Transparent Amorphous Oxide Semiconductor as Excellent Thermoelectric Materials
Abstract
1. Introduction
2. Experimental Details
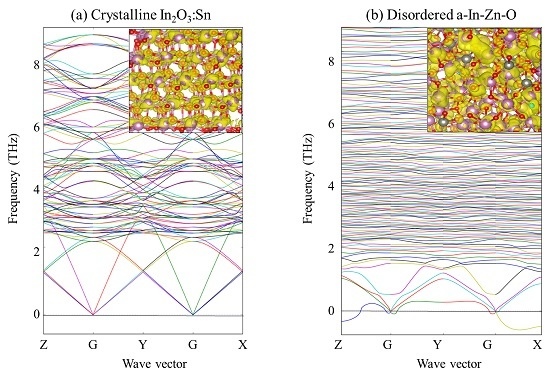
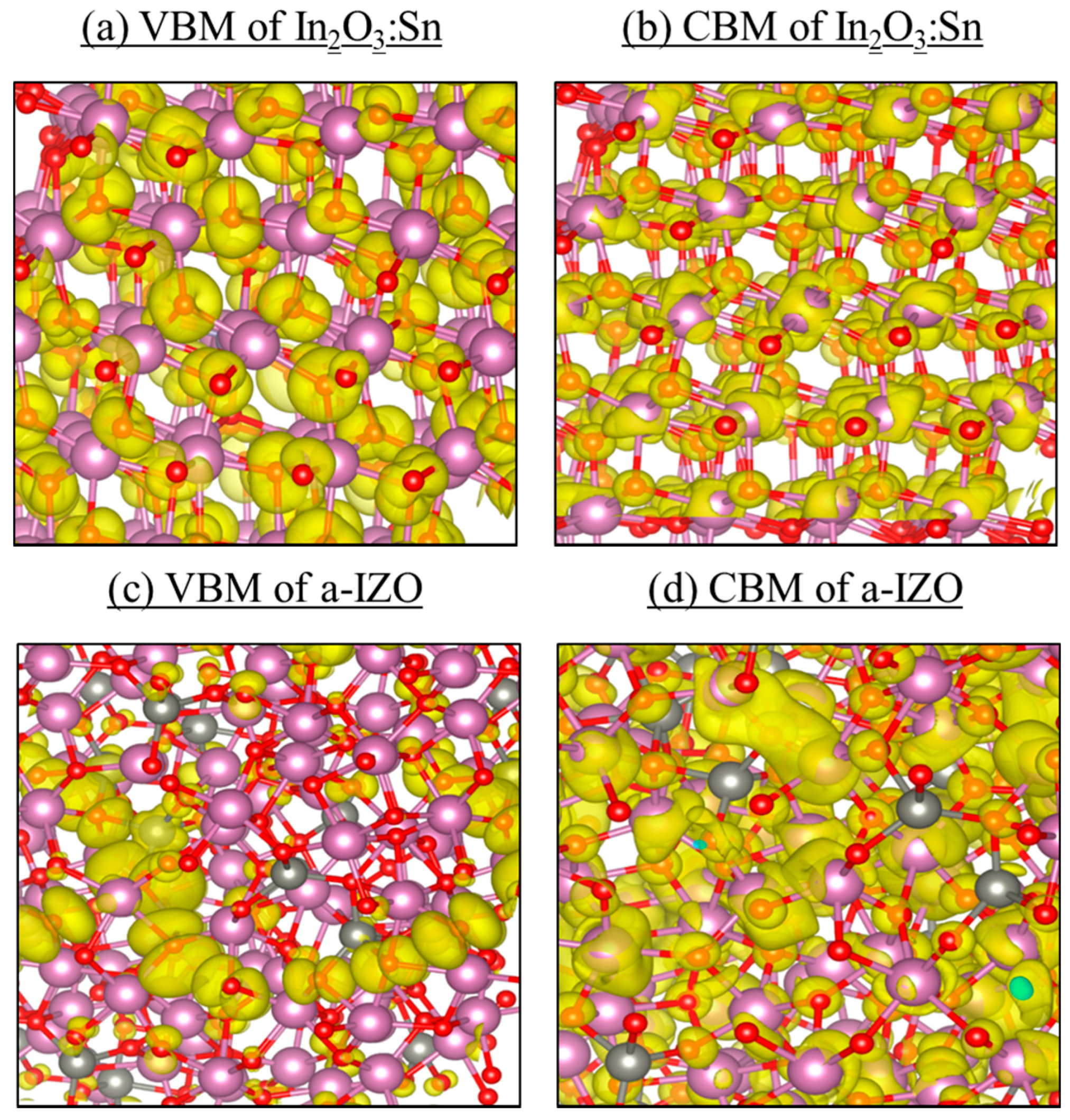
3. First-Principles Atomic Calculations
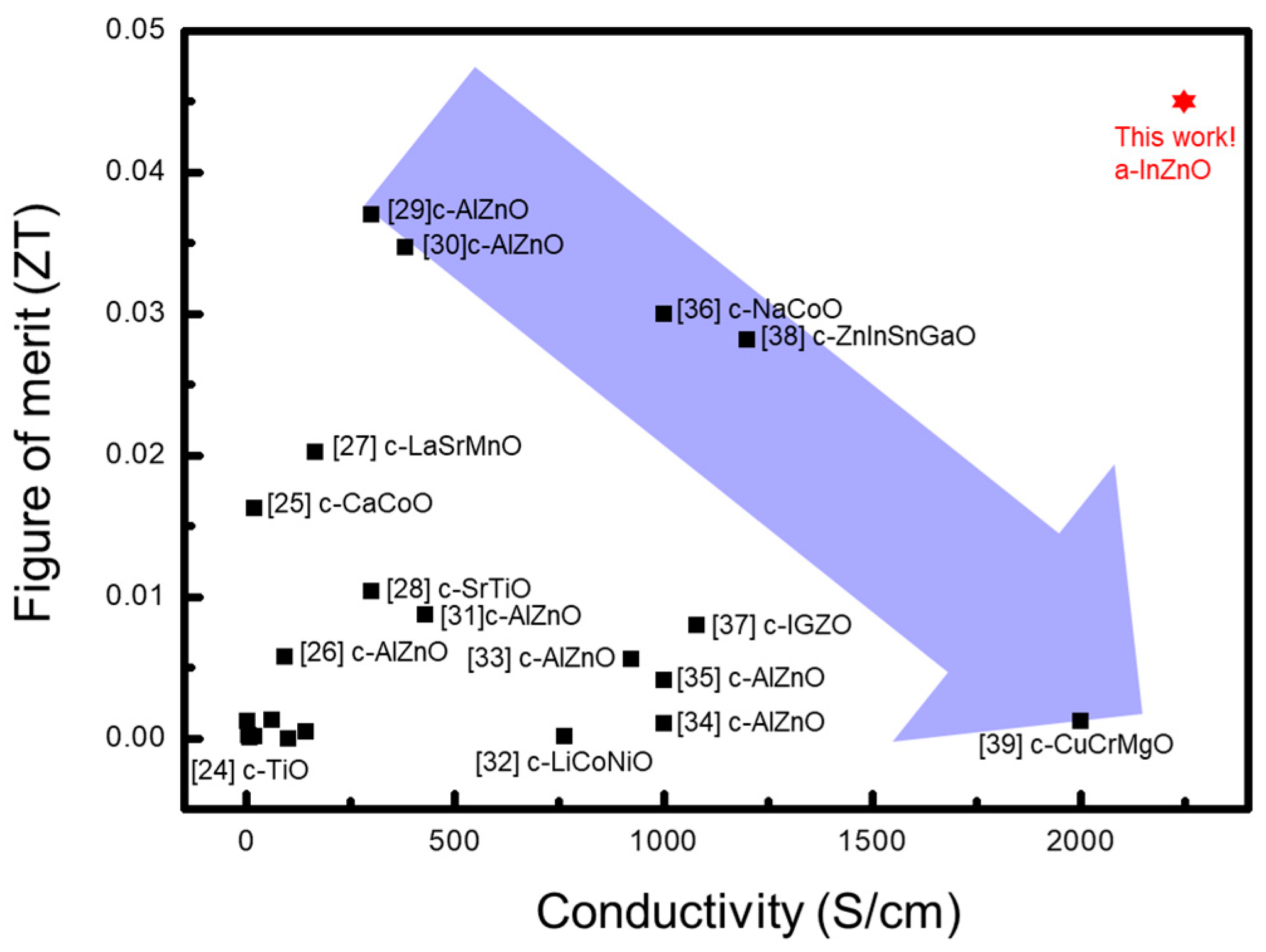
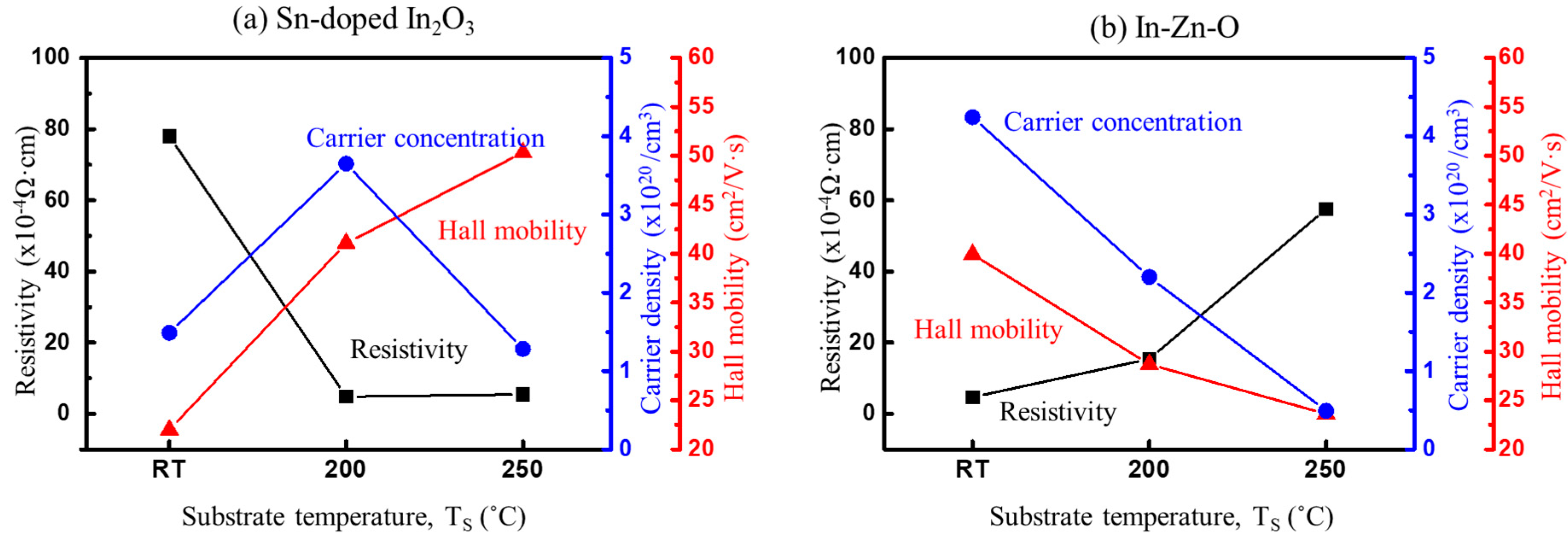
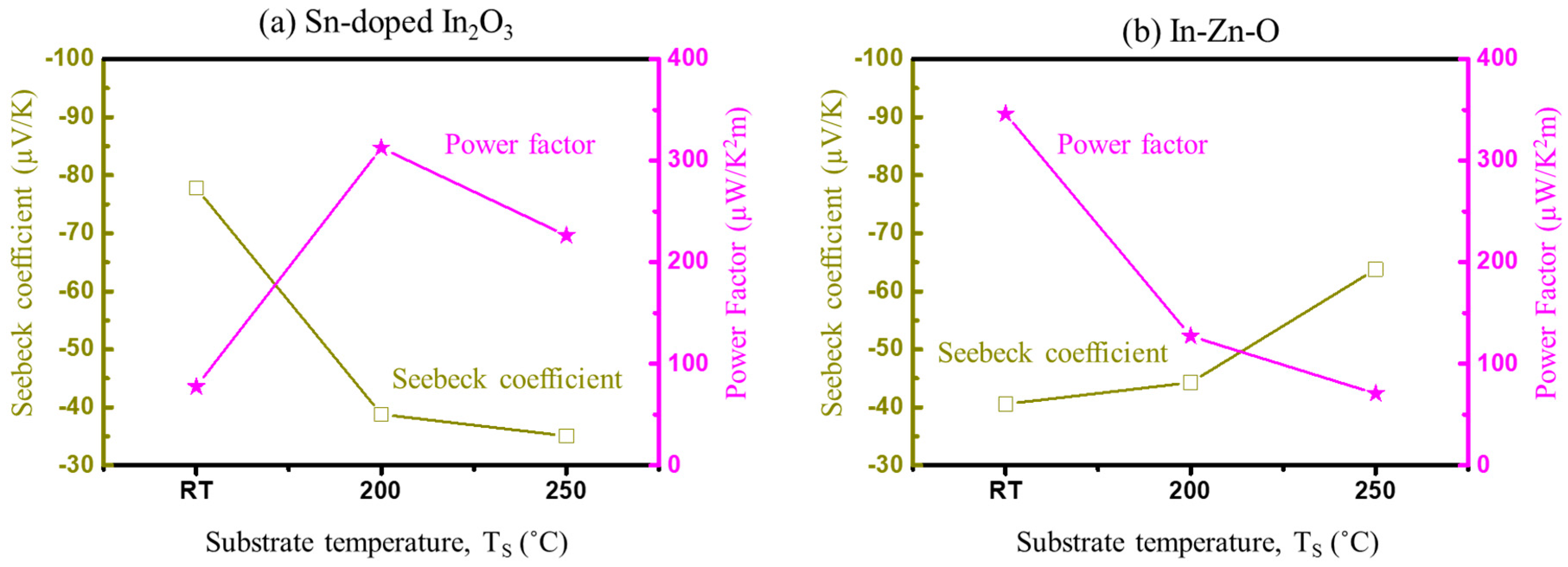
4. Experimental Measurements and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, Y.; Tan, Q.; Zhu, J.; Li, S.; Liu, C.; Lei, Y.; Li, L. Thermoelectric properties of amorphous Zr–Ni–Sn thin films deposited by magnetron sputtering. J. Electron. Mater. 2015, 44, 1957–1962. [Google Scholar] [CrossRef]
- Kim, D.-H.; Byon, E.; Lee, G.H.; Cho, S. Effect of deposition temperature on the structural and thermoelectric properties of bismuth telluride thin films grown by co-sputtering. Thin Solid Films 2006, 510, 148–153. [Google Scholar] [CrossRef]
- Yoo, I.-J.; Song, Y.; Lim, D.C.; Myunug, N.V.; Lee, K.H.; Oh, M.; Lee, D.; Kim, Y.D.; Kim, S.; Choa, Y.H.; et al. Thermoelectric characteristics of Sb2Te3 thin films formed via surfactant-assisted electrodeposition. J. Mater. Chem. A 2013, 1, 5430–5435. [Google Scholar] [CrossRef]
- Liu, H.; Fang, L.; Wu, F.; Tian, D.; Li, W.; Lu, Y.; Kong, C.; Zhang, S. Thermoelectric and magneto-thermoelectric properties of Ga-doped ZnO thin films by RF magnetron sputtering. Surf. Rev. Lett. 2014, 21, 1450033. [Google Scholar] [CrossRef]
- Fang, L.; Yang, X.F.; Peng, L.P.; Zhou, K.; Wu, F.; Huang, Q.L.; Kong, C.Y. Thermoelectric and magnetothermoelectric properties of In-doped nano-ZnO thin films prepared by RF magnetron sputtering. J. Supercond. Nov. Magn. 2010, 23, 889–892. [Google Scholar] [CrossRef]
