Reversible Switching of Icing Properties on Pyroelectric Polyvenylidene Fluoride Thin Film Coatings
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Preparation
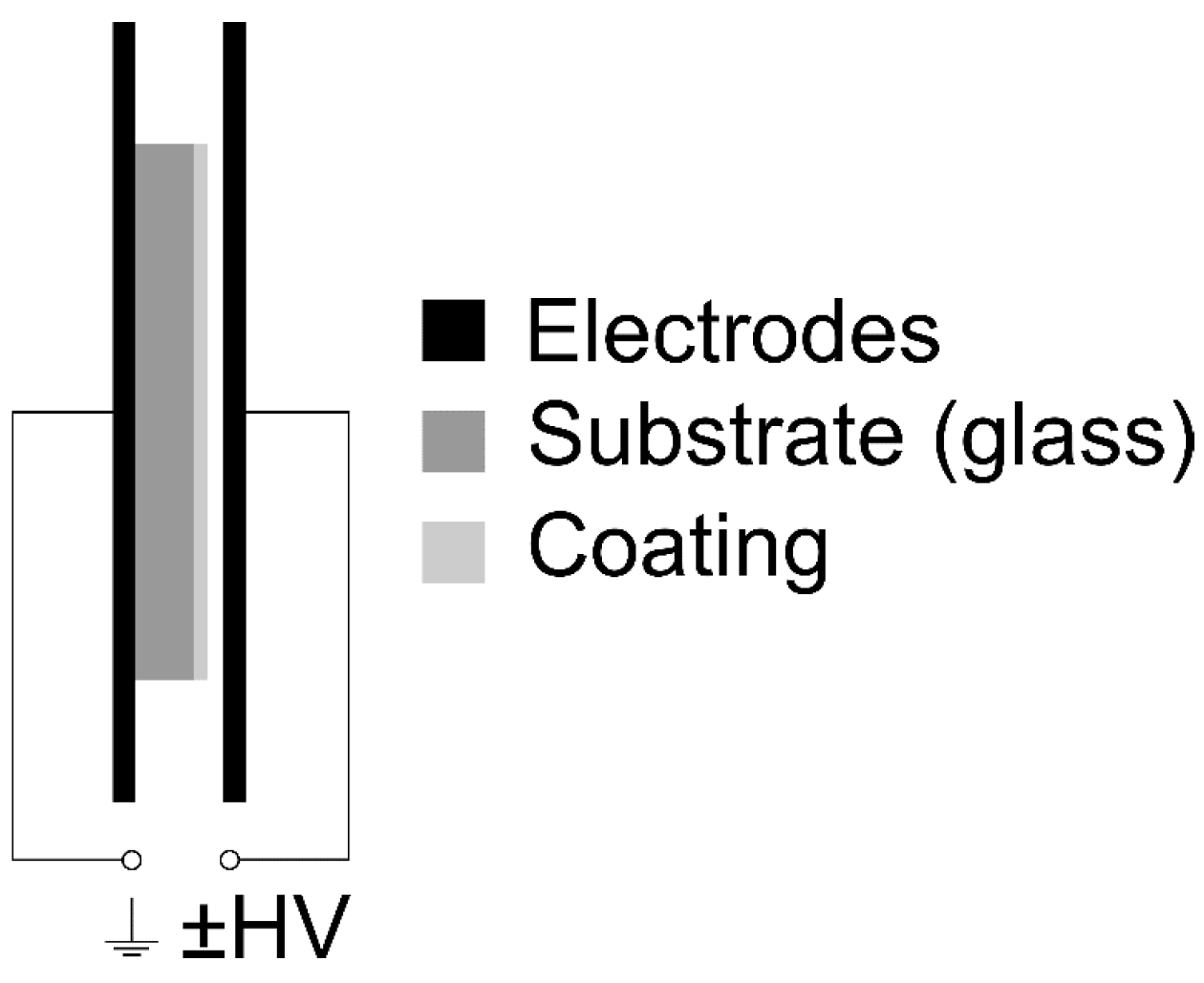
