Rational Construction of LaFeO3 Perovskite Nanoparticle-Modified TiO2 Nanotube Arrays for Visible-Light Driven Photocatalytic Activity
Abstract
:1. Introduction
2. Materials and Methods
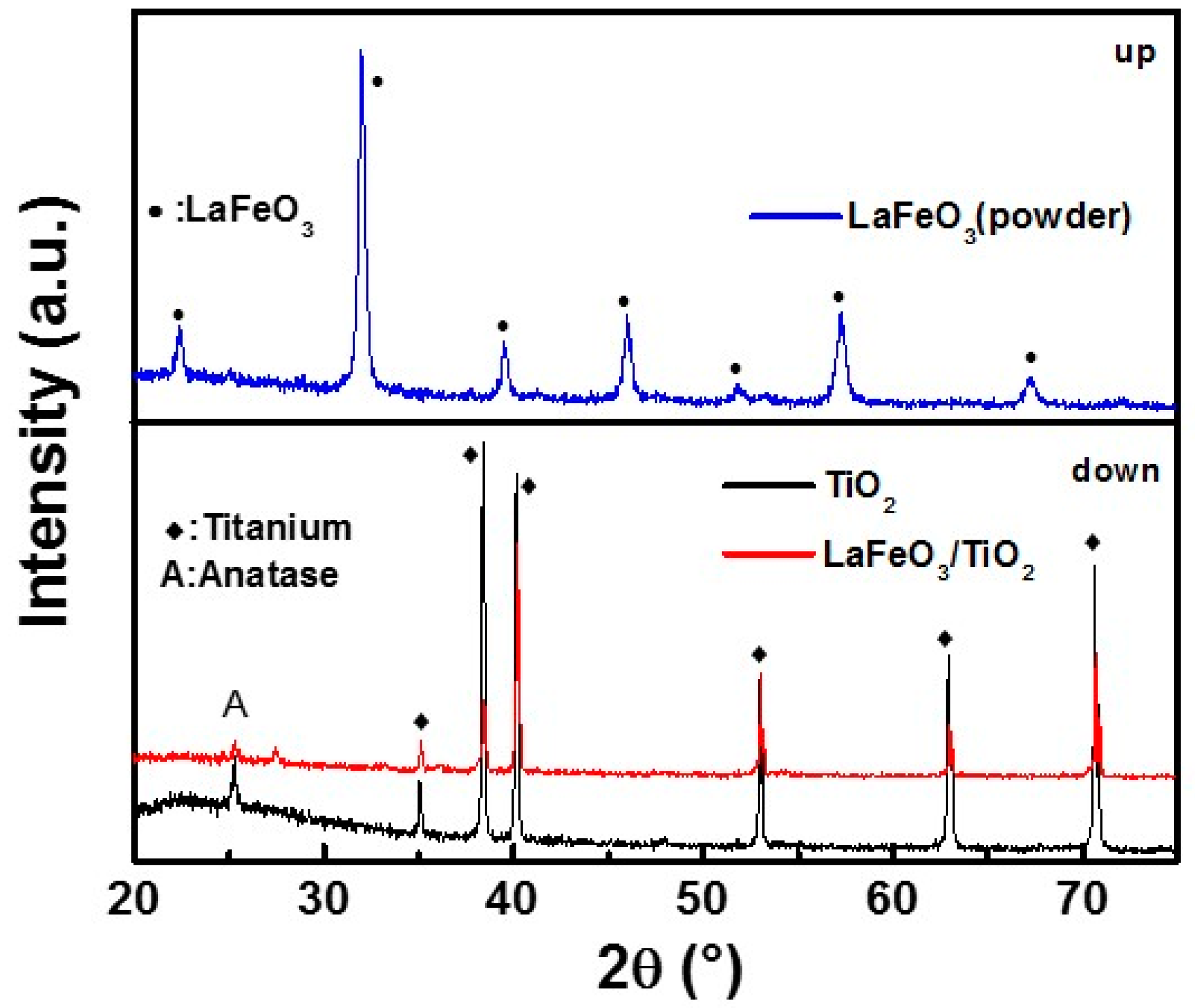
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, M.; Iocozzia, J.; Sun, L.; Lin, C.; Lin, Z. Inorganic-modified semiconductor TiO2 nanotube arrays for photocatalysis. Energy Environ. Sci. 2014, 7, 2182–2202. [Google Scholar] [CrossRef]
- Tang, Y.; Jiang, Z.; Xing, G.; Li, A.; Kanhere, P.D.; Zhang, Y.; Sum, T.C.; Li, S.; Chen, X.; Dong, Z.; et al. Efficient Ag@AgCl cubic cages photocatalyst profited from ultrafast plasmon-induced electron transfer process. Adv. Funct. Mater. 2013, 23, 2932. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Malyi, O.I.; Bucher, N.; Xia, H.; Xi, S.; Zhu, Z.; Lv, Z.; Li, W.; Wei, J.; et al. Identifying the origin and contribution of surface storage in TiO2(B) nanotube electrode by in-situ dynamic valence state monitoring. Adv. Mater. 2018, 30, 1802200. [Google Scholar] [CrossRef] [PubMed]
- Riboni, F.; Nguyen, N.T.; So, S.; Schmuki, P. Aligned metal oxide nanotube arrays: Key-aspects of anodic TiO2 nanotube formation and properties. Nanoscale Horizons 2016, 1, 445–466. [Google Scholar] [CrossRef]
- Ge, M.-Z.; Cao, C.-Y.; Li, S.-H.; Tang, Y.-X.; Wang, L.-N.; Qi, N.; Huang, J.-Y.; Zhang, K.-Q.; Al-Deyab, S.S.; Lai, Y.K. In situ plasmonic Ag nanoparticle anchored TiO2 nanotube arrays as visible-light-driven photocatalysts for enhanced water splitting. Nanoscale 2016, 8, 5226–5234. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Shen, J.; Zhang, X.; Ng, Y.H.; Huang, J.; Guo, W.; Lin, C.; Lai, Y. Light-driven sustainable hydrogen production utilizing TiO2 nanostructures: A review. Small Methods 2018, 2, 1800184. [Google Scholar] [CrossRef]
- Ye, M.; Gong, J.; Lai, Y.; Lin, C.; Lin, Z. High-efficiency photoelectrocatalytic hydrogen generation enabled by palladium quantum dots-sensitized TiO2 nanotube arrays. J. Am. Chem. Soc. 2012, 134, 15720–15723. [Google Scholar] [CrossRef] [PubMed]
- Macak, J.M.; Zlamal, M.; Krysa, J.; Schmuki, P. Self-organized TiO2 nanotube layers as highly efficient photocatalysts. Small 2007, 3, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Huang, H.; Zhang, Y.; Chan, H.L.; Zhou, L. Highly ordered nanoporous TiO2 and its photocatalytic properties. Electrochem. Commun. 2007, 9, 2854–2858. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.-Q.; Al-Deyab, S.S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Ge, M.; Li, Q.; Cao, C.; Huang, J.; Li, S.; Zhang, S.; Chen, Z.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. One-dimensional TiO2 nanotube photocatalysts for solar water splitting. Adv. Sci. 2017, 4, 1600152. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, D.; Kim, D.; Schmuki, P. TiO2 nanotubes, nanochannels and mesosponge: Self-organized formation and applications. Nano Today 2013, 8, 235–264. [Google Scholar] [CrossRef]
