Recent Studies of Semitransparent Solar Cells
Abstract
:1. Introduction
2. Figure of Merits for Characterizing Semitransparent Solar Cells
2.1. Average Visible Light Transmittance
2.2. Transparency Color Perception
2.3. Corresponding Color Temperature
2.4. Color Rendering Index
3. Semitransparent Organic Solar Cells
3.1. Transparent Conductive Electrodes for Semitransparent Organic Solar Cells
3.1.1. Transparent Conducting Polymers
3.1.2. Carbon Nanotubes
3.1.3. Graphene
3.1.4. Metal Nanowires
3.2. High Color Neutral Perception
3.2.1. Photoactive Layer Materials
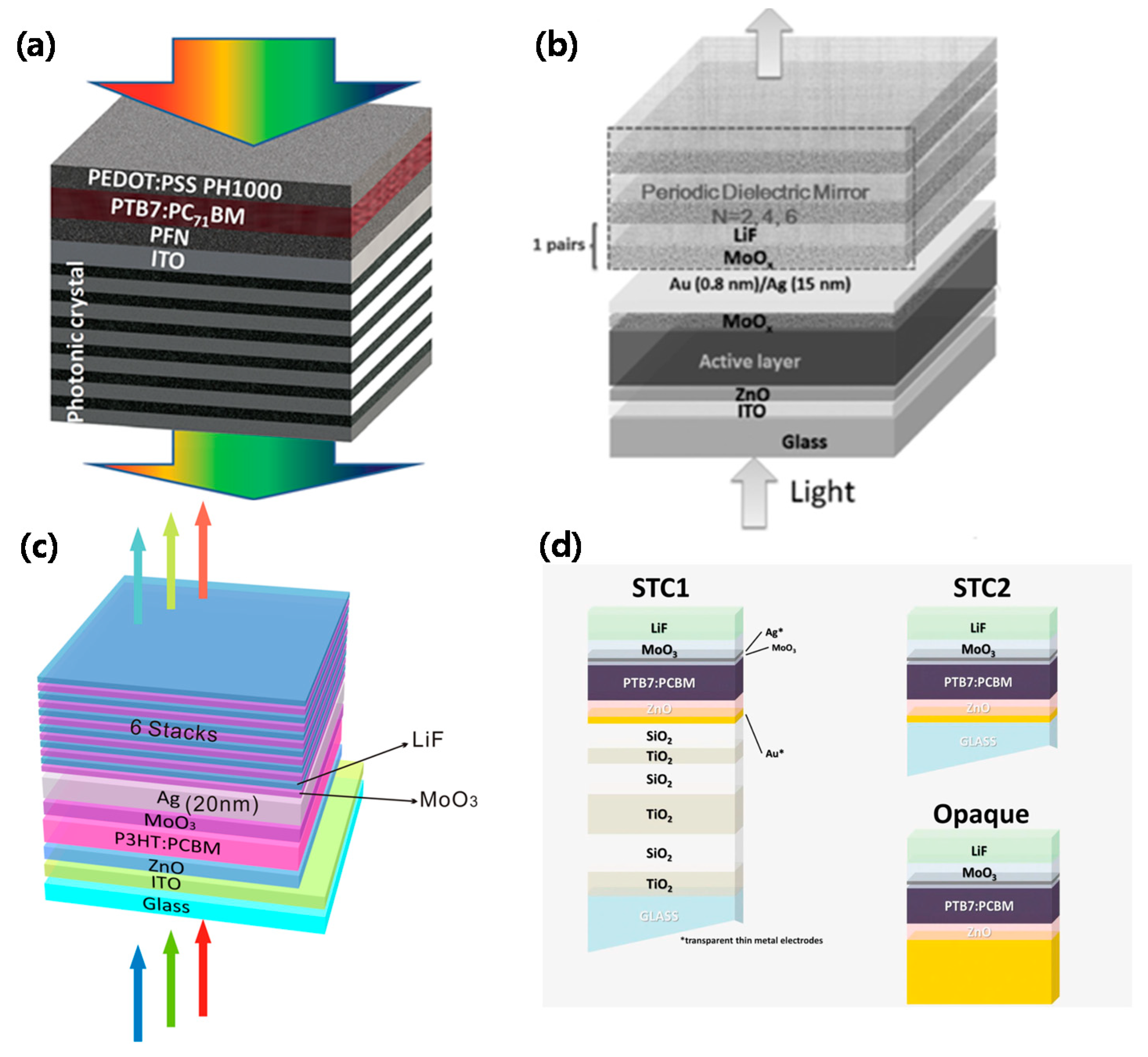
3.2.2. Photonic Crystals
3.2.3. Tandem Cells
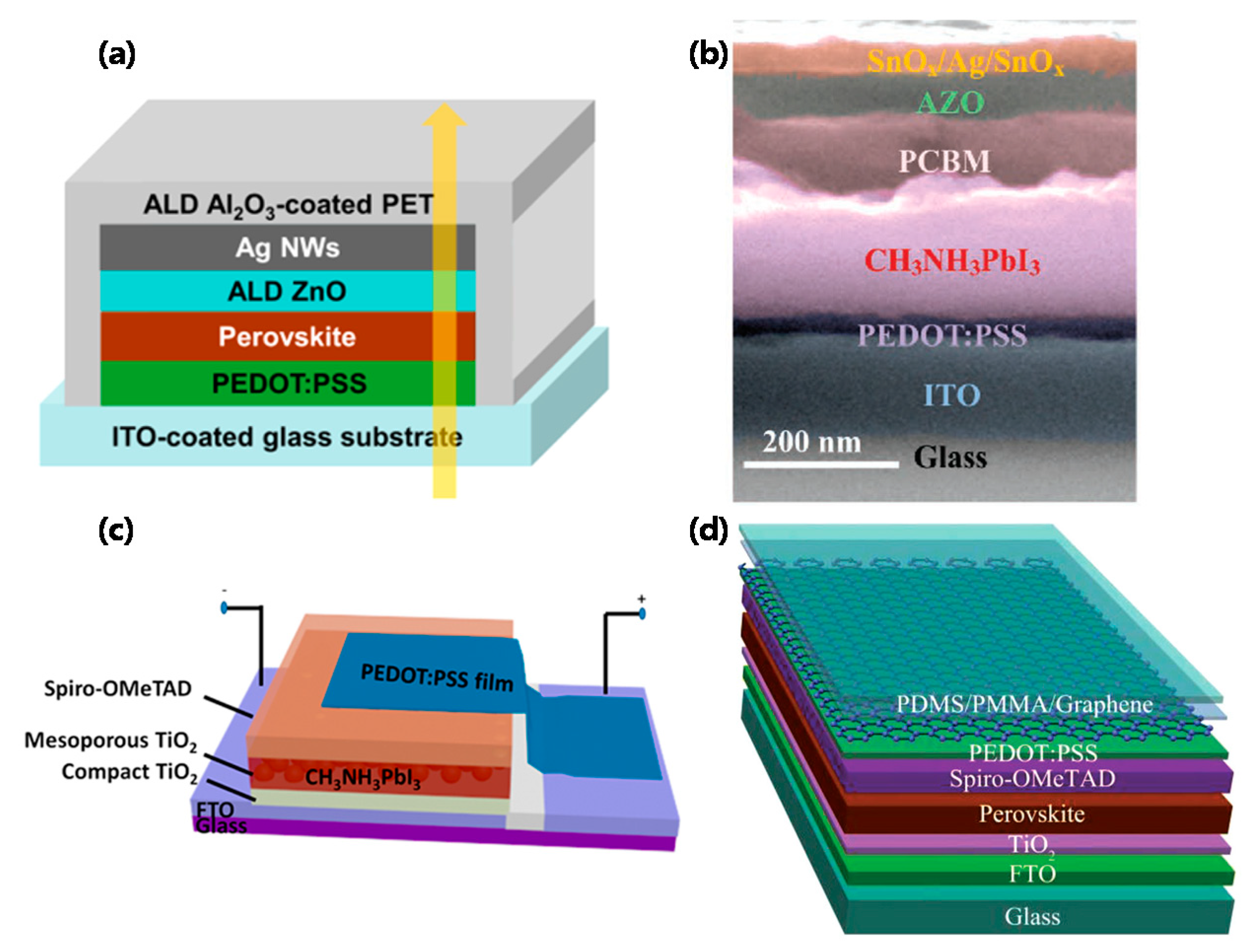
4. Semitransparent Perovskite Solar Cells
4.1. Single-Junction Cells
4.1.1. General Trend
4.1.2. Use of TCO Electrodes
4.1.3. Use of One or Both TCO—Free Electrodes
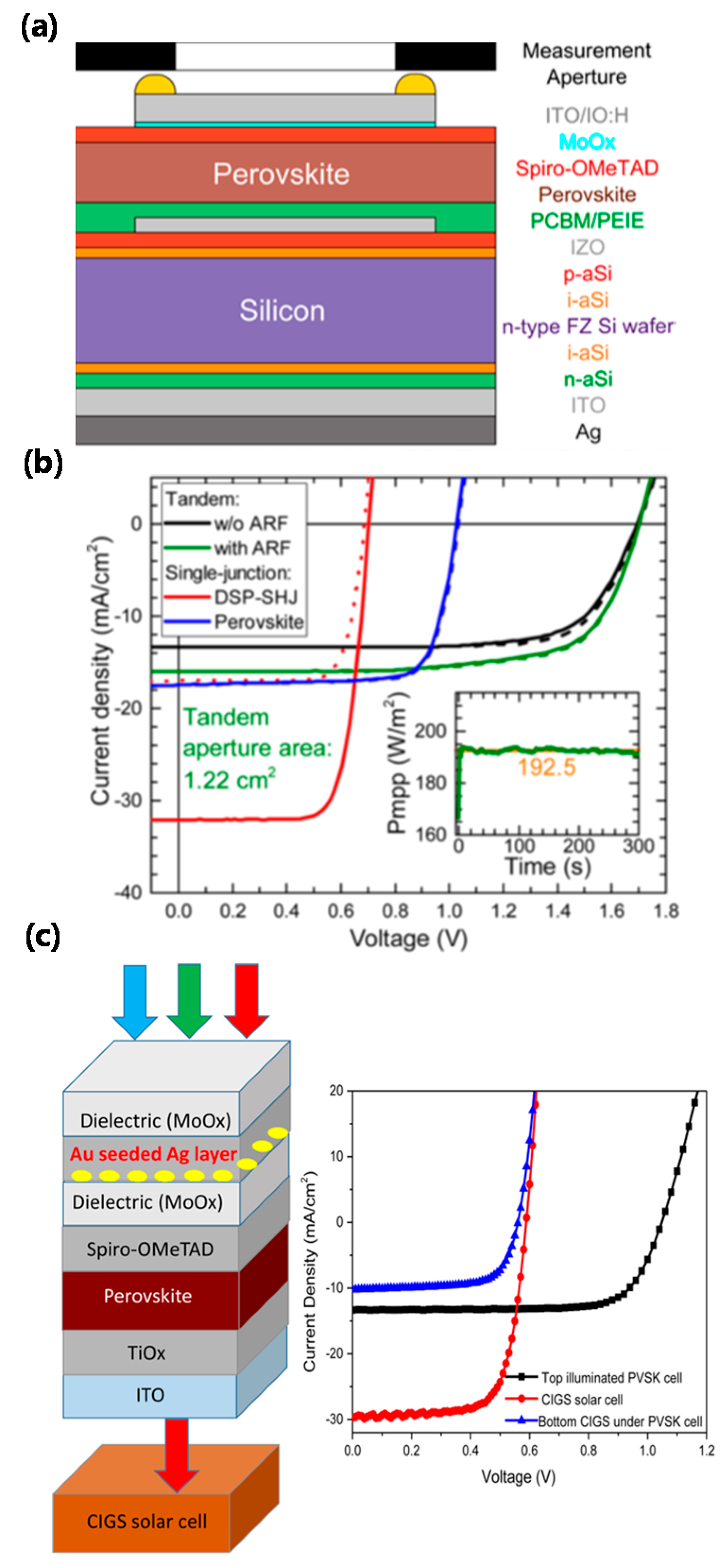
4.2. Multi-Junction Cells
5. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Gaur, A.; Tiwari, G.N. Performance of photovoltaic modules of different solar cells. Sol. Energy 2013, 2013, 734581. [Google Scholar] [CrossRef]
- Green, M.A.; Hishikawa, Y.; Dunlop, E.D.; Levi, D.H.; Hohl-Ebinger, J.; Ho-Baillie, A.W.Y. Solar cell efficiency tables. Prog. Photovolt. Res. Appl. 2018, 26, 427–436. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Brittman, S.; Adhyaksa, G.W.P.; Garnett, E.C. The expanding world of hybrid perovskites: Materials properties and emerging applications. MRS Commun. 2015, 5, 7–26. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Martinson, A.B.F.; Elam, J.W.; Liu, J.; Pellin, M.J.; Marks, T.J.; Hupp, J.T. Radial electron collection in dye-sensitized solar cells. Nano Lett. 2008, 8, 2862–2866. [Google Scholar] [CrossRef] [PubMed]
- Bagnis, D.; Beverina, L.; Huang, H.; Silvestri, F.; Yao, Y.; Yan, H.; Pagani, G.A.; Marks, T.J.; Facchetti, A. Marked alkyl- vs. alkenyl-substitutent effects on squaraine dye solid-state structure, carrier mobility, and bulk-heterojunction solar cell efficiency. J. Am. Chem. Soc. 2010, 132, 4074–4075. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Prabakaran, K.; Lee, B.; Chang, S.H.; Harutyunyan, B.; Guo, P.; Butler, M.R.; Timalsina, A.; Bedzyk, M.J.; Ratner, M.A.; et al. Metal-free tetrathienoacene sensitizers for high-performance dye sensitized solar cells. J. Am. Chem. Soc. 2015, 137, 4414–4423. [Google Scholar] [CrossRef] [PubMed]
- Yella, A.; Lee, H.-W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.-G.; Yeh, C.-Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrinsensitized solar cells with cobalt (II/III)-based redox electrolyte exceed 12% efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, P.; Baig, H.; Mallick, T.K.; Siviter, J.; Montecucco, A.; Lib, W.; Paul, M.; Sweet, T.; Gaoc, M.; Knox, A.R.; et al. Enhancing the efficiency of transparent dye-sensitized solar cells using concentrated light. Sol. Energy Mater. Sol. Cells 2018, 175, 29–34. [Google Scholar] [CrossRef]
- Yoon, S.; Tak, S.; Kim, J.; Jun, Y.; Kang, K.; Park, J. Application of transparent dye-sensitized solar cells to building integrated photovoltaic systems. Build. Environ. 2011, 46, 1899–1904. [Google Scholar] [CrossRef]
- Chen, D.; Huang, F.; Cheng, Y.B.; Caruso, R.A. Mesoporous anatase TiO2 beads with high surface areas and controllable pore sizes: A superior candidate for high-performance dye-sensitized solar cells. Adv. Mater. 2009, 21, 2206–2210. [Google Scholar] [CrossRef]
- Li, D.; Qin, D.; Deng, M.; Luo, Y.; Meng, Q. Optimization the solid-state electrolytes for dye-sensitized solar cells. Energy Environ. Sci. 2009, 2, 283–291. [Google Scholar] [CrossRef]
- Chueh, C.-C.; Chien, S.-C.; Yip, H.-L.; Salinas, J.F.; Li, C.-Z.; Chen, K.-S.; Chen, F.-C.; Chen, W.-C.; Jen, A.K.-Y. Toward high-performance semi-transparent polymer solar cells: Optimization of ultra-thin light absorbing layer and transparent cathode architecture. Adv. Energy Mater. 2013, 3, 417–423. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, S.; Zhao, W.; Yao, H.; Hou, J. Highly efficient 2D-conjugated benzodithiophene-based photovoltaic polymer with linear alkylthio side chain. Chem. Mater. 2014, 26, 3603–3605. [Google Scholar] [CrossRef]
- Park, N.-G. Perovskite solar cells: An emerging photovoltaic technology. Mater. Today 2015, 18, 65–72. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting tin and lead iodide perovskites with organic cations: Phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef] [PubMed]
- Miyata, A.; Mitioglu, A.; Plochocka, P.; Portugall, O.; Wang, J.T.-W.; Stranks, S.D.; Snaith, H.J.; Nicholas, R.J. Direct mesasurement of the exciton binding energy and effective masses for charge carriers in organic-inorganic tri-halide perovskites. Nat. Phys. 2015, 11, 582–587. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, K. Organic–inorganic hybrid lead halide perovskites for optoelectronic and electronic applications. Chem. Soc. Rev. 2016, 45, 655–689. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jasieniak, J.J. Semi-transparent solar cells. J. Phys. D Appl. Phys. 2017, 50, 093001. [Google Scholar] [CrossRef]
