Investigation on the Cathodic Protection Effect of Low Pressure Cold Sprayed AlZn Coating in Seawater via Numerical Simulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fundamentals of Cathodic Protection Numerical Simulation
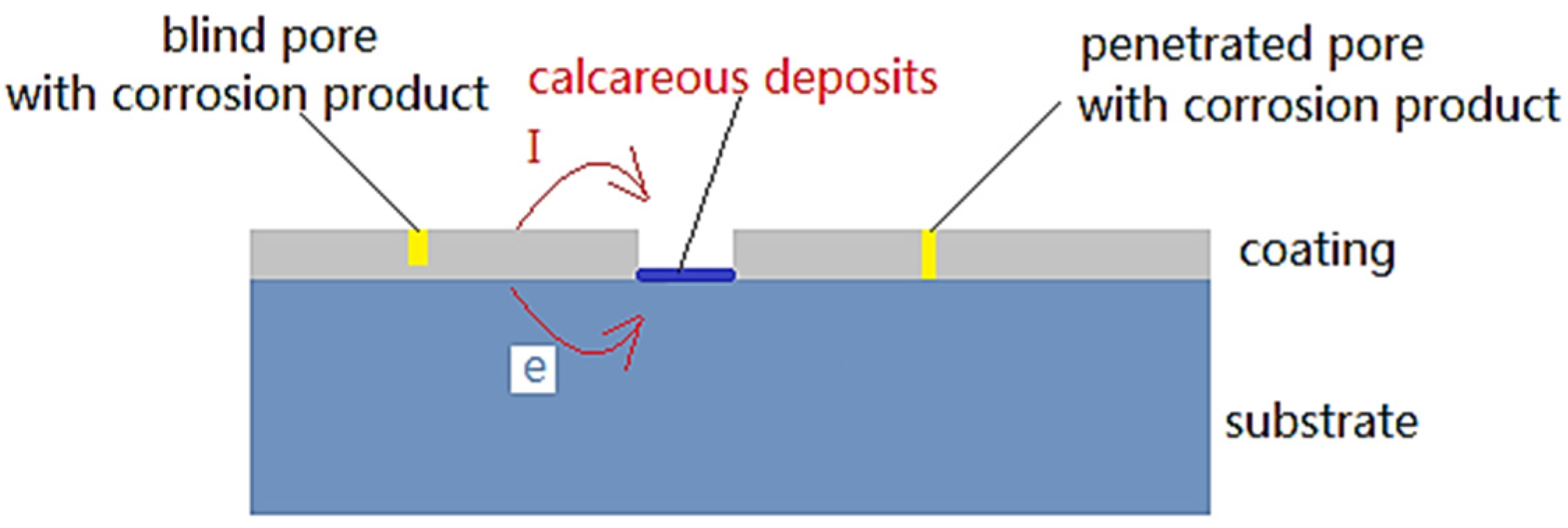
