Interlayers Applied to CVD Diamond Deposition on Steel Substrate: A Review
Abstract
1. Introduction
2. Overview of the State of the Art for CVD Diamond Growth on Steel Substrates
3. Interlayers Applied to CVD Diamond Deposition Especially Suited to Mitigate Thermal Stress
3.1. Multilayer Structures
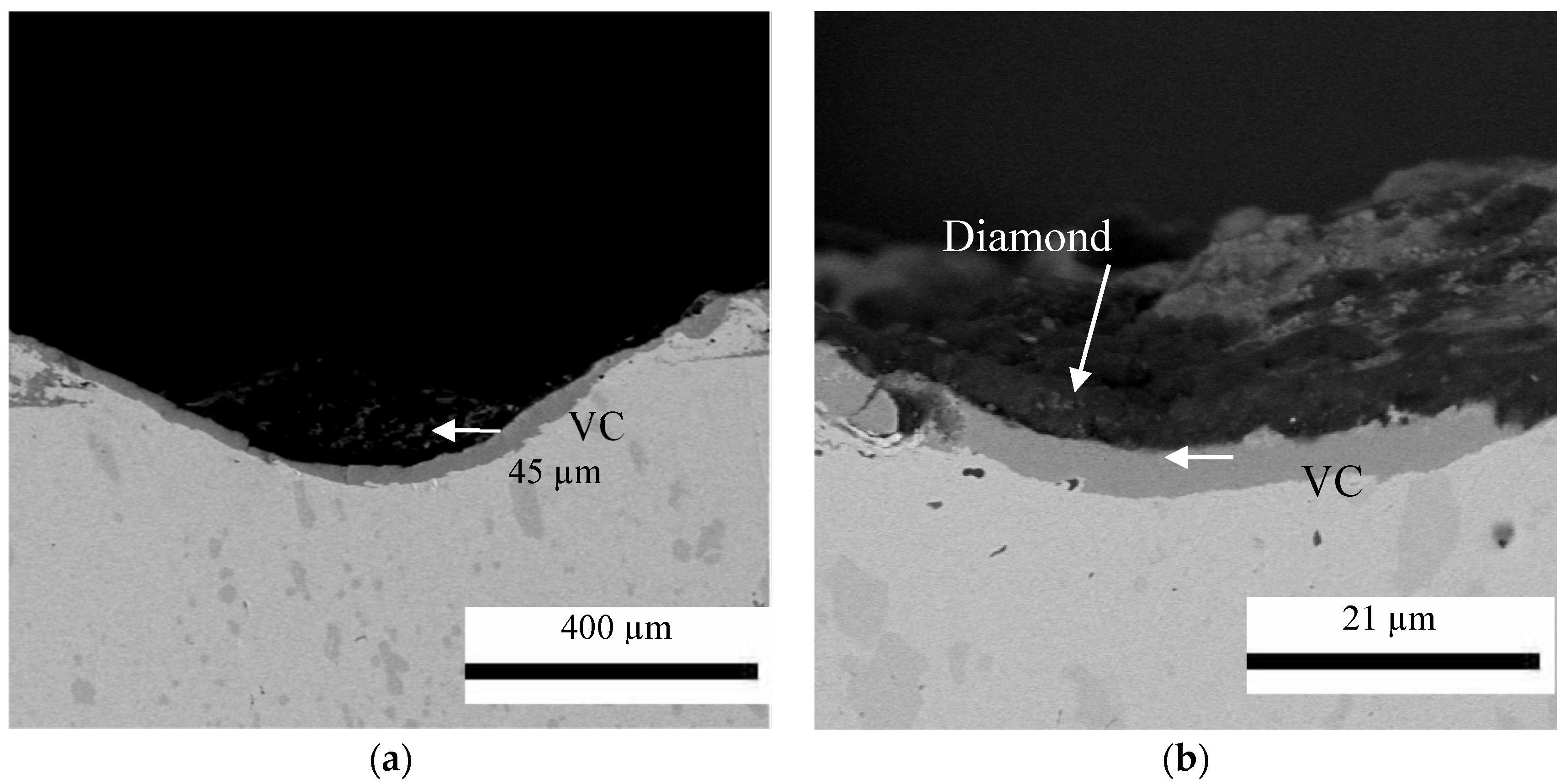
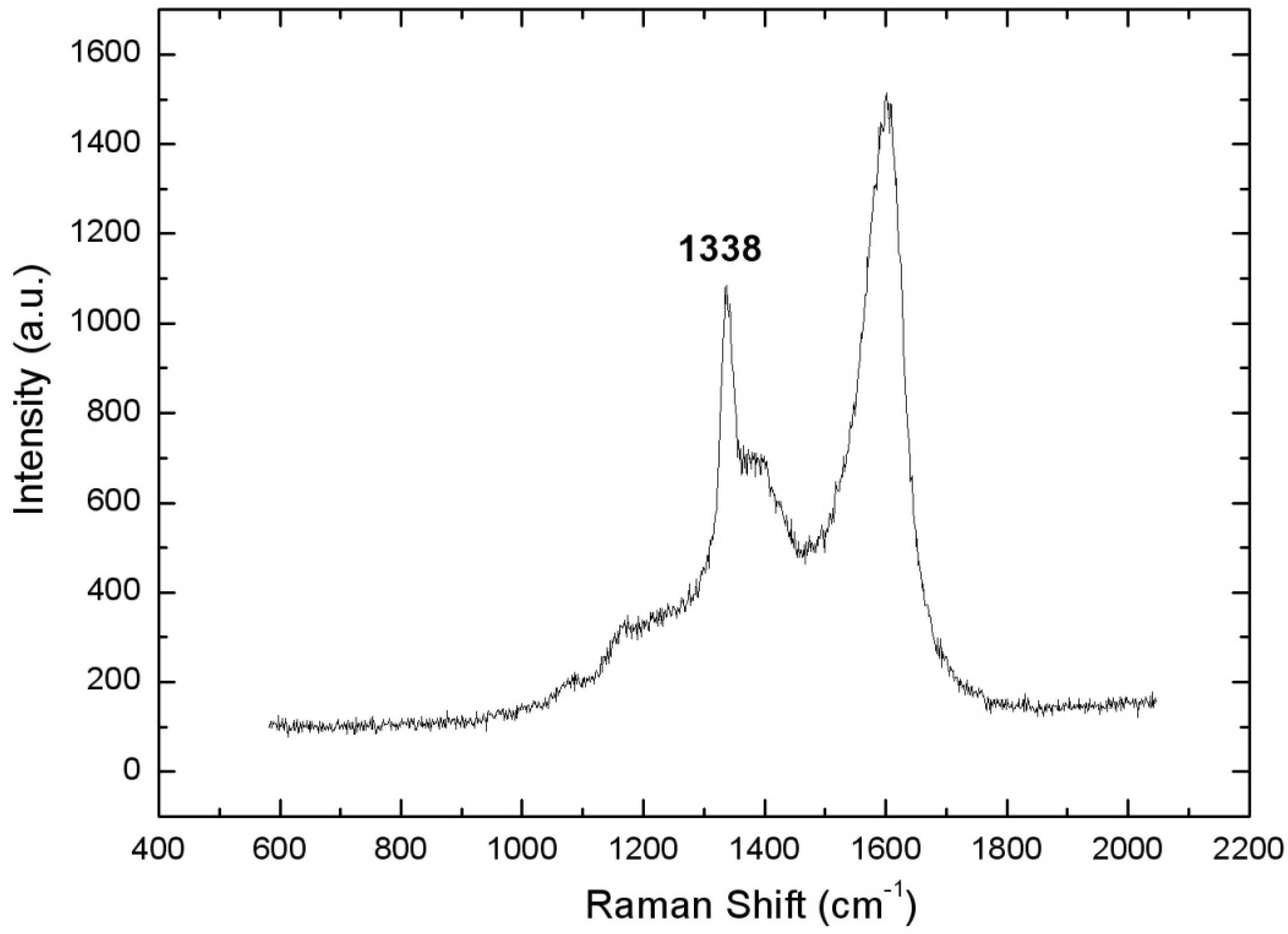
3.2. Thermodiffused Vanadium Carbide Coating (TDVC)
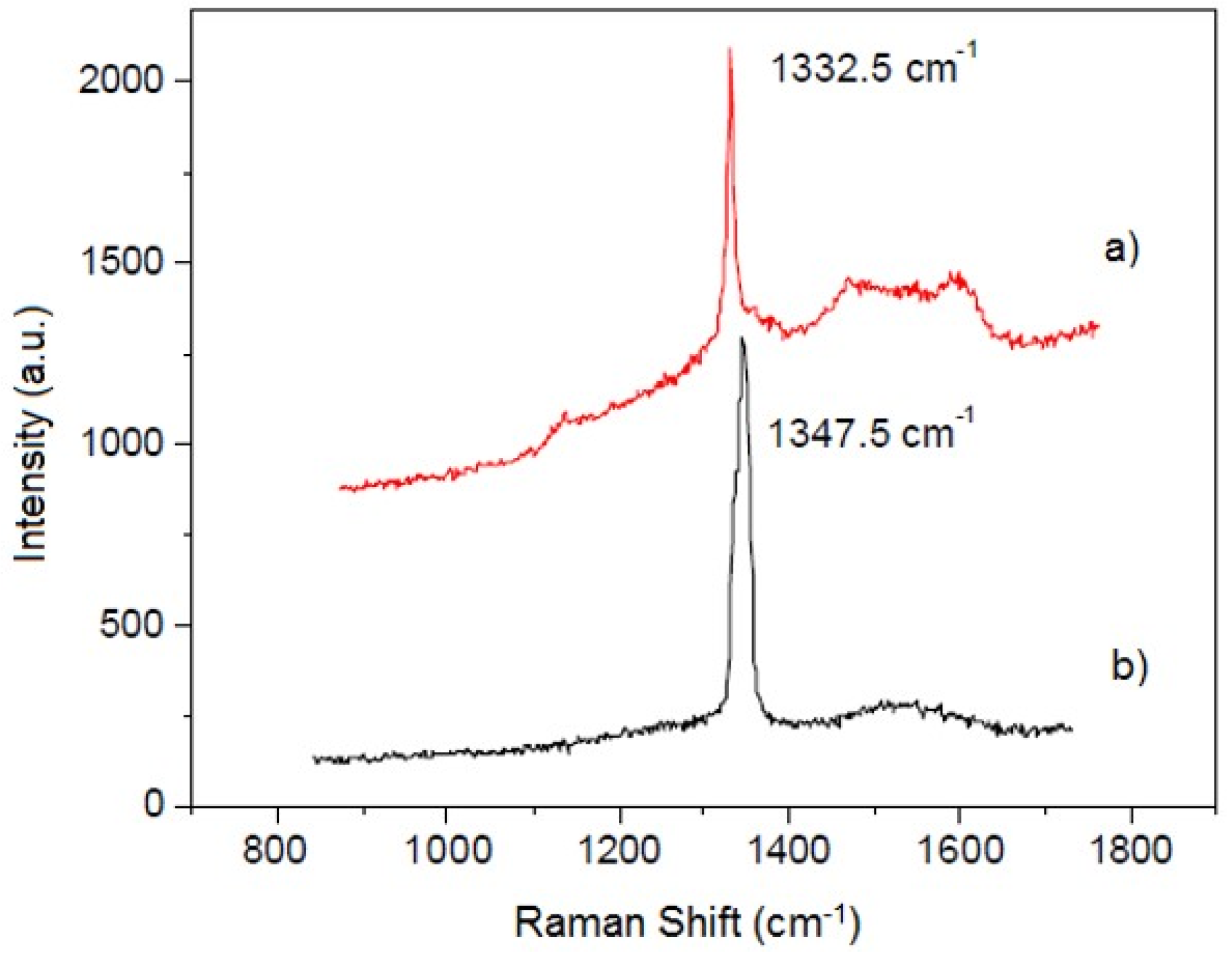
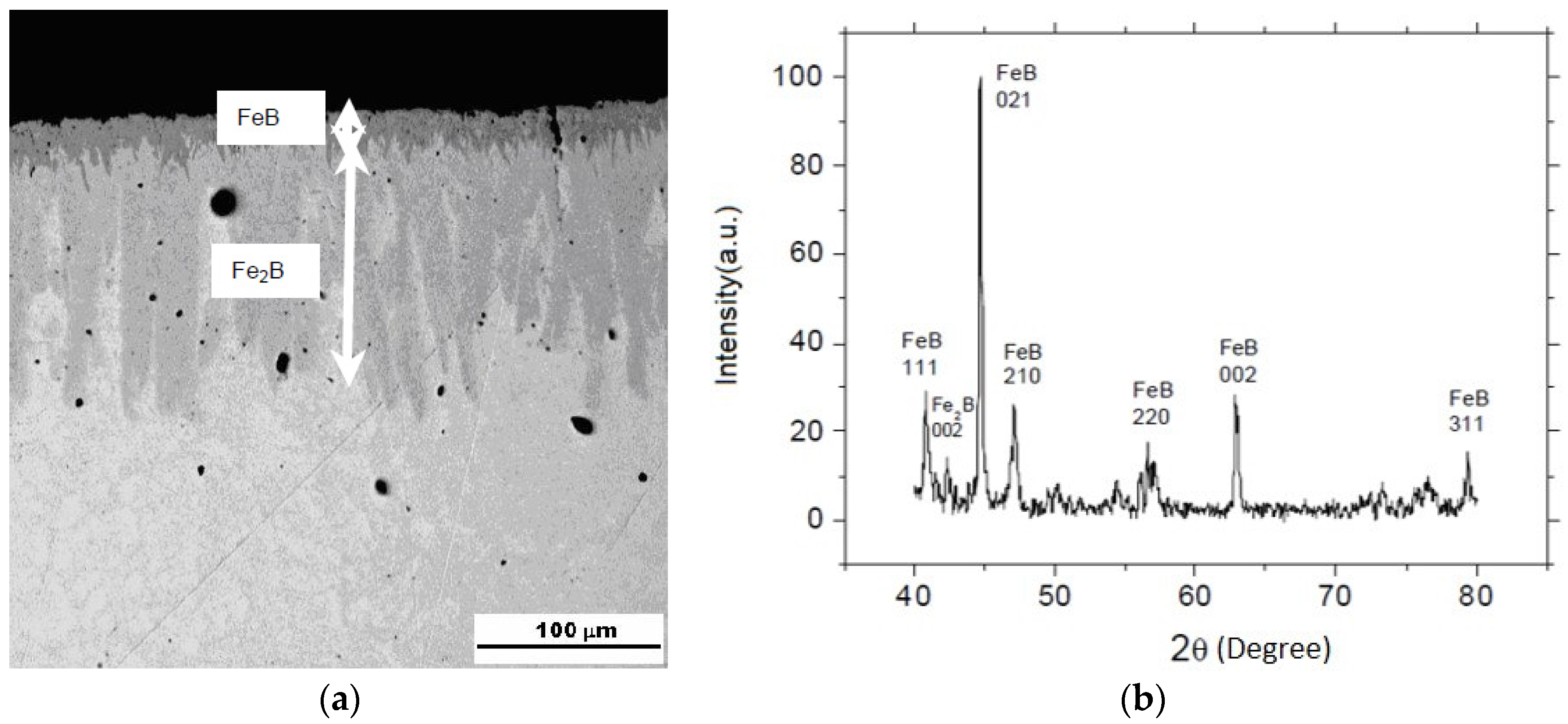
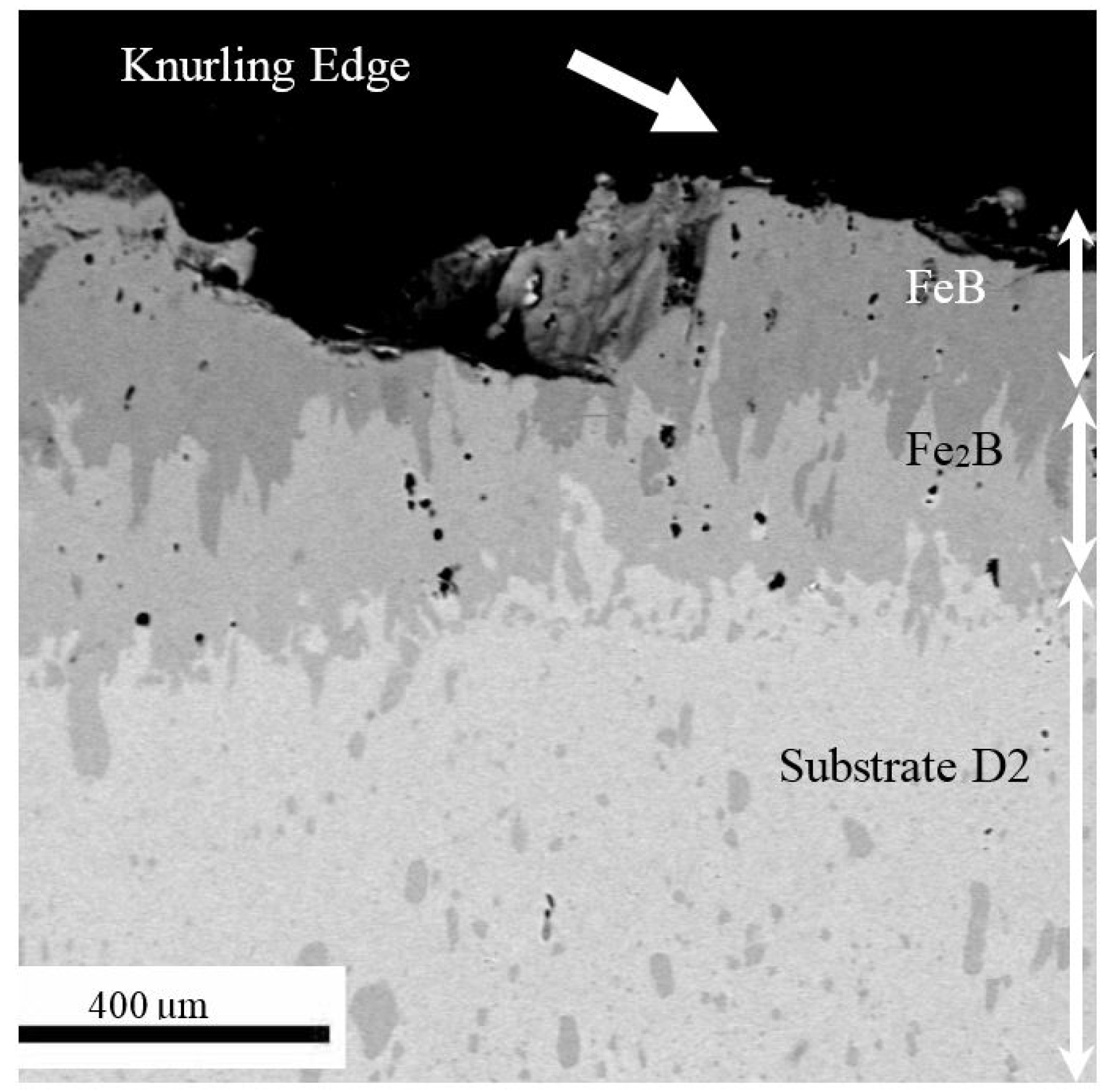
3.3. Iron Borides
3.4. Multilayers with Fe2B to Mitigate Thermal Stress
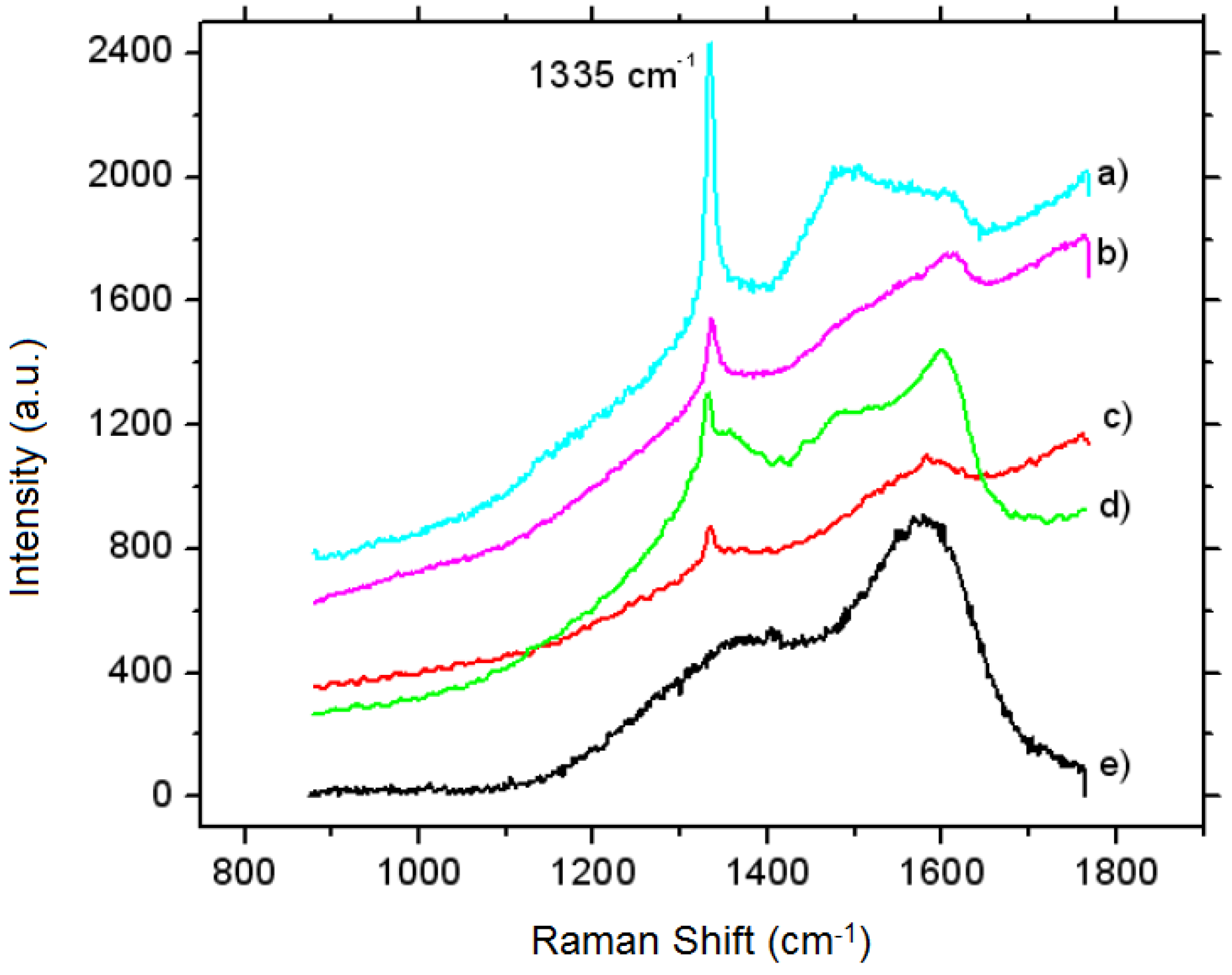
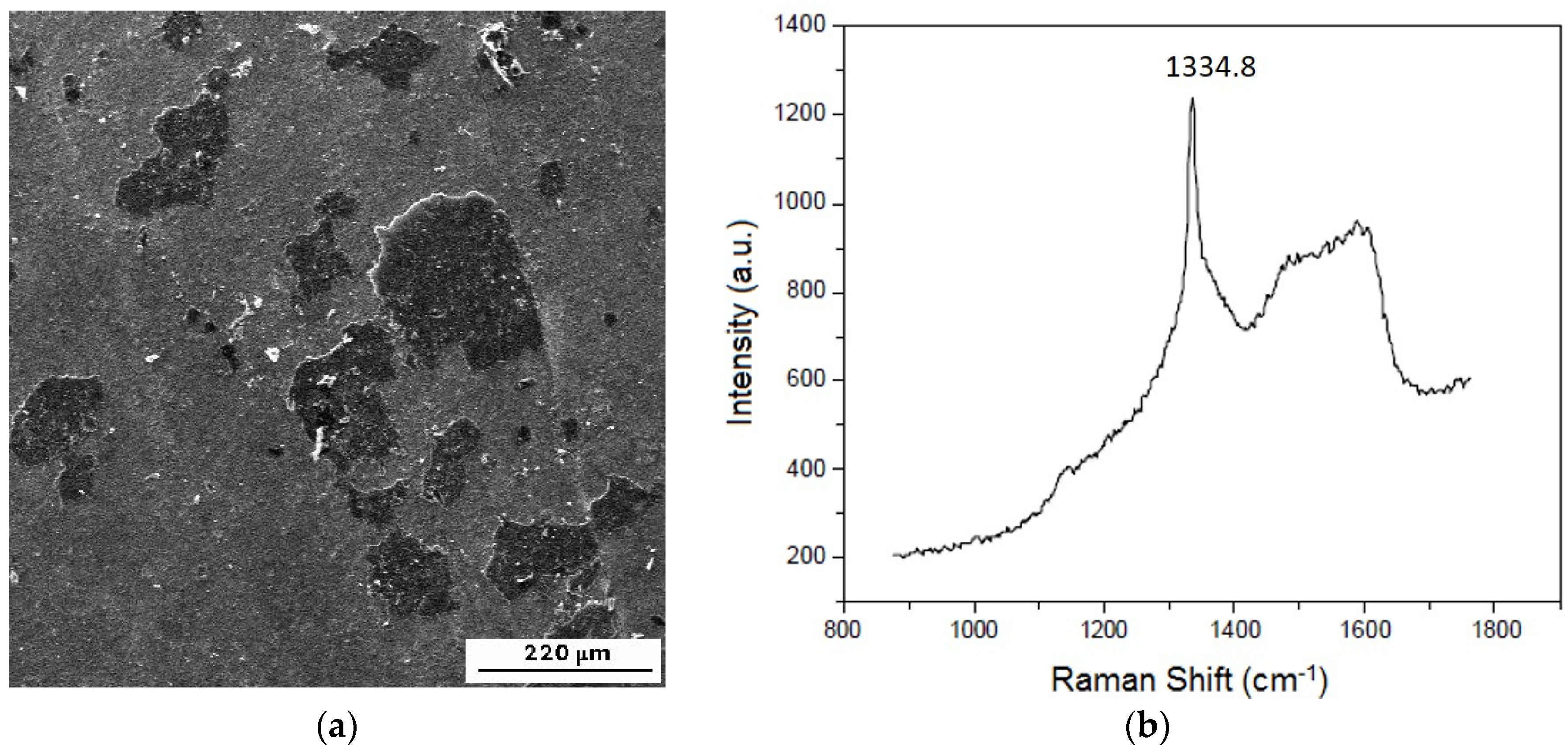
3.4.1. The Multilayer of Fe2B with a Controlled Thin FeB Layer
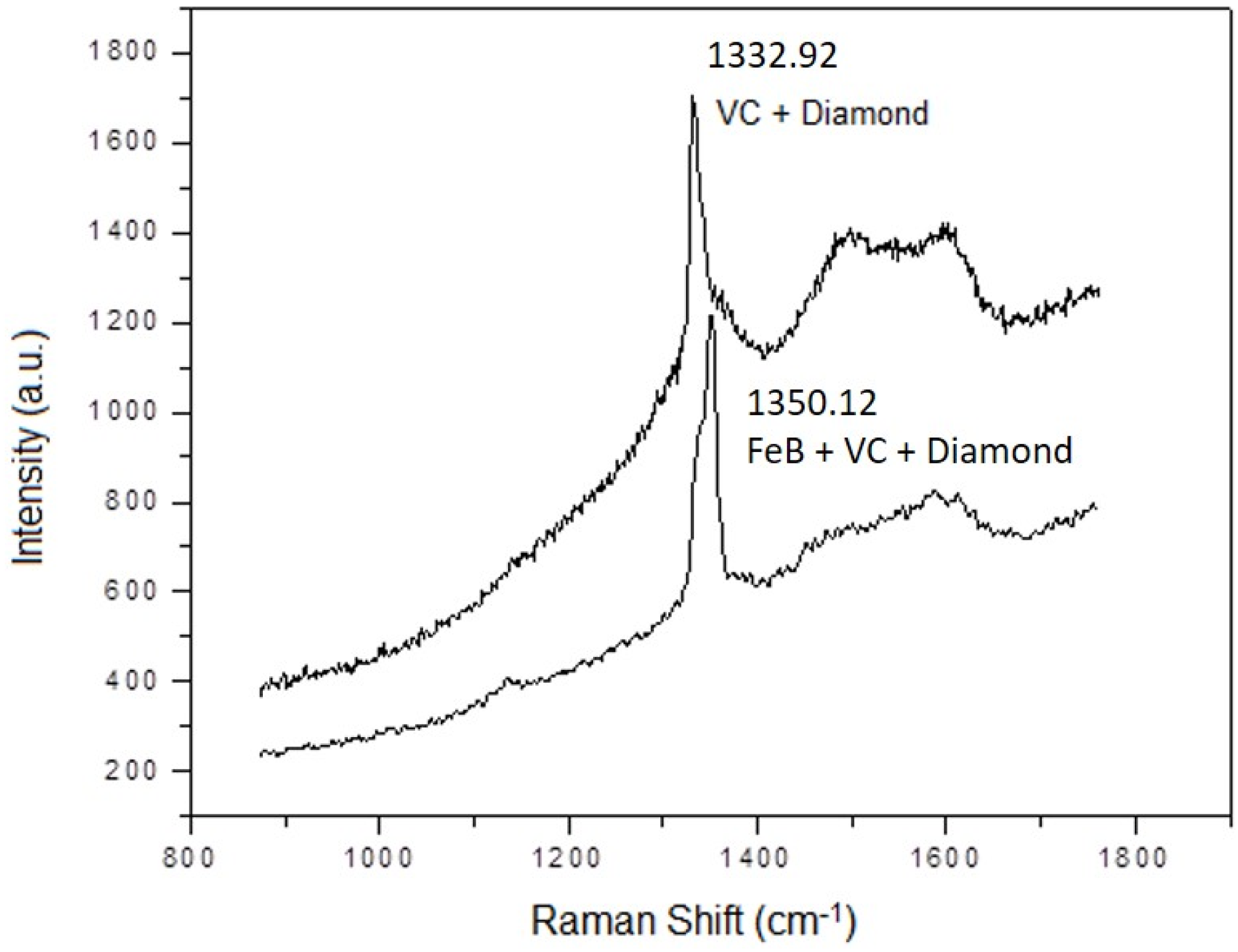
3.4.2. The Multilayer of Fe2B with Vanadium Carbide Layer
3.5. Mechanical Interlocking Applied to Relief Residual Stresses in Highly Stressed Diamond Films
3.6. Chromium Carbides
3.7. Laser Cladding
3.7.1. Laser Cladding of SiC Layers
3.7.2. Laser Cladding of VC Layers (LCVC)
4. Concluding Remarks and Perspectives
Acknowledgments
Conflicts of Interest
References
- Matsumoto, S.; Sato, Y.; Tsutsumi, M.; Setaka, N. Growth of diamond particles from methane-hydrogen gas. J. Mater. Sci. 1982, 17, 3106–3112. [Google Scholar] [CrossRef]
- Badzian, A.; Badzian, T. Diamond homoepitaxy by chemical vapor deposition. Diam. Relat. Mater. 1993, 2, 147–157. [Google Scholar] [CrossRef]
- Lee, S.-T.; Lin, Z.; Jiang, X. CVD diamond films: Nucleation and growth. Mater. Sci. Eng. R Rep. 1999, 25, 123–154. [Google Scholar] [CrossRef]
- Kobashi, K. Diamond-structure and CVD growth. In Diamond Films; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Miranzo, P.; Osendi, M.I.; Garcia, E.; Fernandes, A.J.S.; Silva, V.A.; Costa, F.M.; Silva, R.F. Thermal conductivity enhancement in cutting tools by chemical vapor deposition diamond coating. Diam. Relat. Mater. 2002, 11, 703–707. [Google Scholar] [CrossRef]
- Campos, R.A.; Contin, A.; Trava-Airoldi, V.J.; Moro, J.R.; Barquete, D.M.; Corat, E.J. CVD Diamond Films Growth on Silicon Nitride Inserts (Si3N4) with High Nucleation Density by Functionalization Seeding. Mater. Sci. Forum 2012, 727–728, 1433–1438. [Google Scholar] [CrossRef]
- Ying, Y.; Ying, W.; Li, Q.; Meng, D.; Ren, G.; Yan, R.; Peng, X. Recent advances of nanomaterial-based membrane for water purification. Appl. Mater. Today 2017, 7, 144–158. [Google Scholar] [CrossRef]
- Zeng, A.; Neto, V.F.; Gracio, J.J.; Fan, Q.H. Diamond-like carbon (DLC) films as electrochemical electrodes. Diam. Relat. Mater. 2014, 43, 12–22. [Google Scholar] [CrossRef]
- Kato, H.; Hees, J.; Hoffmann, R.; Wolfer, M.; Yang, N.; Yamasaki, S.; Nebel, C.E. Electrochemistry communications diamond foam electrodes for electrochemical applications. Electrochem. Commun. 2013, 33, 88–91. [Google Scholar] [CrossRef]