- Xue, Q.; Xia, R.; Brabec, C.J.; Yip, H.-L. Recent advances in semi-transparent polymer and perovskite solar cells for power generating window applications. Energy Environ. Sci. 2018, 11, 1688–1709. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Cui, C.; Li, Y. Flexible and semitransparent organic solar cells. Adv. Energy Mater. 2018, 8, 1701791. [Google Scholar] [CrossRef]
- Lee, K.-T.; Guo, L.J.; Park, H.J. Neutral- and multi-colored semitransparent perovskite solar cells. Molecules 2016, 21, 475. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cheun, H.; Choi, S.; Fuentes-Hernandez, C.; Kippelen, B. Optimization of a polymer top electrode for inverted semitransparent organic solar cells. Org. Electron. 2011, 12, 827–831. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, F.; Barrau, S.; Tian, W.; Inganäs, O.; Zhang, F. Inverted and transparent polymer solar cells prepared with vacuum-free processing. Sol. Energy Mater. Sol. Cells 2009, 93, 497–500. [Google Scholar] [CrossRef]
- Dong, Q.; Zhou, Y.; Pei, J.; Liu, Z.; Li, Y.; Yao, S.; Zhang, J.; Tian, W. All-spin-coating vacuum-free processed semi-transparent inverted polymer solar cells with PEDOT:PSS anode and PAH-D interfacial layer. Org. Electron. 2010, 11, 1327–1331. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, B.; Qin, F.; Wu, Y.; Meng, W.; Dai, S.; Zhou, Y.; Zhan, X. Semitransparent, non-fullerene and flexible all-plastic solar cells. Polymer 2016, 107, 108–112. [Google Scholar] [CrossRef]
- Kim, Y.H.; Müller-Meskamp, L.; Zakhidov, A.A.; Sachse, C.; Meiss, J.; Bikova, J.; Cook, A.; Zakhidov, A.A.; Leo, K. Semi-transparent small molecule organic solar cells with laminated free-standing carbon nanotube top electrodes. Sol. Energy Mater. Sol. Cells 2012, 96, 244–250. [Google Scholar] [CrossRef]
- Xia, X.; Wang, S.; Jia, Y.; Bian, Z.; Wu, D.; Zhang, L.; Cao, A.; Huang, C. Infrared-transparent polymer solar cells. J. Mater. Chem. 2010, 20, 8478–8482. [Google Scholar] [CrossRef]
- Jeon, I.; Delacou, C.; Kaskela, A.; Kauppinen, E.I.; Maruyama, S.; Matsuo, Y. Metal-electrode-free window-like organic solar cells with p-doped carbon nanotube thin-film electrodes. Sci. Rep. 2016, 6, 31348. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-Y.; Tu, K.-H.; Yu, C.-C.; Li, S.-S.; Hwang, J.-Y.; Lin, C.-C.; Chen, K.-H.; Chen, L.-C.; Chen, H.-L.; Chen, C.-W. Top laminated graphene electrode in a semitransparent polymer solar cell by simultaneous thermal annealing/releasing method. ACS Nano 2011, 5, 6564–6570. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, J.; Sun, Z.-H.; Tai, G.; Lau, S.-P.; Yan, F. The application of highly doped single-layer graphene as the top electrodes of semitransparent organic solar cells. ACS Nano 2012, 6, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; You, P.; Liu, S.; Yan, F. Neutral-color semitransparent organic solar cells with all-graphene electrodes. ACS Nano 2015, 9, 12026–12034. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Choy, W.C.H.; Zhang, D.; Xie, F.; Xin, J.; Leung, C.W. Semitransparent organic solar cells with hybrid monolayer graphene/metal grid as top electrodes. Appl. Phys. Lett. 2013, 102, 113303. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.H.; Jang, C.W.; Lee, H.S.; Seo, S.W.; Choi, S.-H. Semitransparent flexible organic solar cells employing doped-graphene layers as anode and cathode electrodes. ACS Appl. Mater. Interfaces 2018, 10, 3596–3601. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Connor, S.T.; Cui, Y.; Peumans, P. Semitransparent organic photovoltaic cells with laminated top electrode. Nano Lett. 2010, 10, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Krantz, J.; Stubhan, T.; Richter, M.; Spallek, S.; Litzov, I.; Matt, G.J.; Spiecker, E.; Brabec, C.J. Spray-coated silver nanowires as top electrode layer in semitransparent P3HT:PCBM-based organic solar cell devices. Adv. Funct. Mater. 2013, 23, 1711–1717. [Google Scholar] [CrossRef]
- Guo, F.; Zhu, X.; Forberich, K.; Krantz, J.; Stubhan, T.; Salinas, M.; Halik, M.; Spallek, S.; Butz, B.; Spiecker, E.; et al. ITO-free and fully solution-processed semitransparent organic solar cells with high fill factors. Adv. Energy Mater. 2013, 3, 1062–1067. [Google Scholar] [CrossRef]
- Yim, J.H.; Joe, S.-Y.; Pang, C.; Lee, K.M.; Jeong, H.; Park, J.-Y.; Ahn, Y.H.; de Mello, J.C.; Lee, S. Fully solution-processed semitransparent organic solar cells with a silver nanowire cathode and a conducting polymer anode. ACS Nano 2014, 8, 2857–2863. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Dou, L.; Zhu, R.; Chung, C.-H.; Song, T.-B.; Zheng, Y.B.; Hawks, S.; Li, G.; Weiss, P.S.; Yang, Y. Visibly transparent polymer solar cells produced by solution processing. ACS Nano 2012, 6, 7185–7190. [Google Scholar] [CrossRef] [PubMed]