2.2. Optical Microscopy
2.3. Measurement
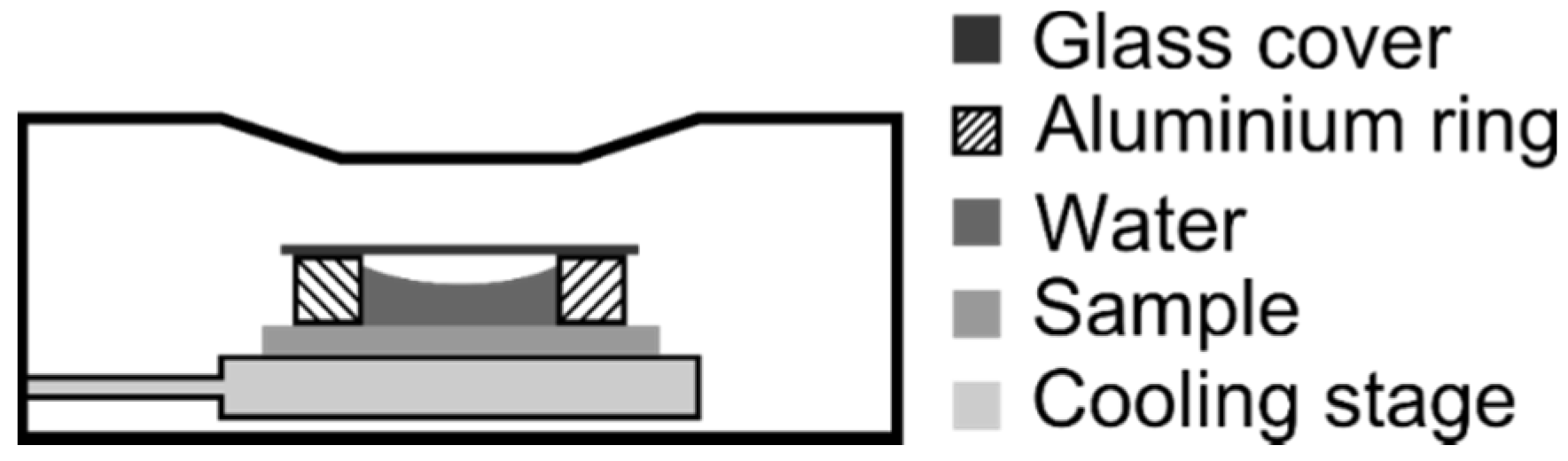