- Sajad, Y.; Pettes, M.T. Nanoscale self-assembly of thermoelectric materials: A review of chemistry-based approaches. Nanotechnology 2018, 29, 432001. [Google Scholar] [CrossRef] [PubMed]
- Harman, T.C.; Taylor, P.J.; Walsh, M.P.; LaForge, B.E. Quantum dot superlattice thermoelectric materials and devices. Science 2002, 297, 2229–2232. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.F.; Loo, S.; Guo, F.; Chen, W.; Dyck, J.S.; Uher, C.; Hogan, T.; Polychroniadis, E.K.; Kanatzidis, M.G. Cubic AgPbmSbTe2+m: Bulk thermoelectric materials with high figure of merit. Science 2004, 303, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Dughaish, Z.H. Lead telluride as a thermoelectric material for thermoelectric power generation. Phys. B Condens. Matter 2002, 322, 205–223. [Google Scholar] [CrossRef]
- Lan, Y.; Minnich, A.J.; Chen, G.; Ren, Z. Enhancement of thermoelectric figure-of-merit by a bulk nanostructuring approach. Adv. Funct. Mater. 2010, 20, 357–376. [Google Scholar] [CrossRef]
- Kuo, C.H.; Jeng, M.S.; Ku, J.R.; Wu, S.K.; Chou, Y.W.; Hwang, C.S. p-Type PbTe thermoelectric bulk materials with nanograins fabricated by attrition milling and spark plasma sintering. J. Electron. Mater. 2009, 38, 1956–1961. [Google Scholar] [CrossRef]
- Poudel, B.; Hao, Q.; Ma, Y.; Lan, Y.; Minnich, A.; Yu, B.; Yan, X.; Wang, D.; Muto, A.; Vashaee, D.; et al. High-thermoelectric performance of nanostructured bismuth antimony telluride bulk alloys. Science 2008, 320, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.F.; Nan, C.W.; Zhou, M.; Liu, W.; Zhang, B.P.; Kita, T. High-performance Ag0.8Pb18+xSbTe20 thermoelectric bulk materials fabricated by mechanical alloying and spark plasma sintering. Appl. Phys. Lett. 2006, 88, 092104. [Google Scholar] [CrossRef]
