Novel Development of Biocompatible Coatings for Bone Implants
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.1.1. Bone Implants
2.1.2. Preparation of Ultrafine Coating Powder
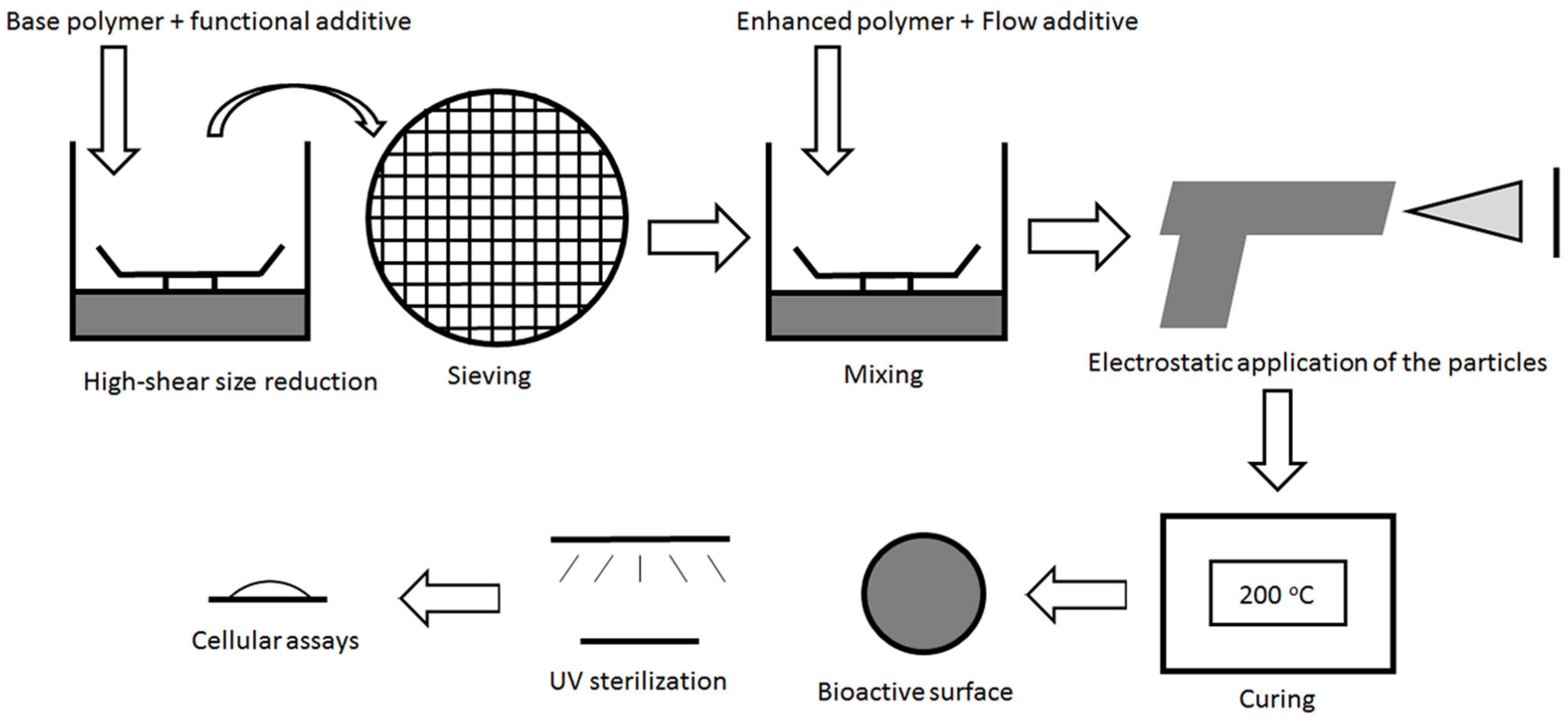
| Coating | Base Polymer | Flow Additive (wt %) | Bioactive Agent (wt %) | Bioactive Calcium (mmol/100g) | Bioactive Phosphate (mmol/100g) |
|---|---|---|---|---|---|
| PPC (polymeric powder coating) | Polyester resin | nTiO2 (0.5%) | None | 0.00 | 0.00 |
| PPC + 1% CaO | CaO (1%) | 17.83 | 0.00 | ||
| PPC + 5% CaO | CaO (5%) | 89.16 | 0.00 | ||
| PPC + 5% CaP | Ca3(PO4)2 (5%) | 0.29 | 0.29 |
2.1.3. Powder Coating for the Modification of Biomaterial
2.2. Physical Characterizations
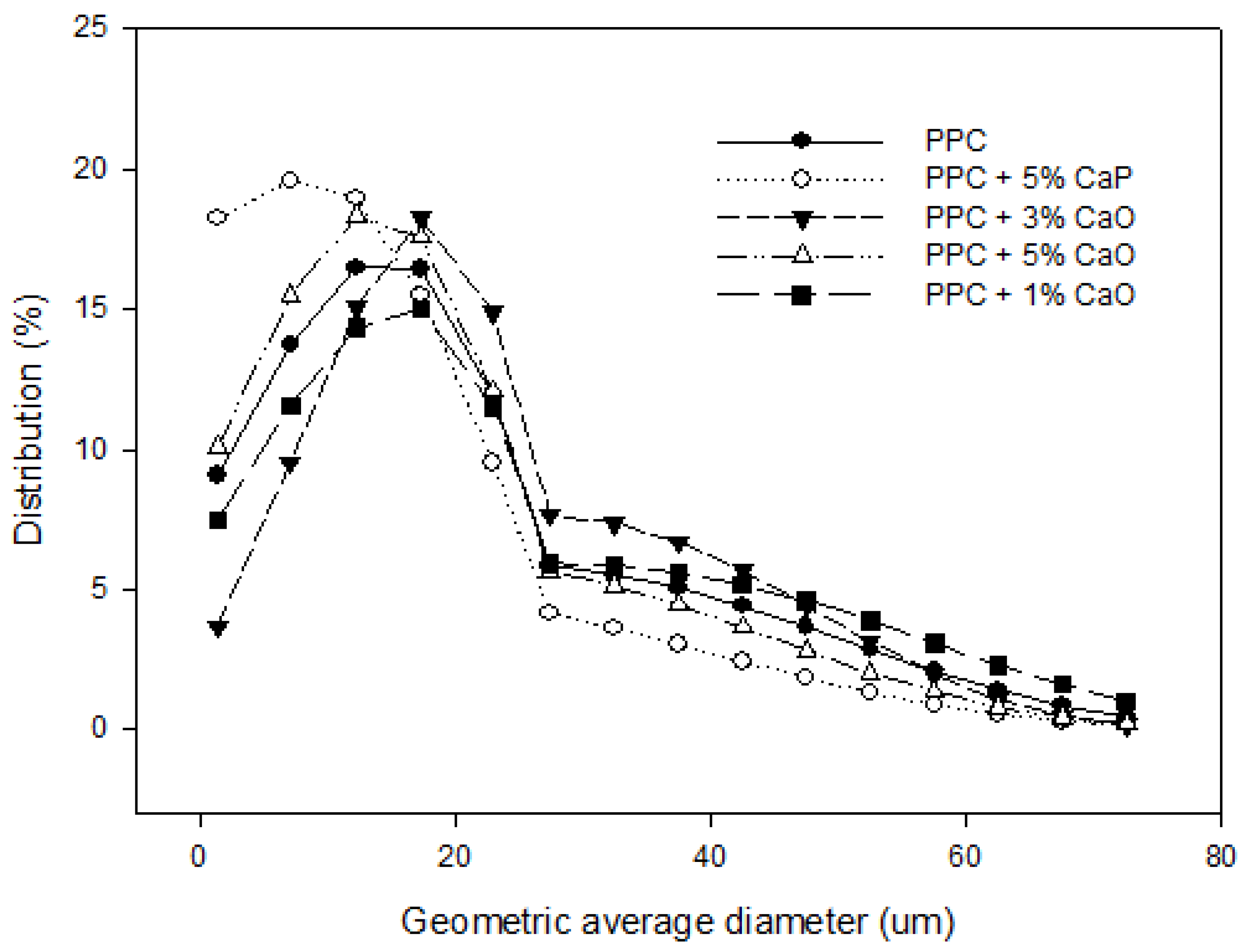
2.2.1. Particle Size Analysis
2.2.2. Surface Analysis
2.2.3. Hydrophilicity Assay
2.2.4. Surface Roughness Analysis
2.3. Biocompatibility
2.3.1. Protein Localization and Visualization
2.3.2. Optical and Scanning Electron Microscopy
2.4. Mineralization Assays
