Raman Spectroscopy for Reliability Assessment of Multilayered AlCrN Coating in Tribo-Corrosive Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Materials
2.3. Evaluation of Coating Reliability
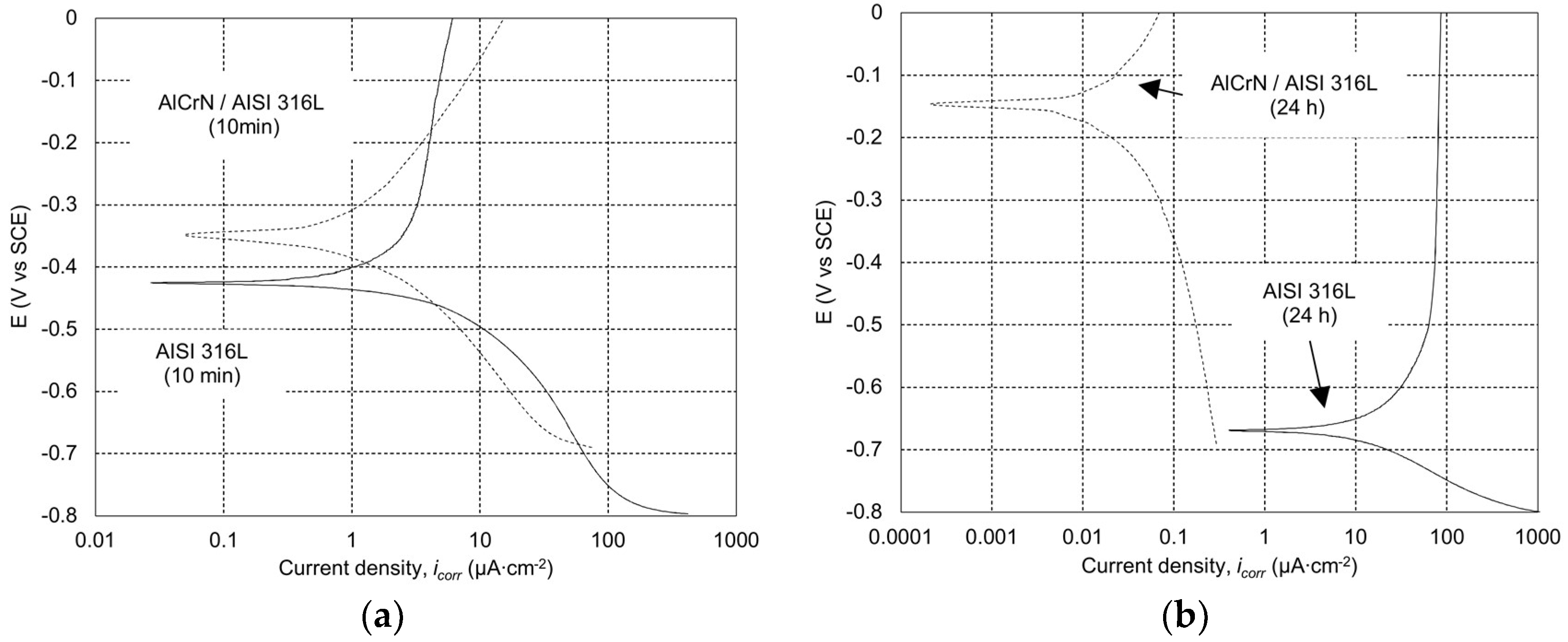
2.3.1. Potentiodynamic Polarization Test
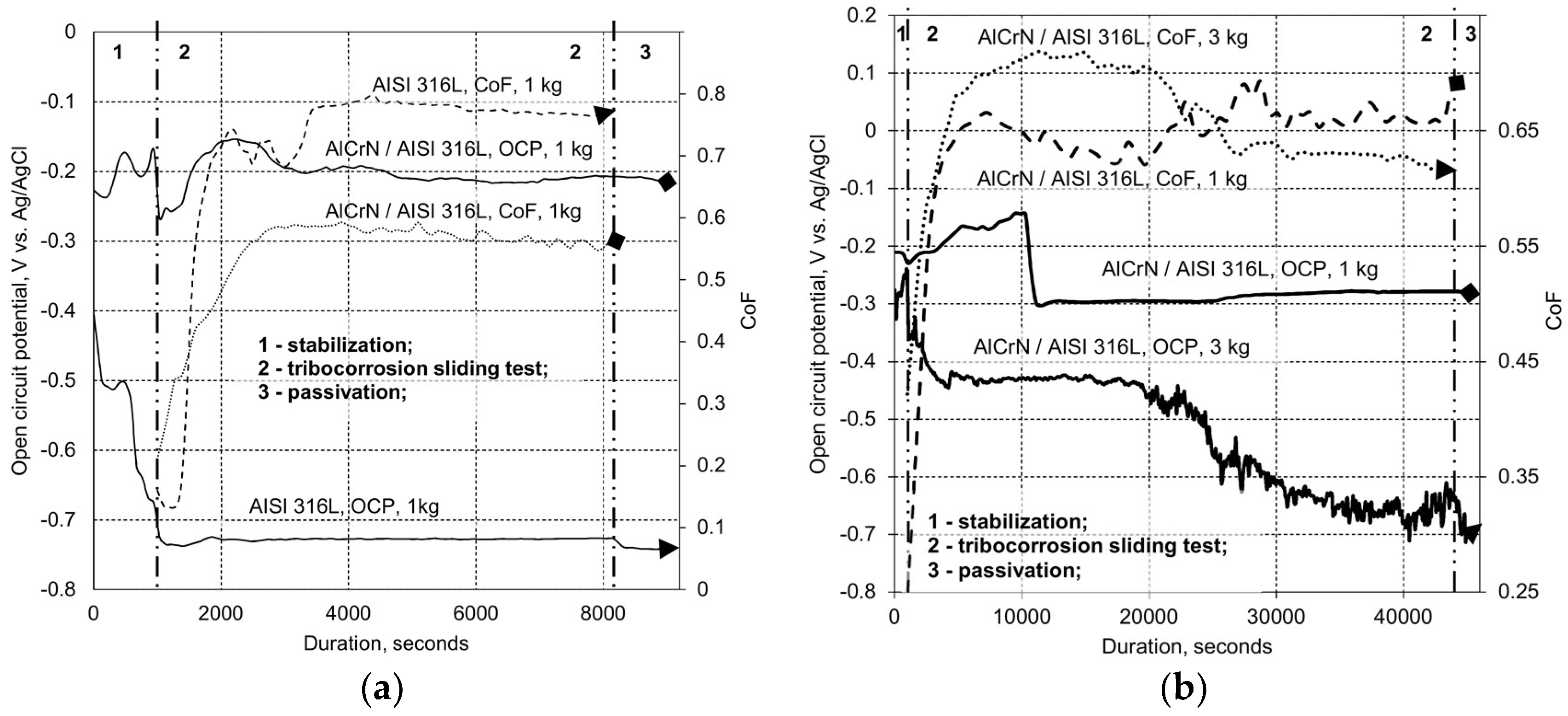
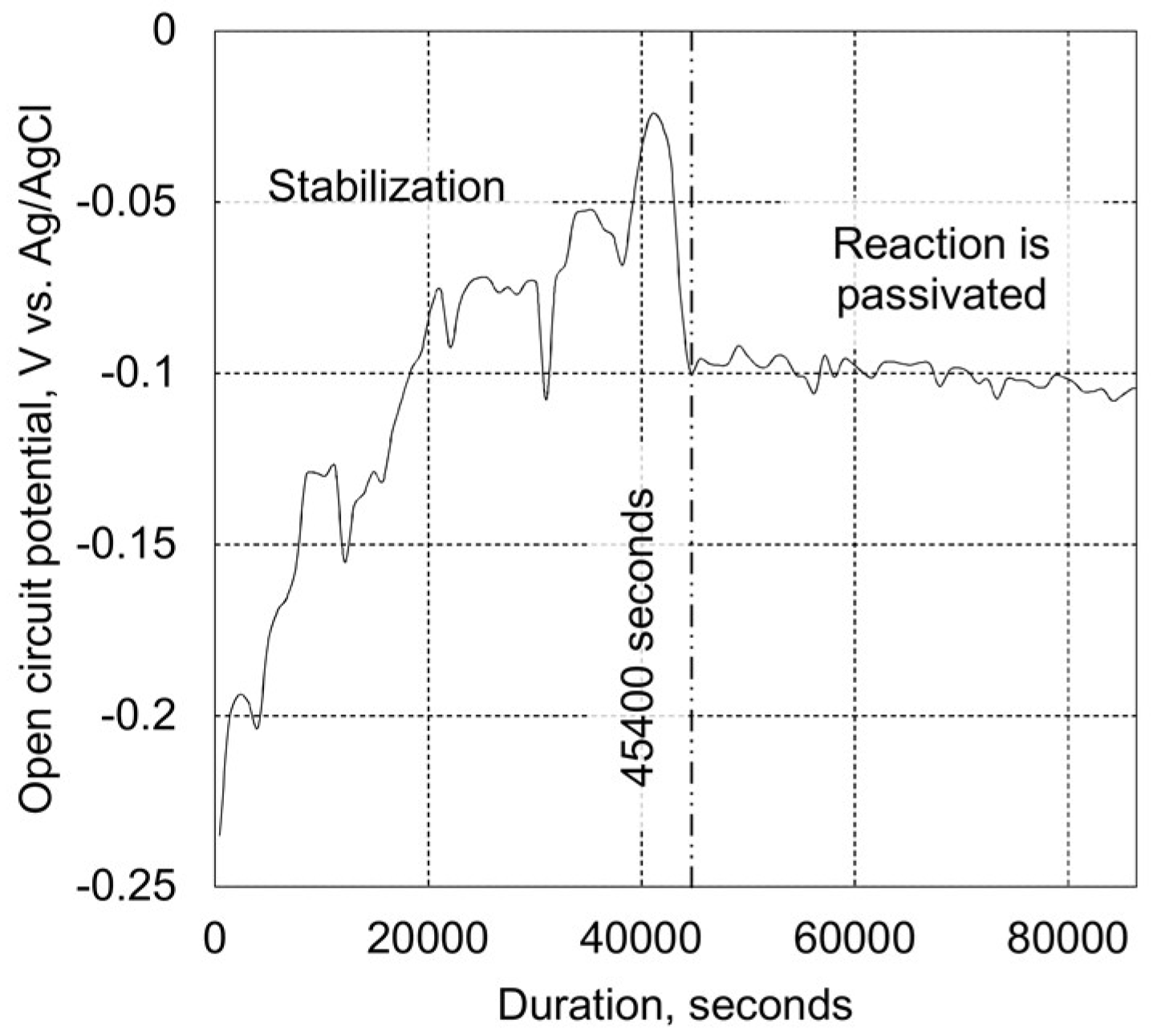
2.3.2. Tribo-Corrosion at Open Circuit Potential
3. Results and Discussion
3.1. Potentiodynamic Polarization Test of Statically Corroded Uncoated and Coated SS AISI 316L
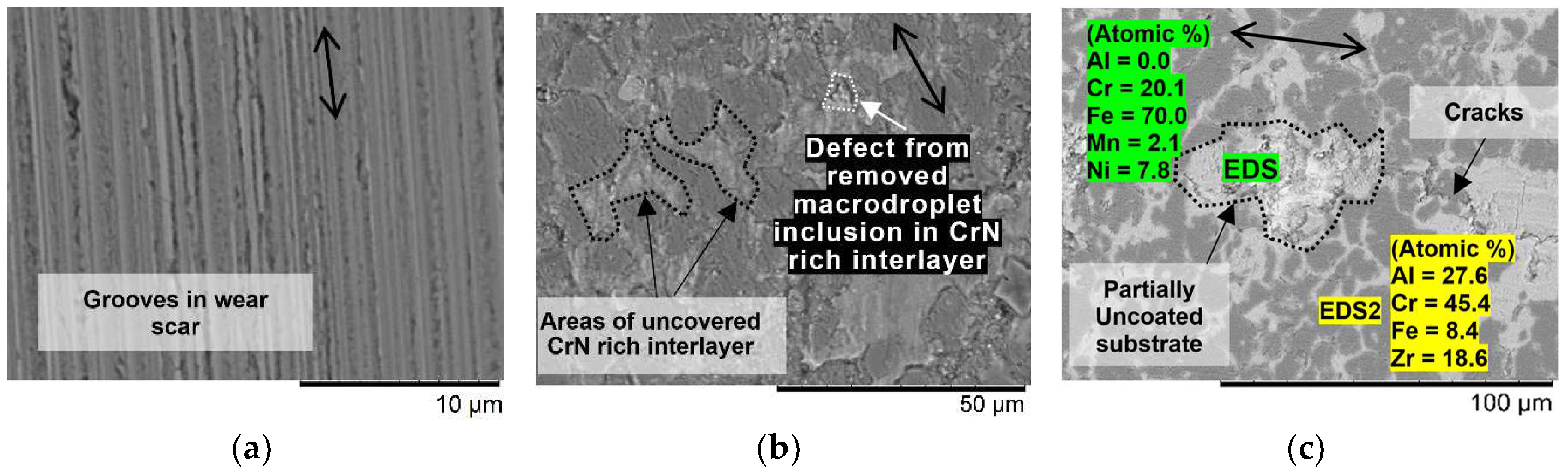
3.2. Tribo-Corrosive Wear Test of Uncoated and Coated SS AISI 316L
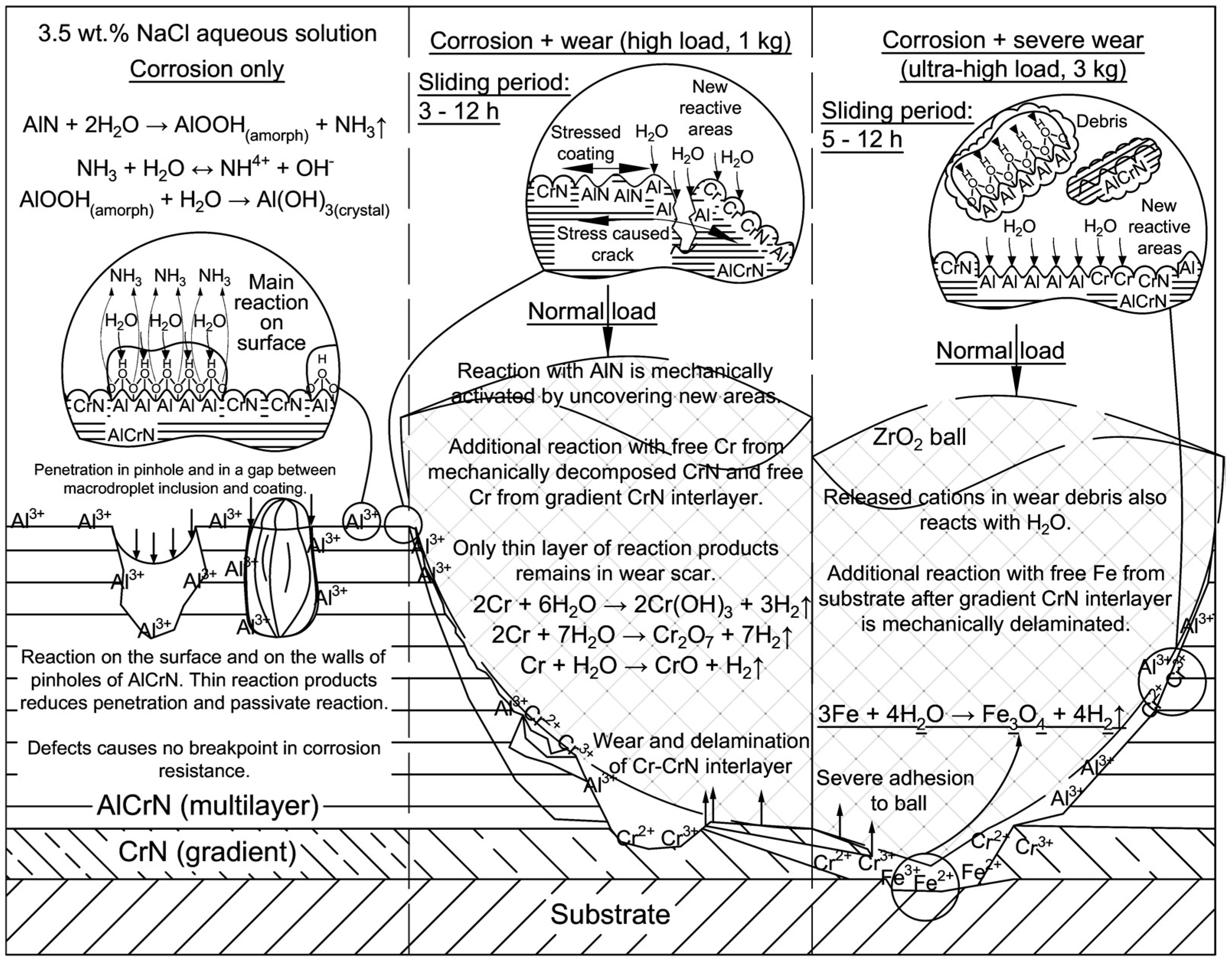
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wood, R.J.K. Tribo-corrosion of coatings: A review. J. Phys. D: Appl. Phys. 2007, 40, 5502–5521. [Google Scholar] [CrossRef]
- Holovenko, Y.; Antonov, M.; Kollo, L.; Hussainova, I. Friction studies of metal surfaces with various 3D printed patterns tested in dry sliding conditions. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2018, 232, 43–53. [Google Scholar] [CrossRef]
- Ricker, R.E.; Papavinasam, S.; Berke, N.; Brossia, S.; Dean, S.W. On using laboratory measurements to predict corrosion service lives for engineering applications. J. ASTM Int. 2008, 5, 1–10. [Google Scholar] [CrossRef]