- Nazari, M.; Hancock, B.L.; Anderson, J.; Hobart, K.D.; Feygelson, T.I.; Tadjer, M.J.; Pate, B.B.; Anderson, T.J.; Piner, E.L.; Holtz, M.W.; et al. Optical characterization and thermal properties of CVD diamond films for integration with power electronics. Solid State Electron. 2017, 136, 12–17. [Google Scholar] [CrossRef]
- Fu, J.; Wang, F.; Zhu, T.; Wang, W.; Liu, Z.; Li, F.; Liu, Z.; Denu, G.A.; Zhang, J.; Wang, H.-X.; et al. Single crystal diamond cantilever for micro-electromechanical systems. Diam. Relat. Mater. 2017, 73, 267–272. [Google Scholar] [CrossRef]
- Shikata, S. Single crystal diamond wafers for high power electronics. Diam. Relat. Mater. 2016, 65, 168–175. [Google Scholar] [CrossRef]
- Ralchenko, V.G.; Smolin, A.A.; Pereverzev, V.G.; Obraztsova, E.D.; Korotoushenko, K.G.; Konov, V.I.; Lakhotkin, Y.V.; Loubnin, E.N. Diamond deposition on steel with CVD tungsten intermediate layer. Diam. Relat. Mater. 1995, 4, 754–758. [Google Scholar] [CrossRef]
- Lorenz, H.P. Investigation of TiN as an interlayer for diamond deposition on steel. Diam. Relat. Mater. 1995, 4, 1088–1092. [Google Scholar] [CrossRef]
- Li, X.-J.; He, L.-L.; Li, Y.-S.; Yang, Q.; Hirose, A. TEM interfacial characterization of cvd diamond film grown on Al inter-layered steel substrate. Diam. Relat. Mater. 2014, 50, 103–109. [Google Scholar] [CrossRef]
- Buijnsters, J.G.; Shankar, P.; Van Enckevort, W.J.P.; Schermer, J.J.; Ter Meulen, J.J. The applicability of ultra thin silicon films as interlayers for CVD diamond deposition on steels. Phys. Stat. Sol. 2003, 195, 383–395. [Google Scholar] [CrossRef]
- Buijnsters, J.G.; Shankar, P.; Gopalakrishnan, P.; Van Enckevort, W.J.P.; Schermer, J.J.; Ramakrishnan, S.S.; Meulen, J.J.T. Diffusion-modified boride interlayers for chemical vapour deposition of low-residual-stress diamond films on steel substrates. Thin Solid Films 2003, 426, 85–93. [Google Scholar] [CrossRef]
- Lux, B.; Haubner, R. Diamond substrate interactions and the adhesion of diamond coatings. Pure Appl. Chem. 1994, 66, 1783–1788. [Google Scholar] [CrossRef]
- Endler, I.; Leonhardt, A.; Scheibe, H.-J.; Born, R. Interlayers for diamond deposition on tool materials. Diam. Relat. Mater. 1996, 5, 299–303. [Google Scholar] [CrossRef]
- Gowri, M.; Li, H.; Schermer, J.J.; van Enckevort, W.J.P.; ter Meulen, J.J. Direct deposition of diamond films on steel using a three-step process. Diam. Relat. Mater. 2006, 15, 498–501. [Google Scholar] [CrossRef]
- Li, Y.-S.; Tang, Y.; Yang, Q.; Xiao, C.; Hirose, A. Growth and adhesion failure of diamond thin films deposited on stainless steel with ultra-thin dual metal interlayers. Appl. Surf. Sci. 2010, 256, 7653–7657. [Google Scholar] [CrossRef]
- Wild, C.; Kohl, R.; Herres, N.; Müller-Sebert, W.; Koidl, P. Oriented CVD diamond films: Twin formation, structure and morphology. Diam. Relat. Mater. 1994, 3, 373–381. [Google Scholar] [CrossRef]
