1. Introduction
The self-cleaning quality of textile materials is among the most demanding functions today [
1]. The fabric surface refining and its low surface energy, acquired through nano-coatings and alternative procedures, can help in retarding dirt, grease, oils and water over the surface to keep it clean for longer durations. Previously, the most commonly employed agents for this purpose were wax, silicones, polyfluorinated carbons and polyvinylchloride [
2]. Recently, nanomaterials as coatings over the textile surfaces have been introduced due to their improved results for syntheses and application. These materials have been prepared through plasma treatment, wet chemical etching, vapor deposition and sol-gel methods. For TiO
2 nanoparticles, sol-gel method is the most frequently used technique due to ease of manufacturing and for obtaining high performance nanoparticles [
3].
In parallel, the self-cleaning can also be achieved by modifying the fabric conditions in accordance with this requirement. TiO
2 nanoparticles are presumed to be bound with cotton through ester bonding over the non-homogeneous irregular structure of the cotton making it more self-cleaning, ultraviolet irradiation resistant and antibacterial in nature [
4,
5,
6]. Similar properties were achieved for polyester nonwoven structures coated with titananium dioxide nanoparticles by reactive sputtering methods [
7]. If an easy route prepared in laboratory and/or in industry maintains such surfaces, it can be a new exciting phenomenon due to its endless applications [
8]. The refining of textile surfaces and loading them with nanoparticles for both natural and synthetic fibers have been evaluated, but textiles with various structures are yet to be examined.
In this research work, the manufactured substrates were: 100% polyester (A), blends of viscose and modacrylic (B) and blends of viscose and high performance polyethylene (C), details given in
Table 1. The modacrylic fibers are manufactured fibers with fiber-forming substance containing synthetic polymer of less than 65%, but minimum 35% by weight of acrylonitrile and High performance polyethylene fibers under consideration are a commercial product
Dyneema by DSM High Performance Fibers in the Netherlands [
9]. These structures, if coated with hydrophobic finishes, could be used for many domestic and commercial applications with enhanced shelf-life and water-repellent, dirt-resistant and hence self-cleaning efficiency [
10].
2. Materials and Methods
All chemicals were used as received and/or mentioned if otherwise. Titanium tetraisopropoxide (97%), polyvinylpyrolidone (99%), surfactant triton-X100 were obtained from Sigma Aldrich (Munich, Germany), whereas nitric acid, hydrochloric acid, caustic soda and distilled water were obtained from Ittehad Chemicals (Sheikhupura, Pakistan). The purity of inorganic acids and bases was tested by simple gravimetric methods before applying for certain purposes. All solutions were made in distilled water of electrical conductivity below 3 μs/cm. Fibers of high performance polyethylene, viscose the regenerated cellulosic fibers from cotton and modacrylic fiber was purchased from local textile mill in Faisalabad, Pakistan.
2.1. Yarn and Fabric Formation
Three types of yarns with different composition but of same linear density, i.e., 37 tex, were spun on a ring spinning machine. These yarns were then converted to three woven fabric samples using a lab scale rapier weaving machine. To keep the areal density of each fabric sample almost the same, the number of ends and picks per centimeter of each fabric were kept at 32 and 16, respectively, and the weave design of each fabric was selected as 3/1 Z-twill. The yarn and fabric specifications are given in
Table 1.
2.2. Pretreatment of Fabric Surfaces
The fabrics were desized and scoured according to standard methods before the application of nanoparticles [
11]. For the freshly woven fabrics, the proper desizing process was established. For the pretreatment process, scouring was carried out to make the fabrics in the best position for carrying the finish application on them. Initially, both polyvinyl alcohol and polyvinyl acetate were used in sizing process of yarns. The desizing and scouring processes were performed on the substrates to get the fabric ready for other treatments. The scouring was accomplished in alkaline media of NaOH and was later neutralized in presence of acid. For scouring, nonionic detergent WOB was additionally added alongside NaOH [
12].