- Fischer, A.; Mischler, S. Tribocorrosion: Fundamentals, materials and applications. J. Phys. D Appl. Phys. 2006, 39, 3120–3219. [Google Scholar] [CrossRef]
- Antonov, M.; Afshari, H.; Baronins, J.; Adoberg, E.; Raadik, T.; Hussainova, I. The effect of temperature and sliding speed on friction and wear of Si3N4, Al2O3, and ZrO2 balls tested against AlCrN PVD coating. Tribol. Int. 2018, 118, 500–514. [Google Scholar] [CrossRef]
- Stack, M.M.; Antonov, M.M.; Hussainova, I. Some views on the erosion–corrosion response of bulk chromium carbide based cermets. J. Phys. D Appl. Phys. 2006, 39, 3165–3174. [Google Scholar] [CrossRef]
- Larroumet, D.; Greenfield, D.; Akid, R.; Yarwood, J. Raman spectroscopic studies of the corrosion of model iron electrodes in sodium chloride solution. J. Raman Spectrosc. 2007, 38, 1577–1585. [Google Scholar] [CrossRef]
- Fenker, M.; Balzer, M.; Kappl, H. Corrosion protection with hard coatings on steel: Past approaches and current research efforts. Surf. Coat. Technol. 2014, 257, 182–205. [Google Scholar] [CrossRef]
- Dinu, M.; Mouele, E.; Parau, A.; Vladescu, A.; Petrik, L.; Braic, M. Enhancement of the corrosion resistance of 304 stainless steel by Cr–N and Cr(N,O) coatings. Coatings 2018, 8, 132. [Google Scholar] [CrossRef]