- Maisch, P.; Tam, K.C.; Lucera, L.; Egelhaafa, H.-J.; Scheiber, H.; Maier, E.; Brabec, C.J. Inkjet printed silver nanowire percolation networks as electrodes for highly efficient semitransparent organic solar cells. Org. Electron. 2016, 38, 139–143. [Google Scholar] [CrossRef]
- Colsmann, A.; Puetz, A.; Bauer, A.; Hanisch, J.; Ahlswede, E.; Lemmer, U. Efficient semi-transparent organic solar cells with good transparency color perception and rendering properties. Adv. Energy Mater. 2011, 1, 599–603. [Google Scholar] [CrossRef]
- Chen, K.-S.; Salinas, J.-F.; Yip, H.-L.; Huo, L.; Hou, J.; Jen, A.K.Y. Semi-transparent polymer solar cells with 6% PCE, 25% average visible transmittance and a color rendering index close to 100 for power generating window applications. Energy Environ. Sci. 2012, 5, 9551–9557. [Google Scholar] [CrossRef]
- Wong, Y.Q.; Meng, H.-F.; Wong, H.Y.; Tan, C.S.; Wu, C.-Y.; Tsai, P.-T.; Chang, C.-Y.; Horng, S.-F.; Zan, H.-W. Efficient semitransparent organic solar cells with good color perception and good color rendering by blade coating. Org. Electron. 2017, 43, 196–206. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, Z.; Zhang, C.; Zhang, J.; Hu, Q.; Vergote, T.; Liu, F.; Russell, T.P.; Zhu, X. Efficient semitransparent solar cells with high NIR responsiveness enabled by a small-bandgap electron acceptor. Adv. Mater. 2017, 29, 1606574. [Google Scholar] [CrossRef] [PubMed]
- Czolk, J.; Landerer, D.; Koppitz, M.; Nass, D.; Colsmann, A. Highly efficient, mechanically flexible, semi-transparent organic solar cells doctor bladed from non-halogenated solvents. Adv. Mater. Technol. 2016, 1, 1600184. [Google Scholar] [CrossRef]
- Song, Y.; Chang, S.; Gradecak, S.; Kong, J. Visibly-transparent organic solar cells on flexible substrates with all-graphene electrodes. Adv. Energy Mater. 2016, 6, 1600847. [Google Scholar] [CrossRef]
- Upama, M.B.; Wright, M.; Elumalai, N.K.; Mahmud, M.A.; Wang, D.; Xu, C.; Uddin, A. High-efficiency semitransparent organic solar cells with non-fullerene acceptor for window application. ACS Photonics 2017, 4, 2327–2334. [Google Scholar] [CrossRef]
- Yin, Z.; Wei, J.; Chen, S.-C.; Cai, D.; Ma, Y.; Wang, M.; Zheng, Q. Long lifetime stable and efficient semitransparent organic solar cells using a ZnMgO-modified cathode combined with a thin MoO3/Ag anode. J. Mater. Chem. A 2017, 5, 3888–3899. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, C.; Yao, H.; Zhu, J.; Wang, Y.; Jia, G.; Gao, F.; Hou, J. Efficient semitransparent organic solar cells with tunable color enabled by an ultralow-bandgap nonfullerene acceptor. Adv. Mater. 2017, 29, 1703080. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Shen, L.; Shen, P.; Meng, F.; Long, Y.; Wang, Y.; Lv, T.; Ruan, S.; Chen, G. Simultaneous improvement in efficiency and transmittance of low bandgap semitransparent polymer solar cells with one-dimensional photonic crystals. Sol. Energy Mater. Sol. Cells 2013, 117, 198–202. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Z.; Cai, C.; Liu, Z.; Lin, Y.; Zheng, W.; Yang, J.; Hou, L.; Cao, Y. Colorful semitransparent polymer solar cells employing a bottom periodic one-dimensional photonic crystal and a top conductive PEDOT:PSS layer. J. Mater. Chem. A 2016, 4, 11821–11828. [Google Scholar] [CrossRef]
- Xu, G.; Shen, L.; Cui, C.; Wen, S.; Xue, R.; Chen, W.; Chen, H.; Zhang, J.; Li, H.; Li, Y.; et al. High-performance colorful semitransparent polymer solar cells with ultrathin hybrid-metal electrodes and fine-tuned dielectric mirrors. Adv. Funct. Mater. 2017, 27, 1605908. [Google Scholar] [CrossRef]
- Zhang, D.-D.; Jiang, X.-C.; Wang, R.; Xie, H.-J.; Ma, G.-F.; Ou, Q.-D.; Chen, Y.-L.; Li, Y.-Q.; Tang, J.-X. Enhanced performance of semitransparent inverted organic photovoltaic devices via a high reflector structure. ACS Appl. Mater. Interfaces 2013, 5, 10185–10190. [Google Scholar] [CrossRef] [PubMed]
- Pastorelli, F.; Romero-Gomez, P.; Betancur, R.; Martinez-Otero, A.; Mantilla-Perez, P.; Bonod, N.; Martorell, J. Enhanced light harvesting in semitransparent organic solar cells using an optical metal cavity configuration. Adv. Energy Mater. 2014, 5, 1400614. [Google Scholar] [CrossRef]
- Meiss, J.; Menke, T.; Leo, K.; Uhrich, C.; Gnehr, W.-M.; Sonntag, S.; Pfeiffer, M.; Riede, M. Highly efficient semitransparent tandem organic solar cells with complementary absorber materials. Appl. Phys. Lett. 2011, 99, 043301. [Google Scholar] [CrossRef]
- Tang, Z.; George, Z.; Ma, Z.; Bergqvist, J.; Tvingstedt, K.; Vandewal, K.; Wang, E.; Andersson, L.M.; Andersson, M.R.; Zhang, F.; et al. Semi-transparent tandem organic solar cells with 90% internal quantum efficiency. Adv. Energy Mater. 2012, 2, 1467–1476. [Google Scholar] [CrossRef]
