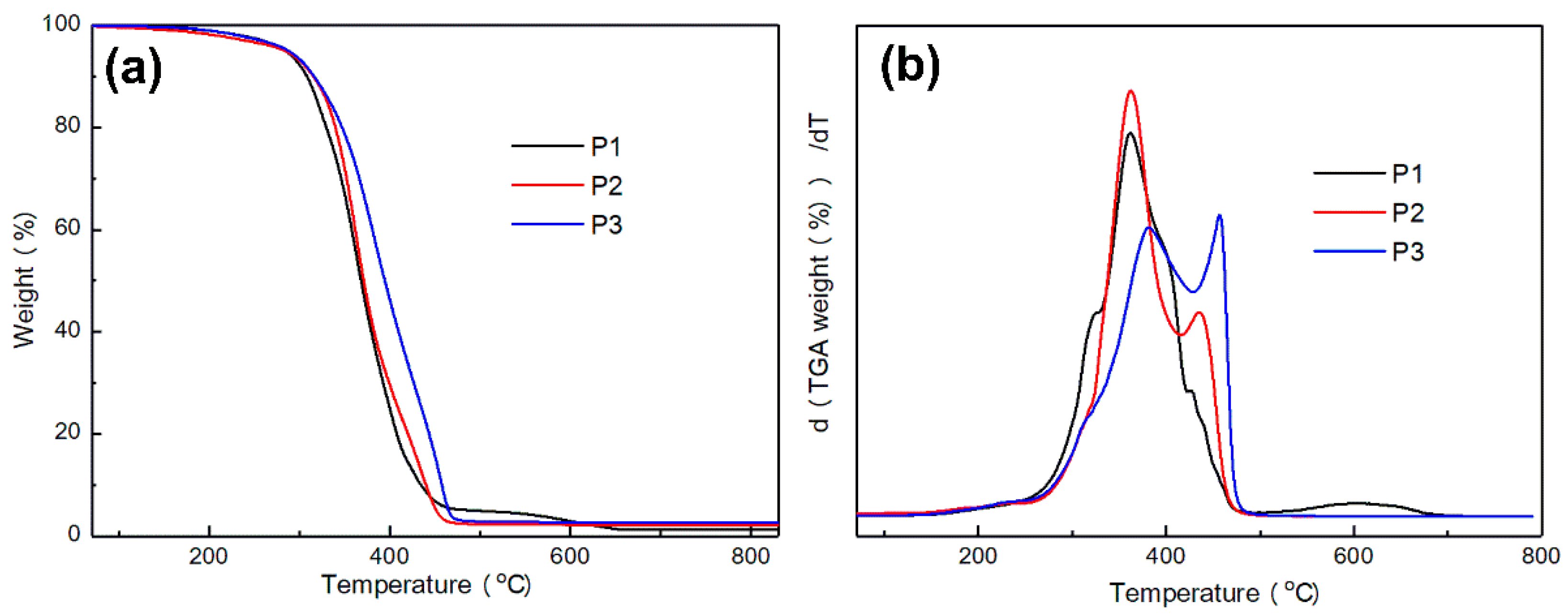
3.3.3. TGA Analysis
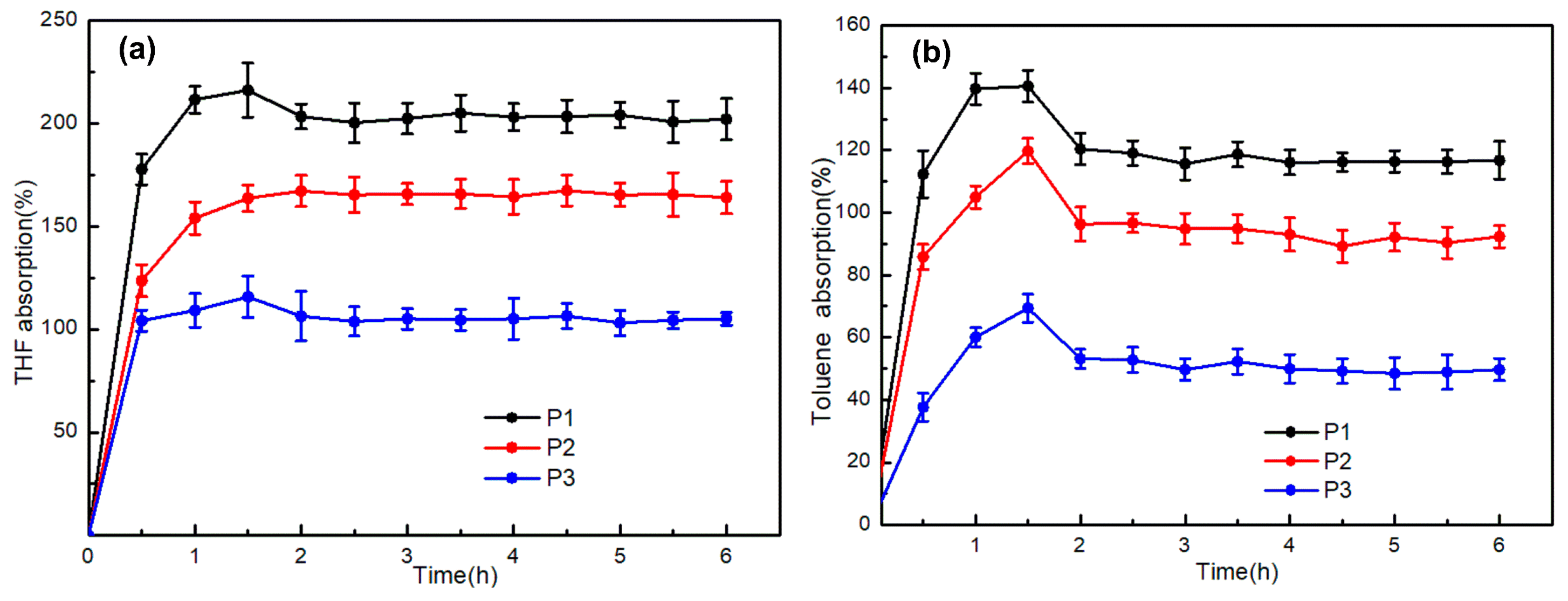
3.3.4. Solvent-Swelling
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, Q.; Liu, X.; Zhang, R.; Zhu, J.; Jiang, Y. Synthesis and properties of full bio-based thermosetting resins from rosin acid and soybean oil: The role of rosin acid derivatives. Green Chem. 2013, 15, 1300–1310. [Google Scholar] [CrossRef]
- Adekunle, K.F.; Åkesson, D.; Skrifvars, M.O.V. Synthesis of reactive soybean oils for use as a biobased thermoset resins in structural natural fiber composites. J. Appl. Polym. Sci. 2010, 115, 3137–3145. [Google Scholar] [CrossRef]
- Bakare, F.O.; Åkesson, D.; Skrifvars, M.; Bashir, T.; Ingman, P.; Srivastava, R. Synthesis and characterization of unsaturated lactic acid based thermoset bio-resins. Eur. Polym. J. 2015, 67, 570–582. [Google Scholar] [CrossRef]
- Shang, L.; Zhang, X.; Zhang, M.; Jin, L.; Liu, L.; Xiao, L.; Li, M.; Ao, Y. A highly active bio-based epoxy resin with multi-functional group: Synthesis, characterization, curing and properties. J. Mater. Sci. 2018, 53, 5402–5417. [Google Scholar] [CrossRef]
- Narute, P.; Rao, G.R.; Misra, S.; Palanisamy, A. Modification of cottonseed oil for amine cured epoxy resin: Studies on thermo-mechanical, physico-chemical, morphological and antimicrobial properties. Prog. Org. Coat. 2015, 88, 316–324. [Google Scholar] [CrossRef]
- Mutlu, H.; Meier, M.A.R.; Metzger, J.O.; Meier, M.A.R. Castor oil as a renewable resource for the chemical industry. Eur. J. Lipid Sci. Technol. 2010, 112, 10–30. [Google Scholar] [CrossRef]
- Fu, C.; Hu, X.; Yang, Z.; Shen, L.; Zheng, Z. Preparation and properties of waterborne bio-based polyurethane/siloxane cross-linked films by an in situ sol–gel process. Prog. Org. Coat. 2015, 84, 18–27. [Google Scholar] [CrossRef]