3. Results
3.1. Surface Characterization
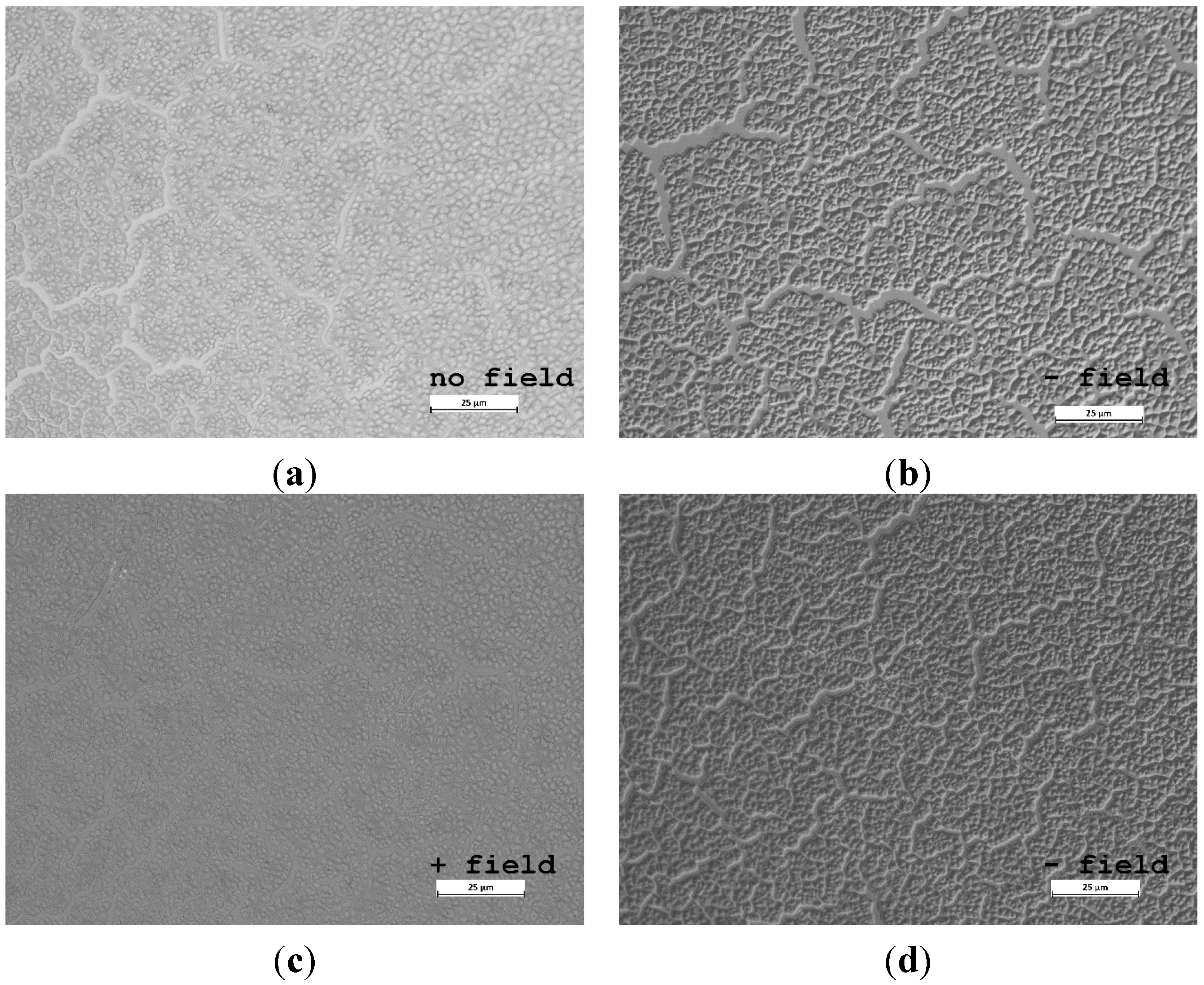