2.3. Synthesis and Characterization of Nanoparticles
The sol-gel method was used to prepare nanoparticles according to a procedure already reported in the literature [
13]. In a typical experiment, TiO
2 nano-sol aqueous solution was prepared by the stirring of 1% nitric acid in 300 mL distilled water at room temperature, after which 1% of precursor titanium tetraisopropoxide was added and stirred the solution for 22 h. The reaction mixture was also added with 0.1% of surfactant triton-X100 to keep the particles dispersed. The sol-gel solution was cleaned three times with distilled water, and then sonication was carried out for 20 min. The suspended particles were filtered followed by separation through centrifugation at 5000 rpm for 30 min. The wet particles were dried in oven at 60 °C for 16 h to get completely dried nanoparticles. The nanoparticles prepared through this method were further characterized via XRD and DLS before applying to fabric structures.
PANalytical (London, UK) made X’Pert Powder equipped with radiation, λ = 0.1540 nm or 1.5 Å Cu X-ray generator sources with applied voltage 45 kV and current 40 mA was used for X-ray crystallinity analysis of nanoparticles. The ultra-refined solid nanoparticles of TiO2 were subjected to analysis for X-ray diffraction at a set angle between 10° and 80° with 2 min scan speed at sampling pitch of 0.01°. The size of the nanoparticles and their distribution was measured through Zetasizer Nano ZS by Malvern (London, UK). An appropriate aqueous solution of nanoparticles was subjected for the measurement of the Doppler-shifted frequency spectrum of scattered laser light performance.
2.4. Printing and Coating of Fabric Samples
For a comparative analysis of the effects of coatings, the portions of all fabric samples were also subjected to printing before application of nano- or commercial coatings. The samples were printed through screen printing by using methotrexate (MTX) as a binder and common printing pigment Neolan Blue 2G (Aglia, Czech Republic). Pad-dry-cure method was used for the application of the nanoparticles of three different concentrations of 1%, 1.5% and 2% of TiO2 nanoparticles by weight of the fabric samples. For 1% TiO2 coating, the weighed fabric sample was poured in 300 mL of distilled water, 15 g of polyvinylpyrolidone and 3 g of TiO2 nanoparticle powder. One gram of binder was also added in the solution mixture for proper attachment of nanoparticles to the fabric. The specimen was dried at 110 °C for 2 min and was cured for 1 min at the consistent temperature of 120 °C. To establish the comparison of self-coated TiO2 in the laboratory with commercially available hydrophobic coating, Rucogaurd AFR from Rudolf, Germany was used and was treated with 2% by weight of the fabric. The procedure for treatment such as padding, drying and curing was kept the same for all the samples.
2.5. Characterization of the Treated Fabric Samples
The coated sample was kept at ambient conditions before characterization and standard methods were used for the characterization of coated samples. Air permeability was evaluated [
14]. The pressure differential of 100 Pa at test area 20 cm
2 was set and the results were reported in mm/s. The action of self-cleaning was calculated by a method of coffee stain test. Six percent coffee (Nestle, pure) solution was prepared for spotting the substrate samples [
15]. The samples were stained with coffee solution, which was allowed to spread and dry. The stain was divided into two parts. One part was covered with black paper and the other half was exposed to light for almost 24 h. Exposed and unexposed stain parts were compared. To calculate photo degradation of coffee stain, X-rite spectrophotometer UV-2800 (Zhengzhou, China) was used for measuring the difference. The self-cleaning activity of the coffee stain was measured by
K/
S value of the exposed and unexposed part.
K/
S is a function of color depth and is represented by the equation of Kubelka and Munk (Equation (1)) [
16].
where
R is the reflectance of the dyed fabric,
K is the sorption coefficient, and
S is the scattering coefficient. The UV-protection of the fabric samples were calculated in accordance to AATCC-183 test method [
17]. This method was used to evaluate the transmittance value of the UV radiation through fabrics by the help of UV-vis spectrophotometer. M550 double beam scanning UV-vis spectrophotometer (Spectronic Camspec Ltd., Leeds, UK) was used. UPF and UV profile values of uncoated and coated fabric samples were analyzed against each other. An average of four scans was used for final measurements of each sample. The nanoparticle treated fabric was fixed on the specimen stub by the help of double sided adhesive tape and covered with gold in as putter coater and analyzed by means of FEI SEM Quanta 250 (Thermo Fisher Scientific, Hillsboro, OR, USA). The contact angle of all treated and untreated fabrics was calculated through sessile drop method through contact angle goniometry (DSA 100, Krüss, Hamburg, Germany) [
18]. The distilled water (with 4 KL volume) was dropped to the surface and image was captured using adjacent camera. The different measurements were conducted at different locations to calculate the contact angle as average of five measurements.
