Process and Formulation Strategies to Improve Adhesion of Nanoparticulate Coatings on Stainless Steel
Abstract
1. Basics and State of the Art
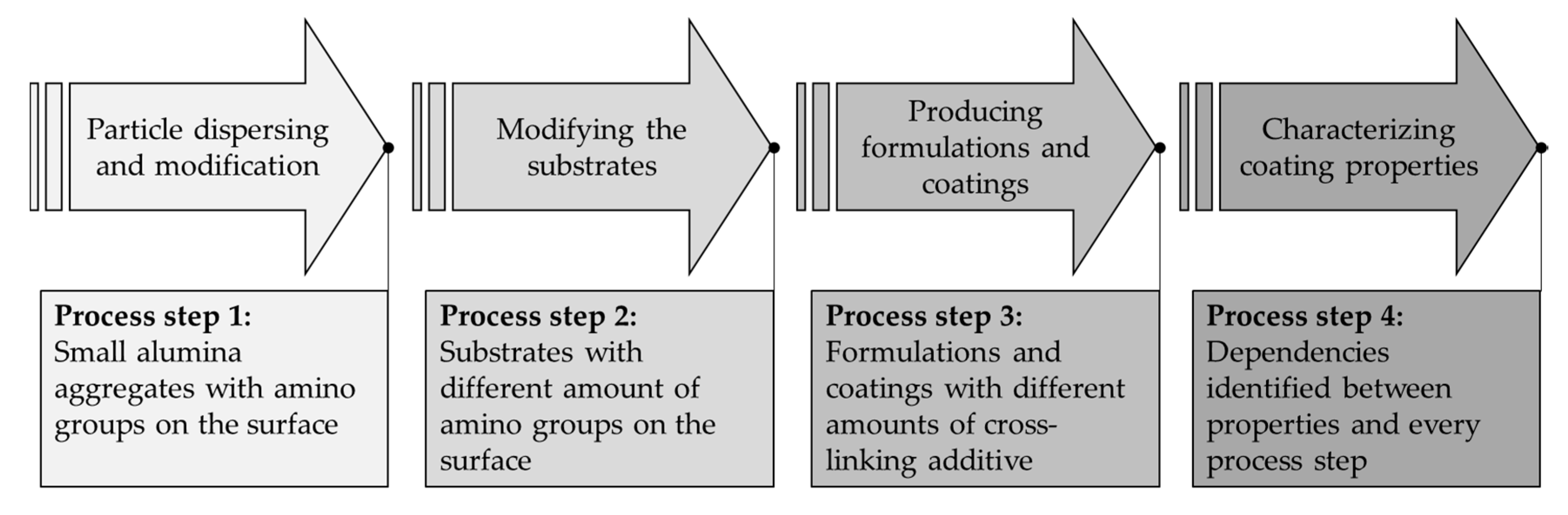
2. Materials and Methods
2.1. Step 1: Modifying the Substrates
2.2. Step 2: Particle Dispersing and Modification
2.3. Step 3: Producing the Formulations and Coatings
2.4. Step 4: Characterizing Coating Properties
3. Results
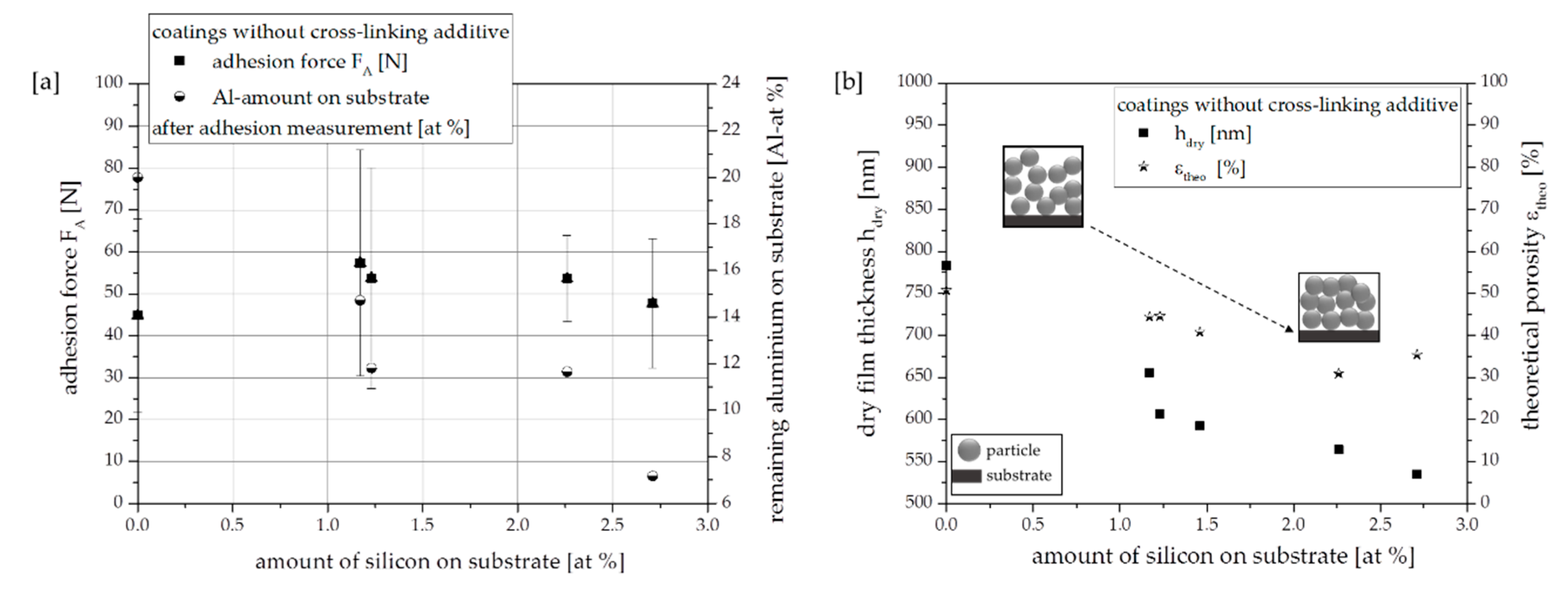
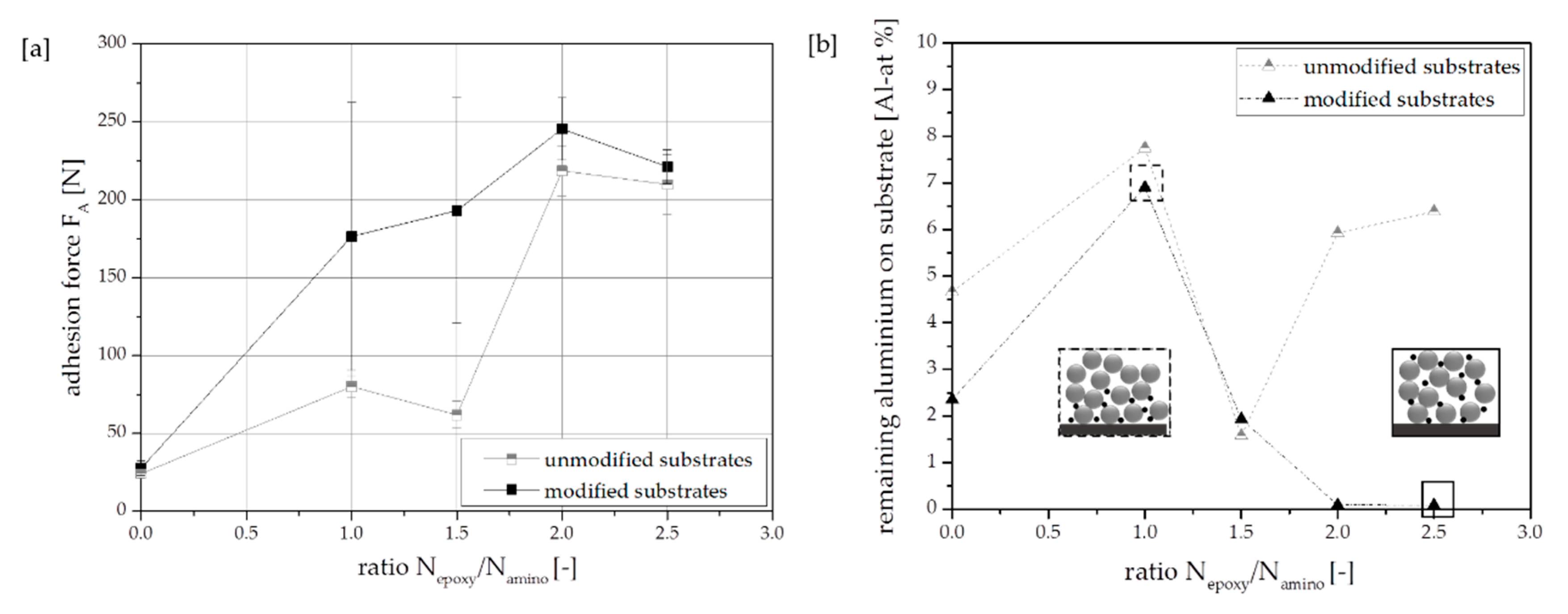
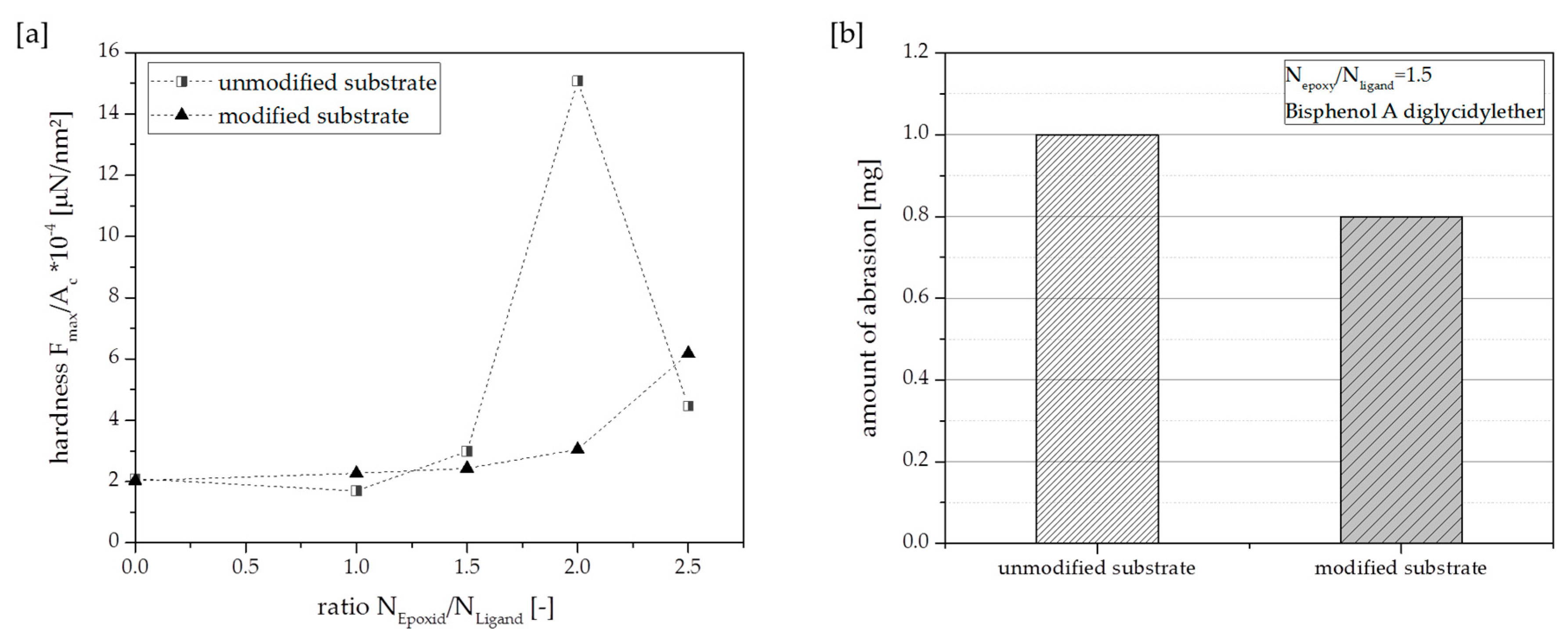
3.1. Effect of Substrates with Different Amount of Modification Ligand
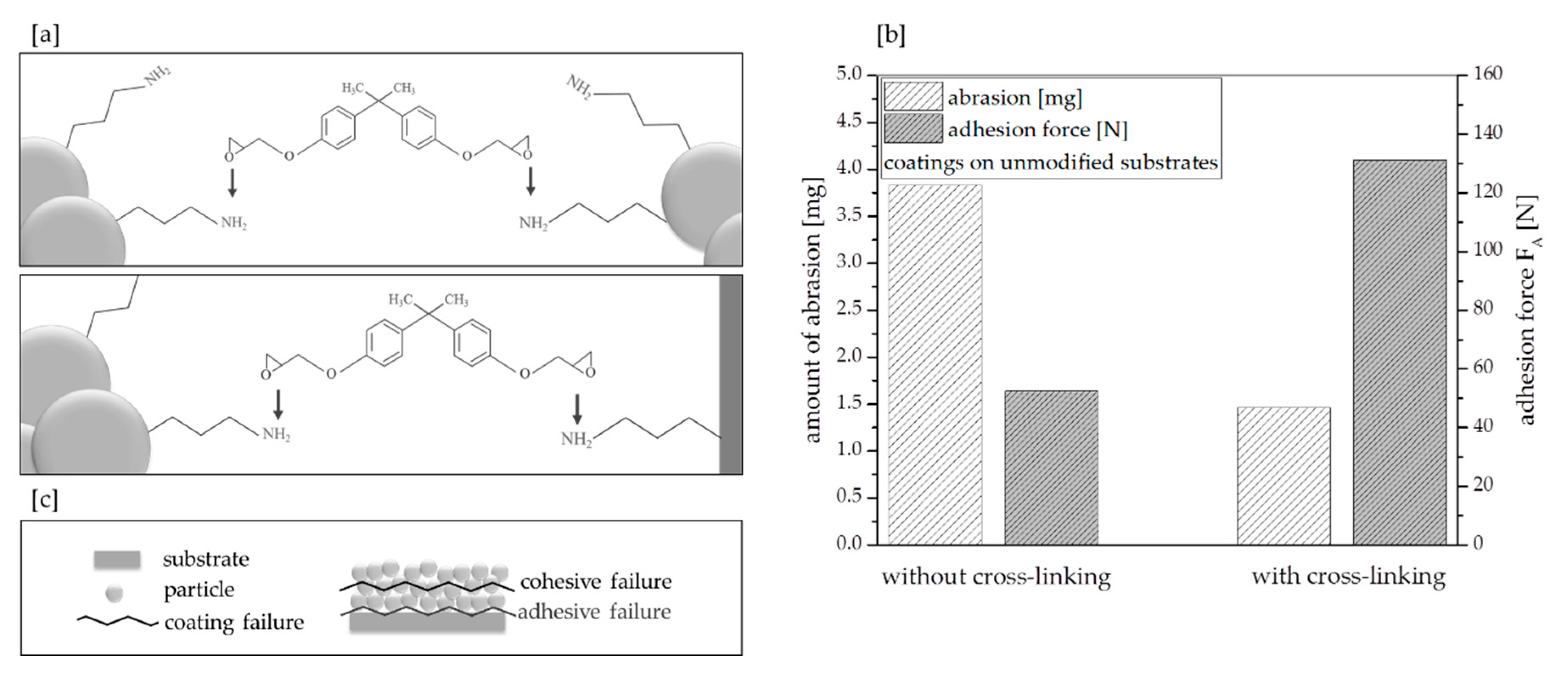
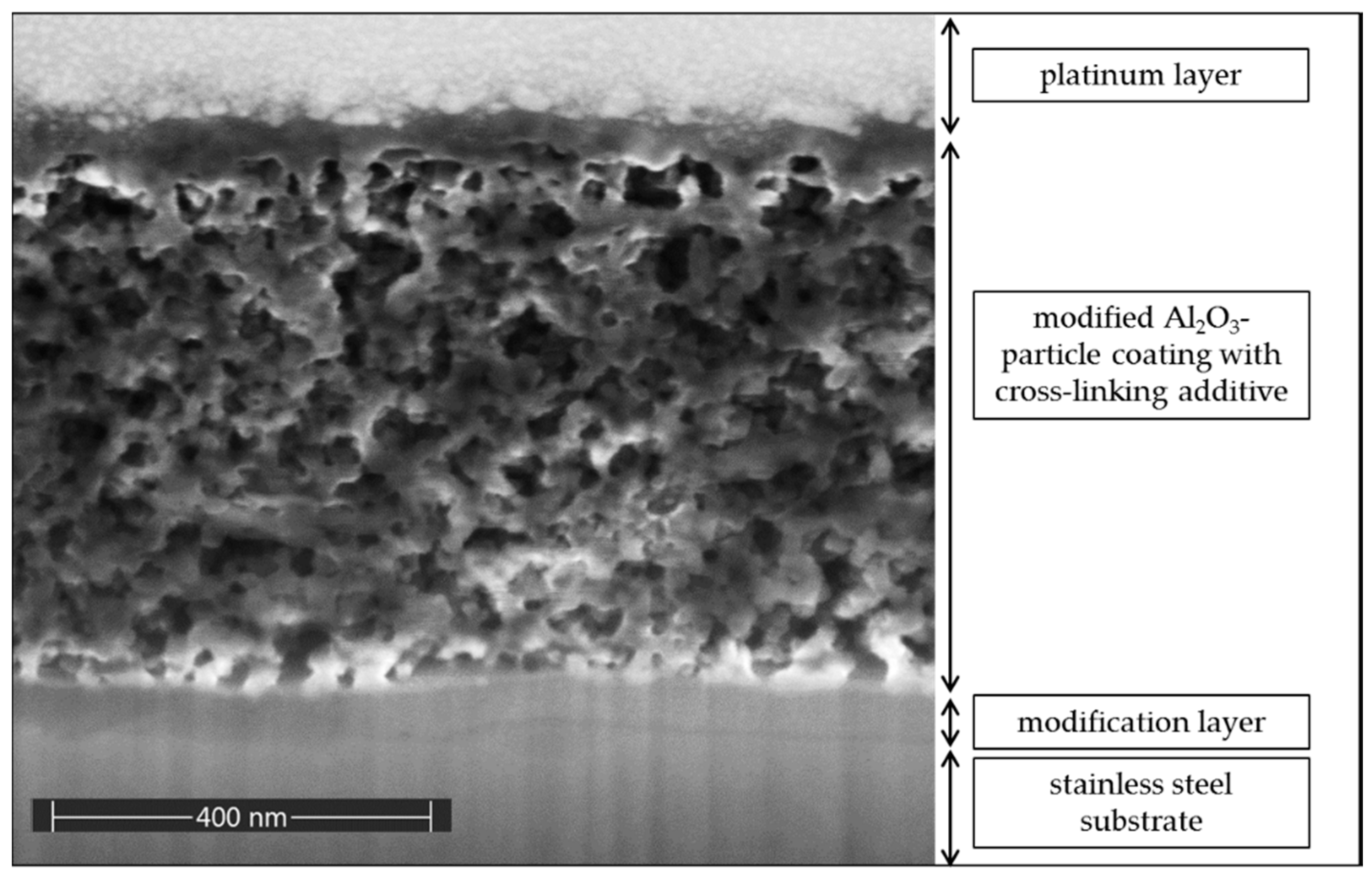
3.2. Effect of Cross-Linking Additive Concentration
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kamegawa, T.; Shimizu, Y.; Yamashita, H. Superhydrophobic surfaces with photocatalytic self-cleaning properties by nanocomposite coating of TiO2 and polytetrafluoroethylene. Adv. Mater. 