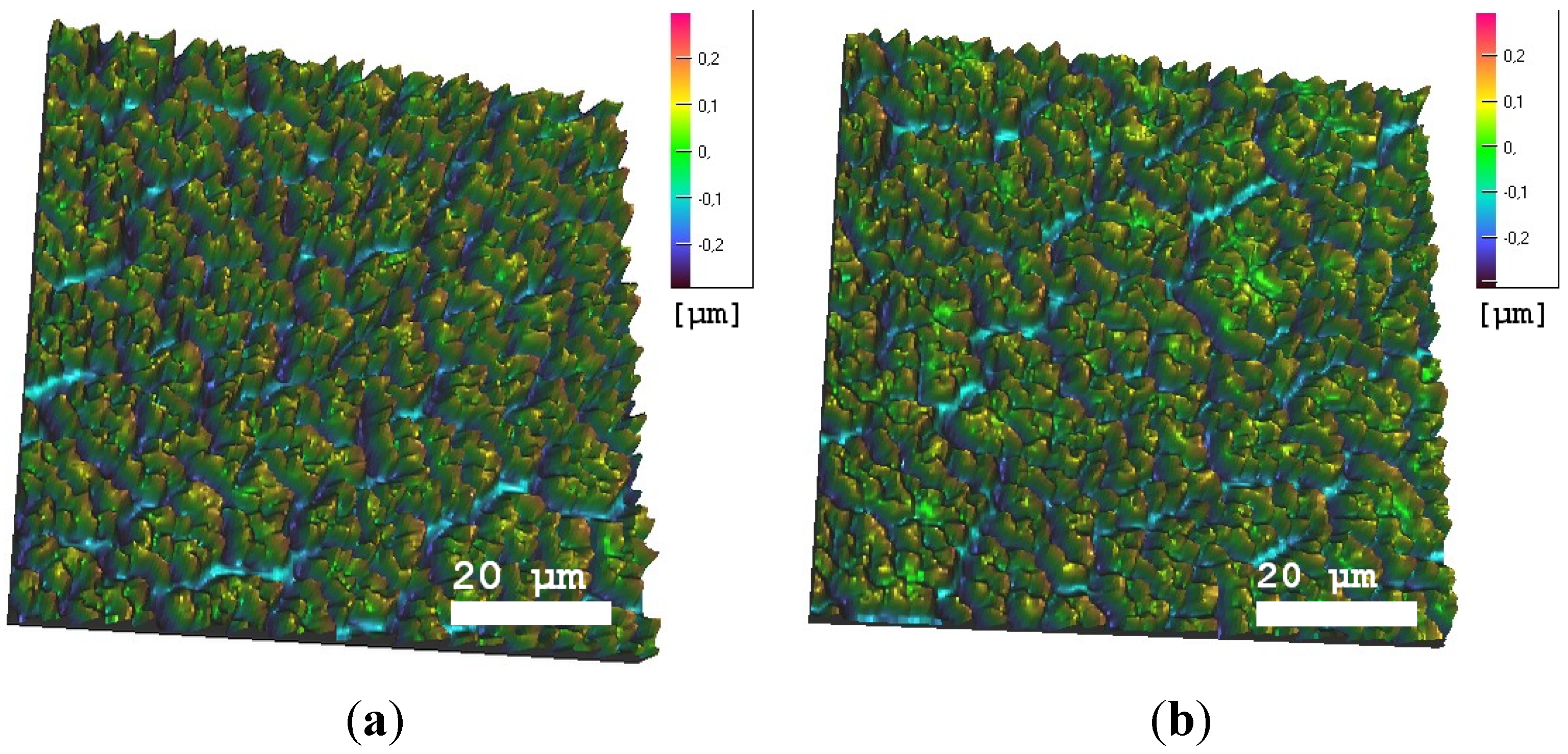
3.2. Freezing Experiments
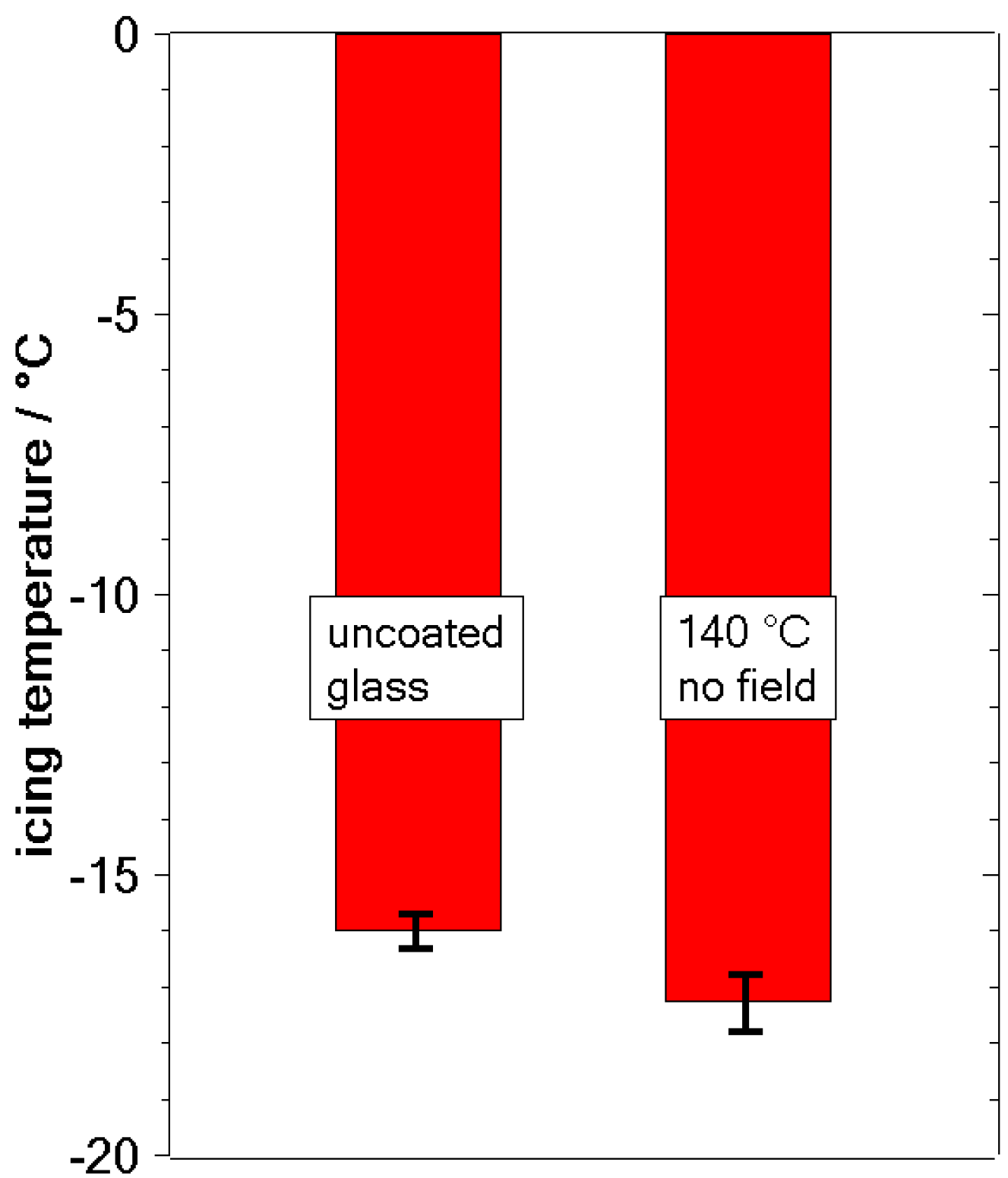