- Gutsev, D.; Antonov, M.; Hussainova, I.; Grigoriev, A.Y. Effect of SiO2 and PTFE additives on dry sliding of NiP electroless coating. Tribol. Int. 2013, 65, 295–302. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Feng, Y.; Chen, S.; Wan, Q.; Zhu, J. Electrochemical characterization of AlTiN, AlCrN and AlCrSiWN coatings. Int. J. Refract. Met. Hard Mater. 2015, 53, 68–73. [Google Scholar] [CrossRef]
- Baronins, J.; Podgursky, V.; Antonov, M.; Bereznev, S.; Hussainova, I. Electrochemical behaviour of TiCN and TiAlN gradient coatings prepared by lateral rotating cathode arc PVD technology. Key Eng. Mater. 2016, 721, 414–418. [Google Scholar] [CrossRef]
- Kaindl, R.; Franz, R.; Soldan, J.; Reiter, A.; Polcik, P.; Mitterer, C.; Sartory, B.; Tessadri, R.; O’Sullivan, M. Structural investigations of aluminum-chromium-nitride hard coatings by Raman micro-spectroscopy. Thin Solid Films 2006, 515, 2197–2202. [Google Scholar] [CrossRef]
- Johnson, D.F.; Jiang, D.E.; Carter, E.A. Structure, magnetism, and adhesion at Cr/Fe interfaces from density functional theory. Surf. Sci. 2007, 601, 699–705. [Google Scholar] [CrossRef]
- Lukaszkowicz, K.; Sondor, J.; Balin, K.; Kubacki, J. Characteristics of CrAlSiN + DLC coating deposited by lateral rotating cathode arc PVD and PACVD process. Appl. Surf. Sci. 2014, 312, 126–133. [Google Scholar] [CrossRef]
- Rana, D.; Mandal, B.M.; Bhattacharyya, S.N. Analogue calorimetric studies of blends of poly(vinyl ester)s and polyacrylates. Macromolecules 1996, 29, 1579–1583. [Google Scholar] [CrossRef]