2012, 24, 3697–3700. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, H.; Taghavinia, N.; Alamdari, E.K. Self cleaning TiO2 coating on polycarbonate: Surface treatment, photocatalytic and nanomechanical properties. Surf. Coat. Technol. 2010, 204, 1562–1568. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Bharathidasan, T.; Basu, B.J. Superhydrophobic sol–gel nanocomposite coatings with enhanced hardness. Appl. Surf. Sci. 2011, 257, 10421–10426. [Google Scholar] [CrossRef]
- Barth, N.; Schilde, C.; Kwade, A. Influence of particle size distribution on micromechanical properties of thin nanoparticulate coatings. Phys. Procedia 2013, 40, 9–18. [Google Scholar] [CrossRef][Green Version]
- Barth, N.; Schilde, C.; Kwade, A. Influence of electrostatic particle interactions on the properties of particulate coatings of titanium dioxide. J. Colloid Interface Sci. 2014, 420, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Barth, N.; Zimmermann, M.; Becker, A.E.; Graumann, T.; Garnweitner, G.; Kwade, A. Influence of TiO2 nanoparticle synthesis on the properties of thin coatings. Thin Solid Films 2015, 574, 20–27. [Google Scholar] [CrossRef]
- Hesselbach, J.; Barth, N.; Lippe, K.; Schilde, C.; Kwade, A. Process chain and characterisation of nanoparticle enhanced composite coatings. Adv. Powder Technol. 2015, 26, 1624–1632. [Google Scholar] [CrossRef]
- Hesselbach, J.; Kockmann, A.; Lüke, S.; Overbeck, A.; Garnweitner, G.; Schilde, C.; Kwade, A. Enhancement of the mechanical properties of nanoparticulate thin coatings via surface modification and cross-linking additive. Chem. Eng. Technol. 2017, 40, 1561–1568. [Google Scholar] [CrossRef]
- Kockmann, A.; Hesselbach, J.; Zellmer, S.; Kwade, A.; Garnweitner, G. Facile surface tailoring of metal oxide nanoparticles via a two-step modification approach. RSC Adv. 2015, 5, 60993–60999. [Google Scholar] [CrossRef]