3. Results and Discussion
The three stages mechanism for designed fabrics was to evaluate the properties of self-designed fabric, the nanoparticles synthesized in our laboratory and the samples coated with these nanoparticles and with Rucogaurd AFR. First, the designed fabrics were manufactured on automatic air-jet looms after selecting the yarns for specific applications and general fabric characteristics were evaluated for all samples (A–C). Such structures were chosen by keeping in mind the upholstery applications of the fabric for exterior use including applications in geo-textile, buildtech, agritech and home textile applications [
9]. All fabrics have the areal density around 200 g/m
2, which makes them mechanically strong fabrics for such applications.
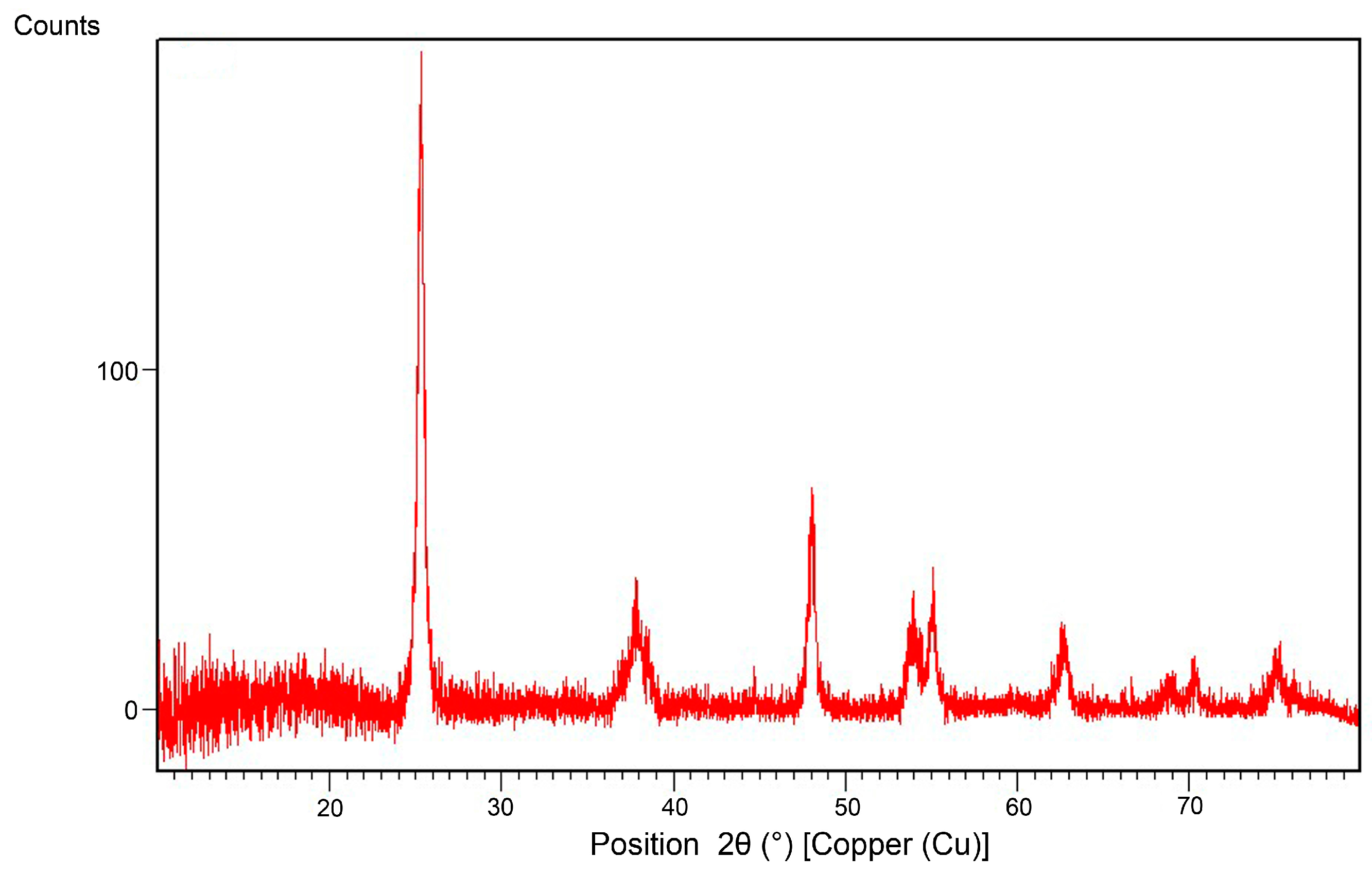
Secondly, the oven dried powdered nanoparticles prepared by sol-gel method in our laboratory were characterized through X-ray diffraction and five distinguished peaks at 25.50°, 38.02°, 47.90°, 54.20° and 62.40° were detected for TiO
2 nanoparticles (cf.