- Benedek, I.; Feldstein, M.M. Fundamentals of pressure sensitivity. In Handbook of Pressure-Sensitive Adhesives and Products; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Kawate, M.; Kimura Hashimoto, A.; Suzuki, T. Oxidation resistance of Cr1−xAlxN and Ti1−xAlxN films. Surf. Coat. Technol. 2003, 165, 163–167. [Google Scholar] [CrossRef]
- Samani, M.K.; Chen, G.C.K.; Ding, X.Z.; Zeng, X.T. Thermal conductivity of CrAlN and TiAlN coatings deposited by lateral rotating cathode arc. Key Eng. Mater. 2010, 447–448, 705–709. [Google Scholar] [CrossRef]
- Nohava, J.; Dessarzin, P.; Karvankova, P.; Morstein, M. Characterization of tribological behavior and wear mechanisms of novel oxynitride PVD coatings designed for applications at high temperatures. Tribol. Int. 2015, 81, 231–239. [Google Scholar] [CrossRef]
- Somiya, S.; Aldinger, F.; Spriggs, R.M.; Uchino, K.; Koumoto, K.; Kaneno, M. Handbook of Advanced Ceramics: Materials, Applications, Processing, and Properties. In Handbook of Advanced Ceramics; Elsevier: New York, NY, USA, 2003. [Google Scholar]
- ASTM G59-97e1 Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements; ASTM International: West Conshohocken, PA, USA, 1997.
- Bowen, P.; Highfield, J.G.; Mocellin, A.; Ring, T.A. Degradation of aluminum nitride powder in an aqueous environmet. J. Am. Ceram. Soc. 1990, 73, 724–728. [Google Scholar] [CrossRef]