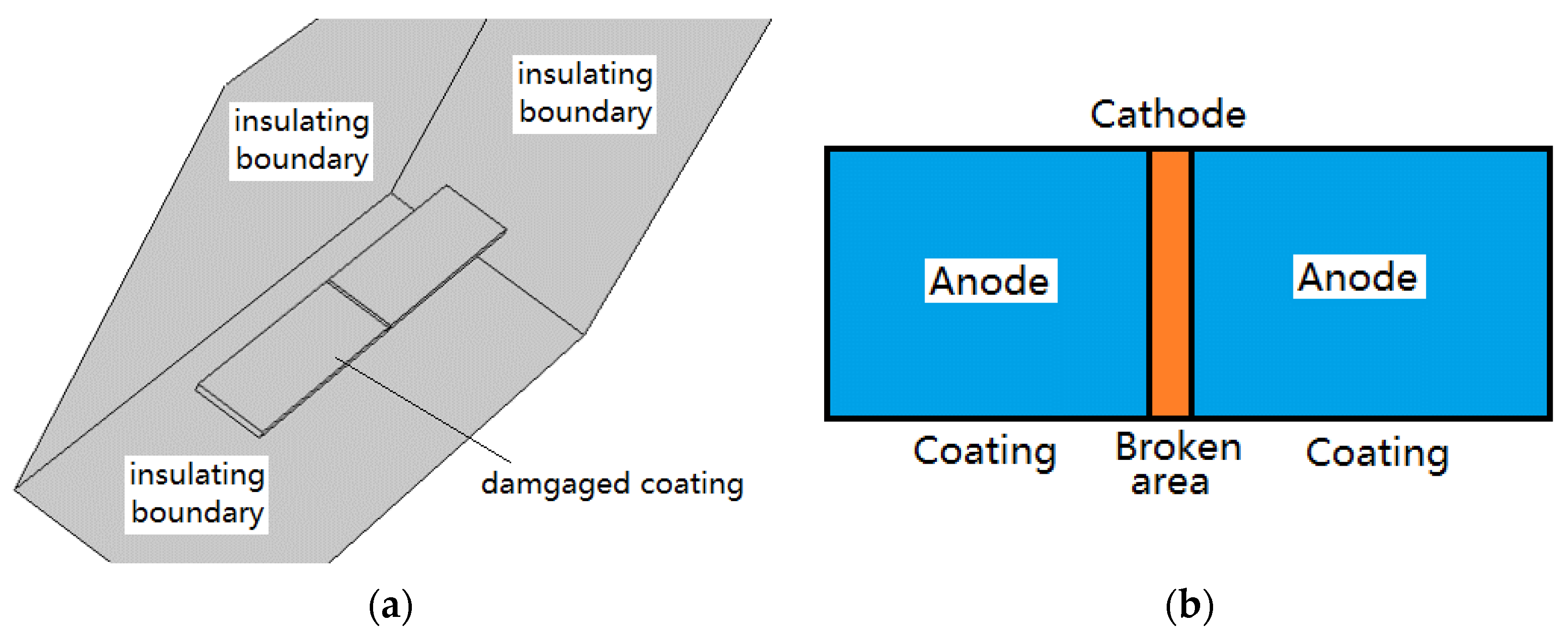
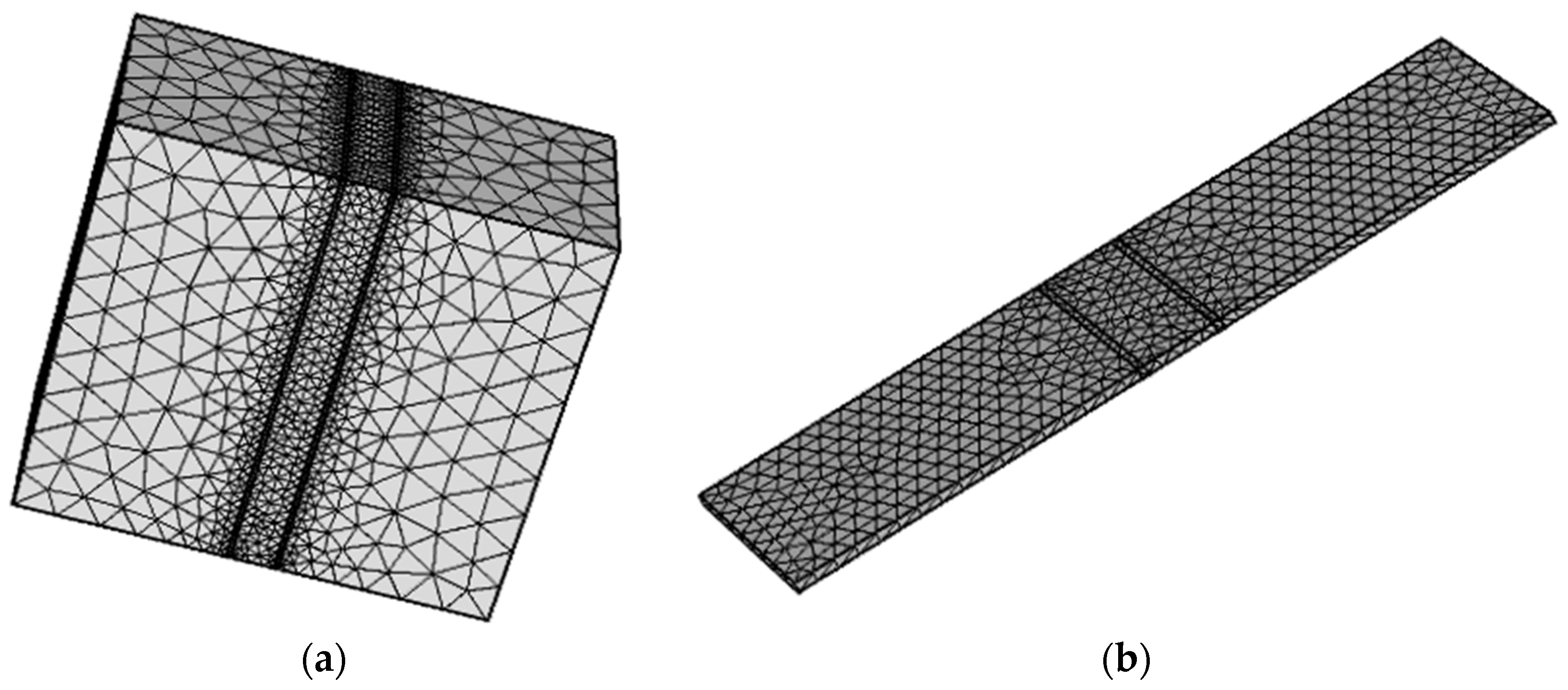
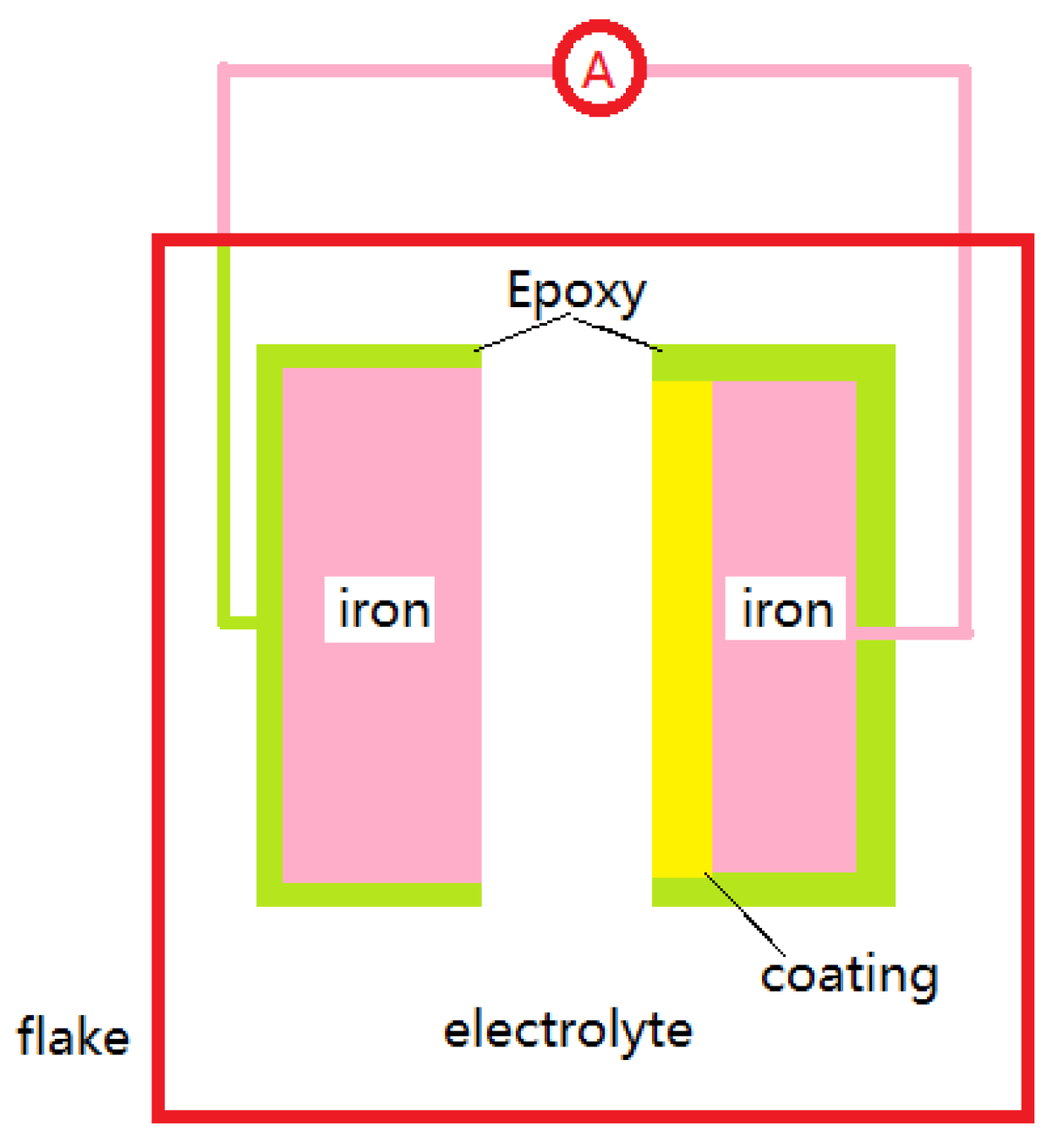
2.2. Physical Model
2.3. Boundary Condition and Data Collection
2.4. Validation of Simulation Results
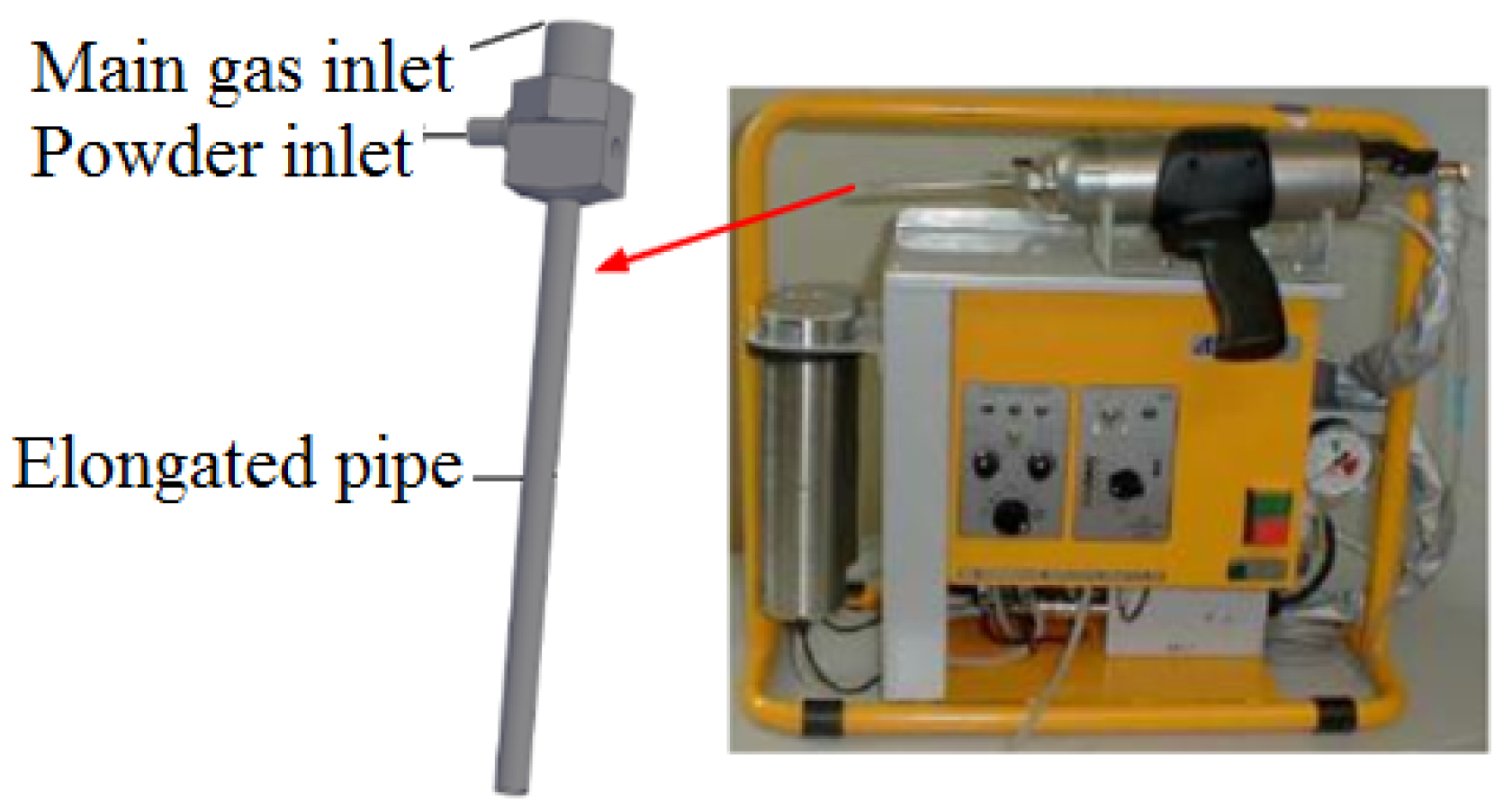