- Lai, Y.; Huang, J.; Zhang, H.; Subramaniam, V.-P.; Tang, Y.-X.; Gong, D.-G.; Sundarb, L.; Sun, L.; Chen, Z.; Lin, C.J. Nitrogen-doped TiO2 nanotube array films with enhanced photocatalytic activity under various light sources. J. Hazard. Mater. 2010, 184, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.Z.; Cai, J.S.; Iocozzia, J.; Cao, C.; Huang, J.; Zhang, X.; Shen, J.; Wang, S.; Zhang, S.; Zhang, K.-Q.; et al. A review of TiO2 nanostructured catalysts for sustainable H2 generation. Int. J. Hydrog. Energy 2017, 42, 8418–8449. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Tu, W.; Ye, J.; Zou, Z. State-of-the-art progress in diverse heterostructured photocatalysts toward promoting photocatalytic performance. Adv. Funct. Mater. 2015, 25, 998–1013. [Google Scholar] [CrossRef]
- Jia, L.; Li, J.; Fang, W. Enhanced visible-light active C and Fe co-doped LaCoO3 for reduction of carbon dioxide. Catal. Commun. 2009, 11, 87–90. [Google Scholar] [CrossRef]
- Wheeler, G.P.; Choi, K.-S. photoelectrochemical properties and stability of nanoporous p-Type LaFeO3 photoelectrodes prepared by electrodeposition. ACS Energy Lett. 2017, 2, 2378–2382. [Google Scholar] [CrossRef]
- Dhinesh Kumar, R.; Thangappan, R.; Jayavel, R. Enhanced visible light photocatalytic activity of LaMnO3 nanostructures for water purification. Res. Chem. Intermed. 2018, 44, 4323–4337. [Google Scholar] [CrossRef]
- Truppi, A.; Petronella, F.; Placido, T.; Striccoli, M.; Agostiano, A.; Curri, M.L.; Comparelli, R. Visible-light-active TiO2-based hybrid nanocatalysts for environmental applications. Catalysts 2017, 7, 100. [Google Scholar] [CrossRef]
- Farhadi, S.; Amini, M.M.; Mahmoudi, F. Phosphotungstic acid supported on aminosilica functionalized perovskite-type LaFeO3 nanoparticles: A novel recyclable and excellent visible-light photocatalyst. RSC Adv. 2016, 6, 102984–102996. [Google Scholar] [CrossRef]
- Guo, M.; Xie, K.; Lin, J.; Yong, Z.; Zhou, L.; Wang, Y.; Huang, H. Design and coupling of multifunctional TiO2 nanotube photonic crystal to nanocrystalline titania layer as semi-transparent photoanode for dye-sensitized solar cell. Energy Environ. Sci. 2012, 5, 9881–9888. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Altomare, M.; Yoo, J.E.; Taccardi, N.; Schmuki, P. Noble metals on anodic TiO2 nanotube mouths: Thermal dewetting of minimal Pt Co-catalyst loading leads to significantly enhanced photocatalytic H2 generation. Adv. Energy Mater. 2016, 6, 1501926. [Google Scholar] [CrossRef]
- Xie, K.; Sun, L.; Wang, C.; Lai, Y.; Wang, M.; Chen, H.; Lin, C. Photoelectrocatalytic properties of Ag nanoparticles loaded TiO2 nanotube arrays prepared by pulse current deposition. Electrochim. Acta 2010, 55, 7211–7218. [Google Scholar] [CrossRef]
- Zhou, K.; Wu, X.; Wu, W.; Xie, J.; Tang, S.; Liao, S. Nanocrystalline LaFeO3 preparation and thermal process of precursor. Adv. Powder Technol. 2013, 24, 359–363. [Google Scholar]