- Li, K.; Shen, Y.; Fei, G.; Wang, H.; Li, J. Preparation and properties of castor oil/pentaerythritol triacrylate-based UV curable waterborne polyurethane acrylate. Prog. Org. Coat. 2015, 78, 146–154. [Google Scholar] [CrossRef]
- Cvengroš, J.; Paligová, J.; Cvengrošová, Z. Properties of alkyl esters base on castor oil. Eur. J. Lipid Sci. Technol. 2010, 108, 629–635. [Google Scholar] [CrossRef]
- Xiao, W.; He, P.; He, B. Improving the toughness and flame retardancy of cured epoxy resin with brominated castor oil. J. Appl. Polym. Sci. 2010, 86, 2530–2534. [Google Scholar] [CrossRef]
- Mather, B.D.; Viswanathan, K.; Miller, K.M.; Long, T.E. Michael addition reactions in macromolecular design for emerging technologies. Prog. Polym. Sci. 2006, 31, 487–531. [Google Scholar] [CrossRef]
- Williams, S.R.; Miller, K.M.; Long, T.E. Michael addition reaction kinetics of acetoacetates and acrylates for the formation of polymeric networks. Prog. React. Kinet. Mech. 2007, 32, 165–194. [Google Scholar] [CrossRef]
- Metters, A.; Hubbell, J. Network formation and degradation behavior of hydrogels formed by Michael-type addition reactions. Biomacromolecules 2005, 6, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Arimitsu, K.; Fuse, S.; Kudo, K.; Furutani, M. Imidazole derivatives as latent curing agents for epoxy thermosetting resins. Mater. Lett. 2015, 161, 408–410. [Google Scholar] [CrossRef]
- Gigot, A.; Sangermano, M.; Capozzi, L.C.; Dietliker, K. In-situ synthesis of organic–inorganic coatings via a photolatent base catalyzed Michael-addition reaction. Polymer 2015, 68, 195–201. [Google Scholar] [CrossRef]