2.5. Cellular Assays
2.5.1. Cell Proliferation Assay
2.5.2. Metabolic Activity
2.5.3. Gene Expression
3. Results and Discussion
3.1. Biocompatible Surface Coating
3.2. Physical Characterizations

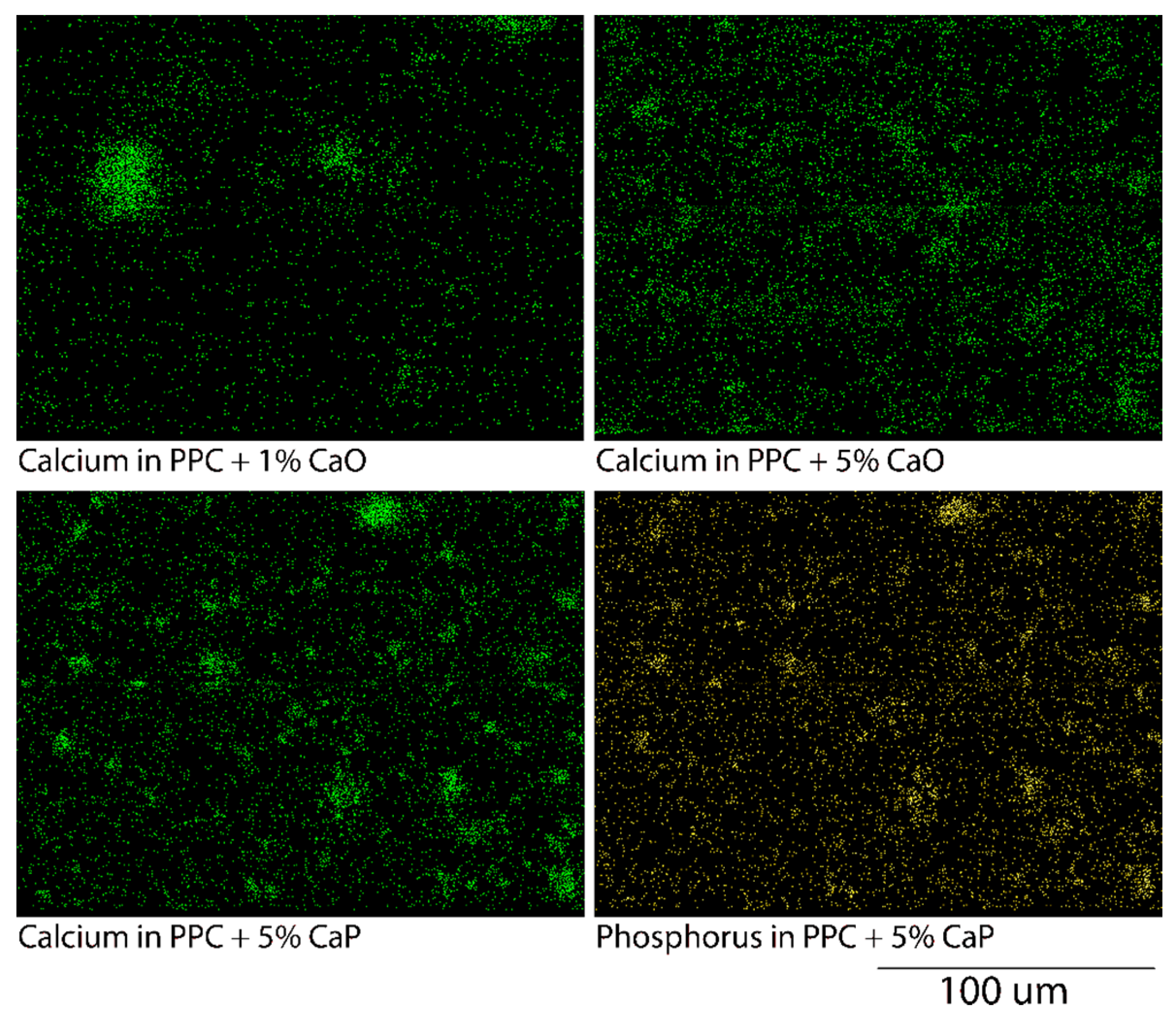
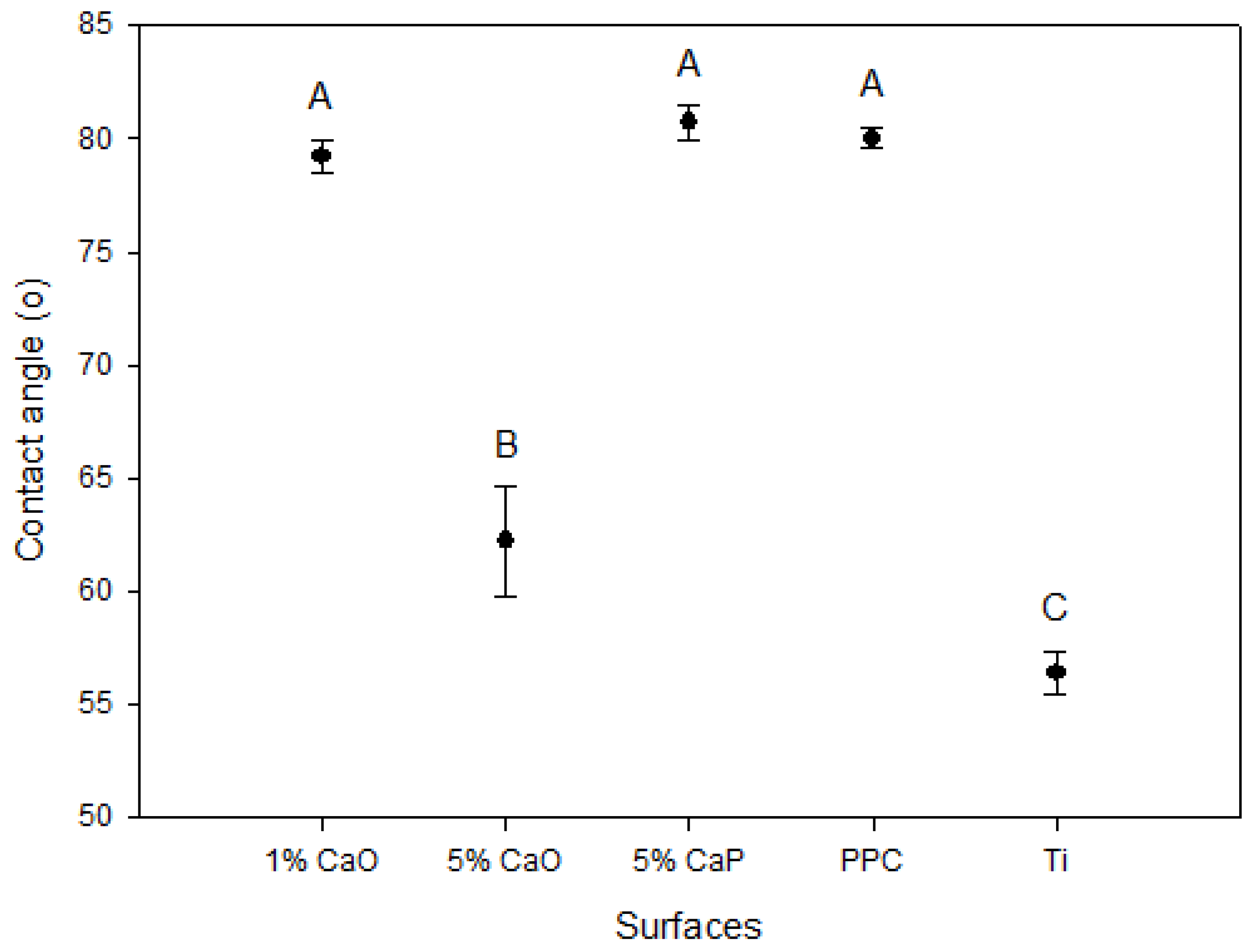
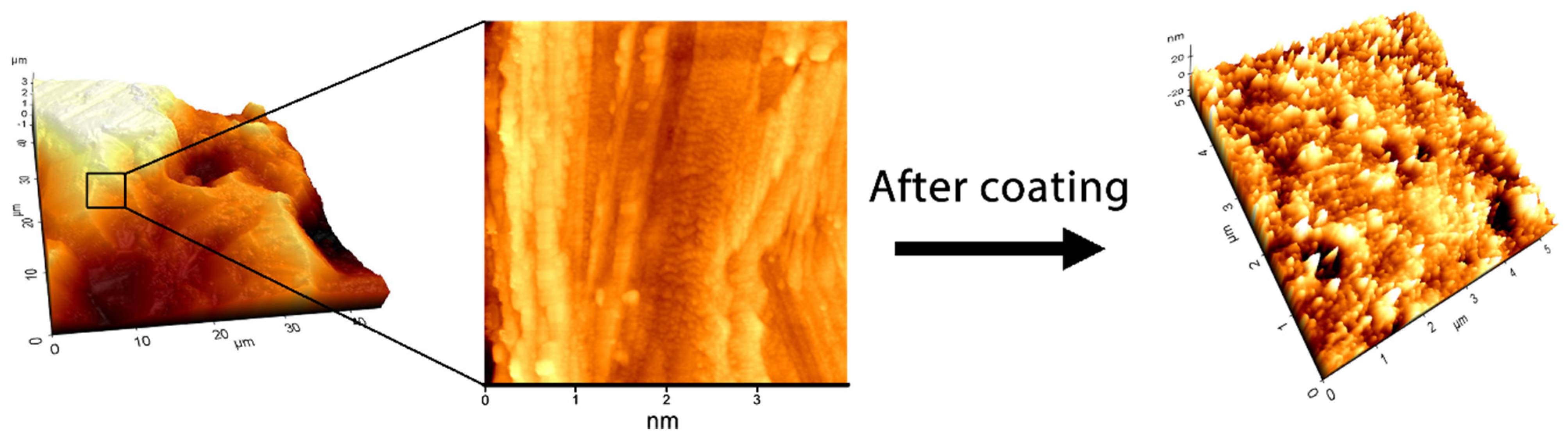
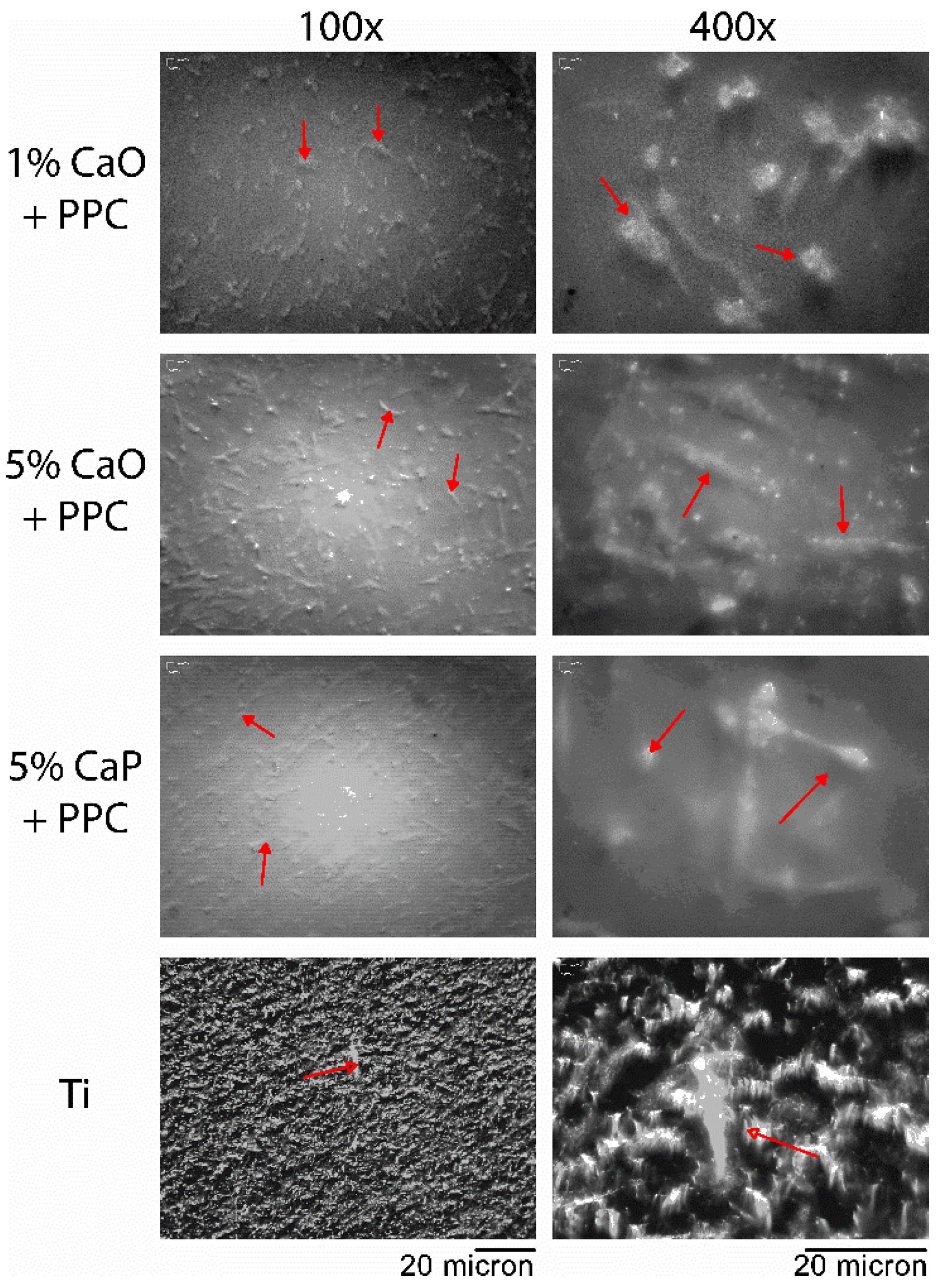
3.3. Biocompatibility


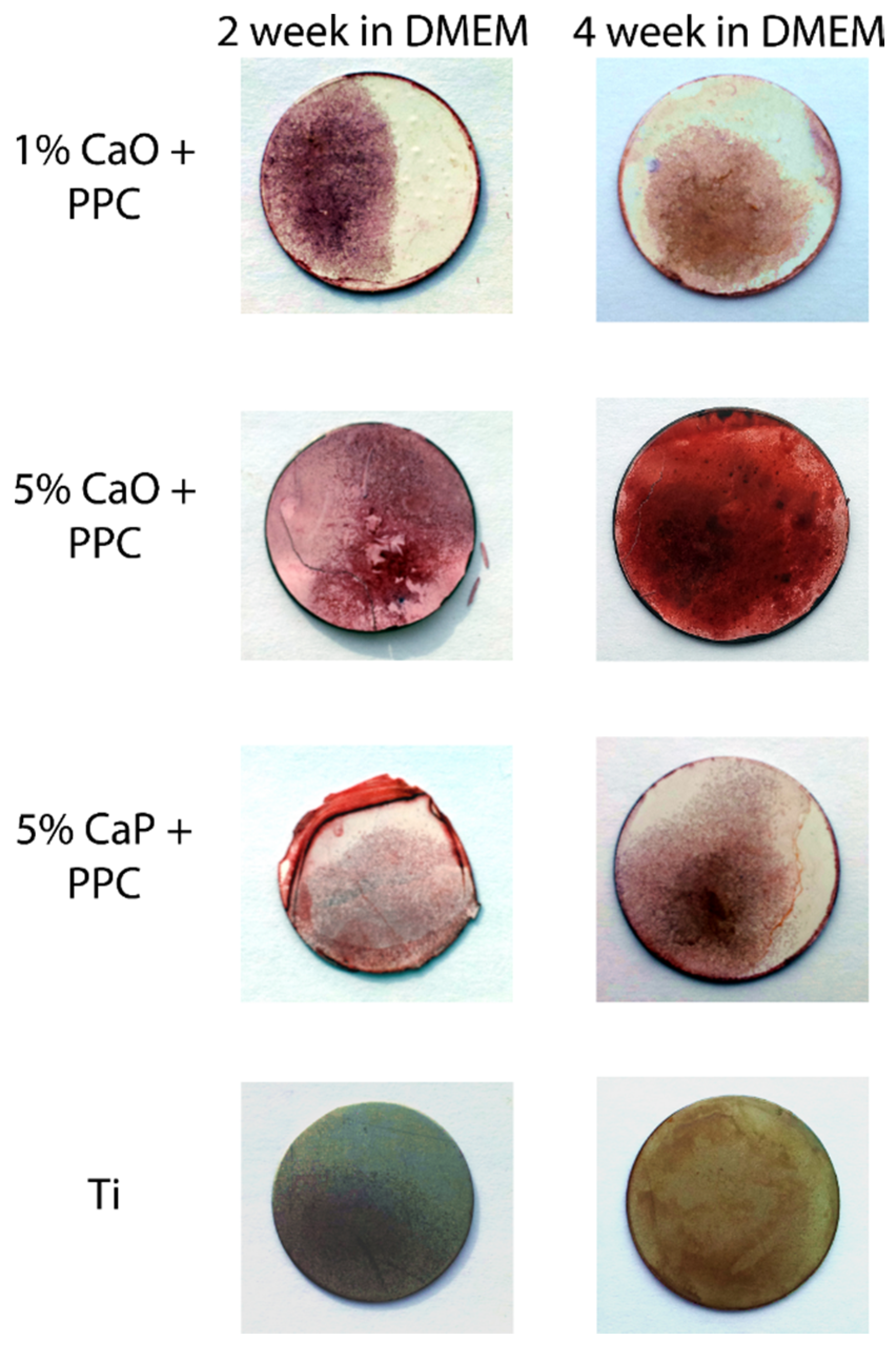
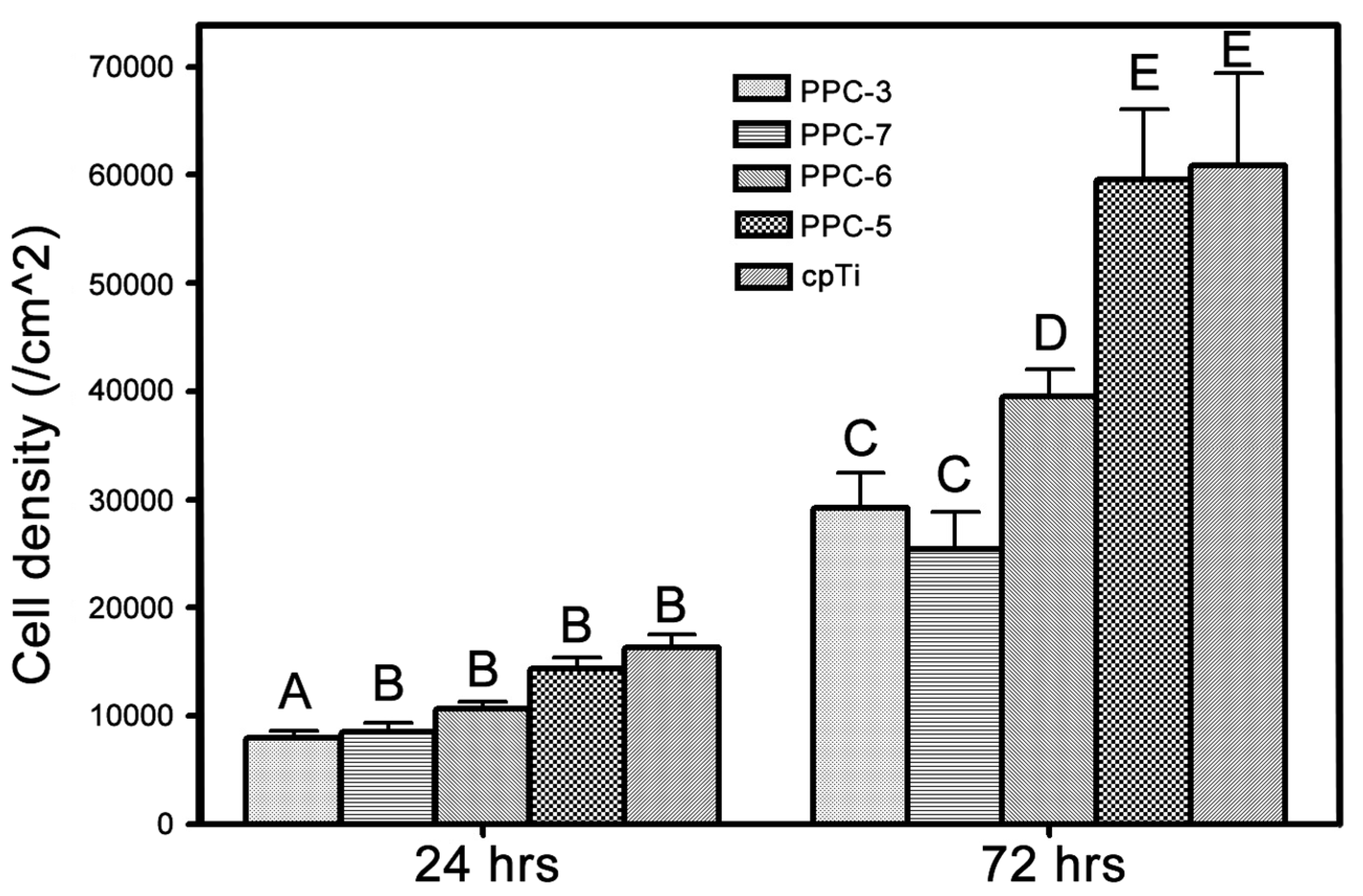
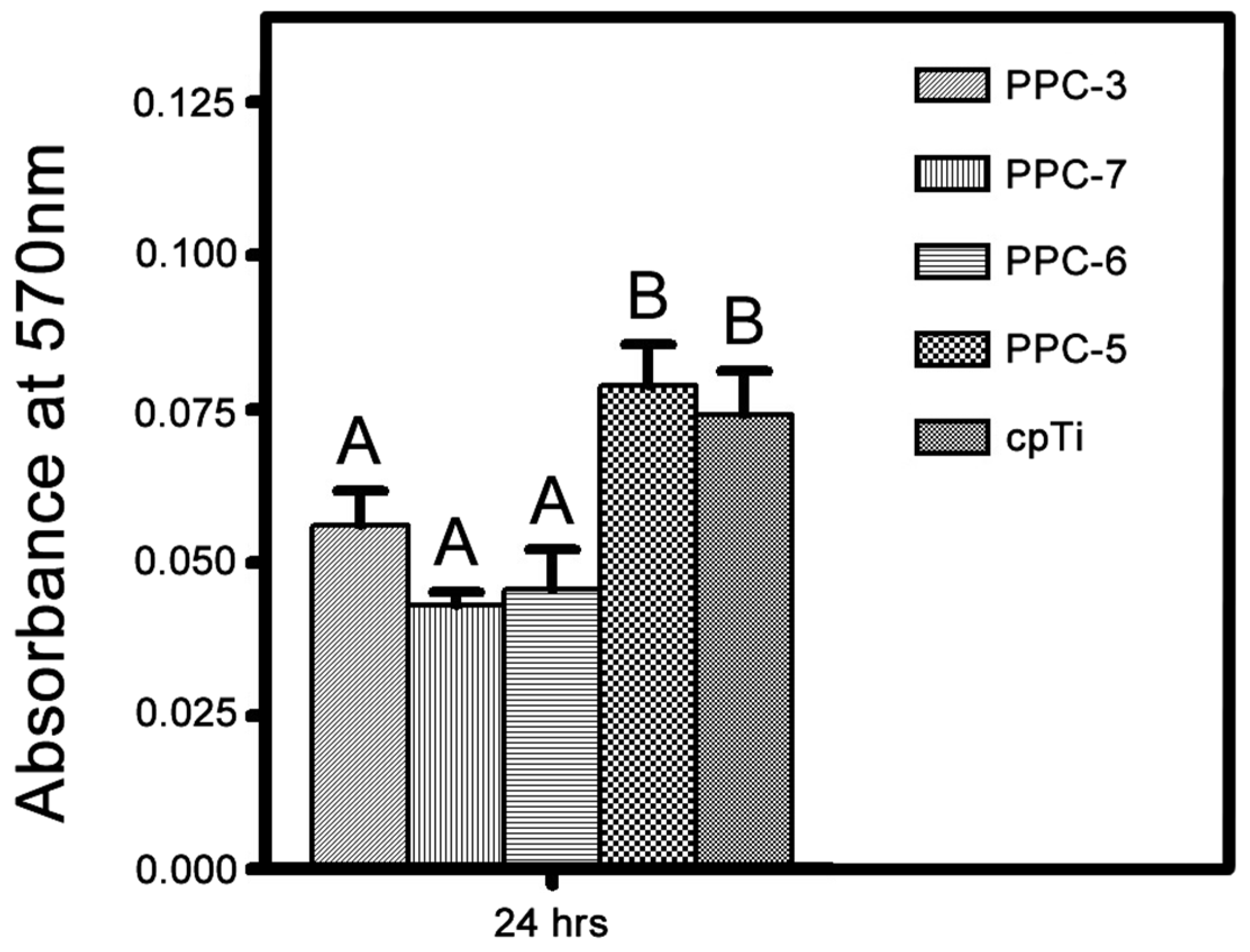
3.4. Cellular Assays
| Surface | Base | Flow Additive | Embedded TiO2 (wt %) | Added nTiO2 (wt %) |
|---|---|---|---|---|
| PPC-1 | Epoxy resin + PTFE | nSiO2 | 0 | 0 |
| PPC-2 | Polyester resin + PTFE | nSiO2 | 25.0 | 0 |
| PPC-3 | Polyester resin + PTFE | nTiO2 | 25.0 | 0.5 |
| PPC-4 | Polyester resin + PTFE | nTiO2 | 25.0 | 2.0 |
| PPC-5 | Polyester resin | nTiO2 | 25.0 | 0.5 |
| PPC-6 | Polyester resin + PTFE | nTiO2 | 25.0 | 0.5 |
| PPC-7 | Polyester resin + PTFE | nTiO2 | 25.0 | 0.5 |