- Blackburn, J.L.; Ferguson, A.J.; Cho, C.; Grunlan, J.C. Carbon-nanotube-based thermoelectric materials and devices. Adv. Mater. 2018, 30, 1704386. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.J.; Leonard, F.; Stavila, V.; Foster, M.E.; Spataru, C.D.; Jones, R.E.; Foley, B.M.; Hopkins, P.E.; Allendorf, M.D.; Talin, A.A. Thin film thermoelectric metal–organic framework with high Seebeck coefficient and low thermal conductivity. Adv. Mater. 2015, 27, 3453–3459. [Google Scholar] [CrossRef]
- Catellani, A.; Ruini, A.; Nardelli, M.B.; Calzolari, A. Unconventional co-existence of plasmon and thermoelectric activity in In:ZnO nanowires. RSC Adv. 2015, 5, 44865–44872. [Google Scholar] [CrossRef]
- Castro, M.V.; Tavares, C.J. Dependence of Ga-doped ZnO thin film properties on different sputtering process parameters: Substrate temperature, sputtering pressure and bias voltage. Thin Solid Films 2015, 586, 13–21. [Google Scholar] [CrossRef]
- Granqvist, C.G.; Hultaker, A. Transparent and conducting ITO films: New developments and applications. Thin Solid Films 2002, 411, 1–5. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Noh, Y.-Y. Optical and thermal properties of large-area OLED lightings with metallic grids. Org. Electron. 2012, 13, 184–194. [Google Scholar] [CrossRef]
- Hosono, H. Ionic amorphous oxide semiconductors: Material design, carrier transport, and device application. J. Non-Cryst. Sol. 2006, 352, 851–858. [Google Scholar] [CrossRef]