- Wild, C.; Koidl, P.; Müller-Sebert, W. Chemical vapour deposition and characterization of smooth {100}-faceted diamond films. Diam. Relat. 1993, 2, 158–168. [Google Scholar] [CrossRef]
- Haubner, R.; Lux, B. Diamond deposition on steel substrates using intermediate layers. Int. J. Refract. Metals Hard Mater. 2006, 24, 380–386. [Google Scholar] [CrossRef]
- Chandran, M.; Hoffman, A. Diamond film deposition on WC–Co and steel substrates with a CrN interlayer for tribological applications. J. Phys. D Appl. Phys. 2016, 49, 213002. [Google Scholar] [CrossRef]
- Azina, C.; Wang, M.-M.; Feuillet, E.; Constantin, L.; Mortaigne, B.; Geffroy, P.; Lu, Y.-F.; Silvain, J. Surface & Coatings Technology Improved adhesion of polycrystalline diamond films on copper/carbon composite surfaces due to in situ formation of mechanical gripping sites. Surf. Coat. Technol. 2017, 321, 1–7. [Google Scholar] [CrossRef]
- Barquete, D.M.; Corat, E.J.; Campos, R.A.; Neto, C.M.; Trava-Airoldi, V.J. Thermodiffused vanadium carbide interface for diamond films on steel and cemented carbides substrates. Surf. Eng. 2010, 26, 506–510. [Google Scholar] [CrossRef]
- Contin, A.; de Vasconcelos, G.; Barquete, D.M.; Campos, R.A.; Trava-Airoldi, V.J.; Corat, E.J. Laser cladding of SiC multilayers for diamond deposition on steel substrates. Diam. Relat. Mater. 2016, 65, 105–114. [Google Scholar] [CrossRef]
- Damm, D.D.; Contin, A.; Trava-airoldi, V.J.; Barquete, D.M.; Corat, E.J. Synthesis of Vanadium Interface for HFCVD Diamond Deposition on Steel Surface. Mater. Res. 2017, 10–15. [Google Scholar] [CrossRef]
- Velázquez, R.; Neto, V.F.; Uppireddi, K.; Weiner, B.R.; Morell, G. Fabrication of Nanodiamond Coating on Steel. Coatings 2013, 3, 243–252. [Google Scholar] [CrossRef]
- Ager, J.W., III; Drory, M.D. Quantitative measurement of residual biaxial stress by Raman spectroscopy in diamond grown on a Ti alloy by chemical vapor deposition. Phys. Rev. B 1993, 48, 2601. [Google Scholar] [CrossRef]
- Fayer, A.; Glozman, O.; Hoffman, A. Deposition of continuous and well adhering diamond films on steel. Appl. Phys. Lett. 1995, 67, 2299. [Google Scholar] [CrossRef]
- Buijnsters, J.G.; Shankar, P.; Fleischer, W.; Van Enckevort, W.J.P.; Schermer, J.J.; Ter Meulen, J.J. CVD diamond deposition on steel using arc-plated chromium nitride interlayers. Diam. Relat. Mater. 2002, 11, 536–544. [Google Scholar] [CrossRef]
- Li, X.; Ye, J.; Zhang, H.; Feng, T.; Chen, J.; Hu, X. Sandblasting induced stress release and enhanced adhesion strength of diamond films deposited on austenite stainless steel. Appl. Surf. Sci. 2017, 412, 366–373. [Google Scholar] [CrossRef]