- Yusoff, A.R.B.M.; Lee, S.J.; Shneider, F.K.; da Silva, W.J.; Jang, J. High-performance semitransparent tandem solar cell of 8.02% conversion efficiency with solution-processed graphene mesh and laminated Ag nanowire top electrodes. Adv. Energy Mater. 2014, 4, 1301989. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Zuo, L.; Yip, H.-L.; Li, C.-Z.; Li, Y.; Hsu, C.-S.; Cheng, Y.-J.; Chen, H.; Jen, A.K.-Y. Highly efficient polymer tandem cells and semitransparent cells for solar energy. Adv. Energy Mater. 2013, 4, 1301645. [Google Scholar] [CrossRef]
- Chen, C.-C.; Dou, L.; Gao, J.; Chang, W.-H.; Li, G.; Yang, Y. High-performance semi-transparent polymer solar cells possessing tandem structures. Energy Environ. Sci. 2013, 6, 2714–2720. [Google Scholar] [CrossRef]
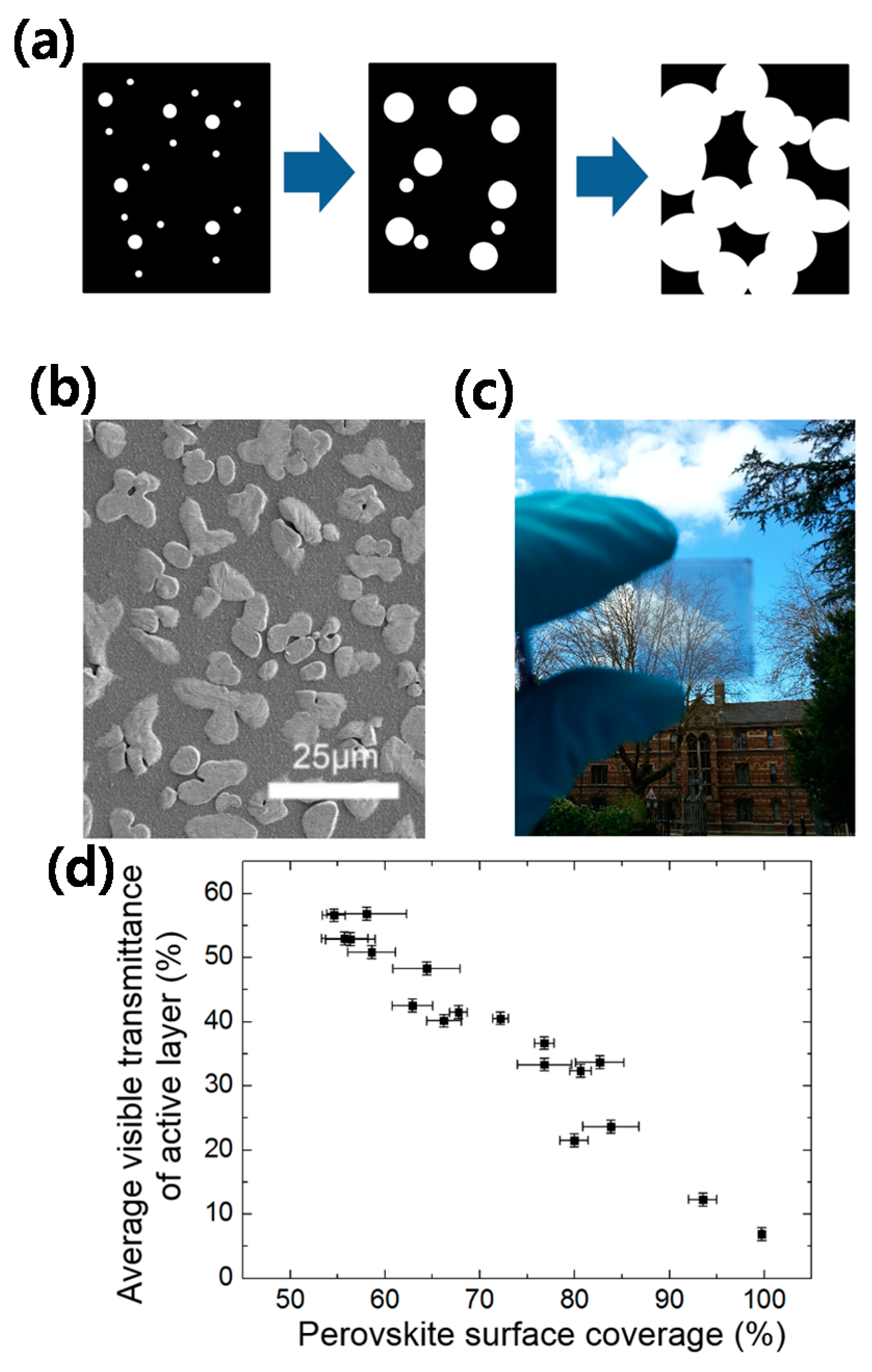
- Eperon, G.E.; Burlakov, V.M.; Goriely, A.; Snaith, H.J. Neutral color semitransparent microstructured perovskite solar cells. ACS Nano 2014, 8, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-X.; Rao, H.-S.; Chen, H.-Y.; Li, W.-G.; Kuang, D.-B.; Su, C.-Y. Ordered macroporous CH3NH3PbI3 perovskite semitransparent film for high-performance solar cells. J. Mater. Chem. A 2016, 4, 15662–15669. [Google Scholar] [CrossRef]
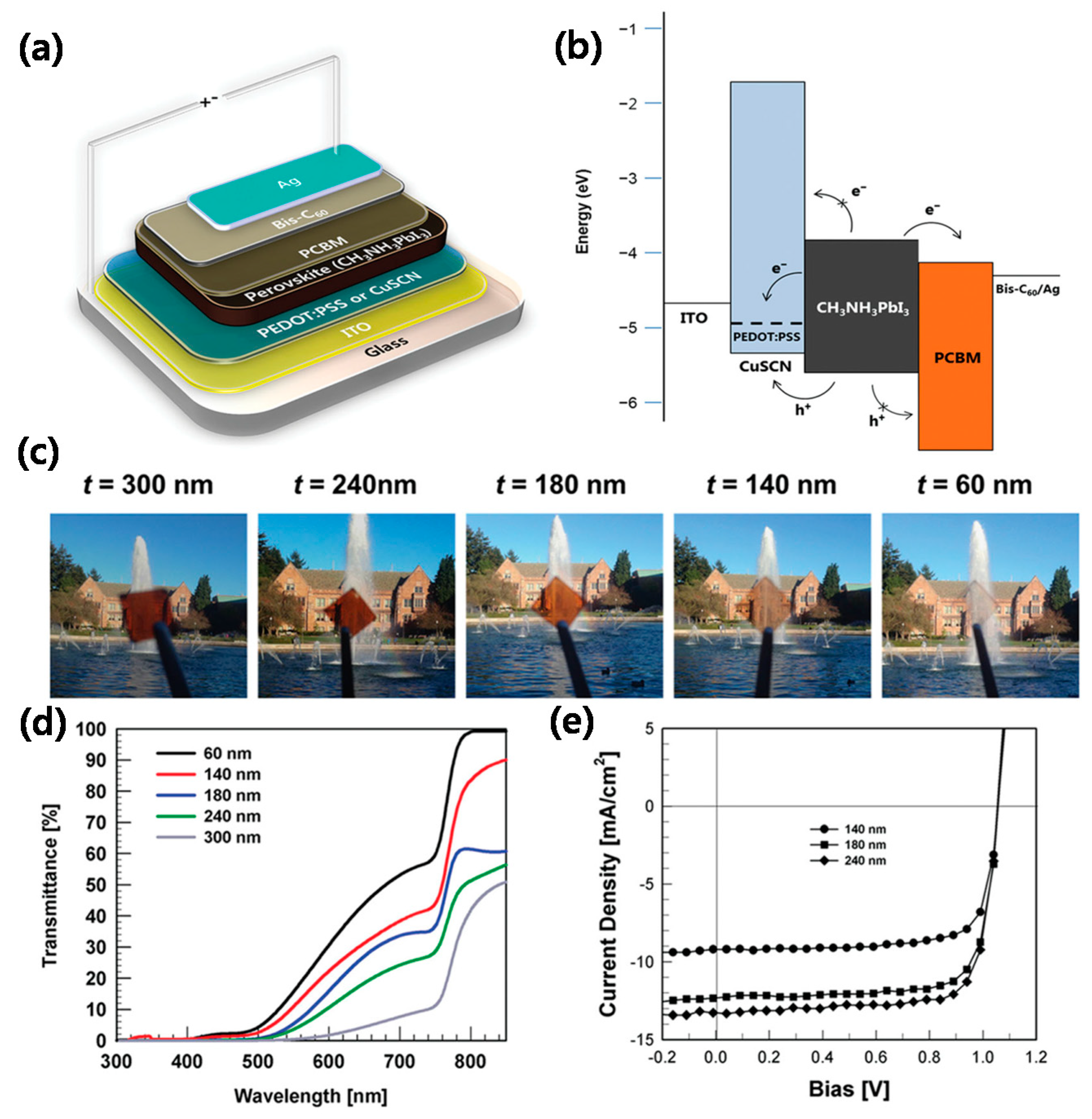
- Jung, J.W.; Chueh, C.-C.; Jen, A.K.-Y. High-performance semitransparent perovskite solar cells with 10% power conversion efficiency and 25% average visible transmittance based on transparent CuSCN as the hole-transporting material. Adv. Energy Mater. 2015, 5, 1500486. [Google Scholar] [CrossRef]
- Roldán-Carmona, C.; Malinkiewicz, O.; Betancur, R.; Longo, G.; Momblona, C.; Jaramillo, F.; Camacho, L.; Bolink, H.J. High efficiency single-junction semitransparent perovskite solar cells. Energy Environ. Sci. 2014, 7, 2968–2973. [Google Scholar] [CrossRef]
- Gaspera, E.D.; Peng, Y.; Hou, Q.; Spiccia, L.; Bach, U.; Jasieniak, J.J.; Cheng, Y.-B. Ultra-thin high efficiency semitransparent perovskitesolarcells. Nano Energy 2015, 13, 249–257. [Google Scholar] [CrossRef]
- Bag, S.; Durstock, M.F. Efficient semi-transparent planar perovskite solar cells using a ‘molecular glue’. Nano Energy 2016, 30, 542–548. [Google Scholar] [CrossRef]
- Wang, Y.; Mahmoudi, T.; Yang, H.-Y.; Bhat, K.S.; Yoo, J.-Y.; Hahn, Y.-B. Fully-ambient-processed mesoscopic semitransparent perovskite solar cells by islands-structure-MAPbI3−xClx-NiO composite and Al2O3/NiO interface engineering. Nano Energy 2018, 49, 59–66. [Google Scholar] [CrossRef]
- Heo, J.H.; Han, H.J.; Lee, M.; Song, M.; Kim, D.H.; Im, S.H. Stable semi-transparent CH3NH3PbI3 planar sandwich solar cells. Energy Environ. Sci. 2015, 8, 2922–2927. [Google Scholar] [CrossRef]
- Fu, F.; Feurer, T.; Jäger, T.; Avancini, E.; Bissig, B.; Yoon, S.; Buecheler, S.; Tiwari, A.N. Low-temperature-processed efficient semi-transparent planar perovskite solar cells for bifacial and tandem applications. Nat. Commun. 2015, 6, 8932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, J.H.; Jang, M.H.; Lee, M.H.; Han, H.J.; Kang, M.G.; Lee, M.L.; Im, S.H. Efficiency enhancement of semi-transparent sandwich type CH3NH3PbI3 perovskite solar cells with island morphology perovskite film by introduction of polystyrene passivation layer. J. Mater. Chem. A 2016, 4, 16324–16329. [Google Scholar] [CrossRef]
- Heo, J.H.; Han, J.; Shin, D.H.; Im, S.H. Highly stable semi-transparent CH3NH3PbI3 sandwich type perovskite solar sub-module with neutral color. Mater. Today Energy 2017, 5, 280–286. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Lee, K.-T.; Huang, W.-K.; Siao, H.-Y.; Chang, Y.-C. High-performance, air-stable, low-temperature processed semitransparent perovskite solar cells enabled by atomic layer deposition. Chem. Mater. 2015, 27, 5122–5130. [Google Scholar] [CrossRef]
- Guo, F.; Azimi, H.; Hou, Y.; Przybilla, T.; Hu, M.; Bronnbauer, C.; Langner, S.; Spiecker, E.; Forberich, K.; Brabec, C.J. High-performance semitransparent perovskite solar cells with solution-processed silver nanowires as top electrodes. Nanoscale 2015, 7, 1642–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Brinkmann, K.O.; Hu, T.; Pourdavoud, N.; Becker, T.; Gahlmann, T.; Heiderhoff, R.; Polywka, A.; Görrn, P.; Chen, Y.; et al. Self-encapsulating thermostable and air-resilient semitransparent perovskite solar cells. Adv. Energy Mater. 2017, 7, 1602599. [Google Scholar] [CrossRef]
- Bu, L.; Liu, Z.; Zhang, M.; Li, W.; Zhu, A.; Cai, F.; Zhao, Z.; Zhou, Y. Semitransparent fully air processed perovskite solar cells. ACS Appl. Mater. Interfaces 2015, 7, 17776–17781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Z.; Li, P.; Ono, L.K.; Qi, Y.; Zhou, J.; Shen, H.; Surya, C.; Zheng, Z. Fully solution-processed TCO-free semitransparent perovskite solar cells for tandem and flexible applications. Adv. Energy Mater. 2018, 8, 1701569. [Google Scholar] [CrossRef]