- Noomen, A. Applications of Michael addition chemistry in coatings technology. Prog. Org. Coat. 1997, 32, 137–142. [Google Scholar] [CrossRef]
- Trevino, A.S.; Trumbo, D.L. Acetoacetylated castor oil in coatings applications. Prog. Org. Coat. 2002, 44, 49–54. [Google Scholar] [CrossRef]
- Pan, X.; Nelson, T.J.; Webster, D.C. Novel biobased dual-cure coating system. Prog. Org. Coat. 2012, 73, 344–354. [Google Scholar] [CrossRef]
- Paramarta, A.; Webster, D.C. The exploration of Michael-addition reaction chemistry to create high performance, ambient cure thermoset coatings based on soybean oil. Prog. Org. Coat. 2017, 108, 59–67. [Google Scholar] [CrossRef]
- Liu, Z.; Shao, P.; Huang, Z.; Liu, B.; Chen, T.; Qin, J. Two-photon absorption enhancement induced by aggregation due to intermolecular hydrogen bonding in V-shaped 2-hydroxypyrimidine derivatives. Chem. Commun. 2008, 2260–2262. [Google Scholar] [CrossRef]
- Whittington, C.P.; Daily, L.A.; Miller, K.M. Crosslinked imidazolium-containing polyester networks containing a pendant imidazolium group: Swelling studies and thermal properties. Polymer 2014, 55, 3320–3329. [Google Scholar] [CrossRef]
- Kim, S.; Miller, K.M. Synthesis and thermal analysis of crosslinked imidazolium-containing polyester networks prepared by Michael addition polymerization. Polymer 2012, 53, 5666–5674. [Google Scholar] [CrossRef]
- Jena, K.; Raju, K. Synthesis and characterization of hyperbranched polyurethane hybrids using tetraethoxysilane (TEOS) as cross-linker. Ind. Eng. Chem. Res. 2008, 47, 9214–9224. [Google Scholar] [CrossRef]
- Ni, H.; Skaja, A.D.; Soucek, M.D. Acid-catalyzed moisture-curing polyurea/polysiloxane ceramer coatings. Prog. Org. Coat. 2000, 40, 175–184. [Google Scholar] [CrossRef]