- Basnar, B.; Litschauer, M.; Strasser, G.; Neouze, M.-A. Analyzing imidazolium bridging in nanoparticle networks covalently linked to silicon substrates. J. Phys. Chem. C 2012, 116, 9343–9350. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee, Y.-J.; Park, Y.S.; Ha, K.; Yoon, K.B. Monolayer assembly of zeolite crystals on glass with fullerene as the covalent linker. J. Am. Chem. Soc. 2000, 122, 5201–5209. [Google Scholar] [CrossRef]
- He, H.-X.; Zhang, H.; Li, Q.G.; Zhu, T.; Li, S.; Liu, Z.-F. Fabrication of designed architectures of au nanoparticles on solid substrate with printed self-assembled monolayers as templates. Langmuir 2000, 16, 3846–3851. [Google Scholar] [CrossRef]
- Stratmann, M. Chemically modified metal surfaces—A new class of composite materials. Adv. Mater. 1990, 2, 191–195. [Google Scholar] [CrossRef]
- Yoon, K.B. Organization of zeolite microcrystals for production of functional materials. Acc. Chem. Res. 2007, 40, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Hull, T.; Colligon, J.; Hill, A. Measurement of thin film adhesion. Vacuum 1987, 37, 327–330. [Google Scholar] [CrossRef]