- Nomura, K.; Ohta, H.; Takagi, A.; Kamiya, T.; Hirano, M.; Hosono, H. Room-temperature fabrication of transparent flexible thin-film transistors using amorphous oxide semiconductors. Nature 2004, 432, 488–492. [Google Scholar] [CrossRef]
- Carey, P.G.; Smith, P.M.; Theiss, S.D.; Wickboldt, P. Polysilicon thin film transistors fabricated on low temperature plastic substrates. J. Vac. Sci. Technol. A 1999, 17, 1946–1949. [Google Scholar] [CrossRef]
- Yang, C.-S.; Smith, L.L.; Arthur, C.B.; Parsons, G.N. Stability of low-temperature amorphous silicon thin film transistors formed on glass and transparent plastic substrates. J. Vac. Sci. Technol. B 2000, 18, 683–689. [Google Scholar] [CrossRef]
- Hong, M.-H.; Jung, S.-Y.; Ha, T.-J.; Seo, W.-S.; Lim, Y.S.; Shin, S.; Cho, H.H.; Park, H.-H. Thermoelectric properties of mesoporous TiO2 thin films through annealing temperature and ratio of surfactant. Surf. Coat. Technol. 2013, 231, 370–373. [Google Scholar] [CrossRef]
- Aksit, M.; Kolli, S.K.; Slauch, I.M.; Robinson, R.D. Misfit layered Ca3Co4O9 as a high figure of merit p-type transparent conducting oxide film through solution processing. Appl. Phys. Lett. 2014, 104, 161901. [Google Scholar] [CrossRef]
- Saini, S.; Mele, P.; Honda, H.; Suzuki, T.; Matsumoto, K.; Miyazaki, K.; Ichinose, A.; Molina Luna, L.; Carlini, R.; Tiwari, A. Effect of self-grown seed layer on thermoelectric properties of ZnO thin films. Thin Solid Films 2016, 605, 289–294. [Google Scholar] [CrossRef]
- Jha, P.; Sands, T.D.; Jackson, P.; Bomberger, C.; Favaloro, T.; Hodson, S.; Zide, J.; Xu, X.; Shakouri, A. Cross-plane thermoelectric transport in p-type La0.67Sr0.33MnO3/LaMnO3 oxide metal/semiconductor superlattices. J. Appl. Phys. 2013, 113, 193702. [Google Scholar] [CrossRef]