4. Conclusions
Conflicts of Interest
References
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [PubMed]
- Hou, N.Y.; Zhu, J.; Zhang, H.; Perinpanayagam, H. Ultrafine calcium-titania-polyester dry powder coatings promote human mesenchymal cell attachment and biomineralization. Surf. Coat. Technol. 2014, 251, 177–185. [Google Scholar] [CrossRef]
- Shi, W.; Mozumder, M.S.; Zhang, H.; Zhu, J.; Perinpanayagam, H. MTA-enriched nanocomposite TiO2-polymeric powder coatings support human mesenchymal cell attachment and growth. Biomed. Mater. 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-W.; Kim, H.-E.; Salih, V.; Knowles, J.C. Hydroxyapatite and titania sol-gel composite coatings on titanium for hard tissue implants: Mechanical and in vitro biological performance. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 72, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ducheyne, P.; Cuckler, J.M. Bioactive ceramic prosthetic coatings. Clin. Orthop. 1992, 276, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kim, H.-W.; Lee, E.-J.; Li, L.-H.; Kim, H.-E. Hydroxyapatite-TiO2 hybrid coating on Ti implants. J. Biomater. Appl. 2006, 20, 195–208. [Google Scholar] [CrossRef] [PubMed]
- McPherson, E.J.; Dorr, L.D.; Gruen, T.A.; Saberi, M.T. Hydroxyapatite-coated proximal ingrowth femoral stems. A matched pair control study. Clin. Orthop. 1995, 315, 223–230. [Google Scholar] [PubMed]
- Ramires, P.A.; Romito, A.; Cosentino, F.; Milella, E. The influence of titania/hydroxyapatite composite coatings on in vitro osteoblasts behaviour. Biomaterials 2001, 22, 1467–1474. [Google Scholar] [CrossRef]
- Gomes, P.S.; Botelho, C.; Lopes, M.A.; Santos, J.D.; Fernandes, M.H. Evaluation of human osteoblastic cell response to plasma-sprayed silicon-substituted hydroxyapatite coatings over titanium substrates. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Leyland, A.; Matthews, A. Deposition of layered bioceramic hydroxyapatite/TiO2 coatings on titanium alloys using a hybrid technique of micro-arc oxidation and electrophoresis. Surf. Coat. Technol. 2000, 125, 407–414. [Google Scholar] [CrossRef]