- Yang, T.; Wei, Q.; Qi, Y.; Wang, Y.; Xie, Y.; Luo, J.; Yu, Z. Microstructure evolution of thermal spray WC–Co interlayer during hot filament chemical vapor deposition of diamond thin films. J. Alloys Compd. 2015, 639, 659–668. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y.; Wan, B.; Yang, L.; Yang, Q. Deposition of diamond coatings on Fe-based substrates with Al and Al/AlN interlayers. Surf. Coat. Technol. 2015, 284, 139–144. [Google Scholar] [CrossRef]
- Fan, Q.H.; Fernandes, A.; Gracio, J. Diamond coating on steel with a titanium interlayer. Diam. Relat. Mater. 1998, 7, 603–606. [Google Scholar] [CrossRef]
- Kundrt, V.; Zhang, X.; Cooke, K.; Sun, H.; Sullivan, J.; Ye, H. A novel Mo-W interlayer approach for CVD diamond deposition on steel. AIP Adv. 2015, 5. [Google Scholar] [CrossRef]
- Bareiß, J.C.; Hackl, G.; Popovska, N.; Rosiwal, S.M.; Singer, R.F. CVD diamond coating of steel on a CVD-TiBN interlayer. Surf. Coat. Technol. 2006, 201, 718–723. [Google Scholar] [CrossRef]
- Negrea, G.; Vermesan, G. Investigations of diamond layers growth on steel. J. Optoelectron. Adv. Mater. 2000, 2, 698–703. [Google Scholar] [CrossRef]
- Silva, F.J.G.; Fernandes, A.J.S.; Costa, F.M.; Teixeira, V.; Baptista, A.P.M.; Pereira, E. Tribological behaviour of CVD diamond films on steel substrates. Wear 2003, 255, 846–853. [Google Scholar] [CrossRef]
- Polini, R.; Mantini, F.P.; Braic, M.; Amar, M.; Ahmed, W.; Taylor, H. Effects of Ti- and Zr-based interlayer coatings on the hot filament chemical vapour deposition of diamond on high speed steel. Thin Solid Films 2006, 494, 116–122. [Google Scholar] [CrossRef]
- Li, Y.S.; Ma, H.T.; Yang, L.Z.; Yang, Q.; Hirose, A. Enhanced diamond CVD synthesis on steel substrates modi fi ed by ion beam implantation and sputtering of Al. Surf. Coat. Technol. 2012, 207, 328–333. [Google Scholar] [CrossRef]
- Li, X.J.; He, L.L.; Li, Y.S.; Yang, Q.; Hirose, A. Direct Coating Adherent Diamond Films on Fe-Based Alloy Substrate: The Roles of Al, Cr in Enhancing Interfacial Adhesion and Promoting Diamond Growth. ACS Appl. Mater. Interfaces 2013, 5, 7370–7378. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Tang, Y.; Maley, J.; Sammynaiken, R.; Regier, T.; Xiao, C.; Hirose, A. Ultrathin W-Al dual interlayer approach to depositing smooth and adherent nanocrystalline diamond films on stainless steel. Appl. Mater. Interfaces 2010, 2, 335–338. [Google Scholar] [CrossRef] [PubMed]
- de Resende, W.D.; Corat, E.J.; Trava-airoldi, V.J.; Leite, N.F. Multi-layer structure for chemical vapor deposition diamond on electroplated diamond tools. Diam. Relat. Mater. 2001, 10, 332–336. [Google Scholar] [CrossRef]
- Lin, C.R.; Kuo, C.T. High adhesion and quality diamond films on steel substrate. Diam. Relat. Mater. 1998, 7, 903–907. [Google Scholar] [CrossRef]
- Sikder, A.K.; Sharda, T.; Misra, S.; Chandrasekaram, D.; Selvam, P. Chemical vapour deposition of diamond on stainless steel: The effect of Ni-diamond composite coated buffer layer. Diam. Relat. Mater. 1998, 7, 1010–1013. [Google Scholar] [CrossRef]