Figure 1). All respective peaks show the presence of amorphous TiO
2 nanoparticles at solid state and the hydrophobicity and/or self-cleaning efficiency was measured via static angle measurement and coffee stain method. The data were in agreement with those reported by other authors [
7].
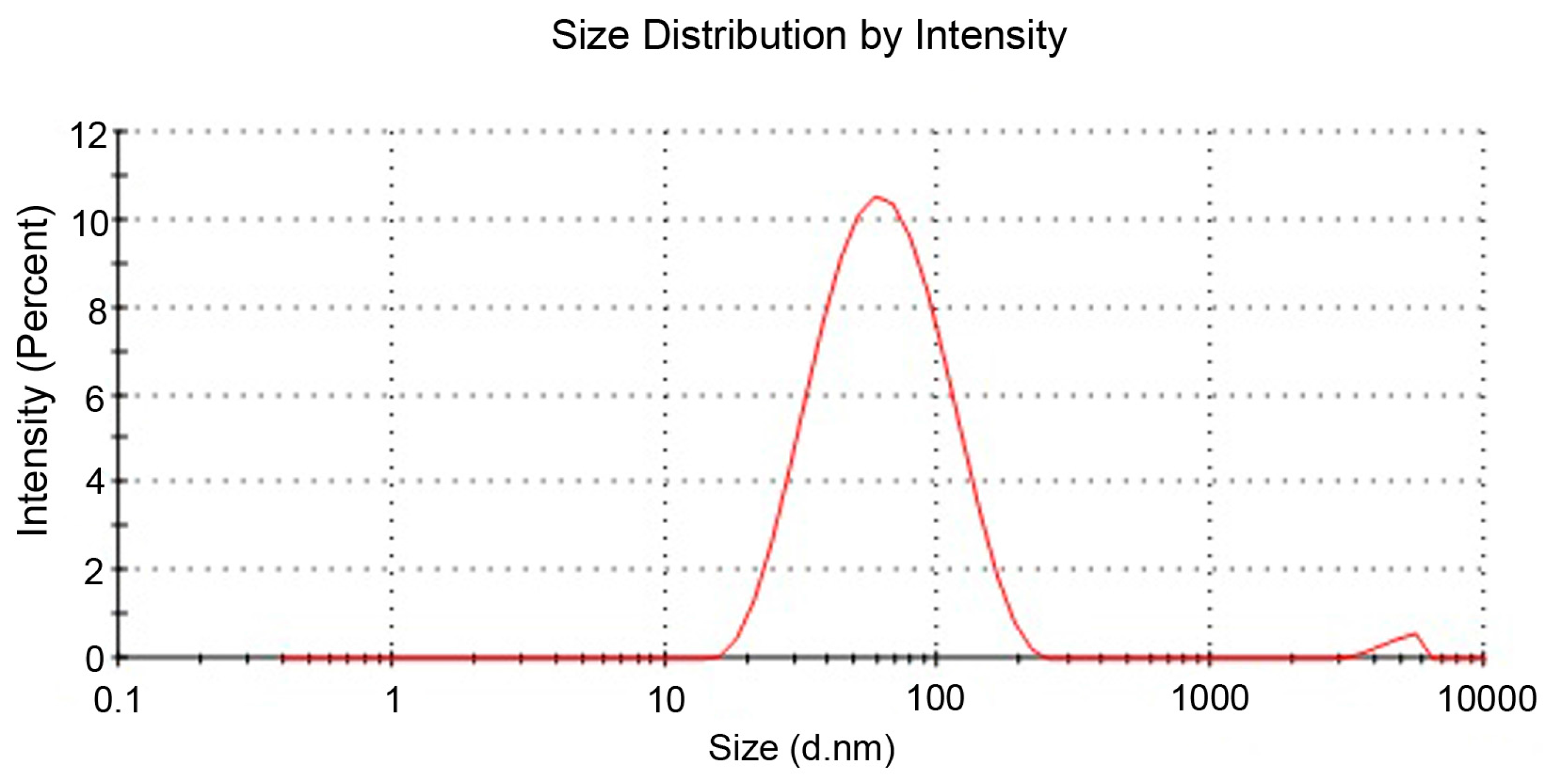
After the confirmation of formation of nanoparticles, the size and distribution of nanoparticles were analyzed. Therefore, the dynamic light scattering (DLS) technique helped in obtaining the average size of 68 nm (cf.
Figure 2). Finally, the spreading of nanoparticles over the textile surface was also evaluated through SEM images at different resolutions.
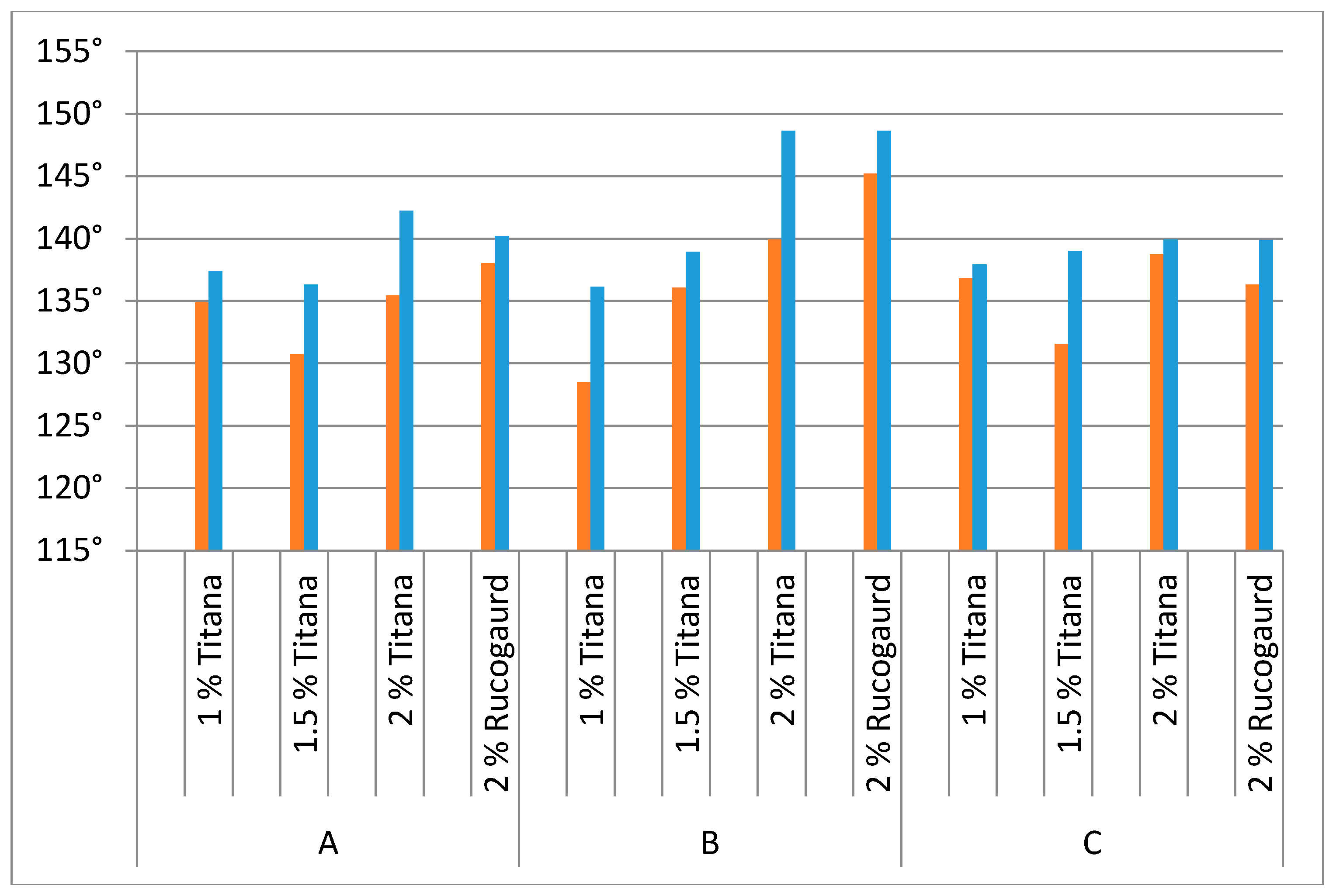
Finally, the coating of nanoparticles was accomplished via pad-dry-cure method and textile performance properties including air permeability, UV protection analysis, hydrophobicity through contact angle and surface of fabric via scanning electron microscope (SEM) was evaluated. The hydrophobic characteristic was evaluated through statistic contact angle measurement (
Table 2) and it was observed that the hydrophobicity is directly related to both the percentage of TiO
2 nanoparticle concentrations and on printing applied over the surfaces. For all samples, the printed version had higher values of contact angle than the unprinted ones due to extra layer of pigment and binder (cf.