- Kasuga, T.; Nogami, M.; Niinomi, M.; Hattori, T. Bioactive calcium phosphate invert glass-ceramic coating on β-type Ti–29Nb–13Ta–4.6Zr alloy. Biomaterials 2003, 24, 283–290. [Google Scholar] [CrossRef]
- Anitua, E.; Prado, R.; Orive, G.; Tejero, R. Effects of calcium-modified titanium implant surfaces on platelet activation, clot formation, and osseointegration. J. Biomed. Mater. Res. A 2015, 103, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Mendes, V.C.; Moineddin, R.; Davies, J.E. Discrete calcium phosphate nanocrystalline deposition enhances osteoconduction on titanium-based implant surfaces. J. Biomed. Mater. Res. A 2009, 90, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Jung, U.-W.; Kim, C.-S.; Jung, S.-M.; Lee, I.-S.; Choi, S.-H. Influence of nanocoated calcium phosphate on two different types of implant surfaces in different bone environment: An animal study. Clin. Oral Implants Res. 2013, 24, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Poulos, N.M.; Rodriguez, N.A.; Lee, J.; Rueggeberg, F.A.; Schupbach, P.; Hall, J.; Susin, C.; Wikesjö, U.M. Evaluation of a novel calcium phosphate-coated titanium porous oxide implant surface: A study in rabbits. Int. J. Oral Maxillofac. Implants 2011, 26, 731–738. [Google Scholar] [PubMed]
- Aniket, A.El-G. Electrophoretic deposition of bioactive silica-calcium phosphate nanocomposite on Ti-6Al-4V orthopedic implant. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 99, 369–379. [Google Scholar] [CrossRef] [PubMed]
- De Groot, K.; Geesink, R.; Klein, C.P.A.T.; Serekian, P. Plasma sprayed coatings of hydroxylapatite. J. Biomed. Mater. Res. 1987, 21, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Vercaigne, S.; Wolke, J.G.C.; Naert, I.; Jansen, J.A. Histomorphometrical and mechanical evaluation of titanium plasma-spray-coated implants placed in the cortical bone of goats. J. Biomed. Mater. Res. 1998, 41, 41–48. [Google Scholar] [CrossRef]
- Van Dijk, K.; Schaeken, H.G.; Wolke, J.C.; Marée, C.H.; Habraken, F.H.; Verhoeven, J.; Jansen, J.A. Influence of discharge power level on the properties of hydroxyapatite films deposited on Ti–6A1–4V with RF magnetron sputtering. J. Biomed. Mater. Res. 1995, 29, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, M.; Klinge, B.; Dérand, T. The biocompatibility (cell culture and histologic study) of hydroxy-apatite-coated implants created by ion beam dynamic mixing. Clin. Oral Implants Res. 1996, 7, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, S.; Søballe, K.; Josephsen, K.; Hansen, E.S.; Bünger, C. Role of different loading conditions on resorption of hydroxyapatite coating evaluated by histomorphometric and stereological methods. J. Orthop. Res. 1996, 14, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, H. Fluidization Additives to Fine Powders. 2004. Available online: http://www.google.com/patents?id=SqsQAAAAEBAJ (accessed on 1 August 2015).