- Trava-airoldi, V.J.; Corat, E.J.; Leite, N.F.; Nono, M.C.; Ferreira, N.G.; Baranauskas, V. CVD diamond burrs—Development and applications. Diam. Relat. Mater. 1996, 5, 857–860. [Google Scholar] [CrossRef]
- Campos, R.A.; Contin, A.; Trava-Airoldi, V.J.; Barquete, D.M.; Corat, E.J.; Canale, L.; Dean, S.W. CVD of alternated MCD and NCD films on cemented carbide inserts. J. ASTM Int. 2011, 8, 103242. [Google Scholar] [CrossRef]
- Biesuz, M.; Sglavo, V.M. Chromium and vanadium carbide and nitride coatings obtained by TRD techniques on UNI 42CrMoS4 (AISI 4140) steel. Surf. Coat. Technol. 2016, 286, 319–326. [Google Scholar] [CrossRef]
- Wu, L.; Yao, T.; Wang, Y.; Zhang, J.; Xiao, F.; Liao, B. Understanding the mechanical properties of vanadium carbides: Nano-indentation measurement and first-principles calculations. J. Alloys Compd. 2013, 548, 60–64. [Google Scholar] [CrossRef]
- Chicco, B.; Borbidge, W.E.; Summerville, E. Experimental study of vanadium carbide and carbonitride coatings. Mater. Sci. Eng. A 1999, 266, 62–72. [Google Scholar] [CrossRef]
- Chong, X.-Y.; Jiang, Y.; Zhou, R.; Feng, J. RSC Advances Electronic structures mechanical and thermal properties of V-C binary compounds. RSC Adv. 2014, 4, 44959–44971. [Google Scholar] [CrossRef]
- Chong, X.; Jiang, Y.; Zhou, R.; Feng, J. The effects of ordered carbon vacancies on stability and thermo-mechanical properties of V8C7 compared with VC. Sci. Rep. 2016, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, A.; D’Alessio, L.; De Maria, G. Physiso-Chemical and Thermophysical Properties of Cubic Binary Carbides. Cryst. Res. Technol. 1998, 33, 341–374. [Google Scholar] [CrossRef]
- Arai, T.; Harper, S. Thermoreactive deposition diffusion process. Heat Treat. ASM Int. USA 1991, 4, 448–453. [Google Scholar]
- Aghaie-Khafri, M.; Daemi, N. Characterization of vanadium carbide coating deposited by borax salt bath process. Adv. Mater. Res. 2012, 1, 233–243. [Google Scholar] [CrossRef]
- Arai, T.; Fujita, H.; Sugimoto, Y.; Ohta, Y. Diffusion carbide coatings formed in molten borax systems. J. Mater. Eng. 1987, 9, 183–189. [Google Scholar] [CrossRef]
- Kong, D.; Zhou, C. The Surface and Interface Properties of Vanadium Carbide Coating Prepared By Thermal Diffusion Process. J. Adv. Manuf. Syst. 2011, 10, 183–190. [Google Scholar] [CrossRef]
- Rebak, R.B.; Terrani, K.A.; Gassmann, W.P.; Williams, J.B.; Ledford, K.L.; Nuclear, S. Improving Nuclear Power Plant Safety with FeCrAl Alloy Fuel Cladding. MRS Adv. 2017, 1–8. [Google Scholar] [CrossRef]
- Piekarczyk, W.; Yarbrough, W.A. Application of thermodynamics to the examination of the diamond CVD process II. A model of diamond deposition process from hydrocarbon-hydrogen mixtures. J. Cryst. Growth 1991, 108, 583–597. [Google Scholar] [CrossRef]