- Yang, M.; Allen, A.J.; Nguyen, M.T.; Ralston, W.T.; MacLeod, M.J.; DiSalvo, F.J. Corrosion behavior of mesoporous transition metal nitrides. J. Solid State Chem. 2013, 205, 49–56. [Google Scholar] [CrossRef]
- Gomes, A.S.O.; Yaghini, N.; Martinelli, A.; Ahlberg, E. A micro-Raman spectroscopic study of Cr(OH)3 and Cr2O3 nanoparticles obtained by the hydrothermal method. J. Raman Spectrosc. 2017, 48, 1256–1263. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Selvakumar, N.; Deepthi, B.; Rajam, K.S. A comparative study of reactive direct current magnetron sputtered CrAlN and CrN coatings. Surf. Coat. Technol. 2006, 201, 2193–2201. [Google Scholar] [CrossRef]
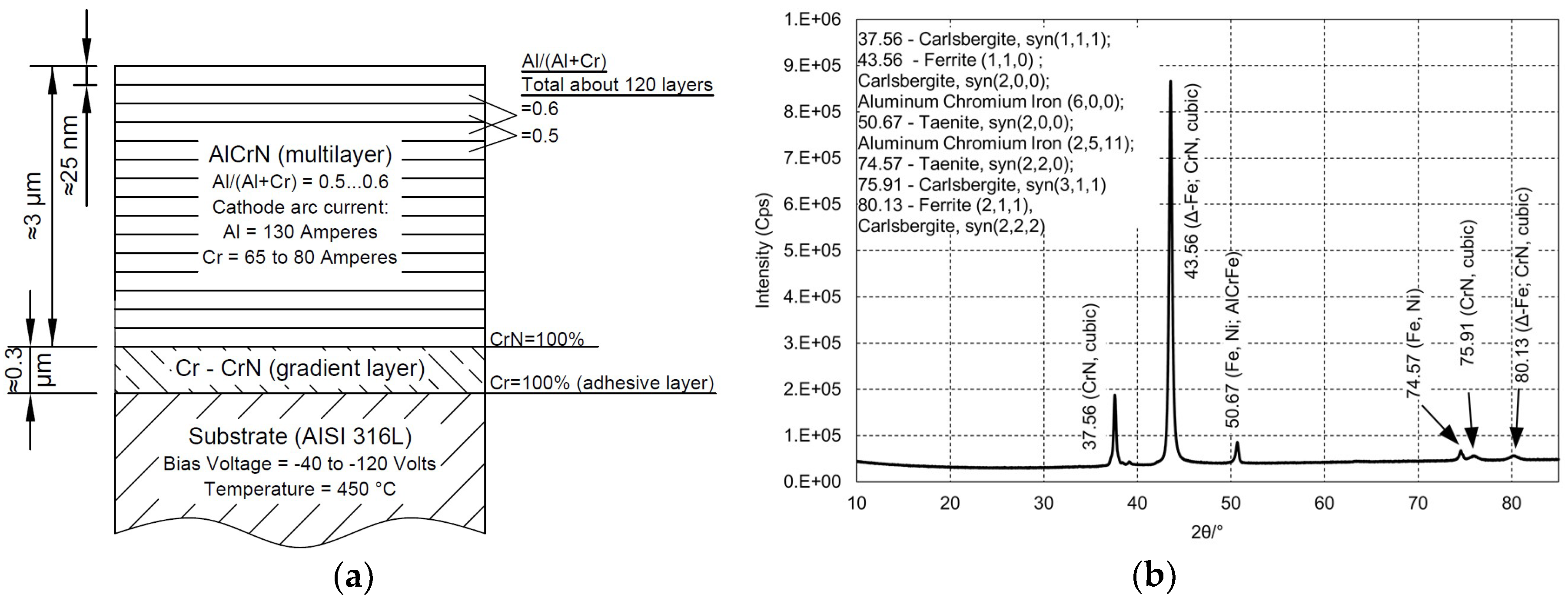
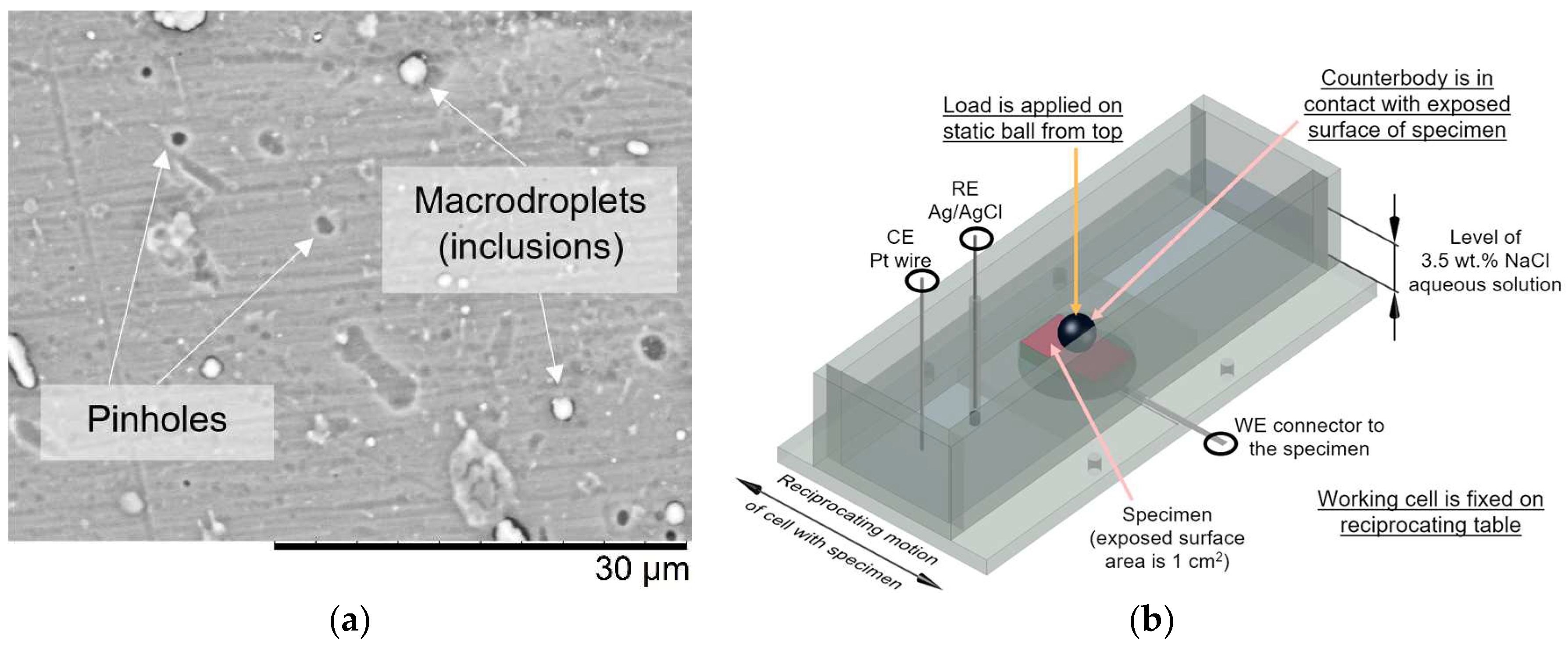
| Properties | AlCrN | YSZ |
|---|---|---|
| Hardness at 20 °C, GPa | 30.6 ± 2.8 [5] * | 10.5 ** |
| Fracture toughness KIC, MPa∙m0.5 | 6.4 [18] | 6.0 ** |
| Young's modulus of elasticity, GPa | 585 ± 54 [5] * | 210 ** |
| Thermal conductivity 20 °C, W m−1∙K−1 | 1.5 [19] | 3 ** |
| Max service temperature, °C | 900 [20] | 1200 ** |
| Density, kg∙m−3 | – | 6000 ** |
| Thermal diffusivity, ×10−6∙m2∙s−1 | – | 0.9 [21] |
| Diameter, mm | – | 10 |
| Task | Method | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visual and Tactile Observation | Optical Microscopy | SEM | 2D Contact Profilometry | 3D Topography | KaloMax Ball Cratering | Adhesion Testing | Scratch Testing | Electrochemical Corrosion Tests | Tribo-Corrosive Test* | EDS | Raman Spectroscopy | XRD | |