- Chen, C.; Eissa, A.M.; Schiller, T.L.; Cameron, N.R. Emulsion-templated porous polymers prepared by thiol-ene and thiol-yne photopolymerisation using multifunctional acrylate and non-acrylate monomers. Polymer 2017, 126, 395–401. [Google Scholar] [CrossRef]
- Anseth, K.S.; Bowman, C.N.; Peppas, N.A. Dynamic mechanical studies of the glass transition temperature of photopolymerized multifunctional acrylates. Polym. Bull. 1993, 31, 229–233. [Google Scholar] [CrossRef]
- Hojabri, L.; Jose, J.; Leao, A.L.; Bouzidi, L.; Narine, S.S. Synthesis and physical properties of lipid-based poly(ester-urethane)s, I: Effect of varying polyester segment length. Polymer 2012, 53, 3762–3771. [Google Scholar] [CrossRef]
- Puskas, J.E.; Kaszas, G.; Chen, C.C.; Kennedy, J.P. New polyisobutylene-based UV-curable flexible coatings. Polym. Bull. 1988, 20, 253–260. [Google Scholar] [CrossRef]
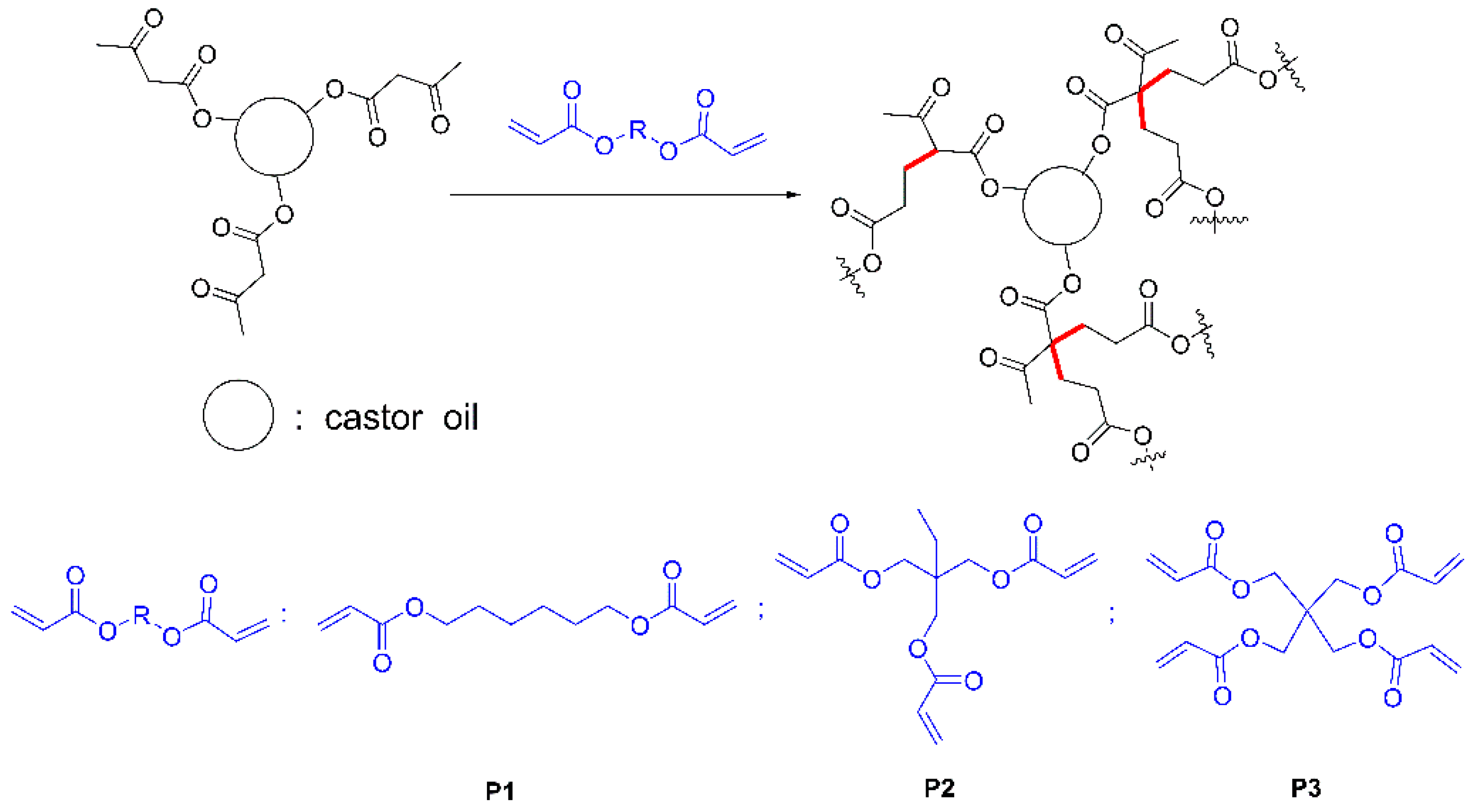
| Sample Code | Acetoacetylated Castor Oil | Cross-Linker | Acetoacetate:Acrylate Ratio |
|---|---|---|---|
| P1 | 1 g (0.84 mmol) | hexamethylene diacrylate (0.428 g, 1.89 mmol) | 1:1.5 |
| P2 | 1 g (0.84 mmol) | trimethylolpropane triacrylate (0.373 g, 1.26 mmol) | 1:1.5 |
| P3 | 1 g (0.84 mmol) | pentaerythritol tetraacrylate (0.333 g, 0.945 mmol) | 1:1.5 |
| Entry | Simple | Catalyst | Cure Time a (h) | Pencil Hardness | THF Swelling (%) | Gel Content (%) |
|---|---|---|---|---|---|---|
| 1 | 1:1.5 | Piperidine (1 wt %) | – | – | – | – |
| 2 | 1:1.5 | DMAP (1 wt %) | 15 | 4B | 265 | 82 |
| 3 | 1:1.5 | DBU (1 wt %) | 12 | 2B | 218 | 90 |
| 4 | 1:1.5 | DBU (2 wt %) | 8 | 2B | 201 | 95 |
| 5 | 1:1.5 | DBU (3 wt %) | 7.5 | 2B | 215 | 92 |
| 6 | 1:1 | DBU (2 wt %) | 7 | 3B | 220 | 91 |
| 7 | 1:2 | DBU (2 wt %) | 10 | 3B | 245 | 86 |