- Li, F.R.; Xu, Y.; Chen, W.; Xie, S.H.; Li, J.Y. Nanotube enhanced carbon grids as top electrodes for fully printable mesoscopic semitransparent perovskite solar cells. J. Mater. Chem. A 2017, 5, 10374–10379. [Google Scholar] [CrossRef]
- You, P.; Liu, Z.; Tai, Q.; Liu, S.; Yan, F. Efficient semitransparent perovskite solar cells with graphene electrodes. Adv. Mater. 2015, 27, 3632–3638. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.; Weng, C.-H.; Walter, A.; Fesquet, L.; Seif, J.P.; Wolf, S.D.; Niesen, B.; Ballif, C. Efficient monolithic perovskite/silicon tandem solar cell with cell area >1 cm2. J. Phys. Chem. Lett. 2016, 7, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Bai, Y.; Yu, Z.; Li, T.; Zheng, X.; Dong, Q.; Shen, L.; Boccard, M.; Gruverman, A.; Holman, Z.; et al. Efficient semitransparent perovskite solar cells for 23.0%-efficiency perovskite/silicon four-terminal tandem cells. Adv. Energy Mater. 2016, 6, 1601128. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Q.; Hsieh, Y.-T.; Song, T.-B.; Marco, N.D.; Zhou, H.; Yang, Y. Multilayer transparent top electrode for solution processed perovskite/Cu(In,Ga)(Se,S)2 four terminal tandem solar cells. ACS Nano 2015, 9, 7714–7721. [Google Scholar] [CrossRef] [PubMed]
- Bailie, C.D.; Christoforo, M.G.; Mailoa, J.P.; Bowring, A.R.; Unger, E.L.; Nguyen, W.H.; Burschka, J.; Pellet, N.; Lee, J.Z.; Grätzel, M.; et al. Semi-transparent perovskite solar cells for tandems with silicon and CIGS. Energy Environ. Sci. 2015, 8, 956–963. [Google Scholar] [CrossRef]
- Drolet, N. Organic Photovoltaic: Efficiency and lifetime challenges for commercial viability. In Proceedings of the 2012 MRS Spring Meeting & Exhibit, San Francisco, CA, USA, 12 April 2012. [Google Scholar]
- Ameri, T.; Dennler, G.; Waldauf, C.; Azimi, H.; Seemann, A.; Forberich, K.; Hauch, J.; Scharber, M.; Hingerl, K.; Brabec, C.J. Fabrication, optical modeling, and color characterization of semitransparent bulk-heterojunction organic solar cells in an inverted structure. Adv. Funct. Mater. 2010, 20, 1592–1598. [Google Scholar] [CrossRef]
- Betancur, R.; Romero-Gomez, P.; Martinez-Otero, A.; Elias, X.; Maymó, M.; Martorell, J. Transparent polymer solar cells employing a layered light-trapping architecture. Nat. Photonics 2013, 7, 995–1000. [Google Scholar] [CrossRef]
- Amara, M.; Mandorlo, F.; Couderc, R.; Gérenton, F.; Lemiti, M. Temperature and color management of silicon solar cells for building integrated photovoltaic. EPJ Photovolt. 2018, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Czolk, J.; Puetz, A.; Kutsarov, D.; Reinhard, M.; Lemmer, U.; Colsmann, A. Inverted semi-transparent polymer solar cells with transparency color rendering indices approaching 100. Adv. Energy Mater. 2013, 3, 386–390. [Google Scholar] [CrossRef]
- Yu, W.; Jia, X.; Yao, M.; Zhu, L.; Long, Y.; Shen, L. Semitransparent polymer solar cells with simultaneously improved efficiency and color rendering index. Phys. Chem. Chem. Phys. 2015, 17, 23732–23740. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Salzman, R.F.; Rand, B.P.; Forrest, S.R. Semitransparent organic photovoltaic cells. Appl. Phys. Lett. 2006, 88, 233502. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Li, G.; Yang, Y. A semi-transparent plastic solar cell fabricated by a lamination process. Adv. Mater. 2008, 20, 415–419. [Google Scholar] [CrossRef]
- Upama, M.B.; Wright, M.; Elumalai, N.K.; Mahmud, M.A.; Wang, D.; Chan, K.H.; Xu, C.; Haque, F.; Uddin, A. High performance semitransparent organic solar cells with 5% PCE using non-patterned MoO3/Ag/MoO3 anode. Curr. Appl. Phys. 2017, 17, 298–305. [Google Scholar]
- Yu, W.; Shen, L.; Long, Y.; Shen, P.; Guo, W.; Chen, W.; Ruan, S. Highly efficient and high transmittance semitransparent polymer solar cells with one-dimensional photonic crystals as distributed Bragg reflectors. Org. Electron. 2014, 15, 470–477. [Google Scholar] [CrossRef]
- Granqvist, C.G. Transparent conductive electrodes for electrochromic devices: A review. Appl. Phys. A 1993, 57, 19–24. [Google Scholar] [CrossRef]
- Alemu, D.; Wei, H.-Y.; Ho, K.-C.; Chu, C.-W. Highly conductive PEDOT: PSS electrode by simple film treatment with methanol for ITO-free polymer solar cells. Energy Environ. Sci. 2012, 5, 9662–9671. [Google Scholar] [CrossRef]
- Kim, Y.H.; Sachse, C.; Machala, M.L.; May, C.; Müller-Meskamp, L.; Leo, K. Highly conductive PEDOT:PSS electrode with optimized solvent and thermal post-treatment for ITO-free organic solar cells. Adv. Funct. Mater. 2011, 21, 1076–1081. [Google Scholar] [CrossRef]