- Ravichandran, J. Thermoelectric and thermal transport properties of complex oxide thin films, heterostructures and superlattices. J. Mater. Res. 2017, 32, 183–203. [Google Scholar] [CrossRef]
- Fan, P.; Li, Y.Z.; Zheng, Z.H.; Lin, Q.Y.; Luo, J.T.; Liang, G.X.; Zhang, M.; Chen, M. Thermoelectric properties optimization of Al-doped ZnO thin films prepared by reactive sputtering Zn–Al alloy target. Appl. Surf. Sci. 2013, 284, 145–149. [Google Scholar] [CrossRef]
- Mele, P.; Saini, S.; Honda, H.; Matsumoto, K.; Miyazaki, K.; Hagino, H.; Ichinose, A. Effect of substrate on thermoelectric properties of Al-doped ZnO thin films. Appl. Phys. Lett. 2013, 102, 253903. [Google Scholar] [CrossRef]
- Saini, S.; Mele, P.; Honda, H.; Matsumoto, K.; Miyazaki, K.; Luna, L.M.; Hopkins, P.E. Influence of postdeposition cooling atmosphere on thermoelectric properties of 2% Al-doped ZnO thin films grown by pulsed laser deposition. J. Electron. Mater. 2015, 44, 1547–1553. [Google Scholar] [CrossRef]
- Robert, R.; Aguirre, M.H.; Bocher, L.; Trottmann, M.; Heiroth, S.; Lippert, T.; Döbeli, M.; Weidenkaff, A. Thermoelectric properties of LaCo1−xNixO3 polycrystalline samples and epitaxial thin films. Solid State Sci. 2008, 10, 502–507. [Google Scholar] [CrossRef]
- Saini, S.; Mele, P.; Honda, H.; Henry, D.J.; Hopkins, P.E.; Molina-Luna, L.; Matsumoto, K.; Miyazaki, K.; Ichinose, A. Enhanced thermoelectric performance of Al-doped ZnO thin films on amorphous substrate. Jpn. J. Appl. Phys. 2014, 53, 060306. [Google Scholar] [CrossRef]