| Preliminary evaluation | + | + | + | ++ | +++ | +++ | +++ | +++ | + | NCH | ++ | ++ | +++ |
| Wear or corrosion rate | - | + | + | +++ | +++ | NCH | NCH | NCH | ++ | +++ | - | - | - |
| Destruction mechanisms | + | + | ++ | ++ | +++ | NCH | NCH | + | + | ++ | + | +++ | ++ |
| Elemental or phase composition of thin tribo-layer | - | - | - SE | NCH | NCH | NCH | NCH | - | 0 | 0 | - | +++ | - |
| Elemental or phase composition of thick (≈>1 um) tribo-layer | - | 0 | + BSE | NCH | NCH | NCH | NCH | - | 0 | 0 | ++ | +++ | ++ |
| In-Situ measurement of corrosion intensity and/or evolution of coating damage | 0 | 0 | NCH | NCH | NCH | NCH | NCH | NCH | +++ | +++ | - | - | + |
| Material | Potentiodynamic Polarization Measurements (From NOVA) | Calculation Results | ||||||
|---|---|---|---|---|---|---|---|---|
| Corrosion Current Density | Polarization Resistance | Calculated Corrosion Potential | Tafel Slope | Tafel Slope | Corrosion Rate | Protective Efficiency | Porosity | |
| icorr | Rpm, Rp | Ecorr calc | |βa| | |βc| | CR | Pi | F | |
| [µA∙cm−2] | [Ω∙cm−2] | [V] | [V∙Decade−1] | [V∙Decade−1] | [mm∙Year−1] | [%] | [%] | |
| AISI 316L, 10 min | 1.9 | 1.43 × 104 | −0.423 | 0.718 | 0.069 | 2.0 × 10−2 | – | – |
| AlCrN/AISI 316L, 10 min | 1.6 | 3.76 × 104 | −0.340 | 0.399 | 0.212 | 1.7 × 10−2 | 15.9 | 29 |