- Yarbrough, W.A.; Stewart, M.A.; Cooper, J.A. Combustion Synthesis of Diamond. Surf. Coat. Technol. 1989, 40, 241–252. [Google Scholar] [CrossRef]
- Yarbrough, W.A.; Badzian, A.R.; Pickrell, Y.L.; Inspektor, A. Diamond Deposition at Low Substrate Temperatures. J. Cryst. Growth 1990, 99, 1177–1182. [Google Scholar] [CrossRef]
- Wright, J.K.; Williamson, R.L.; Maggs, K.L. Finite element analysis of the effectiveness of interlayers in reducing thermal residual stresses in diamond films. Mater. Sci. Eng. A 1994, 187, 87–96. [Google Scholar] [CrossRef]
- Minkevich, A.N. Diffusion boride layers in metals. Metal. Sci. Heat Treat. 1961, 3, 347–351. [Google Scholar] [CrossRef]
- Makyta, M.; Zatko, P.; Bezdicka, P. Electrodeposition of Molybdenum from Molten salts. Chem. Pap. 1993, 47, 28–31. [Google Scholar]
- Ueda, N.; Mizukoshi, T.; Demizu, K.; Sone, T.; Ikenaga, A.; Kawamoto, M. Boriding of nickel by the powder-pack method. Surf. Coat. Technol. 2000, 157, 35–38. [Google Scholar] [CrossRef]
- Sen, S.; Sen, U.; Bindal, C. An approach to kinetic study of borided steels. Surf. Coat. Technol. 2005, 191, 274–285. [Google Scholar] [CrossRef]
- Fichtl, W. Boronizing and its practical applications. Mater. Des. 1981, 2, 276–286. [Google Scholar] [CrossRef]
- Jain, V.; Sundararajan, G. Influence of the pack thickness of the boronizing mixture on the boriding of steel. Surf. Coat. Technol. 2002, 149, 21–26. [Google Scholar] [CrossRef]
- Van Enckevort, I.J.J.; Schermer, J.J.; Buijnsters, J.G.; Shankar, P.; Meulen, J.J.T. Method of Forming a Diamond Coating on an Iron-Based Substrate and Use of an Iron-Based Substrate for Hosting a CVD Diamond Coating. U.S. Patent 7,132,129 B2, 7 November 2006. [Google Scholar]
- Juijerm, P. Diffusion kinetics of different boronizing processes on martensitic stainless steel AISI 420. Kov. Mater. 2014, 52, 231–236. [Google Scholar] [CrossRef]
- Barbieri, F.C. Filmes Intermediários Para a Deposição de Diamantes CVD Sobre Aços. Ph.D. Thesis, National Institute for Space Research, São José dos Campos—SP, 31 August 2007. [Google Scholar]
- Campos, I.; Bautista, O.; Ramírez, G.; Islas, M.; De La Parra, J.; Zúñiga, L. Effect of boron paste thickness on the growth kinetics of Fe2B boride layers during the boriding process. Appl. Surf. Sci. 2005, 243, 429–436. [Google Scholar] [CrossRef]
- Özbek, I.; Konduk, B.A.; Bindal, C.; Ucisik, A.H. Characterization of borided AISI 316L stainless steel implant. Vacuum 2002, 65, 521–525. [Google Scholar] [CrossRef]
- Mougin, J.; Dupeux, M.; Antoni, L.; Galerie, A.A. Adhesion of thermal oxide scales grown on ferritic stainless steels measured using the inverted blister test. Mater. Sci. Eng. A 2003, 359, 44–51. [Google Scholar] [CrossRef]
- Sabioni, A.C.S.; Huntz, A.M.; Philibert, J.; Lesage, B.; Monty, C. Relation between the oxidation growth rate of chromia scales and self-diffusion in Cr2O3. J. Mater. Sci. 1992, 27, 4782–4790. [Google Scholar] [CrossRef]