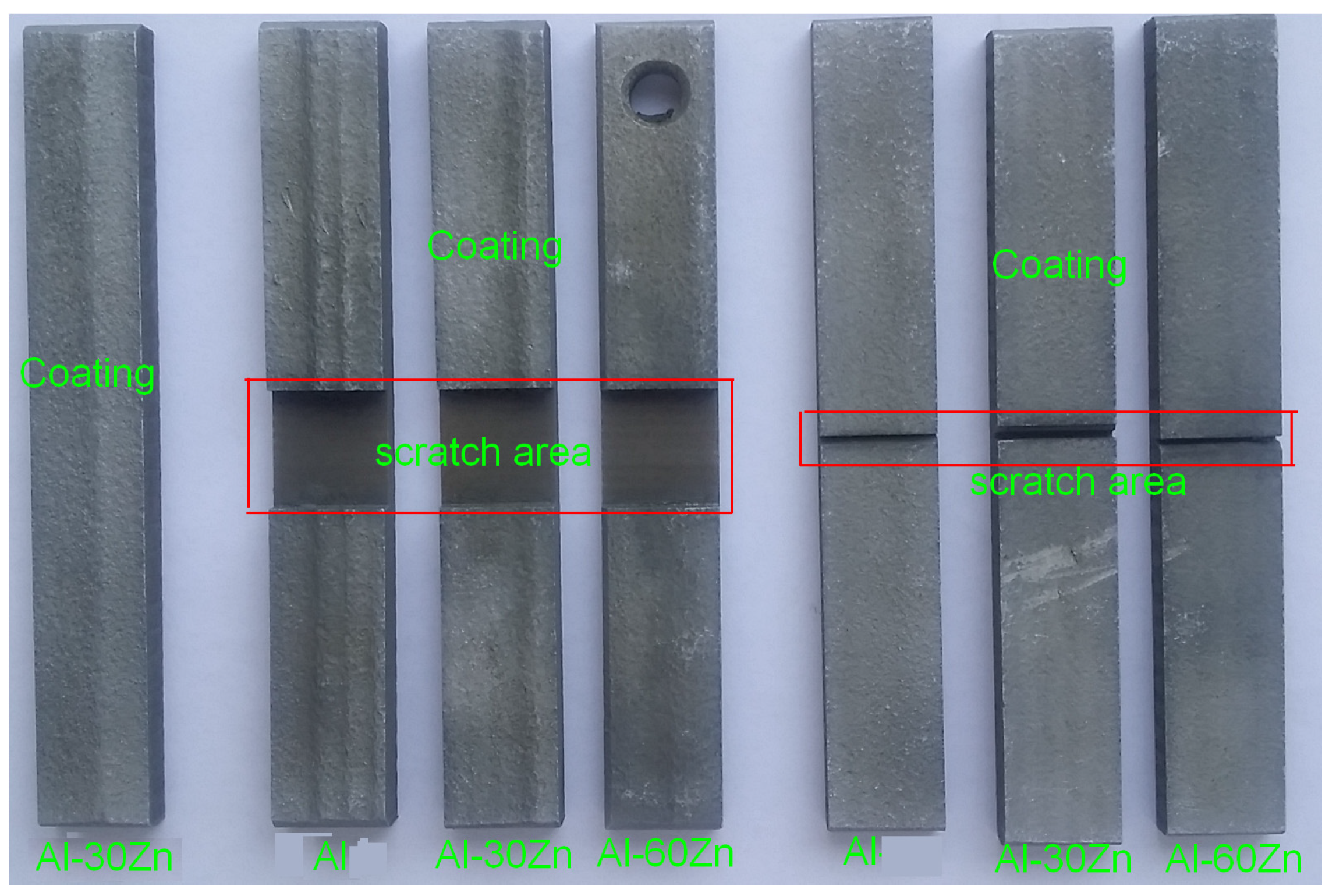
2.4.1. Low-Pressure Cold-Sprayed AlZn Coating Preparation
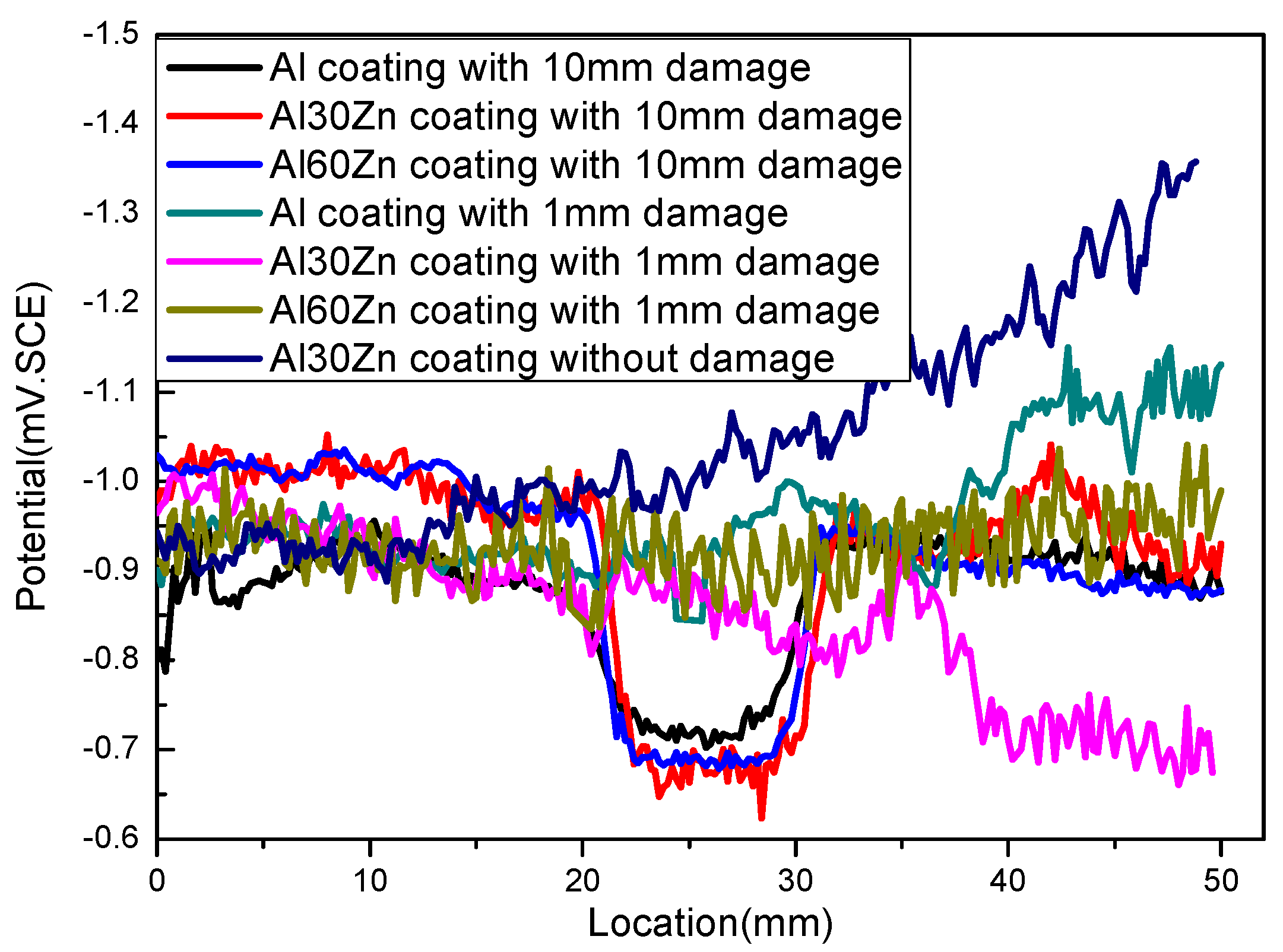
2.4.2. Verification of Simulation Results
3. Results
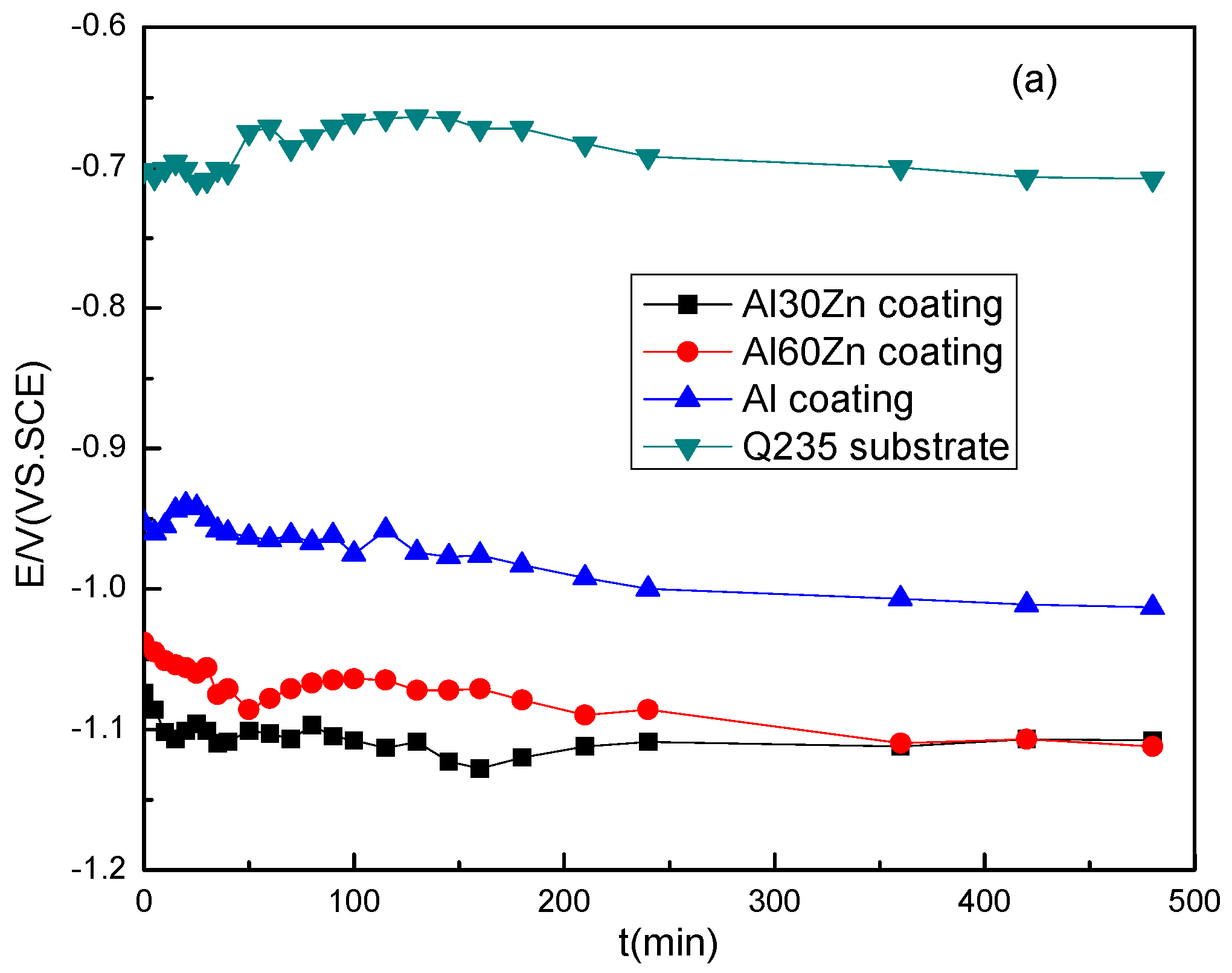