- Park, S.; Vosguerichian, M.; Bao, Z. A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale 2013, 5, 1727–1752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fang, S.; Zakhidov, A.A.; Lee, S.B.; Aliev, A.E.; Williams, C.D.; Atkinson, K.R.; Baughman, R.H. Strong, transparent, multifunctional, carbon nanotube sheets. Science 2005, 309, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Hernandez, Y.; Feng, X.; Müllen, K. Graphene as transparent electrode material for organic electronics. Adv. Mater. 2011, 23, 2779–2795. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Canlier, A.; Kim, G.H.; Choi, J.; Park, M.; Han, S.M. Electrostatic spray deposition of highly transparent silver nanowire electrode on flexible substrate. ACS Appl. Mater. Interfaces 2013, 5, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-W.; Cho, D.-Y.; Kim, J.; Na, S.-I.; Kim, H.-K. Simple brush-painting of flexible and transparent Ag nanowire network electrodes as an alternative ITO anode for cost-efficient flexible organic solar cells. Sol. Energy Mater. Sol. Cells 2012, 107, 348–354. [Google Scholar] [CrossRef]
- Emmott, C.J.M.; Urbina, A.; Nelson, J. Environmental and economic assessment of ITO-free electrodes for organic solar cells. Sol. Energy Mater. Sol. Cells 2012, 97, 14–21. [Google Scholar] [CrossRef]
- Gaynor, W.; Lee, J.-Y.; Peumans, P. Fully solution-processed inverted polymer solar cells with laminated nanowire electrodes. ACS Nano 2010, 4, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, W.; Burkhard, G.F.; McGehee, M.D.; Peumans, P. Smooth nanowire/polymer composite transparent electrodes. Adv. Mater. 2011, 23, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Bormann, L.; Müller-Meskamp, L.; Vandewal, K.; Leo, K. Efficient flexible organic photovoltaics using silver nanowires and polymer based transparent electrodes. Org. Electron. 2016, 36, 68–72. [Google Scholar] [CrossRef]
- Hong, K.; Ham, J.; Kim, B.-J.; Park, J.Y.; Lim, D.C.; Lee, J.Y.; Lee, J.-L. Continuous 1D-metallic microfibers web for flexible organic solar cells. ACS Appl. Mater. Interfaces 2015, 7, 27397–27404. [Google Scholar] [CrossRef] [PubMed]
- Angmo, D.; Andersen, T.R.; Bentzen, J.J.; Helgesen, M.; Søndergaard, R.R.; Jørgensen, M.; Carlé, J.E.; Bundgaard, E.; Krebs, F.C. Roll-to-roll printed silver nanowire semitransparent electrodes for fully ambient solution-processed tandem polymer solar cells. Adv. Funct. Mater. 2015, 25, 4539–4547. [Google Scholar] [CrossRef]
- Geusebroek, J.-M.; van den Boomgaard, R.; Smeulders, A.W.; Gevers, T. Color constancy from physical principles. Pattern Recognit. Lett. 2003, 24, 1653–1662. [Google Scholar] [CrossRef]
- Galagan, Y.; Debije, M.G.; Blom, P.W.M. Semitransparent organic solar cells with organic wavelength dependent reflectors. Appl. Phys. Lett. 2011, 98, 043302. [Google Scholar] [CrossRef] [Green Version]
- Van der Mee, C.; Contu, P.; Pintus, P. One-dimensional photonic crystal design. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 214–225. [Google Scholar] [CrossRef]
- Colodrero, S.; Mihi, A.; Häggman, L.; Ocaña, M.; Boschloo, G.; Hagfeldt, A.; Míguez, H. Porous one-dimensional photonic crystals improve the power-conversion efficiency of dye-sensitized solar cells. Adv. Mater. 2009, 21, 764–770. [Google Scholar] [CrossRef]
- Fink, Y.; Winn, J.N.; Fan, S.; Chen, C.; Michel, J.; Joannopoulos, J.D.; Thomas, E.L. A dielectric omnidirectional refector. Science 1998, 282, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Shen, L.; Long, Y.; Guo, W.; Meng, F.; Ruan, S.; Jia, X.; Ma, H.; Chen, W. Semitransparent polymer solar cells with one-dimensional (WO3/LiF)N photonic crystals. Appl. Phys. Lett. 2012, 101, 153307. [Google Scholar] [CrossRef]
- Yu, W.; Shen, L.; Shen, P.; Long, Y.; Sun, H.; Chen, W.; Ruan, S. Semitransparent polymer solar cells with 5% power conversion efficiency using photonic crystal reflector. ACS Appl. Mater. Interfaces 2014, 6, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Jia, X.; Long, Y.; Shen, L.; Liu, Y.; Guo, W.; Ruan, S. Highly efficient semitransparent polymer solar cells with color rendering index approaching 100 using one-dimensional photonic crystal. ACS Appl. Mater. Interfaces 2015, 7, 9920–9928. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Ruan, S.; Long, Y.; Shen, L.; Guo, W.; Chen, W. Light harvesting enhancement toward low IPCE region of semitransparent polymer solar cells via one-dimensional photonic crystal reflectors. Sol. Energy Mater. Sol. Cells 2014, 127, 27–32. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed]

| Photovoltaic Material | Thickness (μm) | Absorption Properties |
|---|---|---|
| c-Si | 180 | Broad band absorption |
| Absorption coefficient: 102 cm−1 | ||
| Organic material | 0.1 | Confined band absorption |
| Absorption coefficient: 105 cm−1 | ||
| Perovskites | 0.3 | Broad band absorption |
| Absorption coefficient: 105–106 cm−1 |
| Device Structure | Voc (V) | Jsc (mA·cm2) | FF (%) | PCE (%) | AVT (%) | Reference |
|---|---|---|---|---|---|---|
| Indium tin oxide (ITO)/poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS)/PhanQ: phenyl-C61-butyric acid methyl ester (PCBM)/buckminsterfullerene (C60)-surfactant/Ag | 0.84 | 8.0 | 63.0 | 4.2 | 32 | [16] |