- Bull, S.J. Techniques for improving thin film adhesion. Vacuum 1992, 43, 517–520. [Google Scholar] [CrossRef]
- Jacobsson, R. Measurement of the adhesion of thin films. Thin Solid Films 1976, 34, 191–199. [Google Scholar] [CrossRef]
- DIN EN ISO 4624 Beschichtungsstoffe–abreißversuch zur beurteilung der haftfestigkeit; Deutsches Institut für Normung: Berlin, Germany, 2003.
- Landau, L.; Levich, B. Dragging of a liquid by a moving plato. In Dynamics of Curved Fronts, 1st ed.; Pelce, P., Libchaber, A., Eds.; Academic Press: Cambridge, MA, USA, 2012; Volume 141. [Google Scholar]
- Schmidt-Hansberg, B.; Klein, M.F.; Peters, K.; Buss, F.; Pfeifer, J.; Walheim, S.; Colsmann, A.; Lemmer, U.; Scharfer, P.; Schabel, W. In situ monitoring the drying kinetics of knife coated polymer-fullerene films for organic solar cells. J. Appl. Phys. 2009, 106, 124501. [Google Scholar] [CrossRef]
- Barth, N.; Schilde, C.; Kwade, A. Einfluss von prozessparametern auf die mechanischen eigenschaften von nanopartikulären beschichtungen. Chem. Ingenieur Tech. 2012, 84, 328–334. [Google Scholar] [CrossRef]
- Schilde, C.; Westphal, B.; Kwade, A. Effect of the primary particle morphology on the micromechanical properties of nanostructured alumina agglomerates. J. Nanopart. Res. 2012, 14, 745. [Google Scholar] [CrossRef]
- Westphal, B.; Bockholt, H.; Günther, T.; Haselrieder, W.; Kwade, A. Influence of convective drying parameters on electrode performance and physical electrode properties. ECS Trans. 2015, 64, 57–68. [Google Scholar] [CrossRef]
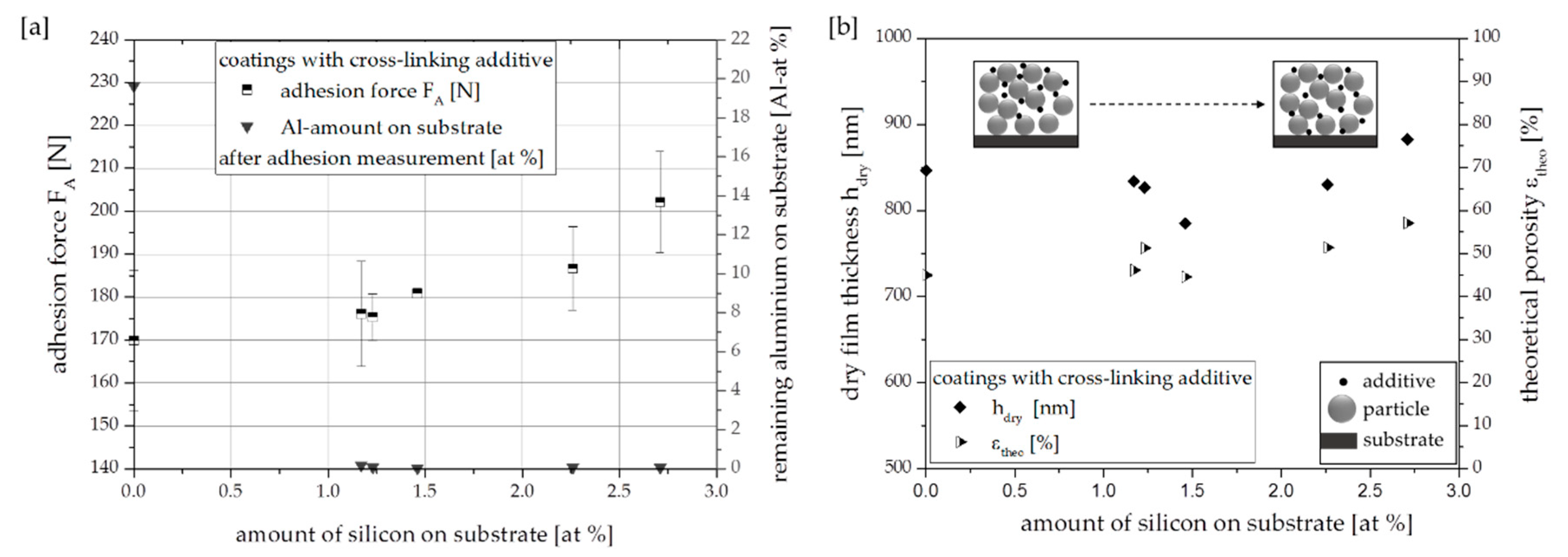
| Coating Speed v (mm/min) | Amount of Silicon (at %) |
|---|---|
| 50 | 1.17 |
| 100 | 1.23 |
| 200 | 1.46 |
| 300 | 2.26 |
| 500 | 2.71 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hesselbach, J.; Böttcher, A.