- Vogel-Schäuble, N.; Romanyuk, Y.E.; Yoon, S.; Saji, K.J.; Populoh, S.; Pokrant, S.; Aguirre, M.H.; Weidenkaff, A. Thermoelectric properties of nanostructured Al-substituted ZnO thin films. Thin Solid Films 2012, 520, 6869–6875. [Google Scholar] [CrossRef]
- Vogel-Schäuble, N.; Jaeger, T.; Romanyuk, Y.E.; Populoh, S.; Mix, C.; Jakob, G.; Weidenkaff, A. Thermal conductivity of thermoelectric Al-substituted ZnO thin films. Phys. Status Solidi Rapid Res. Lett. 2013, 7, 364–367. [Google Scholar] [CrossRef]
- Brinks, P.; Heijmerikx, H.; Hendriks, T.A.; Rijnders, G.; Huijben, M. Achieving chemical stability in thermoelectric NaxCoO2 thin films. RSC Adv. 2012, 2, 6023–6027. [Google Scholar] [CrossRef]
- Tran Nguyen, N.H.; Nguyen, T.H.; Liu, Y.; Aminzare, M.; Pham, A.T.T.; Cho, S.; Wong, D.P.; Chen, K.-H.; Seetawan, T.; Pham, N.K.; et al. Thermoelectric properties of indium and gallium dually doped ZnO thin films. ACS Appl. Mater. Interfaces 2016, 8, 33916–33923. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. In2O3-based multicomponent metal oxide films and their prospects for thermoelectric applications. Solid State Sci. 2016, 52, 141–148. [Google Scholar] [CrossRef]
- Ono, Y.; Satoh, K.-I.; Nozaki, T.; Kajitani, T. Structural, magnetic and thermoelectric properties of delafossite-type oxide, CuCr1−xMgxO2 (0 ≤ x ≤ 0.05). Jpn. J. Appl. Phys. 2007, 46, 1071. [Google Scholar] [CrossRef]
- Seo, D.K.; Shin, S.; Cho, H.H.; Kong, B.H.; Whang, D.M.; Cho, H.K. Drastic improvement of oxide thermoelectric performance using thermal and plasma treatments of the InGaZnO thin films grown by sputtering. Acta Mater. 2011, 59, 6743–6750. [Google Scholar] [CrossRef]