- Zhang, H.; Zhu, J. Method and Apparatus for Uniformly Dispersing Additive Particles in Fine Powders. U.S. Patent 7,878,430, 1 February 2011. [Google Scholar]
- Mozumder, M.S.; Zhu, J.; Perinpanayagam, H. Nano-TiO2 enriched polymeric powder coatings support human mesenchymal cell attachment and growth. J. Biomater. Appl. 2011, 26, 173–193. [Google Scholar] [CrossRef] [PubMed]
- Mozumder, M.S.; Zhu, J.; Perinpanayagam, H. TiO2-enriched polymeric powder coatings support human mesenchymal cell spreading and osteogenic differentiation. Biomed. Mater. 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Mozumder, M.S.; Zhu, J.; Perinpanayagam, H. Titania-polymeric powder coatings with nano-topography support enhanced human mesenchymal cell responses. J. Biomed. Mater. Res. A 2012, 100, 2695–2709. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X. Biomimetic materials for tissue engineering. Emerg. Trends Cell Based Ther. 2008, 60, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.B.; Zaharias, R.; Seabold, D.; Keller, J.; Stanford, C. Differentiation of preosteoblasts is affected by implant surface microtopographies. J. Biomed. Mater. Res. A 2004, 69, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Masaki, C.; Schneider, G.B.; Zaharias, R.; Seabold, D.; Stanford, C. Effects of implant surface microtopography on osteoblast gene expression. Clin. Oral Implants Res. 2005, 16, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.J.; Zaharias, R.S.; Seabold, D.A.; Lafoon, J.; Schneider, G.B. Osteoblast differentiation is enhanced in rotary cell culture simulated microgravity environments. J. Prosthodont. 2007, 16, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J.; Bundy, K.J.; O’Connor, K.; Moses, R.L.; Jacobs, J.J. Evaluation of metallic and polymeric biomaterial surface energy and surface roughness characteristics for directed cell adhesion. Tissue Eng. 2001, 7, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, R.; Rounds, D.E.; Yen, S.P.S.; Rembaum, A. A scanning electron microscope study of cell adhesion and spreading in vitro. Exp. Cell Res. 1974, 88, 327–339. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2000, 22, 87–96. [Google Scholar] [CrossRef]
- Good, R.J. Contact angle, wetting, and adhesion: A critical review. J. Adhes. Sci. Technol. 1992, 6, 1269–1302. [Google Scholar] [CrossRef]
- Hu, M.-X.; Yang, Q.; Xu, Z.-K. Enhancing the hydrophilicity of polypropylene microporous membranes by the grafting of 2-hydroxyethyl methacrylate via a synergistic effect of photoinitiators. J. Membr. Sci. 2006, 285, 196–205. [Google Scholar] [CrossRef]
- Li, J.; Hastings, G.W. Oxide Bioceramics: Inert ceramic materials in medicine and dentistry. In Handbook of Biomaterial Properties; Black, J., Hastings, G., Eds.; Springer: New York, NY, USA, 1998; pp. 340–354. [Google Scholar]
- Deligianni, D.; Katsala, N.; Ladas, S.; Sotiropoulou, D.; Amedee, J.; Missirlis, Y. Effect of surface roughness of the titanium alloy Ti–6Al–4V on human bone marrow cell response and on protein adsorption. Biomaterials 2001, 22, 1241–1251. [Google Scholar] [CrossRef]
- Puchtler, H.; Meloan, S.N.; Terry, M.S. On the history and mechanism of alizarin and alizarin red s stains for calcium. J. Histochem. Cytochem. 1969, 17, 110–124. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, N.Y.; Perinpanayagam, H.; Mozumder, M.S.; Zhu, J. Novel Development of Biocompatible Coatings for Bone Implants. Coatings 2015, 5, 737-757. https://doi.org/10.3390/coatings5040737
Hou NY, Perinpanayagam H, Mozumder MS, Zhu J. Novel Development of Biocompatible Coatings for Bone Implants. Coatings. 2015; 5(4):737-757. https://doi.org/10.3390/coatings5040737
Chicago/Turabian StyleHou, Nicholas Yue, Hiran Perinpanayagam, Mohammad Sayem Mozumder, and Jesse Zhu. 2015. "Novel Development of Biocompatible Coatings for Bone Implants" Coatings 5, no. 4: 737-757. https://doi.org/10.3390/coatings5040737
APA StyleHou, N. Y., Perinpanayagam, H., Mozumder, M. S., & Zhu, J. (2015). Novel Development of Biocompatible Coatings for Bone Implants. Coatings, 5(4), 737-757. https://doi.org/10.3390/coatings5040737