-C.; Kampen, I.; Garnweitner, G.; Schilde, C.; Kwade, A. Process and Formulation Strategies to Improve Adhesion of Nanoparticulate Coatings on Stainless Steel. Coatings 2018, 8, 156. https://doi.org/10.3390/coatings8050156
Hesselbach J, Böttcher A-C, Kampen I, Garnweitner G, Schilde C, Kwade A. Process and Formulation Strategies to Improve Adhesion of Nanoparticulate Coatings on Stainless Steel. Coatings. 2018; 8(5):156. https://doi.org/10.3390/coatings8050156
Chicago/Turabian StyleHesselbach, Jutta, Ann-Christin Böttcher, Ingo Kampen, Georg Garnweitner, Carsten Schilde, and Arno Kwade. 2018. "Process and Formulation Strategies to Improve Adhesion of Nanoparticulate Coatings on Stainless Steel" Coatings 8, no. 5: 156. https://doi.org/10.3390/coatings8050156
APA StyleHesselbach, J., Böttcher, A.-C., Kampen, I., Garnweitner, G., Schilde, C., & Kwade, A. (2018). Process and Formulation Strategies to Improve Adhesion of Nanoparticulate Coatings on Stainless Steel. Coatings, 8(5), 156. https://doi.org/10.3390/coatings8050156