- Endoh, R.; Hirano, T.; Takeda, M.; Oishi, M.; Oka, N.; Shigesato, Y. Thermal conductivity of amorphous indium zinc oxide thin films. In Proceedings of the Transparent Conducting Oxides and Applications, Boston, MA, USA, 29 November–3 December 2010. [Google Scholar]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Anisimov, V.I.; Zaanen, J.; Andersen, O.K. Band theory and Mott insulators: Hubbard U instead of Stoner I. Phys. Rev. B 1991, 44, 943. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scripta Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Byeon, J.; Kim, S.; Lim, J.-H.; Song, J.Y.; Park, S.H.; Song, P. Thermoelectric and electrical properties of micro-quantity Sn-doped amorphous indium-zinc oxide thin films. Jpn. J. Appl. Phys. 2017, 56, 010304. [Google Scholar] [CrossRef]
- Song, P.K.; Shigesato, Y.; Kamei, M.; Yasui, I. Electrical and structural properties of tin-doped indium oxide films deposited by DC sputtering at room temperature. Jpn. J. Appl. Phys. 1999, 38, 2921. [Google Scholar] [CrossRef]
- Kim, H.; Gilmore, C.M.; Pique, A.; Horwitz, J.S.; Mattoussi, H.; Murata, H.; Kafafi, Z.H.; Chrisey, D.B. Electrical, optical, and structural properties of indium-tin-oxide thin films for organic light-emitting devices. J. Appl. Phys. 1999, 86, 6451–6461. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, D.G.; Lee, K.S.; Huh, M.S.; Jeong, S.H.; Kim, K.I.; Seong, T.Y. Plasma damage-free sputtering of indium tin oxide cathode layers for top-emitting organic light-emitting diodes. Appl. Phys. Lett. 2005, 86, 186503. [Google Scholar] [CrossRef]
- Shigesato, Y.; Paine, D.C. A microstructural study of low resistivity tin-doped indium oxide prepared by d.c. magnetron sputtering. Thin Solid Films 1994, 238, 44–50. [Google Scholar] [CrossRef]