| Sample | Cure Time (h) | Pencil Hardness | THF Swelling (%) | Gel Content (%) | |||
|---|---|---|---|---|---|---|---|
| Set to Touch | Tack Free | Dry Hard | Dry Through | ||||
| P1 | 1.5 | 4.2 | 6.5 | 8 | 2B | 201 | 95 |
| P2 | 1.2 | 3.3 | 5.1 | 6 | B | 162 | 94 |
| P3 | 1.1 | 2.8 | 4 | 5 | H | 105 | 97 |
| Sample | tan δ | Tg (at tan δ) | E’ at Tg + 50 °C | T (=Tg + 50 °C) (K) | Crosslinking Density (ve) (at Tg + 50 °C) |
|---|---|---|---|---|---|
| P1 | 1.68 | −22 ℃ | 2.60 MPa | 301 | 346 mol/m3 |
| P2 | 1.18 | −11 ℃ | 3.16 MPa | 312 | 406 mol/m3 |
| P3 | 0.65 | 6 ℃ | 6.55 MPa | 329 | 798 mol/m3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Wang, J.; He, X.; Cao, Z.; Xu, D.; Gao, F.; Zhong, J.; Shen, L. An Ambient Curable Coating Material Based on the Michael Addition Reaction of Acetoacetylated Castor Oil and Multifunctional Acrylate. Coatings 2019, 9, 37. https://doi.org/10.3390/coatings9010037
Wang T, Wang J, He X, Cao Z, Xu D, Gao F, Zhong J, Shen L. An Ambient Curable Coating Material Based on the Michael Addition Reaction of Acetoacetylated Castor Oil and Multifunctional Acrylate. Coatings. 2019; 9(1):37. https://doi.org/10.3390/coatings9010037
Chicago/Turabian StyleWang, Tong, Jianli Wang, Xianfeng He, Zhiyuan Cao, Dongdong Xu, Fei Gao, Jiang Zhong, and Liang Shen. 2019. "An Ambient Curable Coating Material Based on the Michael Addition Reaction of Acetoacetylated Castor Oil and Multifunctional Acrylate" Coatings 9, no. 1: 37. https://doi.org/10.3390/coatings9010037
APA StyleWang, T., Wang, J., He, X., Cao, Z., Xu, D., Gao, F., Zhong, J., & Shen, L. (2019). An Ambient Curable Coating Material Based on the Michael Addition Reaction of Acetoacetylated Castor Oil and Multifunctional Acrylate. Coatings, 9(1), 37. https://doi.org/10.3390/coatings9010037