- Galerie, A.; Toscan, F.; Dupeux, M.; Mougin, J.; Lucazeau, G.; Valot, C.; Huntz, A.-M.; Antoni, L. Stress and adhesion of chromia-rich scales on ferritic stainless steels in relation with spallation. Mater. Res. 2004, 7, 81–88. [Google Scholar] [CrossRef]
- Arai, T.; Endo, J.; Takeda, H. Chromizing and boriding by use of a fluidized bed. Ind. Heat. 1987, 54, 18–19. [Google Scholar]
- Wei, C.-Y.; Chen, F.-S. Characterization on multi-layer fabricated by TRD and plasma nitriding. Mater. Chem. Phys. 2005, 90, 178–184. [Google Scholar] [CrossRef]
- Antonov, M.; Pirso, J. Thermal Shock Resistance of Chromium Carbide-Based Cermets. Encycl. Therm. Stress. 2014, 5128–5135. [Google Scholar] [CrossRef]
- Schwarz, S.; Rosiwal, S.M.; Musayev, Y.; Singer, R.F. High temperature diffusion chromizing as a successful method for CVD-diamond coating of steel-Part II. Diam. Relat. Mater. 2003, 12, 701–706. [Google Scholar] [CrossRef]
- Bareiß, C.; Perle, M.; Rosiwal, S.M.; Singer, R.F. Diamond coating of steel at high temperatures in hot filament chemical vapour deposition (HFCVD) employing chromium interlayers. Diam. Relat. Mater. 2006, 15, 754–760. [Google Scholar] [CrossRef]
- Kosolapova, T.Y. Carbides Properties, Production and Aplications; Plenuss Press: New York, NY, USA, 1971. [Google Scholar]
- Kellermann, K.; Bareiß, C.; Rosiwal, S.M.; Singer, R.F. Well Adherent Diamond Coatings on Steel Substrates. Adv. Eng. Mater. 2008, 10, 657–660. [Google Scholar] [CrossRef]
- Leeds, S.M.; Davis, T.J.; May, P.W.; Pickard, C.D.O.; Ashfold, M.N.R. Use of Different Excitation Wavelengths for the Analysis of CVD Diamond by Laser Raman Spectroscopy. Diam. Relat. Mater. 1988, 7, 233–237. [Google Scholar] [CrossRef]
- Degutis, G.; Pobedinskas, P.; Turner, S.; Lu, Y.G.; Al Riyami, S.; Ruttens, B.; Yoshitake, T.; D’Haen, J.; Haenen, K.; Verbeeck, J.; et al. CVD diamond growth from nanodiamond seeds buried under a thin chromium layer. Diam. Relat. Mater. 2016, 64, 163–168. [Google Scholar] [CrossRef]
- Contin, A.; Alves, K.A.; Campos, R.A.; de Vasconcelos, G.; Damm, D.D.; Trava-airoldi, V.J.; Corat, E.J. Diamond Films on Stainless Steel Substrates with an Interlayer Applied by Laser Cladding. Mater. Res. 2017, 20, 543–548. [Google Scholar] [CrossRef]
- Qin, R.; Zhang, X.; Guo, S.; Sun, B.; Tang, S.; Li, W. Laser cladding of high Co–Ni secondary hardening steel on 18Cr2Ni4WA steel. Surf. Coat. Technol. 2016, 285, 242–248. [Google Scholar] [CrossRef]
- Draper, C.W.; Ewing, C.A. Laser surface alloying: A bibliography. J. Mater. Sci. 1984, 19, 3815–3825. [Google Scholar] [CrossRef]
- Da Silva, L.L.G.; Ueda, M.; Nakazato, R.Z. Enhanced corrosion resistance of AISI H13 steel treated by nitrogen plasma immersion ion implantation. Surf. Coat. Technol. 2007, 201, 8291–8294. [Google Scholar] [CrossRef]