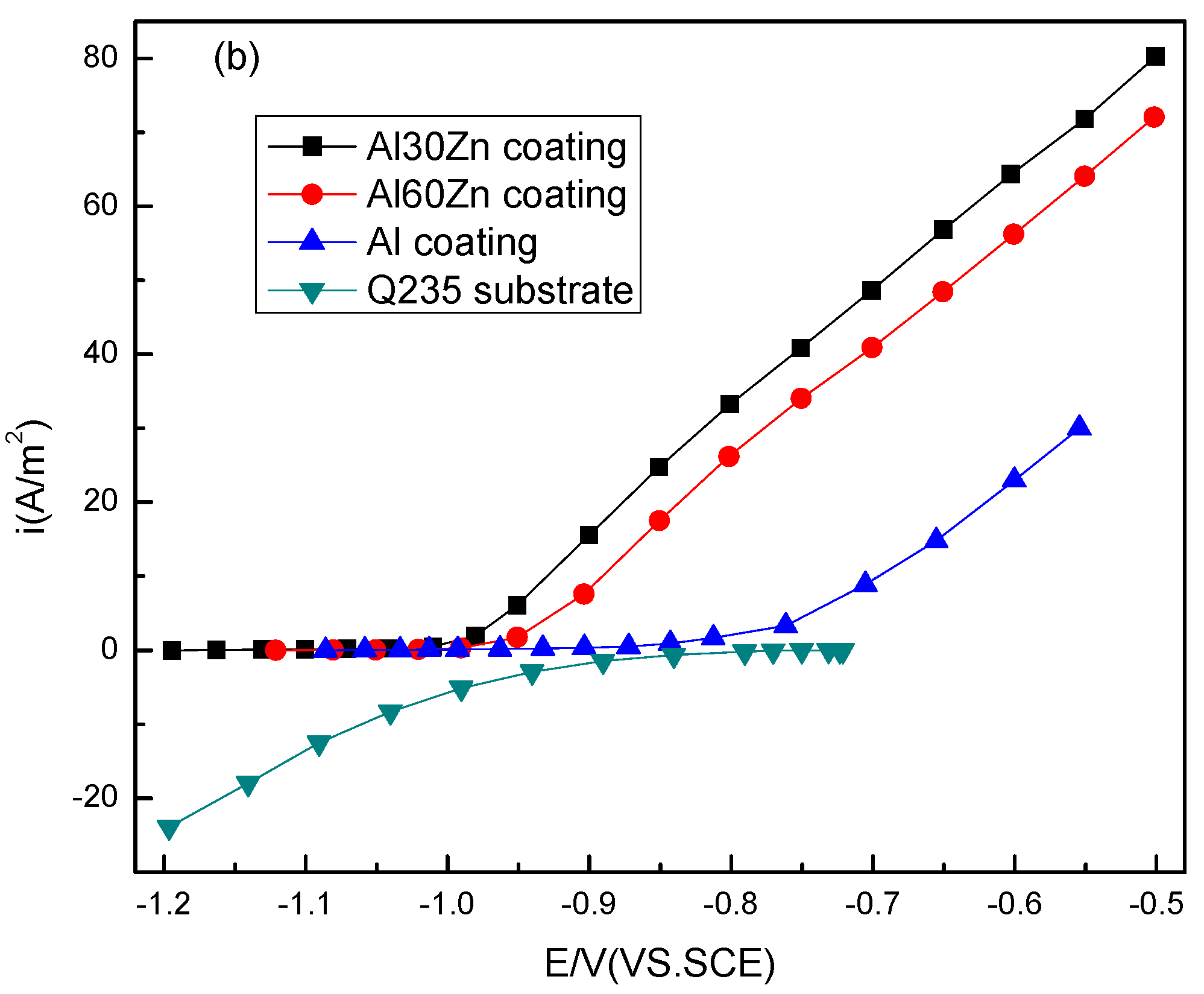
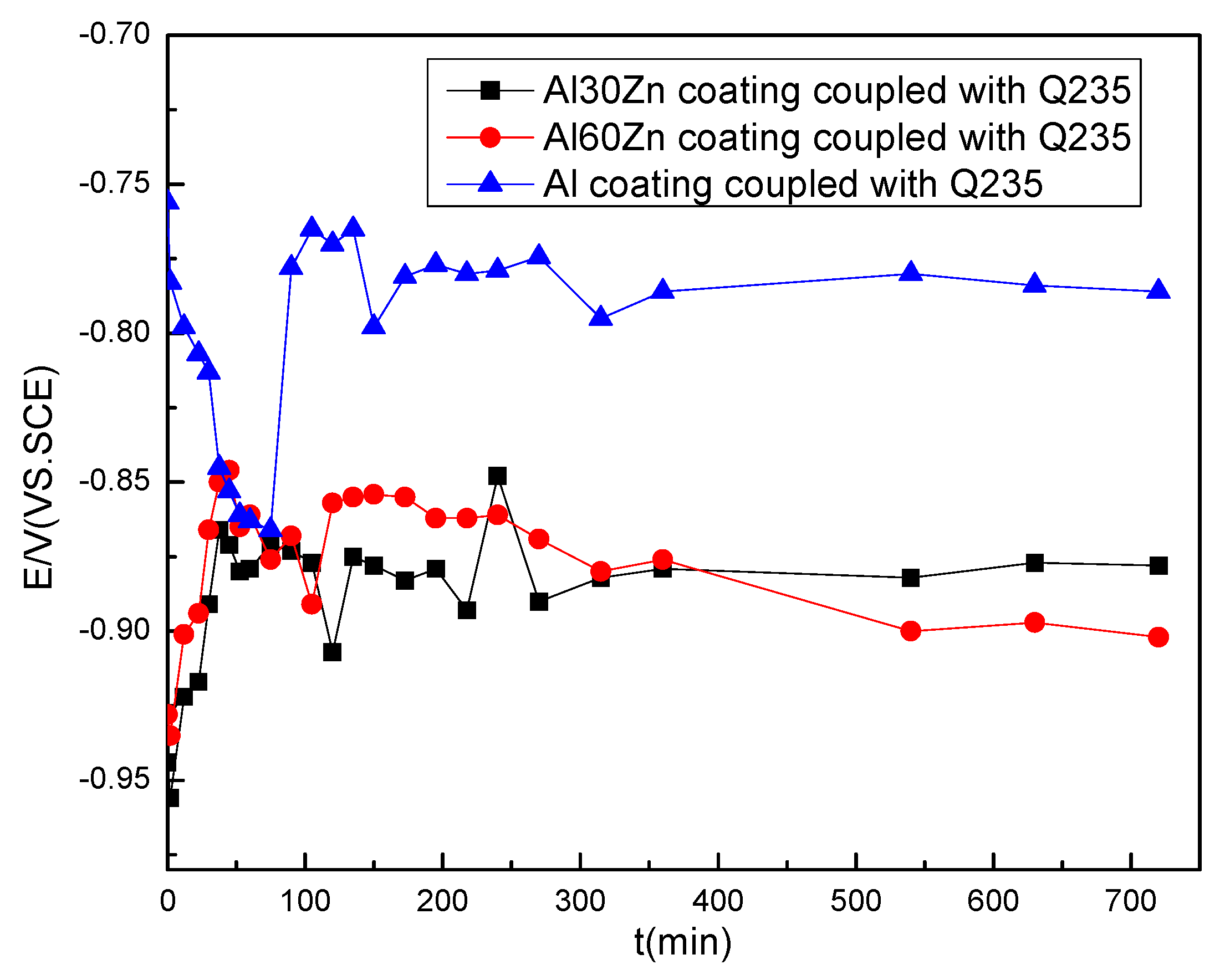
3.1. Open Circuit Potential and Potentiodynamic Polarization Curves
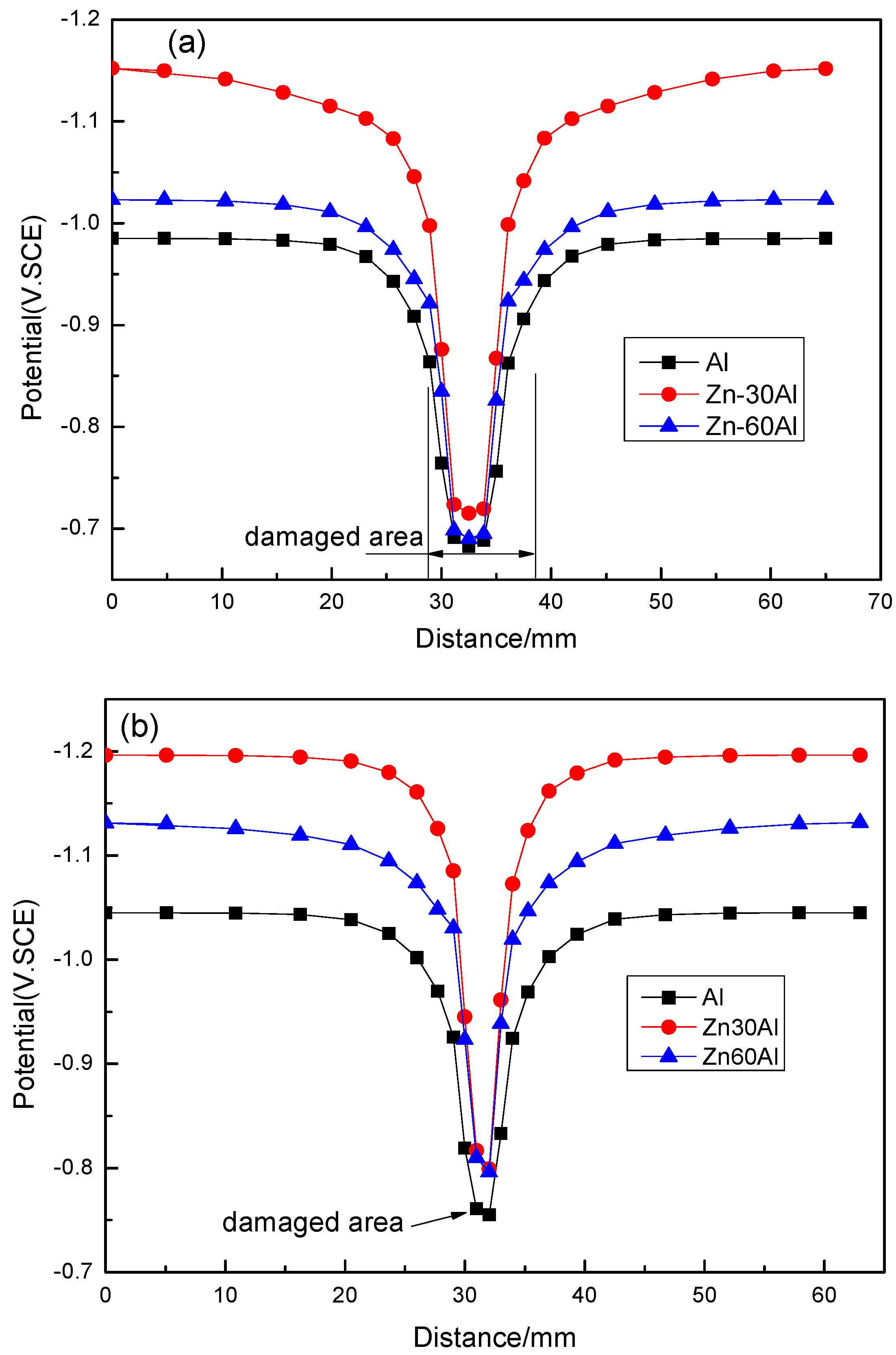
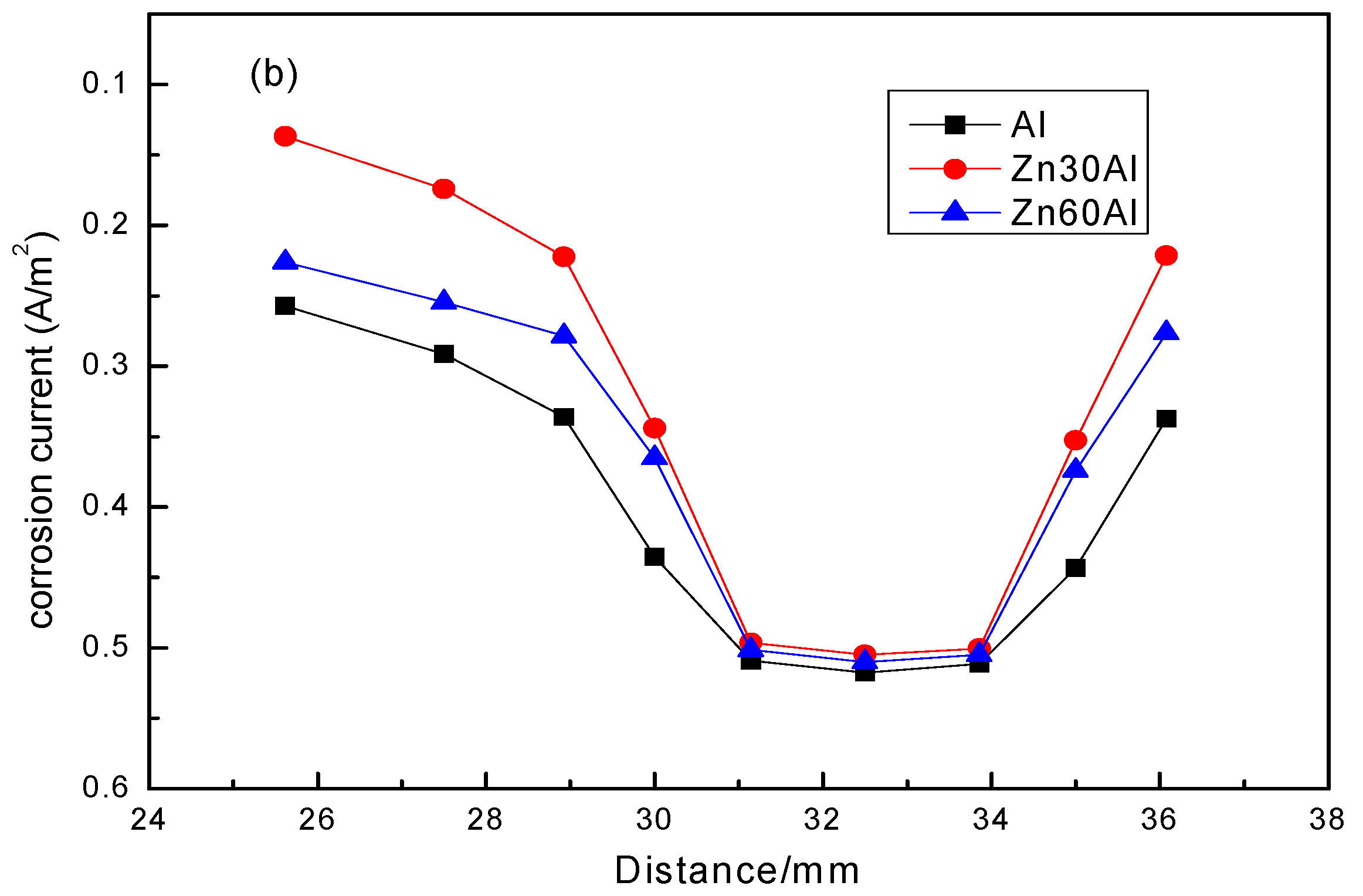
3.2. The Potential Distribution of Different Coatings