- Yaglioglu, B.; Yeom, H.-Y.; Paine, D.C. Crystallization of amorphous In2O3–10 wt.% ZnO thin films annealed in air. Appl. Phys. Lett. 2005, 86, 261908. [Google Scholar] [CrossRef]
- Garf, M.J.; Yip, S.-K.; Sauls, J.A. Electronic thermal conductivity and the Wiedemann-Franz law for unconventional superconductors. Phys. Rev. B 1996, 53, 15147. [Google Scholar] [CrossRef]



| Sample | σ (S/cm) | S (µV/K) | κ (W/mK) | κe (W/mK) | κl (W/mK) | PF (µW/mK) | ZT (S2 σ/κ) |
|---|---|---|---|---|---|---|---|
| c-ITO (TS: 200 °C) | 2061 | 38.803 | 3.432 | 1.515 | 1.916 | 310.384 | 0.027 |
| a-IZO (TS: RT) | 2153 | 40.578 | 2.377 | 1.586 | 0.795 | 354.559 | 0.043 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kim, D.; Byeon, J.; Lim, J.; Song, J.; Park, S.; Park, C.; Song, P. Transparent Amorphous Oxide Semiconductor as Excellent Thermoelectric Materials. Coatings 2018, 8, 462. https://doi.org/10.3390/coatings8120462
Kim S, Kim D, Byeon J, Lim J, Song J, Park S, Park C, Song P. Transparent Amorphous Oxide Semiconductor as Excellent Thermoelectric Materials. Coatings. 2018; 8(12):462. https://doi.org/10.3390/coatings8120462
Chicago/Turabian StyleKim, Seohan, Doukyun Kim, Jayoung Byeon, Jaehong Lim, Jaeyong Song, Sunhwa Park, Chulhong Park, and Pungkeun Song. 2018. "Transparent Amorphous Oxide Semiconductor as Excellent Thermoelectric Materials" Coatings 8, no. 12: 462. https://doi.org/10.3390/coatings8120462
APA StyleKim, S., Kim, D., Byeon, J., Lim, J., Song, J., Park, S., Park, C., & Song, P. (2018). Transparent Amorphous Oxide Semiconductor as Excellent Thermoelectric Materials. Coatings, 8(12), 462. https://doi.org/10.3390/coatings8120462