| AISI 316L, 24 h | 22.1 | 1.71 × 103 | −0.669 | 0.312 | 0.121 | 2.3 × 10−1 | – | – |
| AlCrN/AISI 316L, 24 h | 0.05 | 2.33 × 106 | −0.153 | 0.488 | 0.517 | 4.9 × 10−4 | 99.8 | 0.002 |
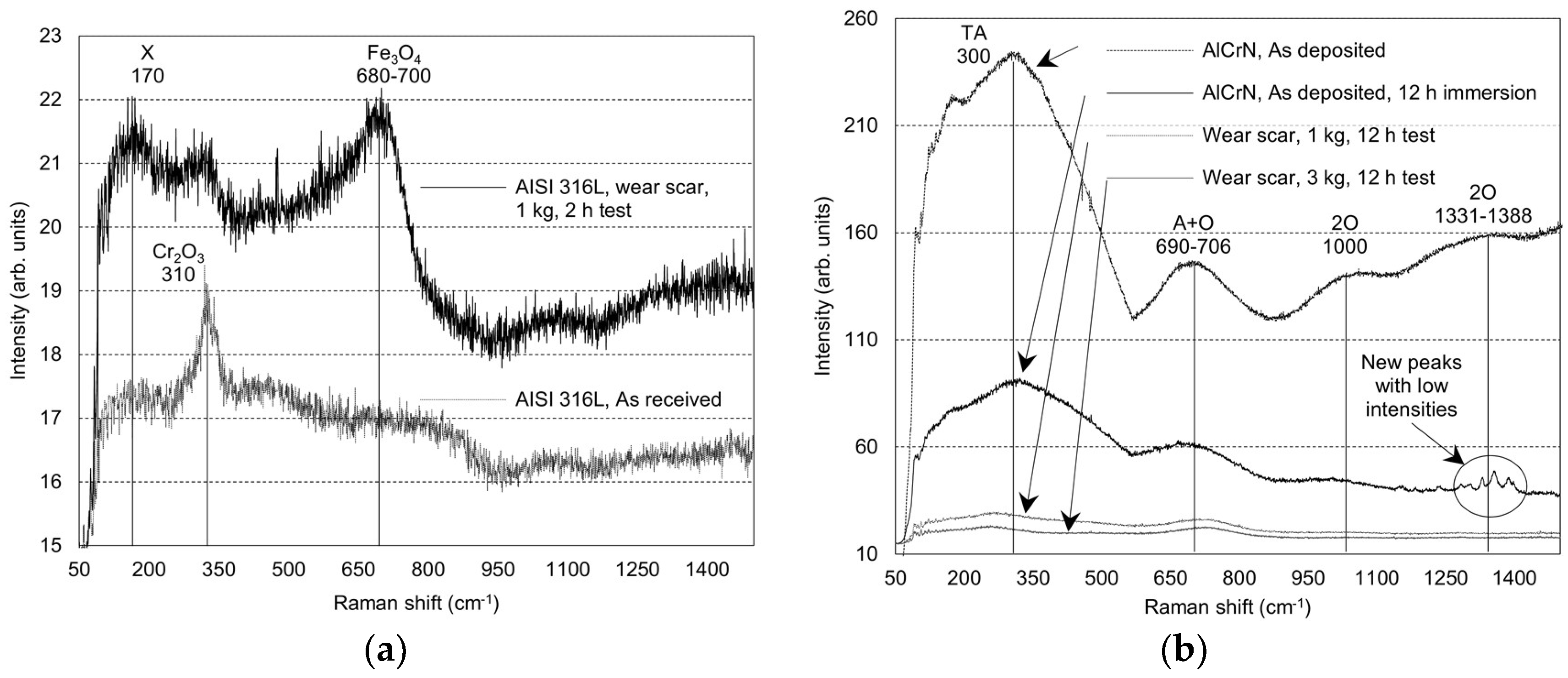
| Material | Test | Peak Position [cm−1] | Peak Assignment | Peak Intensity | Peak Configuration | Comments |
|---|---|---|---|---|---|---|
| AISI 316L | As received | 310 | Cr2O3 | Low | Sharp | Slight oxidation after polishing |
| 24 h static immersion | 680–700 | Fe3O4/γ-Fe2O3 | Low | Broad | Development of Fe based oxides and hydroxides | |
| Tribo-corrosion 2 h, 1 kg | 680–700 | Fe3O4/γ-Fe2O3 | Low | Broad | Development of Fe based oxides and hydroxides | |
| AlCrN PVD on AISI 316L | As deposited | 300 | TA mode-vibration of Cr ions | High | Broad | Cubic CrN structure |
| 690–706 | A+O optic mode-vibration of N ions | High | Broad | Cubic CrN structure | ||
| 1000 | 2 O-second order transition | Low | Broad | Cubic CrN structure | ||
| 1331–1388 | 2 O-second order transition | Low | Broad | Cubic CrN structure | ||
| 12 h static immersion | 300 | TA mode-vibration of Cr ions | High | Broad | * | |
| 690–706 | A+O optic mode-vibration of N ions | High | Broad | * | ||
| 1000 | 2 O-second order transition | Low | Broad | * | ||
| 1331–1388 | - | Low | Sharp | Possible formation of Al based corrosion products | ||