Figure 3). Similarly, in all samples, the amount of TiO
2 predicted the hydrophobic nature of the substrates and the amount of TiO
2 loaded onto the fabric surfaces for 100% polyester fabric A was almost the same. The commercial finish Rucogaurd specifically designed for this particular application showed higher hydrophobic values even at the maximum loading of 2% by weight of nanoparticles except for the fabric substrate C. The maximum possible contact angle was observed in case of 2 wt % of TiO
2 coated nanoparticles over the substrate B whose contact angle was 148.64°.
The value of air permeability of uncoated fabrics is generally higher than the coated due to the absence of binder, etc. However, the air permeability gradually decreased with the increasing concentration of the coating of nanoparticles, as given in
Table 3. The effect may also be due to the extra ratio of binder and other chemicals applied during coatings. The air permeability of all Rucogaurd finished samples was comparable to nano-coated samples. In sum, all samples have high breathability for their use in upholstery applications.
In
Table 4, it is obvious that only the TiO
2 nanoparticles coated fabric samples have self-cleaning activity. Control and Rucoguard AFR coated samples did not show self-cleaning activities. This shows that Rucoguard AFR finish cannot be used for self-cleaning purposes. The
K/
S value of the different test samples are compared against each other and the results are shown in
Table 4. It was obvious from available literature data that increasing the concentration of TiO
2 nanoparticles increases the self-cleaning activity. It was observed that nearly 54%
K/
S value was decreased in modacrylic viscose blend sample B when maximum concentration of TiO
2 (2%) was used.
The test samples of all three fabrics were also analyzed against their protection from ultraviolet radiation (UPF). The UPF values clearly showed that coatings do not significantly vary with light irradiation and were excellent in ultraviolet protection (cf.
Table 5). The control and Rucoguard AFR coated fabric samples have lesser UPF values, but still showed better results overall. These results showed that UPF values of the coated samples are much greater than the uncoated ones.
Finally, the SEM images of the nanocoated fabric were evaluated and all specimens at different resolutions predicted the uniform distribution of nanoparticles over the whole surface of fibers, which caused the irregularity of samples and hence the self-cleaning approach (cf.
Figure 4). The whole compilation of data predicted that the novel structures of fabric loaded with nanoparticles are highly beneficial for ground research and are a good masterpiece for industrial preparations for demanded self-cleaning applications.