| ITO/ZnO/poly(3-hexylthiophene) (P3HT):PCBM/PEDOT:PSS CPP 105D (CPP-PEDOT:PSS) | 0.55 | 7.4 | 58.0 | 2.4 | – | [27] |
| ITO/PEO/APFO3:PCBM/PEDOT:PSS P VP Al 4083 (PEDOT-EL)/PEDOT:PSS | 0.67 | 2.3 | 45.0 | 0.7 | – | [28] |
| ITO/ZnO nanoparticles (NPs)/P3HT:PCBM/poly(allylamine hydrochloride) and dextran(PAH-D)/PEDOT:PSS | 0.59 | 6.7 | 47.3 | 1.9 | – | [29] |
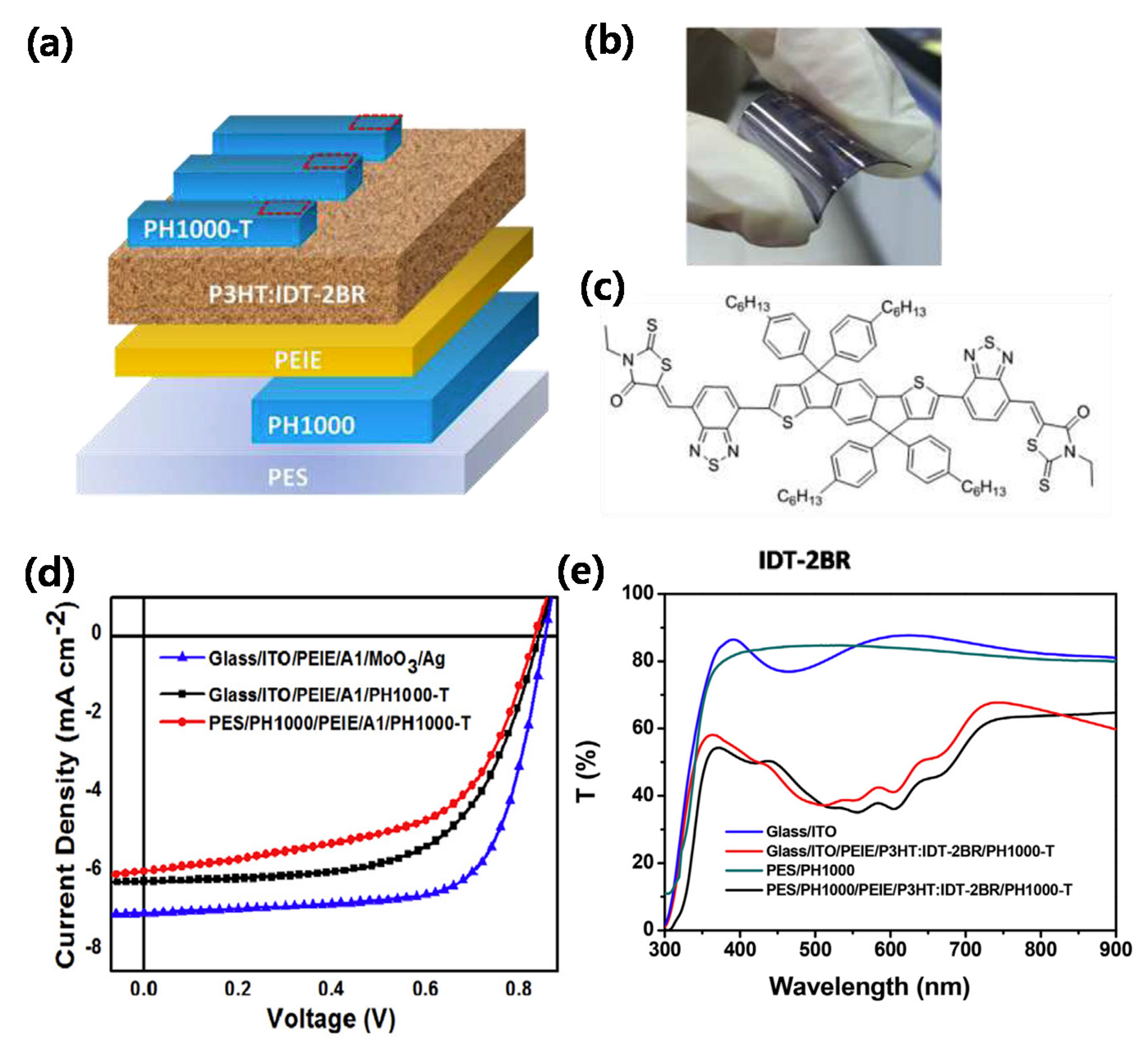
| PEDOT:PSS/P3HT:IDT-2BR/polyethylenimine (PEIE)/PEDOT:PSS | 0.84 | 5.9 | 57.9 | 2.9 | 50 | [30] |
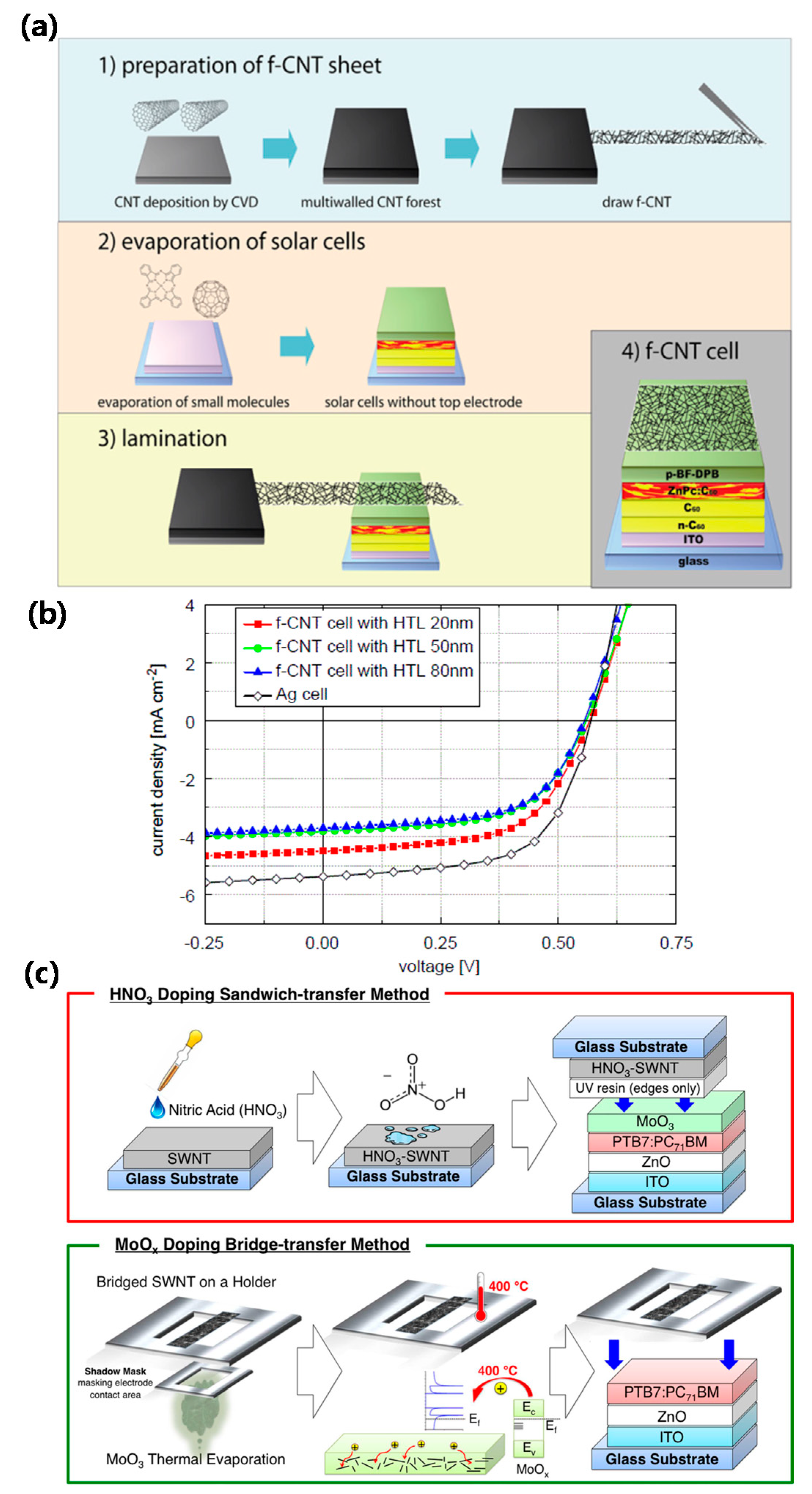
| ITO/n-C60/C60/zinc phthalocyanine (ZnPc):C60/2,20-(perfluoronaphthalene-2,6-diylidene) dima-lononitrile(F6TCNNQ) dopedN,N0-((diphenyl-N,N0-bis)9,9,-dime-thyl-fluoren-2-yl)-benzidine (p-BF-DPB)/carbonnanotube (CNT) | 0.57 | 4.5 | 58.2 | 1.5 | – | [31] |
| ITO/ZnO/P3HT:PCBM/CNT | 0.57 | 10.5 | 40.2 | 2.5 | 80 @ 670 nm | [32] |
| ITO/ZnO/polymer thieno[3,4-b]thiophene/benzodithiophene (PTB7):PCBM/MoOx/HNO3-doped CNT | 0.66 | 6.5 | 40.0 | 1.8 | 80 @ 400 nm | [33] |
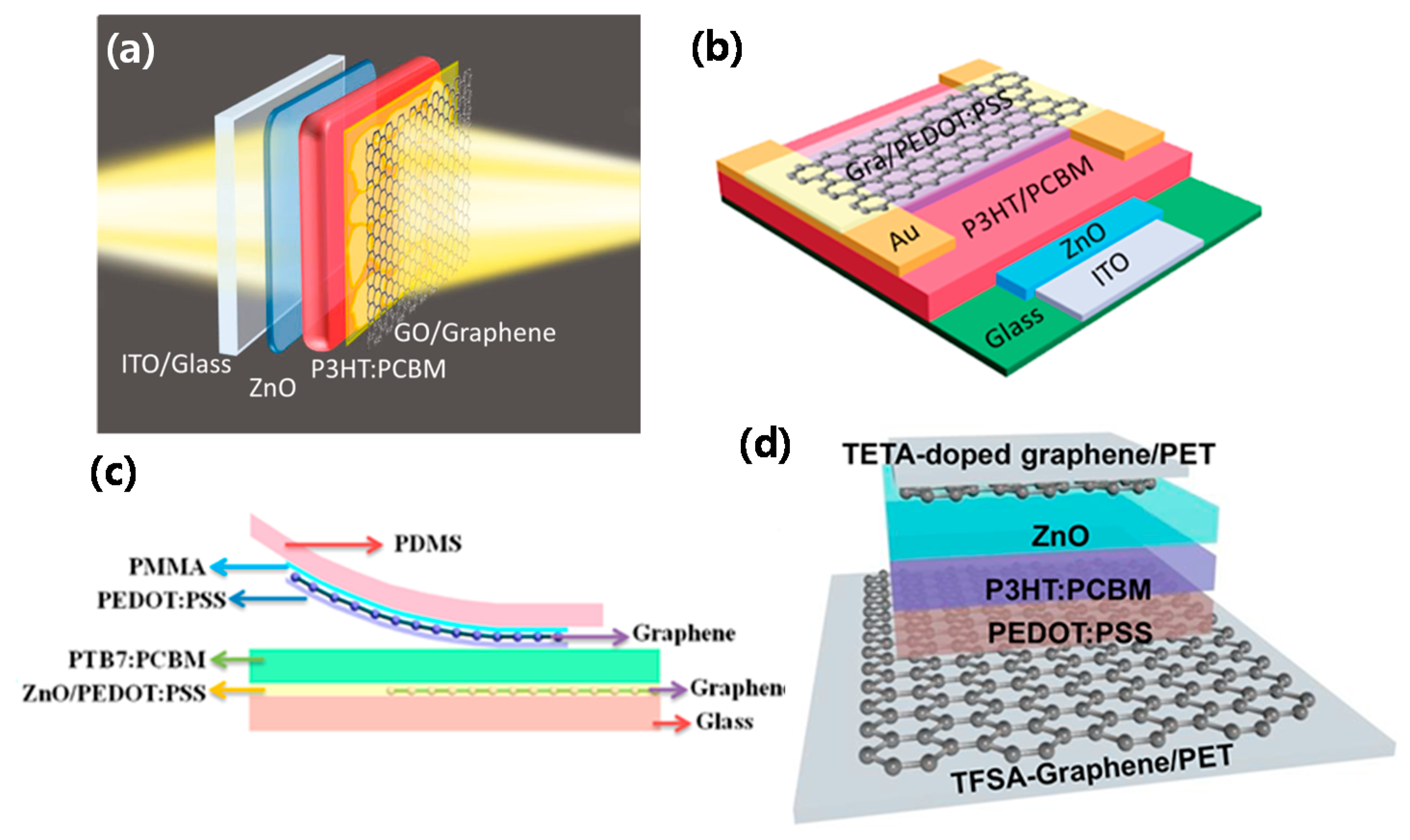
| ITO/ZnO/P3HT:PCBM/graphene oxide (GO)/graphene | 0.54 | 10.5 | 44.0 | 2.5 | 70 @ 650 nm | [34] |
| ITO/ZnO/P3HT:PCBM/PEDOT:PSS/Au-doped Graphene | 0.59 | 10.6 | 43.3 | 2.7 | – | [35] |
| graphene/PEDOT:PSS/PTB7: PhanQ: phenyl-C71-butyric acid methyl ester (PC71BM)/ZnO-NP/PEDOT:PSS/graphene | 0.67 | 12.1 | 41.4 | 3.4 | 40 | [36] |
| ITO/TiO2/P3HT:PCBM/PEDOT:PSS/graphene/Au grid/ | 0.62 | 8.2 | 55.0 | 2.8 | – | [37] |
| Triethylene tetramine (TETA) -doped graphene/ZnO/P3HT:PCBM/ PEDOT:PSS/(trifluoromethanesulfonyl)-amide (TFSA)-doped graphene | 0.62 | 9.8 | 54.5 | 3.3 | 70 @ 650 nm | [38] |
| ITO/copper phthalocyanine (CuPc):C60/bathocuproine (BCP)/Ag/Ag NWs | 0.44 | 1.9 | 55.0 | 0.6 | 26 | [39] |
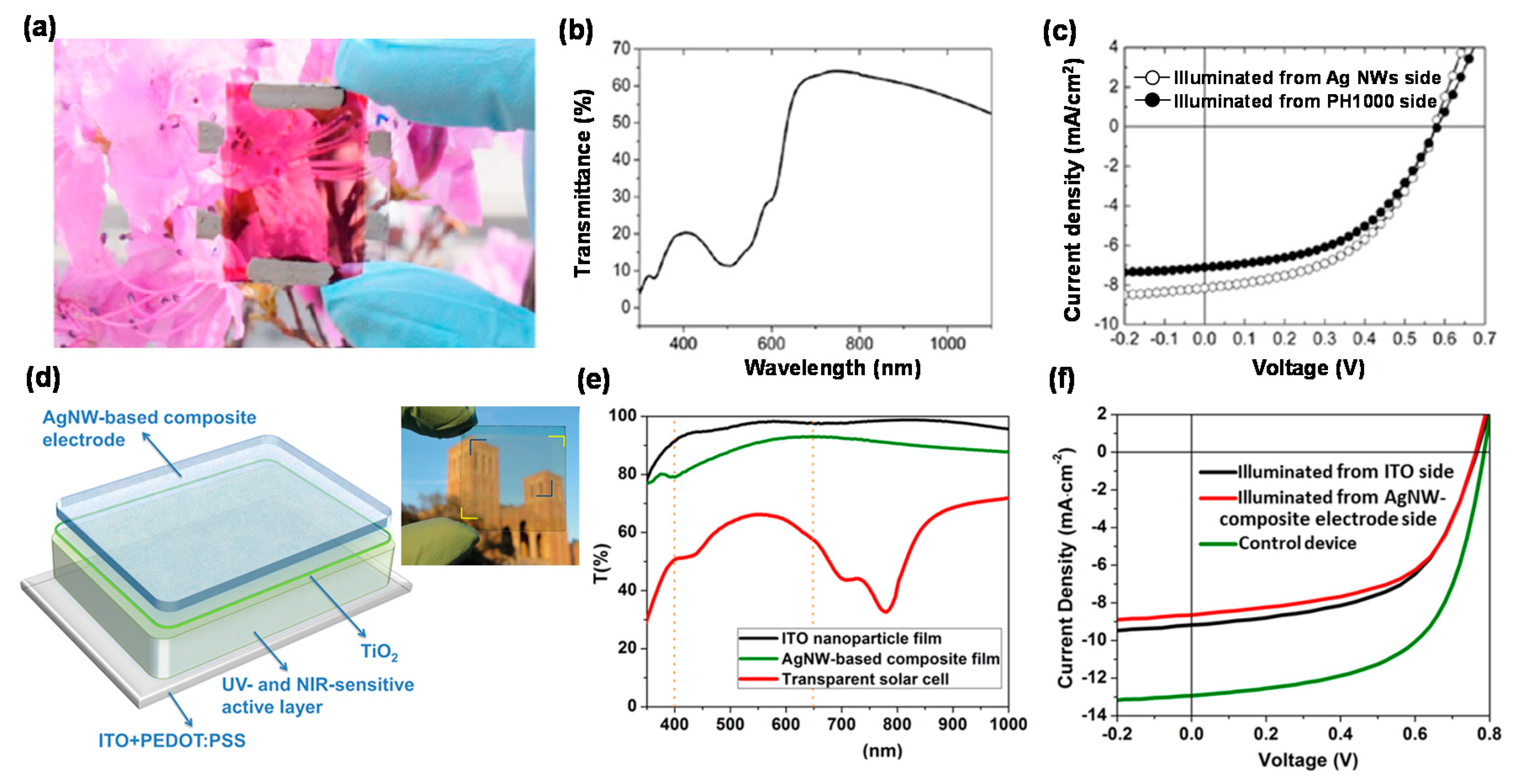
| ITO/AZO/P3HT:PCBM/PEDOT:PSS/Ag NWs | 0.56 | 5.8 | 65.1 | 2.1 | – | [40] |
| Ag NWs/aluminum doped ZnO (AZO)/P3HT: polymer poly[(4,40-bis(2-ethylhexyl) dithieno[3,2-b:2′,3′-d]-silole)-2,6-diyl-alt-(4,7-bis(2-thienyl)-2,1,3-benzothiadiazole)-5,5 ′ -diyl] (Si-PCPDTBT):PCBM/PEDOT:PSS/Ag NWs | 0.57 | 6.4 | 60.0 | 2.2 | 33 | [41] |
| PEDOT:PSS/GO/PEDOT:PSSP3HT:PCBM/ZnO/TiOx/Ag NWs | 0.58 | 8.2 | 49.0 | 2.3 | 60 @ 700 nm | [42] |
| ITO/PEDOT:PSS/poly(2,60-4,8-bis(5-ethylhexylthienyl)benzo- [1,2-b;3,4-b]dithiophene-alt-5-dibutyloctyl-3,6-bis(5-bromothiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4-dione) (PBDTT-DPP):PCBM/TiO2/Ag NWs | 0.77 | 9.3 | 56.2 | 4.0 | 66 @ 550 nm | [43] |
| Ag NWs/PEDOT:PSS/PV2000:PCBM/ZnO/Ag NWs | 0.76 | 10.7 | 52.8 | 4.3 | – | [44] |
| ITO/PEDOT:PSS/poly[(4,4’-bis(2-ethylhexyl)dithieno[3,2-b:2’,3’-d]silole)-2,6-diyl-alt-(2,1,3-benzothiadiazole)-4,7-diyl] (PSBTBT):PCBM/LiCoO2/Al/ZnO:Al | 0.61 | 10.7 | 42.0 | 2.8 | – | [45] |
| ITO/ZnO/Poly[[4,8-bis[5-(2-ethylhexyl)-2-thienyl]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl][2-(2-ethyl-1-oxohexyl)thieno[3,4-b]thiophenediyl]] (PBDTTT-C-T):PC71BM/MoO3/Ag | 0.77 | 12.1 | 61.0 | 5.7 | 28 | [46] |
| ITO/PEDOT:PSS/poly[N-900-hepta-dec- anyl-2,7-carbazole-alt-5,5-(40,70-di-2-thienyl-20,10,30-benzo-thiadiazole)] (PCDTBT):PC71BM/LiF/Al/Ag | 0.76 | 4.6 | 49.0 | 1.7 | 35 | [47] |
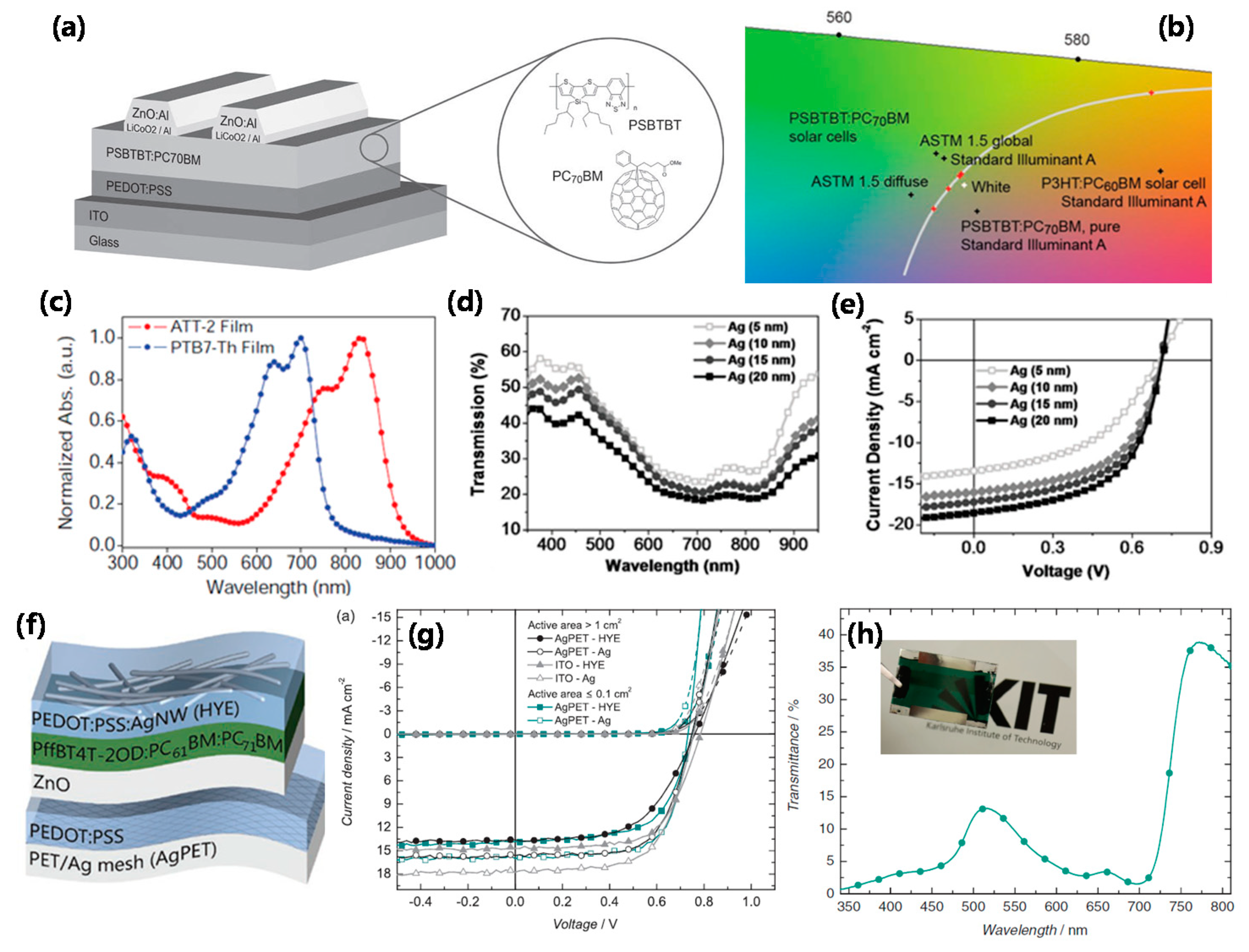
| ITO/ZnO/Poly([2,6′-4,8-di(5-ethylhexylthienyl)benzo[1,2-b;3,3-b]dithiophene]{3-fluoro-2[(2-ethylhexyl)carbonyl]thieno[3,4-b]thiophenediyl}) (PTB7-Th):ATT-2/MoO3/Ag | 0.71 | 16.0 | 55.0 | 6.3 | 45 | [48] |
| ITO/ZnO/ poly[(5,6-difluoro-2,1,3-benzothiadiazol-4,7-diyl)-alt-(3,3″′-di(2-octyldodecyl)-2,2′;5′,2″;5″,2″′-quaterthiophen-5,5″′-diyl)] (PffBT4T-2OD):PCBM:PC71BM/MoO3/Ag | 0.76 | 13.7 | 56.0 | 5.8 | 6 | [49] |
| Graphene/PEDOT:PSS/ZnO/poly[2,7-(5,5-bis-(3,7-dimethyl octyl)-5Hdithieno[3,2-b:20,30-d]pyran)-alt-4,7-(5,6-difluoro-2,1,3-benzothiadiazole)] (PDTP-DFBT):PCBM/MoO3/graphene | 0.67 | 12.4 | 45.0 | 3.7 | 54 @ 500 nm | [50] |
| ITO/ZnO/poly[(2,6-(4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b′]-dithiophene))-alt-(5,5-(1′,3′-di-2-thienyl-5′,7′-bis(2-ethylhexyl)benzo[1′,2′-c:4′,5′-c′]dithiophene-4,8-dione))] (PBDB-T): 9-bis(2-methylene-(3-(1,1-dicyanomethylene)indanone))-5,5,11,11-tetrakis(4-hexylphenyl)dithieno[2,3-d:2′,3′-d′]-s-indaceno[1,2-b:5,6-b′]-dithiophene (ITIC)/MoO3/Ag/MoO3 | 0.88 | 13.8 | 59.8 | 7.3 | 25 | [51] |
| ITO/ZnMgO/PTB7-Th/PC71BM/MoO3/Ag | 0.77 | 14.84 | 59.5 | 6.8 | 22 | [52] |
| ITO/PEDOT:PSS/PTB7-Th:2,2′-((2Z,2′Z)-(((4,4,9-tris(4-hexylphenyl)-9-(4-pentylphenyl)-4,9-dihydro-s-indaceno[1,2-b:5,6-b dithiophene-2,7-diyl) bis(4-((2-ethylhexyl)oxy)thiophene-5,2-diyl)) bis(methanylylidene)) bis(5,6-dichloro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile (IEICO-4Cl)/ poly[9,9-bis[6′-(N,N,Ntrimethylammonium) hexyl]fluorene-alt-co-1,4-phenylene]bromide (PFN-Br)/Au | 0.73 | 19.6 | 59.0 | 8.4 | 26 | [53] |
| FTO/TiO2/(C30H38N2S3Si)n Poly[(4,4′-bis(2-ethylhexyl) dithieno[3,2-b:2′,3′-d]silole)-2,6-diyl-alt-(2,1,3-benzothiadiazole)-4,7-diyl] (PSBTBT):PCBM/WO3/Ag/one-dimensional photonic crystals (1DPCs) | 0.64 | 7.90 | 48.7 | 2.5 | 40 | [54] |