| Tribo-corrosion 12 h, 1 kg | 300 | TA mode-vibration of Cr ions | Low | Broad | * | |
| 690–706 | A+O optic mode-vibration of N ions | Low | Broad | * | ||
| Tribo-corrosion 12 h, 3 kg | 300 | TA mode-vibration of Cr ions | Low | Broad | Formation of corrosion products | |
| 690–706 | A+O optic mode-vibration of N ions | Low | Broad | Formation of corrosion products. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baronins, J.; Antonov, M.; Bereznev, S.; Raadik, T.; Hussainova, I. Raman Spectroscopy for Reliability Assessment of Multilayered AlCrN Coating in Tribo-Corrosive Conditions. Coatings 2018, 8, 229. https://doi.org/10.3390/coatings8070229
Baronins J, Antonov M, Bereznev S, Raadik T, Hussainova I. Raman Spectroscopy for Reliability Assessment of Multilayered AlCrN Coating in Tribo-Corrosive Conditions. Coatings. 2018; 8(7):229. https://doi.org/10.3390/coatings8070229
Chicago/Turabian StyleBaronins, Janis, Maksim Antonov, Sergei Bereznev, Taavi Raadik, and Irina Hussainova. 2018. "Raman Spectroscopy for Reliability Assessment of Multilayered AlCrN Coating in Tribo-Corrosive Conditions" Coatings 8, no. 7: 229. https://doi.org/10.3390/coatings8070229
APA StyleBaronins, J., Antonov, M., Bereznev, S., Raadik, T., & Hussainova, I. (2018). Raman Spectroscopy for Reliability Assessment of Multilayered AlCrN Coating in Tribo-Corrosive Conditions. Coatings, 8(7), 229. https://doi.org/10.3390/coatings8070229