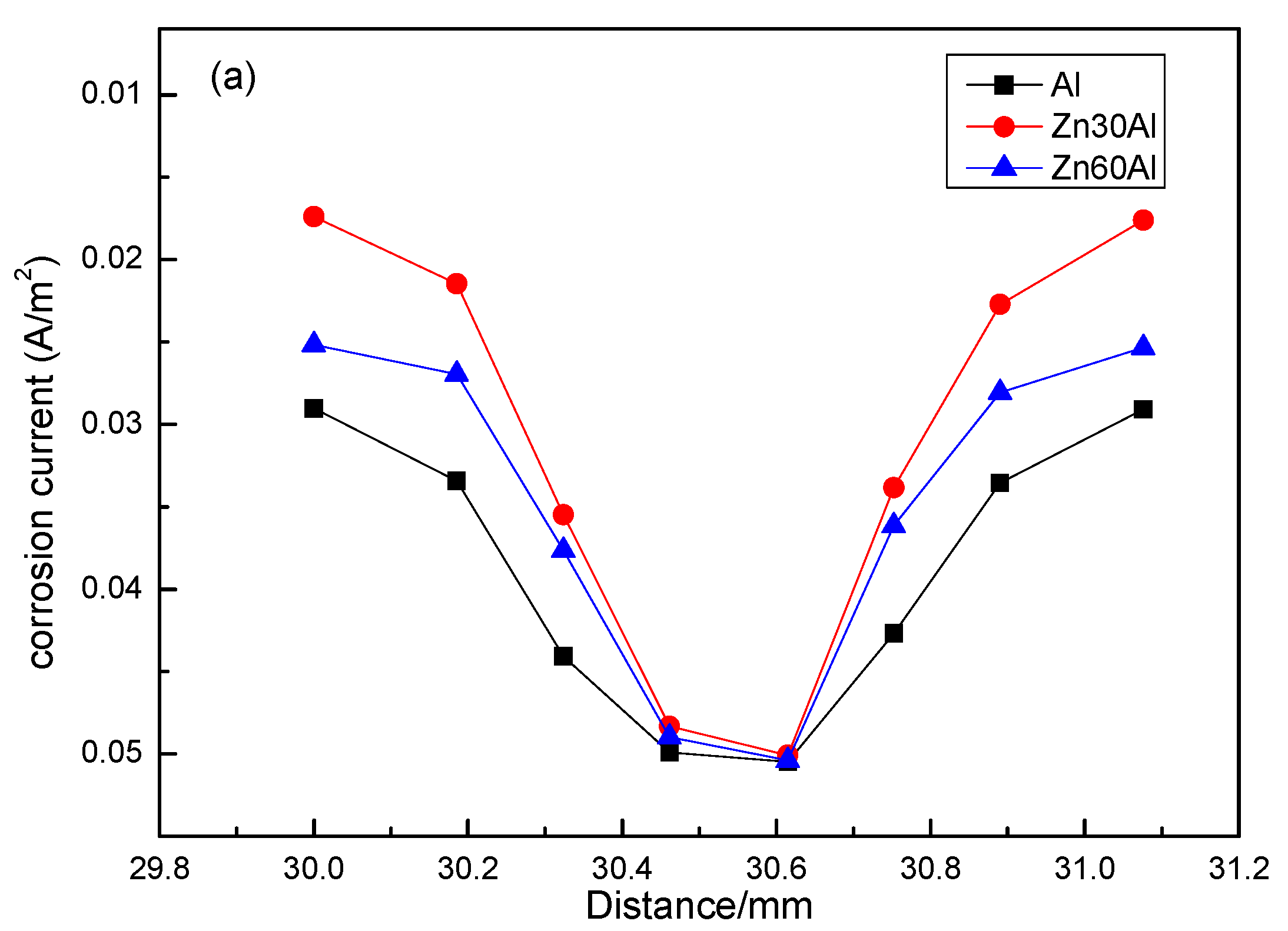
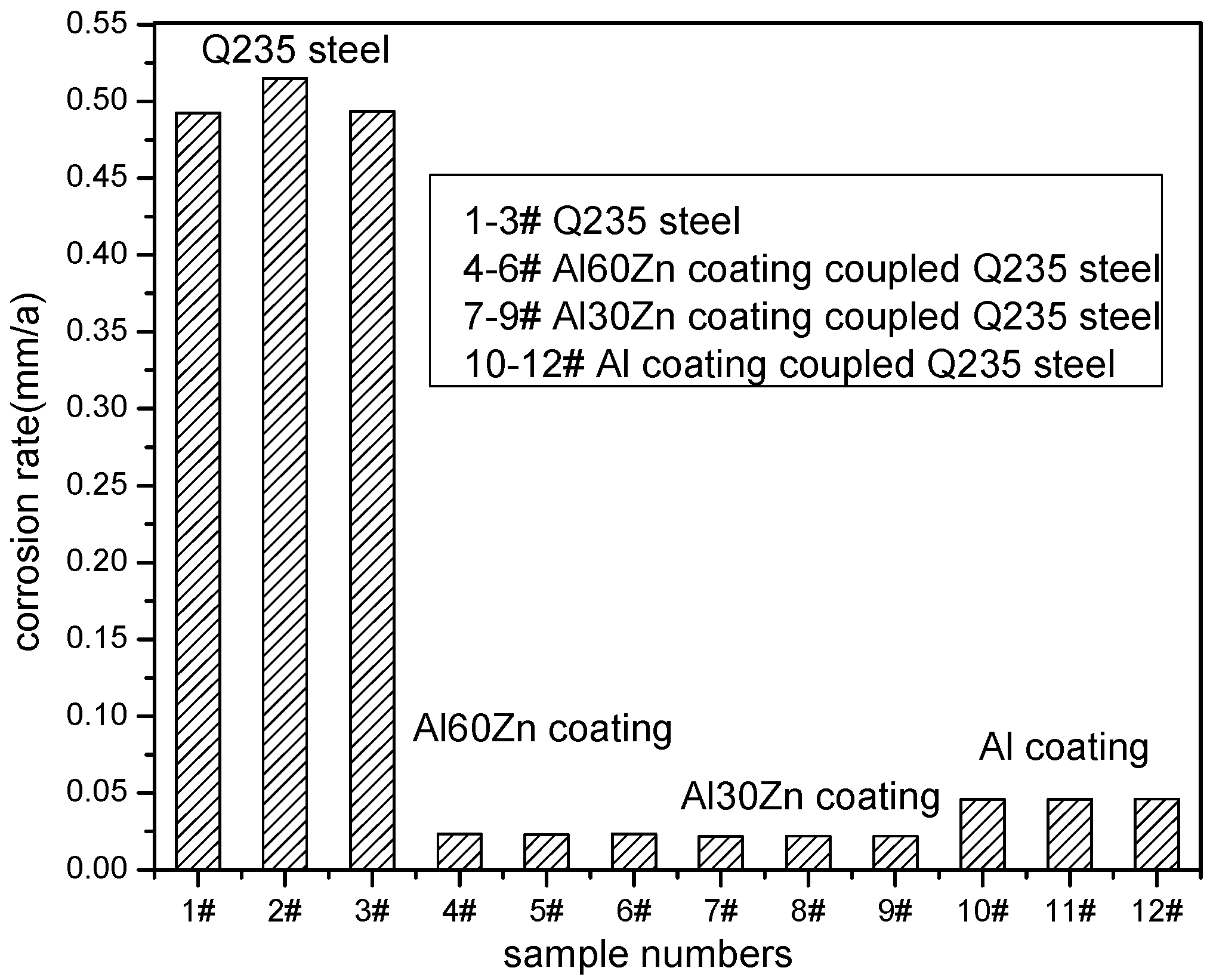
3.3. Residual Corrosion Rate of the Q235 Steel Substrate
4. Discussion
5. Conclusions
- Computational simulation method can be used to clarify the cathodic protection effect of cold-sprayed AlZn coating on steel substrate. The deviation is large in predicting the corrosion rate of coating-protected Q235 steel. More conditions must be taken into consideration to get more accurate results, such as calcareous deposits on steel and corrosion products of coating.
- Cold-sprayed AlZn coating can provide effective cathodic protection for substrate if the coating is in complete form, while it cannot provide effective cathodic protection if the damaged area is larger than 1 mm in width.