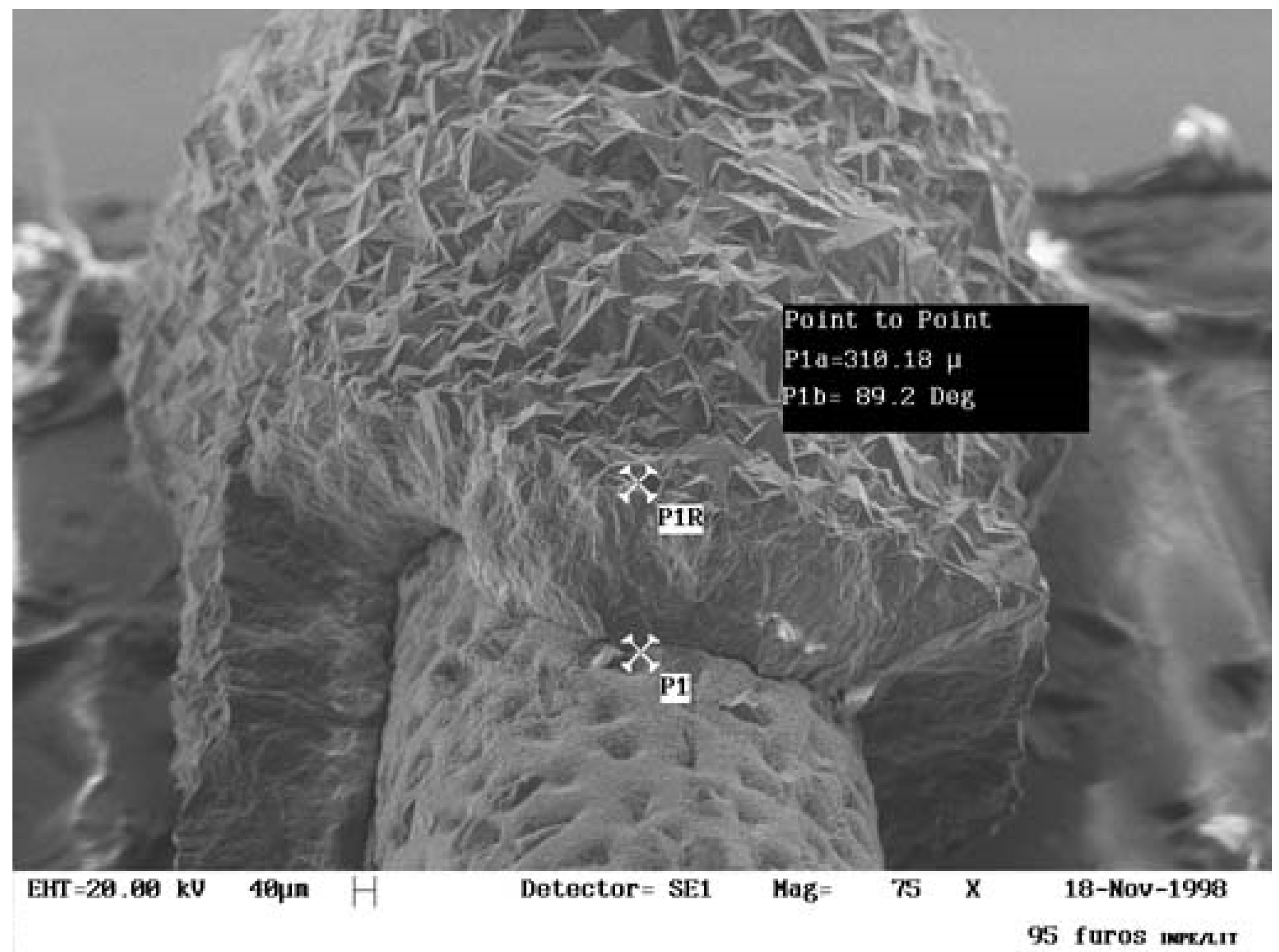
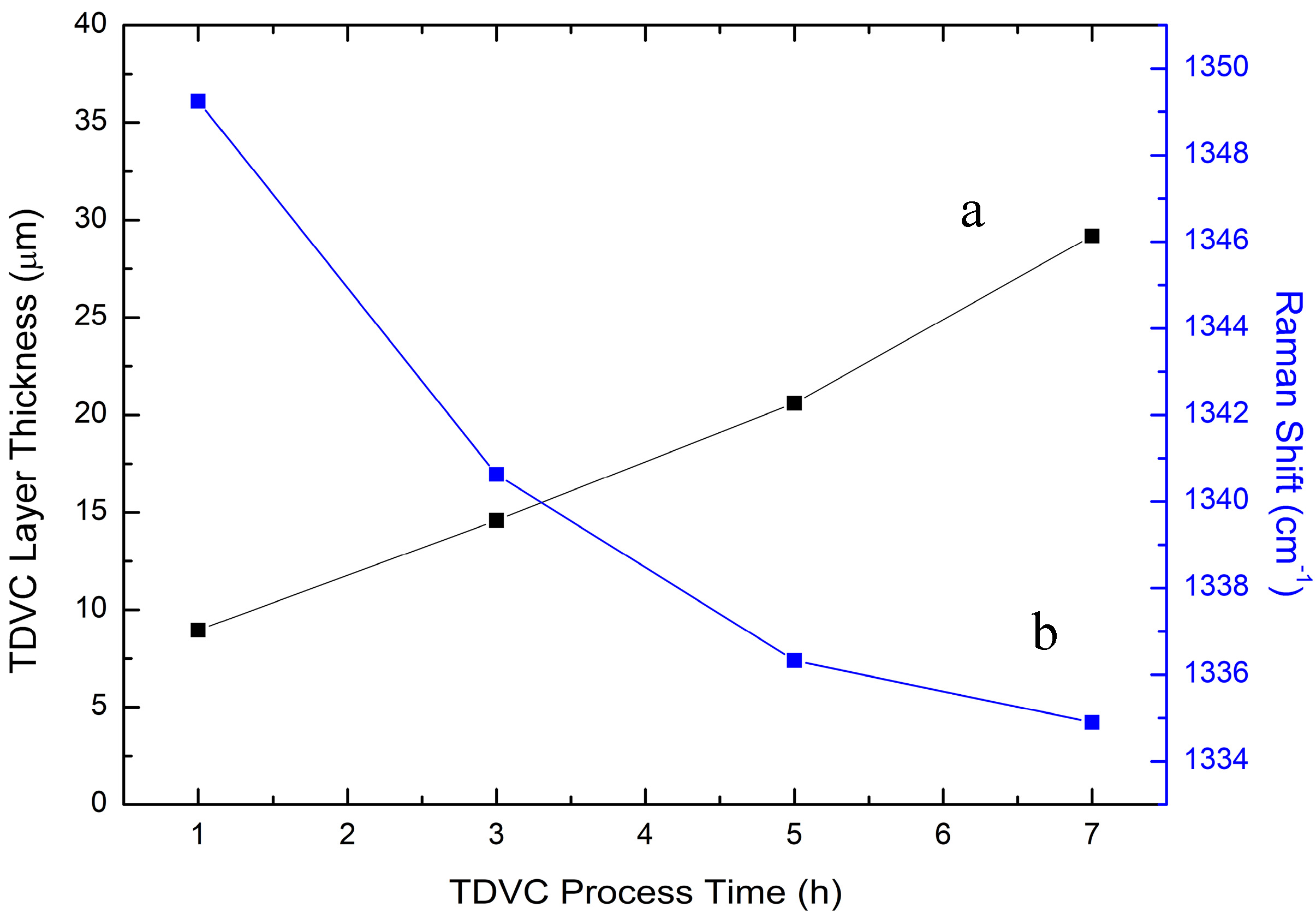
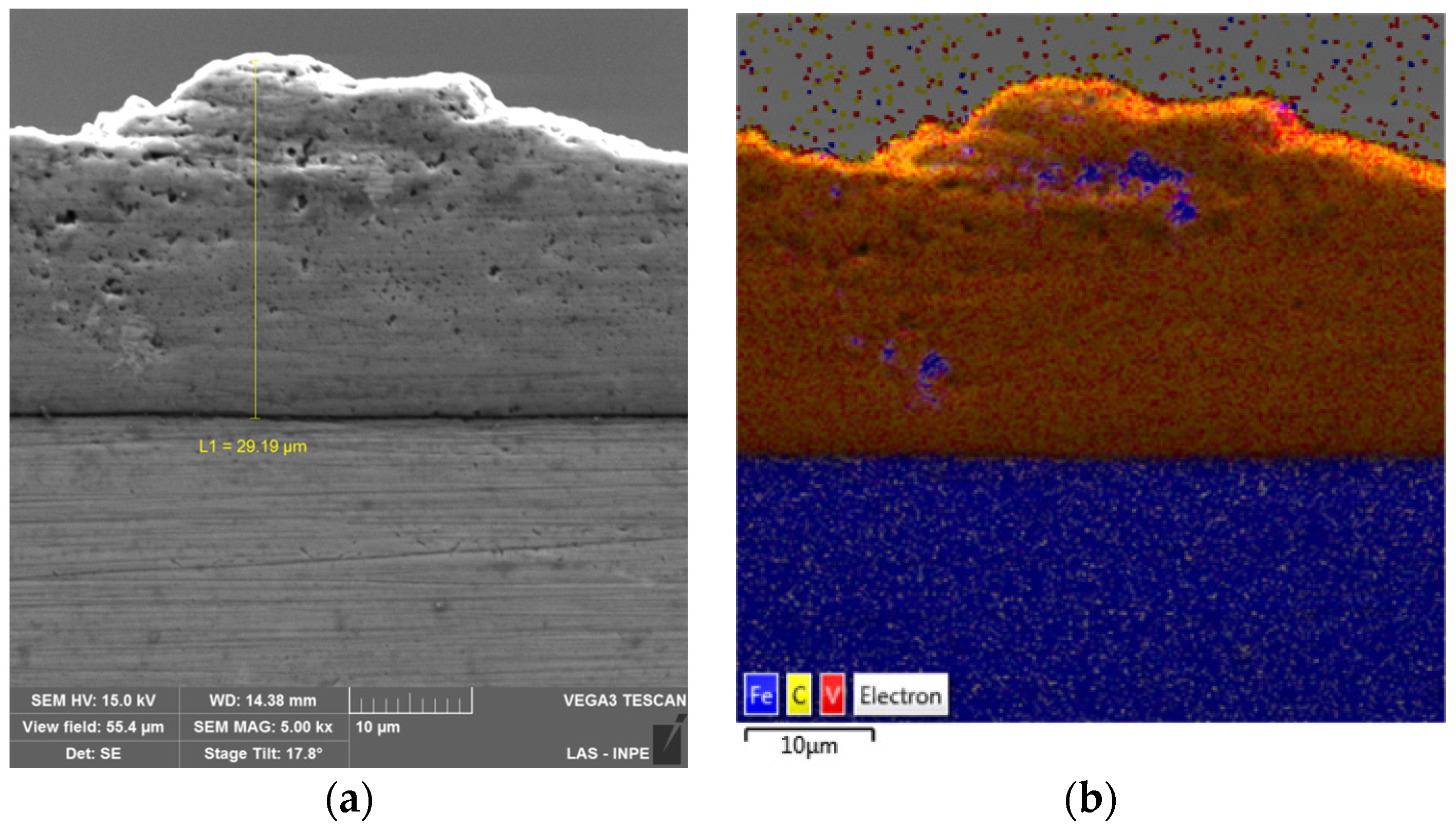
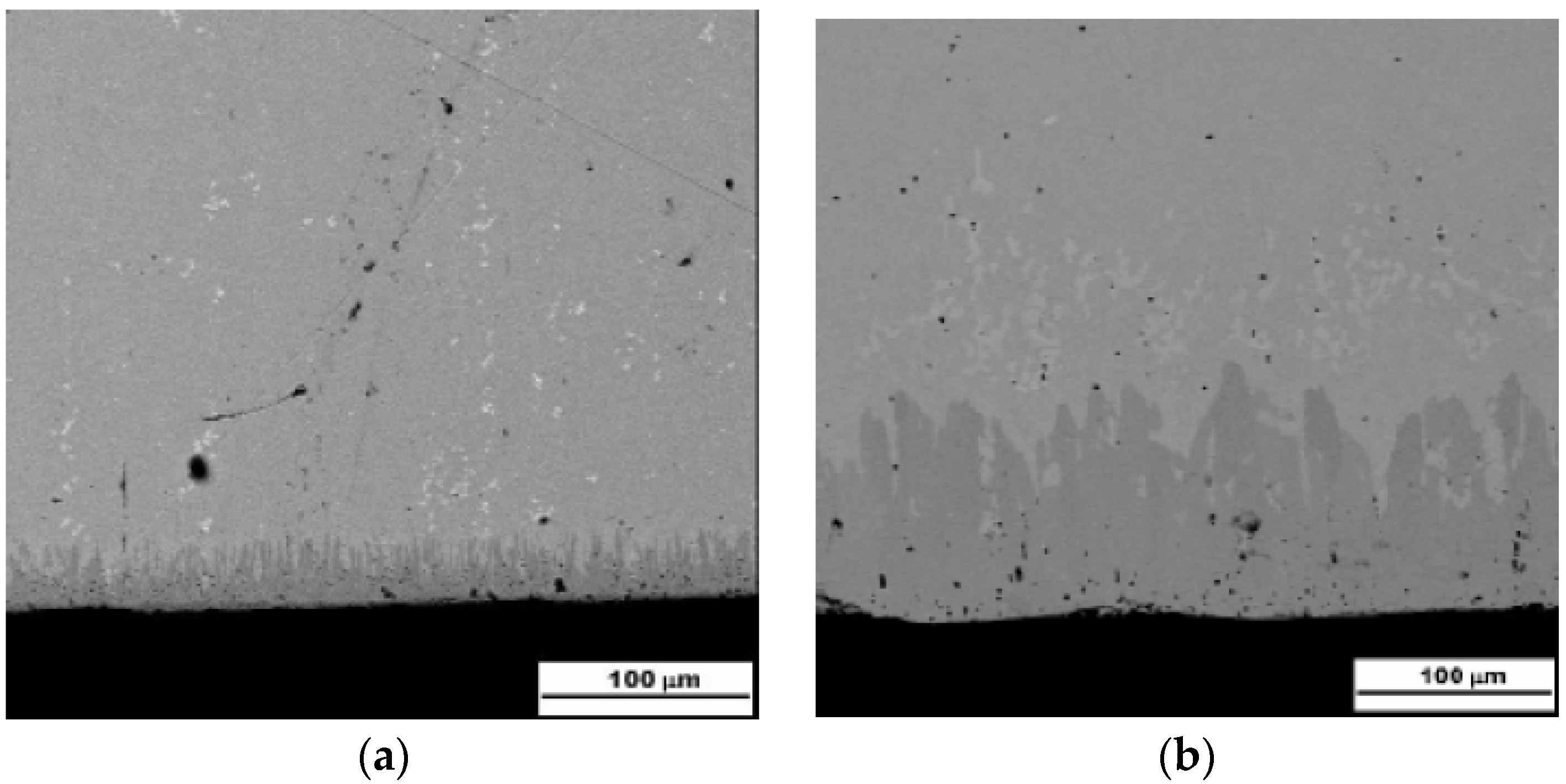
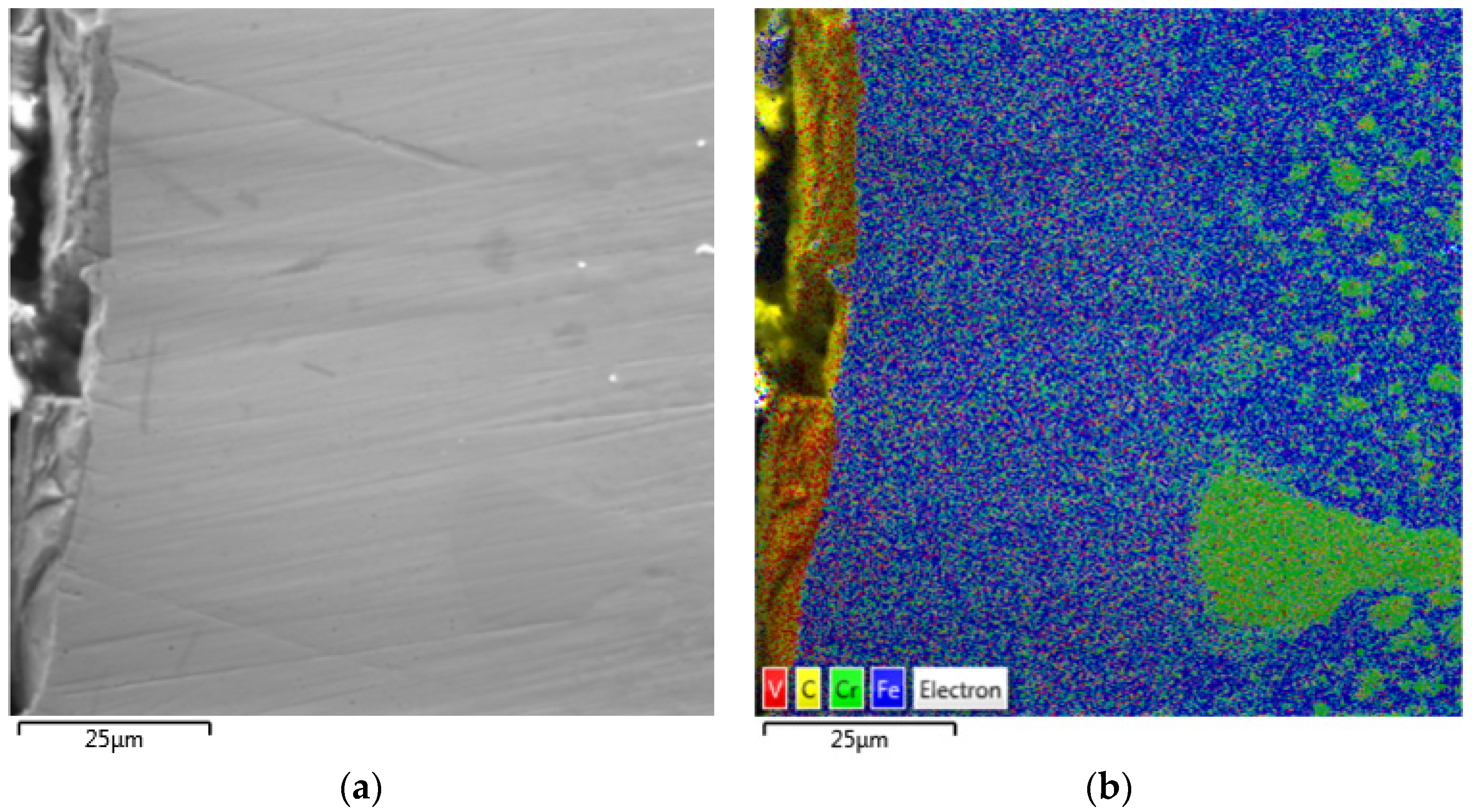
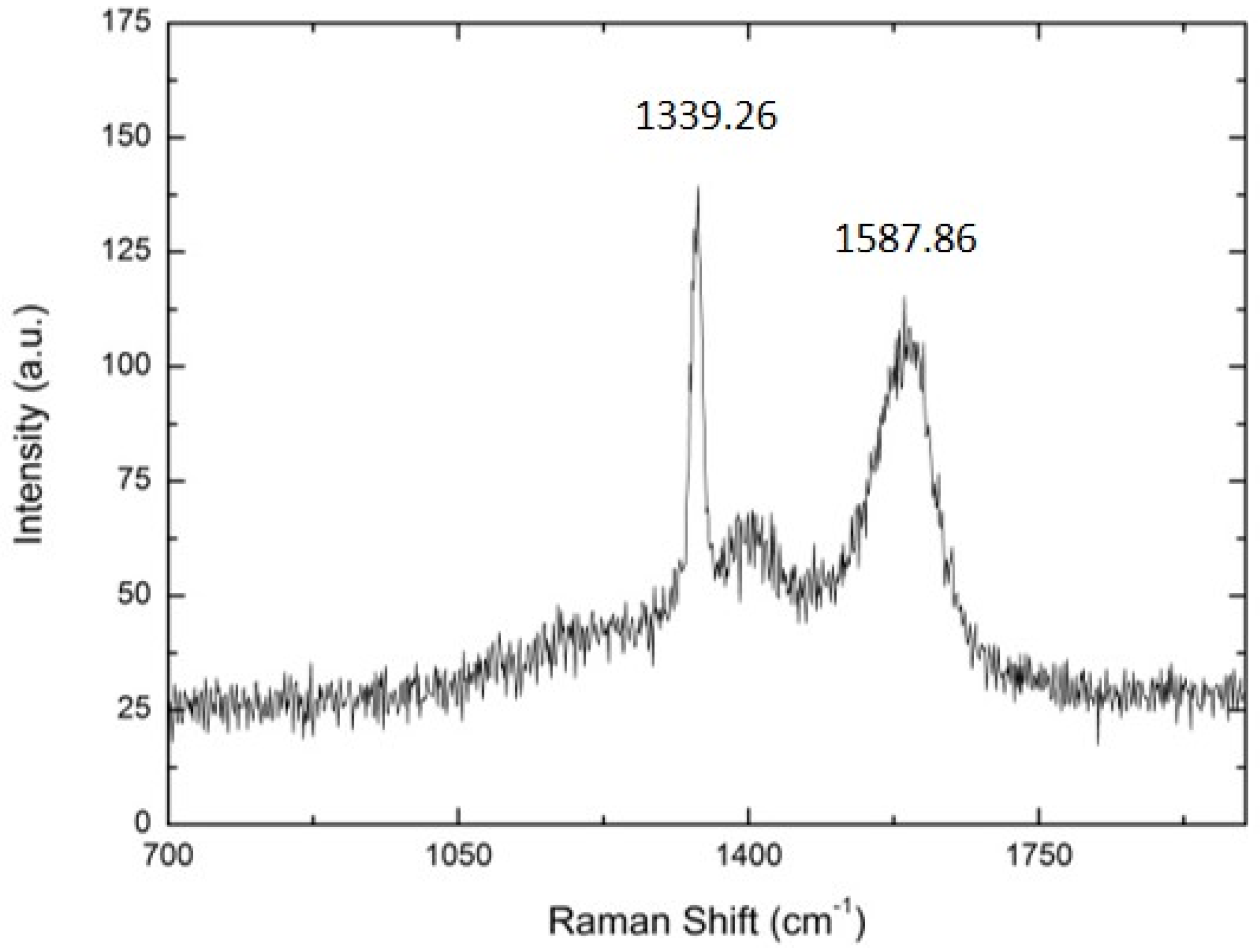
| SUBSTRATE/Intermediate Layer | Intermediate Layer Thickness (μm) | Intermediate Layer Growth Process | Diamond Film Thickness (μm) | Diamond Morphology/Growth Temperature (°C) | Raman Shiift (cm−1) | Ref. |
|---|---|---|---|---|---|---|
| Stainless steel Cr/CrN | 0.2/1.6 | Sputtering | 1 | Nano/630 | 1337 | [34] |
| -Cr-steel/CrN | 10 | Electro plating + Nitriding | 2 | Micro/800 | 1358 | [32] |
| Carbon steel/WC–Co | 200 | HVOF + chemical etching | 5 h—grains of 0.5 μm | Micro/700 | 1332 | [35] |
| Stainless steel/Ti-Al | 0.05 | Sputtering | – | Nano/680 | spallation | [21] |
| Stainless steel/Al–AlN | 6 h deposition p/Al | Sputtering | 6 h Deposition time | Micro/670 | On Al—1332 | [36] |
| 3 h p/Al e AlN | On AlN—1332 | |||||
| HSS/Ti | 2 | Sputtering | 3 h Deposition time | Micro/800 | 1337 | [37] |
| HSS/Mo–W | 1.1 | Sputtering | 0.26 | Nano/785 | 1332 | [38] |
| Cr-Steel/TiBN | 6 | CVD | 3 | Micro/850 | 1338–1349 | [39] |
| HSS/CrN | 4 | Sputtering | 4 h Deposition time | Nano/400 | 1335 | [40] |
| HSS/Ni–Cu–Ti | Ni—4 | Electro plating | 13.5 | Micro | 1339 | [41] |
| Cu—36 | Electro plating | |||||
| Ti—2.5 | PVD | |||||
| Stainless steel/Fe2B | 40 | Thermo diffusion | 3 | Micro/650 | 1333 | [17] |
| HSS/Ti, ZrN | TiC—10 | Cathodic arc | 1.8 | Micro/650 | 1345 | [42] |
| ZrN—10 | Cathodic arc | 1.8 | Micro/650 | spallation | ||
| Stainless steel/CrN | 2.5 | Cathodic arc | Not a continuous film | Micro/650 | 1342 | [33] |
| HSS/CrN | 2.5 | Cathodic arc | 2.5 | Micro/650 | 1345 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damm, D.D.; Contin, A.; Barbieri, F.C.; Trava-Airoldi, V.J.; Barquete, D.M.; Corat, E.J. Interlayers Applied to CVD Diamond Deposition on Steel Substrate: A Review. Coatings 2017, 7, 141. https://doi.org/10.3390/coatings7090141
Damm DD, Contin A, Barbieri FC, Trava-Airoldi VJ, Barquete DM, Corat EJ. Interlayers Applied to CVD Diamond Deposition on Steel Substrate: A Review. Coatings. 2017; 7(9):141. https://doi.org/10.3390/coatings7090141
Chicago/Turabian StyleDamm, Djoille Denner, André Contin, Fernando Cruz Barbieri, Vladimir Jesus Trava-Airoldi, Danilo Maciel Barquete, and Evaldo José Corat. 2017. "Interlayers Applied to CVD Diamond Deposition on Steel Substrate: A Review" Coatings 7, no. 9: 141. https://doi.org/10.3390/coatings7090141
APA StyleDamm, D. D., Contin, A., Barbieri, F. C., Trava-Airoldi, V. J., Barquete, D. M., & Corat, E. J. (2017). Interlayers Applied to CVD Diamond Deposition on Steel Substrate: A Review. Coatings, 7(9), 141. https://doi.org/10.3390/coatings7090141