- Parida, K.M.; Reddy, K.H.; Martha, S.; Das, D.P.; Biswal, N. Fabrication of nanocrystalline LaFeO3: An efficient sol-gel auto-combustion assisted visible light responsive photocatalyst for water decomposition. Int. J. Hydrogen Energy 2010, 35, 12161–12168. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Zhang, L.; Yang, X.; Lu, L.; Wang, X. Preparation and characterization of perovskite LaFeO3 nanocrystals. Mater. Lett. 2006, 60, 1767–1770. [Google Scholar] [CrossRef]
- Yu, J.; Wang, B. Effect of calcination temperature on morphology and photoelectrochemical properties of anodized titanium dioxide nanotube arrays. Appl. Catal. B 2010, 94, 295–302. [Google Scholar] [CrossRef]
- Phoon, B.L.; Lai, C.W.; Pan, G.T.; Yang, T.C.K.; Juan, J.C. One-pot hydrothermal synthesis of strontium titanate nanoparticles photoelectrode using electrophoretic deposition for enhancing photoelectrochemical water splitting. Ceram. Int. 2018, 44, 9923–9933. [Google Scholar] [CrossRef]
- Flynn, B.T.; Zhang, L.; Shutthanandan, V.; Varga, T.; Colby, R.J.; Oleksak, R.P.; Manandhar, S.; Engelhard, M.H.; Chambers, S.A.; Henderson, M.A.; et al. Growth and surface modification of LaFeO3 thin films induced by reductive annealing. Appl. Surf. Sci. 2015, 330, 309–315. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Zhan, W.; Guo, Y.; Guo, Y.; Lu, G.; Baylet, A.; Giroir-Fendler, A. Catalytic oxidation of vinyl chloride emission over LaMnO3 and LaB0.2Mn0.8O3 (B = Co, Ni, Fe) catalysts. Appl. Catal. B 2013, 129, 509–516. [Google Scholar] [CrossRef]
- Simmons, G.W.; Beard, B.C. Characterization of acid-base properties of the hydrated oxides on iron and titanium metal surfaces. J. Phys. Chem. 1987, 91, 1143–1148. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Girija, K.; Hebalkar, N.Y.; Mangalaraj, D.; Viswanathan, C.; Ponpandian, N. Shape evolution of perovskite LaFeO3 nanostructures: A systematic investigation of growth mechanism, properties and morphology dependent photocatalytic activities. RSC Adv. 2013, 3, 7549–7561. [Google Scholar] [CrossRef]
- Lai, Y.; Sun, L.; Chen, Y.; Zhuang, H.; Lin, C.; Chin, J.W. Effects of the structure of TiO2 nanotube array on Ti substrate on its photocatalytic activity. J. Electrochem. Soc. 2006, 153, D123–D127. [Google Scholar] [CrossRef]
- Vijayan, B.K.; Dimitrijevic, N.M.; Wu, J.; Gray, K.A. The effects of Pt doping on the structure and visible light photoactivity of titania nanotubes. J. Phys. Chem. C 2010, 114, 21262–21269. [Google Scholar] [CrossRef]