- The Al30Zn coating has best cathodic protection effect among three kinds of coating. All results did not take the barrier effect of corrosion products into consideration, which will influence the polarization behavior of AlZn coating. Additionally, in the future, the calcareous deposits on the Q235 surface will be taken into account to get more accurate simulation results.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuroda, S.; Takemoto, M.; Kawakita, J. An 18 year exposure test of thermal-sprayed Zn, Al, and Zn-Al coatings in marine environment. Corrosion 2006, 62, 860–863. [Google Scholar] [CrossRef]
- Esfahani, E.; Salimijazi, H.; Golozar, M.; Mostaghimi, J.; Pershin, L. Study of corrosion behavior of arc Sprayed Aluminum Coating on Mild Steel. J. Therm. Spray Technol. 2012, 21, 1195–1202. [Google Scholar] [CrossRef]
- Mcmurray, H.; Magill, S.; Jeffs, B. Scanning reference electrode technique as tool for investigating localised corrosion phenomena in galvanised steels. Ironmak. Steelmak. 1996, 23, 183–188. [Google Scholar]
- Fujita, S.; Mizuno, D. Corrosion and corrosion test methods of zinc coated steel sheets on automobiles. Corros. Sci. 2007, 49, 211–219. [Google Scholar] [CrossRef]
- Yadav, A.; Nishikata, A.; Tsuru, T. Degradation mechanism of galvanized steel in wet–dry cyclic environment containing chloride ions. Corros. Sci. 2004, 46, 361–376. [Google Scholar] [CrossRef]
- Yadav, A.; Nishikata, A.; Tsuru, T. Electrochemical impedance study on galvanized steel corrosion under cyclic wet–dry conditions-influence of time of wetness. Corros. Sci. 2004, 46, 169–181. [Google Scholar] [CrossRef]
- Nishikata, A.; Ichihara, Y.; Tsuru, T. An application of electrochemical impedance spectroscopy to atmospheric corrosion study. Corros. Sci. 1995, 37, 897–911. [Google Scholar] [CrossRef]
- Chung, S.; Sung, S.; Hsien, C.; Hsih, H. Application of EIS to the initial stages of atmospheric zinc corrosion. J. Appl. Electrochem. 2000, 30, 607–615. [Google Scholar] [CrossRef]
- Ohtsuka, T. Corrosion products of zinc coating layer in the presence of NaCl precipitates under atmospheric environments. Univ. Read. 2004, 17, 1149–1152. [Google Scholar]
- Volovitch, P.; Allely, C.; Ogle, K. Understanding corrosion via corrosion product characterization: I. Case study of the role of mg alloying in Zn–Mg coating on steel. Corros. Sci. 2009, 51, 1251–1262. [Google Scholar] [CrossRef]
- Liu, C.S.; Cheng, J.W.; Zheng, J.X. Numerical simulation of gas-particle two phase flow in the process of cold spraying aluminum zinc-base alloy powder. Appl. Mech. Mater. 2010, 37–38, 735–738. [Google Scholar] [CrossRef]
- Liang, Y.L.; Wang, Z.B.; Zhang, J.B. Formation of interfacial compounds and the effects on stripping behaviors of a cold-sprayed Zn–Al coating on interstitial-free steel. Appl. Surf. Sci. 2015, 340, 89–95. [Google Scholar] [CrossRef]
- Li, N.; Li, W.Y.; Yang, X.W.; Alexopoulos, N.D.; Niu, P.L. Effect of powder size on the long-term corrosion performance of pure aluminium coatings on mild steel by cold spraying. Mater. Corros. 2017, 68, 546–551. [Google Scholar] [CrossRef]
- Volovitch, P.; Allely, C.; Ogle, K. Understanding Corrosion via Corrosion Product Characterization: II. Role of Alloying Elements in Improving the Corrosion Resistance of Zn–Al–Mg Coatings on Steel. Corros. Sci. 2011, 53, 2437–2445. [Google Scholar] [CrossRef]
- Durstewitz, C.B.; Almerayacalderon, F.; Jaquez, R.N.; Tiburcio, C.F.; Villafae, A.M. Simulation and modelling of cathodic protection systems by the finite elements and the boundary elements methods. Port. Electrochim. Acta 2005, 23, 123–137. [Google Scholar] [CrossRef]
- Santos, W.; Santiago, J.; Telles, J. Optimal positioning of anodes and virtual sources in the design of cathodic protection systems using the method of fundamental solutions. Eng. Anal. Bound. Elem. 2014, 46, 67–74. [Google Scholar] [CrossRef]
- Xing, S.; Li, Y.; Song, H.; Yan, Y.; Sun, M. Optimization the quantity, locations and output currents of anodes to improve cathodic protection effect of semi-submersible crane vessel. Ocean Eng. 2016, 113, 144–150. [Google Scholar] [CrossRef]
- Wang, W.; Yi, J.; Chen, L.; Li, X. Numerical simulation of transient process for corrosion environment in coating crevice with cathodic protection. J. Comput. Theor. Nanosci. 2012, 9, 1395–1398. [Google Scholar] [CrossRef]
- Miltiadou, P.; Wrobel, L. A BEM-based genetic algorithm for identification of polarization curves in cathodic protection systems. Int. J. Numer. Methods Eng. 2002, 54, 159–174. [Google Scholar] [CrossRef]
- Okada, N.; Takebayashi, M.; Matsumoto, M.; Kimoto, M.; Kodou, T. Numerical analysis of cathodic protection distance for steel-zinc couple under thin layer electrolytes (Surface Treatment and Corrosion). Tetsu Hagane 2006, 92, 667–675. [Google Scholar] [CrossRef]
- Standard DNV-RP-401: Cathodic Protection Design. Available online: http://rules.dnvgl.com/docs/pdf/DNV/codes/docs/2011-04/RP-B401.pdf (accessed on 8 May 2017).
- Yadav, A.; Katayama, H.; Noda, K. Surface potential distribution over a zinc/steel galvanic couple corroding under thin layer of electrolyte. Electrochim. Acta 2007, 52, 3121–3129. [Google Scholar] [CrossRef]
- Ou, K.; Wu, J. Effect of calcareous deposits formation on the hydrogen absorption of steel. Mater. Chem. Phys. 1997, 48, 52–55. [Google Scholar] [CrossRef]
- Mueller, W.A. Theory of the polarization curve technique studying corrosion and electrochemical protection. Can. J. Chem. 2011, 38, 576–587. [Google Scholar] [CrossRef]
- Murakami, D.; Seri, O.; Shimamura, R.; Kimura, M. Polarization curve and its resistance curve of Galvanically-Coupled Al/Cu specimen as simulation of bimetallic corrosion. J. Jpn. Inst. Light Met. 2015, 65, 389–395. [Google Scholar] [CrossRef]
- Montoya, R.; Rendon, O.; Genesca, J. Mathematical simulation of a cathodic protection system by finite element method. Mater. Corros. 2005, 56, 404–411. [Google Scholar] [CrossRef]
| Geometric Parameter | Value |
|---|---|
| Expansion ratio | 3.13 |
| Diameter of nozzle throat | 2.54 |
| Length of converging part | 10 mm |
| Length of diverging part | 10 mm |
| Length of elongated part | 120 mm |
| Standoff distant from nozzle exit to substrate | 20 mm |
| Pressure in prechamber | 0.6 MPa |
| Temperature in prechamber | 686 K |
| Powder feeding rate | 0.51–0.59 g/s |
| Transverse speed of nozzle | 20 mm/s |
| Potential(V) | Corrosion Rate (Experiment Data) | Corrosion Rate (Simulation Results from 2 h) | Deviation (%) |
|---|---|---|---|
| −0.71 | 42.62 | 52.2 | 22.5 |
| −0.78 | 3.93 | 5.02 | 27.7 |
| −0.85 | 1.97 | 2.36 | 19.8 |
| −0.90 | 1.16 | 1.28 | 10.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, G.; Lou, X.; Wang, H.; Li, X.; Xing, L. Investigation on the Cathodic Protection Effect of Low Pressure Cold Sprayed AlZn Coating in Seawater via Numerical Simulation. Coatings 2017, 7, 93. https://doi.org/10.3390/coatings7070093
Huang G, Lou X, Wang H, Li X, Xing L. Investigation on the Cathodic Protection Effect of Low Pressure Cold Sprayed AlZn Coating in Seawater via Numerical Simulation. Coatings. 2017; 7(7):93. https://doi.org/10.3390/coatings7070093
Chicago/Turabian StyleHuang, Guosheng, Xiaodan Lou, Hongren Wang, Xiangbo Li, and Lukuo Xing. 2017. "Investigation on the Cathodic Protection Effect of Low Pressure Cold Sprayed AlZn Coating in Seawater via Numerical Simulation" Coatings 7, no. 7: 93. https://doi.org/10.3390/coatings7070093
APA StyleHuang, G., Lou, X., Wang, H., Li, X., & Xing, L. (2017). Investigation on the Cathodic Protection Effect of Low Pressure Cold Sprayed AlZn Coating in Seawater via Numerical Simulation. Coatings, 7(7), 93. https://doi.org/10.3390/coatings7070093