| PEDO:PSS/PTB7:PC71BM/PEN/ITO/1DPC | 0.77 | 10.03 | 55.6 | 4.3 | 39 | [55] |
| ITO/ZnO/PTB7-Th:PC71BM/MoO3/Ag/6 pairs (MoO3/LiF) | 0.79 | 13.1 | 67.7 | 7.0 | 12 | [56] |
| ITO/ZnO/P3HT:PCBM/MoO3/Ag/6 pairs (MoO3/LiF) | 0.63 | 10.89 | 66.0 | 4.32 | 60 @ 650 nm | [57] |
| 3 pairs (TiO2/SiO2)/ZnO/PTB7:PCBM/2 pairs (MoO3/Ag)/LiF | 0.73 | 10.7 | 67.9 | 5.3 | 21 | [58] |
| ITO/C60:NDN1/C60/F4-ZnPc:C60/5 wt.% Di-NPB:NDP9/10 wt.% Di-NPB:NDP9/C60:NDN1/C60/DCV6T:C60/BPAPF/BPAPF:NDP9/Di-NPB:NDP9/C60:NDN1/Al/Ag/tris(8-hydroxyquinolinato)-aluminum | 1.54 | 5.2 | 61.0 | 4.9 | 24 | [59] |
| ITO/poly(3,3′-([(9′,9′-dioctyl-9H,9′H-[2,2′-bifluorene]-9,9-diyl)bis(4,1-phenylene)]bis(oxy))bis(N,N-dimethylpropan-1-amine))(PFPA-1)/ poly[N,N′-bis(2-hexyldecyl)isoindigo-6,6’-diyl-alt-thiophene-2,5-diyl] P3TI:PC71BM/PEDOT:PSS/ITO/ poly(3,3′-([(9′,9′-dioctyl-9H,9′H- [2,2′-bifluorene]-9,9-diyl)bis(4,1-phenylene)]bis(oxy))bis(N,N-dimethylpropan-1-amine)) (PFPA-1)/ poly[2,3-bis-(3-octyloxyphenyl)quinoxaline-5,8-diyl-alt-thiophene-2,5-diyl] (TQ1):PC71BM/PEDOT:PSS | 1.46 | 4.47 | 67.0 | 4.35 | – | [60] |
| Graphene mesh/PEDOT:PSS/poly[(4,4′-bis(3-ethyl-hexyl)dithieno[3,2-b:′′3′-d]silole)-2,6-diyl-alt-(2,5-(3-(2-ethyl-hexyl)thiophen-2-yl)thiazolo[5,4-d]thiazole] (PSETT): indene-C60-bisadduct (IC60BA)/ZnO/PEDOT:PSS/PBDTT-DPP:PC71BM/TiO2/Ag NWs | 1.62 | 7.62 | 64.2 | 8.02 | 45 | [61] |
| ITO/ZnO/fullerene self-assembled monolayer (C60-SAM)/PCBM/PEDOT:PSS/PEDOT:PSS (PH1000)/ZnO/C60-SAM/PCBM/MoO3/Ag | 1.68 | 5.93 | 68.6 | 8.5 | 40 | [62] |
| ITO/PEDOT:PSS/PBDTT-FDPP-C12:PCBM/PFN/TiO2/PEDOT:PSS/PBDTT-SeDPP:PC71BM/TiO2/Ag NWs | 1.47 | 8.4 | 59.0 | 7.3 | 30 | [63] |
| Device Structure | Voc (V) | Jsc (mA·cm2) | FF (%) | PCE (%) | AVT (%) | Reference |
|---|---|---|---|---|---|---|
| Fluorine-doped tin oxide (FTO)/TiO2/CH3NH3PbI3 (MAPbI3) islands/ 2,20,7,70-tetrakis-(N,N-di-pmethoxyphenylamine)-9,90-spirobifluorene (spiro-OMeTAD)/Au | 0.71 | 8.1 | 61.0 | 3.5 | 22 | [64] |
| FTO/TiO2/polystyrene (PS)/MAPbI3/spiro-OMeTAD/Au | 0.95 | 19.2 | 64.0 | 11.7 | 36 | [65] |
| ITO/copper thiocyanate (CuSCN)/MAPbI3/PCBM/Bis-C60/Ag | 1.06 | 13.0 | 73.0 | 10.7 | 37 | [66] |
| ITO/PEDOT:PSS/poly[N,N0-bis(4-butylphenyl)-N,N0-bis(phenyl)benzidine] (poly-TPD)/MAPbI3/PCBM/Au/LiF | 1.04 | 5.66 | 57.7 | 3.39 | 35.4 | [67] |
| FTO/TiO2/MAPbI3/spiro-OMeTAD/MoO3/Au/MoO3 | 0.72 | 9.7 | 66.0 | 5.3 | 31 | [68] |
| Indium tin oxide (ITO)//PEDOT:PSS/MAPbI3/PCBM/ C60/11-amino-1-undecanethiol hydrochloride (AUH)/Ag | 0.95 | 12.1 | 71.0 | 8.2 | 34 | [69] |
| FTO/TiO2/Al2O3/MAPbI3−xClx-NiO nanoparticles (NPs)/spiro-OMeTAD/Au | 0.79 | 20.62 | 61.8 | 10.1 | 27 | [70] |
| FTO/TiO2/MAPbI3/poly-triarylamine (PTAA)/ poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS)/ITO | 1.10 | 15.2 | 75.0 | 12.6 | 17 | [71] |
| FTO/ZnO/ PhanQ: phenyl-C61-butyric acid methyl ester (PCBM)//MAPbI3/spiro-OMeTAD/MoO3/In2O3:H | 1.10 | 17.4 | 73.6 | 14.2 | 72 @ 800 nm | [72] |
| FTO/TiO2/MAPbI3 islands/PTAA/PEDOT:PSS/ITO | 0.96 | 15.5 | 71.2 | 10.6 | 20.9 | [73] |
| FTO/TiO2/MAPbI3 islands/PTAA/PEDOT:PSS/ITO (sub-module) | 0.98 | 16.52 | 73.8 | 12.0 | 20.1 | [74] |
| ITO/PEDOT:PSS/MAPbI3/ZnO/Ag NWs/Al2O3 | 0.96 | 15.87 | 69.7 | 10.8 | 25.5 | [75] |
| ITO/PEDOT:PSS/MAPbI3/PCBM/ZnO/Ag NWs | 0.96 | 13.18 | 66.8 | 8.5 | 28.4 | [76] |
| ITO/PEDOT:PSS/MAPbI3/PCBM/AZO/SnOx/Ag/SnOx | 0.86 | 18.3 | 72.3 | 11.4 | 70 @ 800 nm | [77] |
| FTO/TiO2/mesoporous (mp)-TiO2/MAPbI3/ spiro-OMeTAD/PEDOT:PSS | 0.94 | 11.4 | 27.3 | 2.9 | 7 | [78] |
| PEDOT:PSS/ZnO/MAPbI3/spiro-OMeTAD/PEDOT:PSS | 1.06 | 19.3 | 68.0 | 13.9 | 60 @ 800 nm | [79] |
| FTO/TiO2/mp-TiO2/carbon mesoscopic/MAPbI3/multiwall carbon nanotubes (MWCNT) | 0.87 | 18.10 | 52.1 | 8.2 | 24 | [80] |
| FTO/TiO2/MAPbI3/spiro-OMeTAD/PEDOT:PSS/graphene | 0.96 | 19.17 | 67.2 | 12.4 | – | [81] |
| ITO/MoOx/spiro-OMeTAD/MAPbI3/PCBM/indium zinc oxide (IZO) | 1.03 | 17.5 | 77.7 | 14.0 | – | [82] |
| ITO/PTAA/MAPbI3/PCBM/ buckminsterfullerene (C60)/ bathocuproine (BCP)/Cu/Au | 1.08 | 20.6 | 74.1 | 16.5 | – | [83] |
| ITO/TiO2/MAPbI3/spiro-OMeTAD/MoOx/Au/Ag/MoOx | 1.05 | 14.6 | 75.1 | 11.5 | – | [84] |
| FTO/TiO2/MAPbI3/spiro-OMeTAD/Ag NWs | 1.03 | 17.5 | 71.0 | 12.7 | – | [85] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, D.H.; Choi, S.-H. Recent Studies of Semitransparent Solar Cells. Coatings 2018, 8, 329. https://doi.org/10.3390/coatings8100329
Shin DH, Choi S-H. Recent Studies of Semitransparent Solar Cells. Coatings. 2018; 8(10):329. https://doi.org/10.3390/coatings8100329
Chicago/Turabian StyleShin, Dong Hee, and Suk-Ho Choi. 2018. "Recent Studies of Semitransparent Solar Cells" Coatings 8, no. 10: 329. https://doi.org/10.3390/coatings8100329
APA StyleShin, D. H., & Choi, S.-H. (2018). Recent Studies of Semitransparent Solar Cells. Coatings, 8(10), 329. https://doi.org/10.3390/coatings8100329