- Yamashita, H.; Harada, M.; Misaka, J.; Takeuchi, M.; Neppolian, B.; Anpo, M. Photocatalytic degradation of organic compounds diluted in water using visible light-responsive metal ion-implanted TiO2 catalysts: Fe ion-implanted TiO2. Catal. Today 2003, 84, 191–196. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, S.; Fu, X.; Xu, Y.J. Synthesis of M@TiO2 (M = Au, Pd, Pt) core-shell nanocomposites with tunable photoreactivity. J. Phys. Chem. C 2011, 115, 9136–9145. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, L.D.; Meng, G.W.; Li, G.H.; Zhang, X.Y.; Liang, C.H.; Zhang, X.Y.; Liang, C.H.; Chen, W.; Wang, S.X. Preparation and photoluminescence of highly ordered TiO2 nanowire arrays. Appl. Phys. Lett. 2001, 78, 1125–1127. [Google Scholar] [CrossRef]
- Xiang, S.; Zhang, Z.; Gong, C.; Wu, Z.; Sun, L.; Ye, C.; Lin, C. LaFeO3 nanoparticle-coupled TiO2 nanotube array composite with enhanced visible light photocatalytic activity. Mater. Lett. 2018, 216, 1–4. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Xie, T.; Wang, D. Low-temperature synthesis and high visible-light-induced photocatalytic activity of BiOI/TiO2 heterostructures. J. Phys. Chem. C 2009, 113, 7371–7378. [Google Scholar] [CrossRef]
- Lin, X.; Xing, J.; Wang, W.; Shan, Z.; Xu, F.; Huang, F. Photocatalytic activities of heterojunction semiconductors Bi2O3/BaTiO3: A strategy for the design of efficient combined photocatalysts. J. Phys. Chem. C 2007, 111, 18288–18293. [Google Scholar] [CrossRef]
- Butler, M.A.; Ginley, D.S. Prediction of flatband potentials at semiconductor-electrolyte interfaces from atomic electronegativities. J. Electrochem. Soc. 1978, 125, 228–232. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, T.; Xu, X.; Lv, N. Fabrication of N-type Fe2O3 and P-type LaFeO3 nanobelts by electrospinning and determination of gas-sensing properties. Sens. Actuators B Chem. 2011, 153, 83–88. [Google Scholar] [CrossRef]
- Khan, M.; Al-Shahry, M.; Ingler, W.B. Efficient photochemical water splitting by a chemically modified n-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Xiang, S.; Ge, M.; Zhang, Z.; Huang, J.; Tang, Y.; Sun, L.; Lin, C.; Lai, Y. Rational Construction of LaFeO3 Perovskite Nanoparticle-Modified TiO2 Nanotube Arrays for Visible-Light Driven Photocatalytic Activity. Coatings 2018, 8, 374. https://doi.org/10.3390/coatings8110374
Yu J, Xiang S, Ge M, Zhang Z, Huang J, Tang Y, Sun L, Lin C, Lai Y. Rational Construction of LaFeO3 Perovskite Nanoparticle-Modified TiO2 Nanotube Arrays for Visible-Light Driven Photocatalytic Activity. Coatings. 2018; 8(11):374. https://doi.org/10.3390/coatings8110374
Chicago/Turabian StyleYu, Jiangdong, Siwan Xiang, Mingzheng Ge, Zeyang Zhang, Jianying Huang, Yuxin Tang, Lan Sun, Changjian Lin, and Yuekun Lai. 2018. "Rational Construction of LaFeO3 Perovskite Nanoparticle-Modified TiO2 Nanotube Arrays for Visible-Light Driven Photocatalytic Activity" Coatings 8, no. 11: 374. https://doi.org/10.3390/coatings8110374
APA StyleYu, J., Xiang, S., Ge, M., Zhang, Z., Huang, J., Tang, Y., Sun, L., Lin, C., & Lai, Y. (2018). Rational Construction of LaFeO3 Perovskite Nanoparticle-Modified TiO2 Nanotube Arrays for Visible-Light Driven Photocatalytic Activity. Coatings, 8(11), 374. https://doi.org/10.3390/coatings8110374