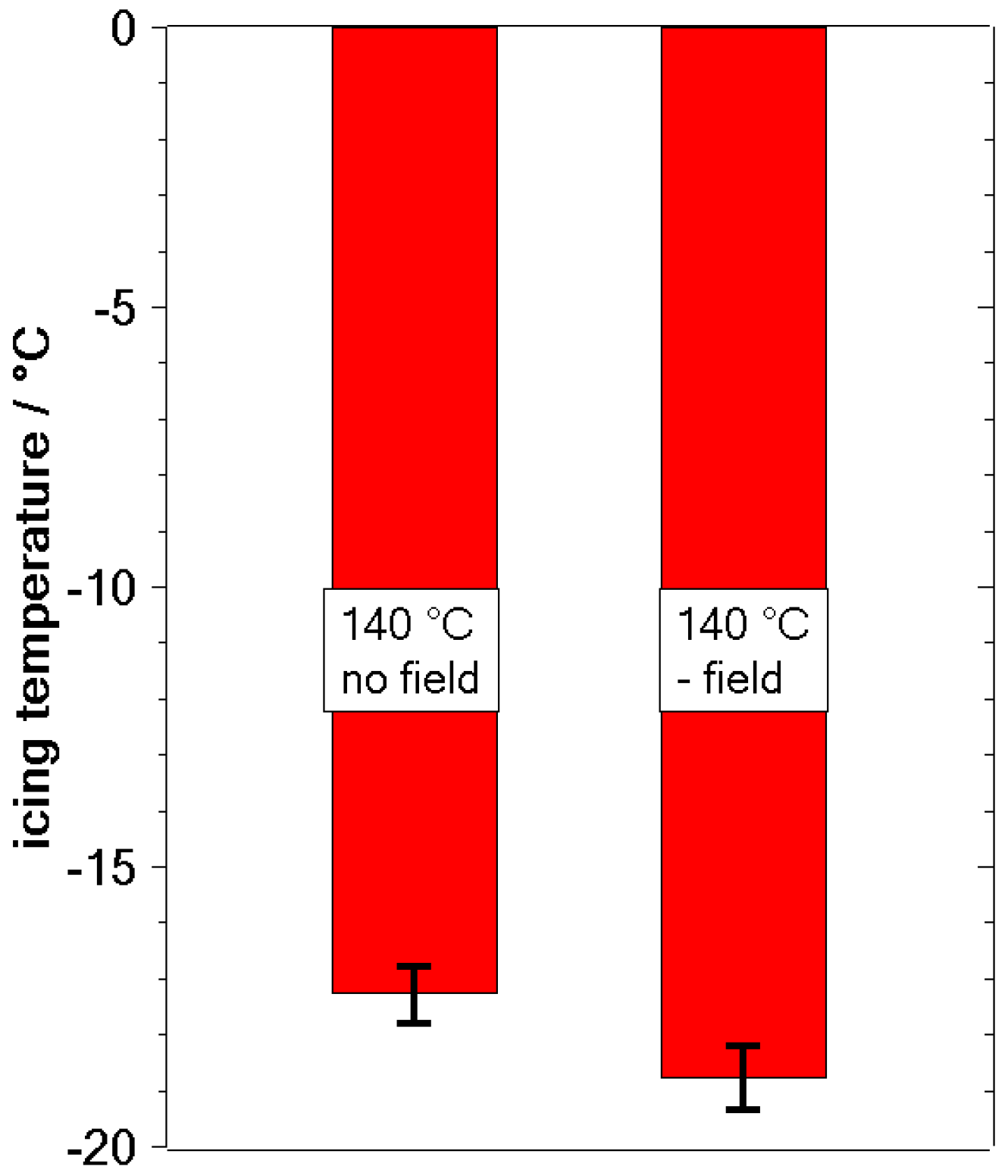
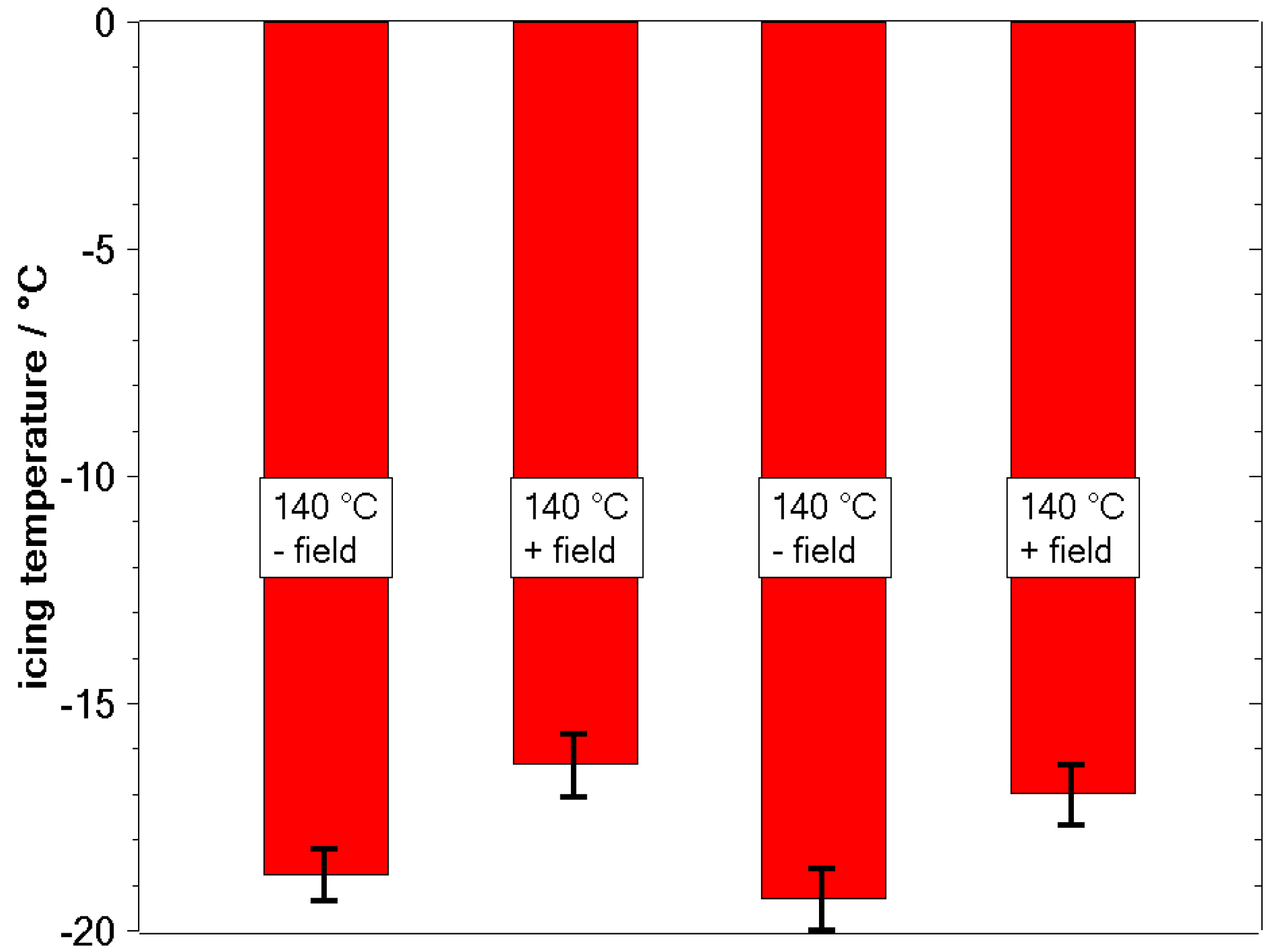
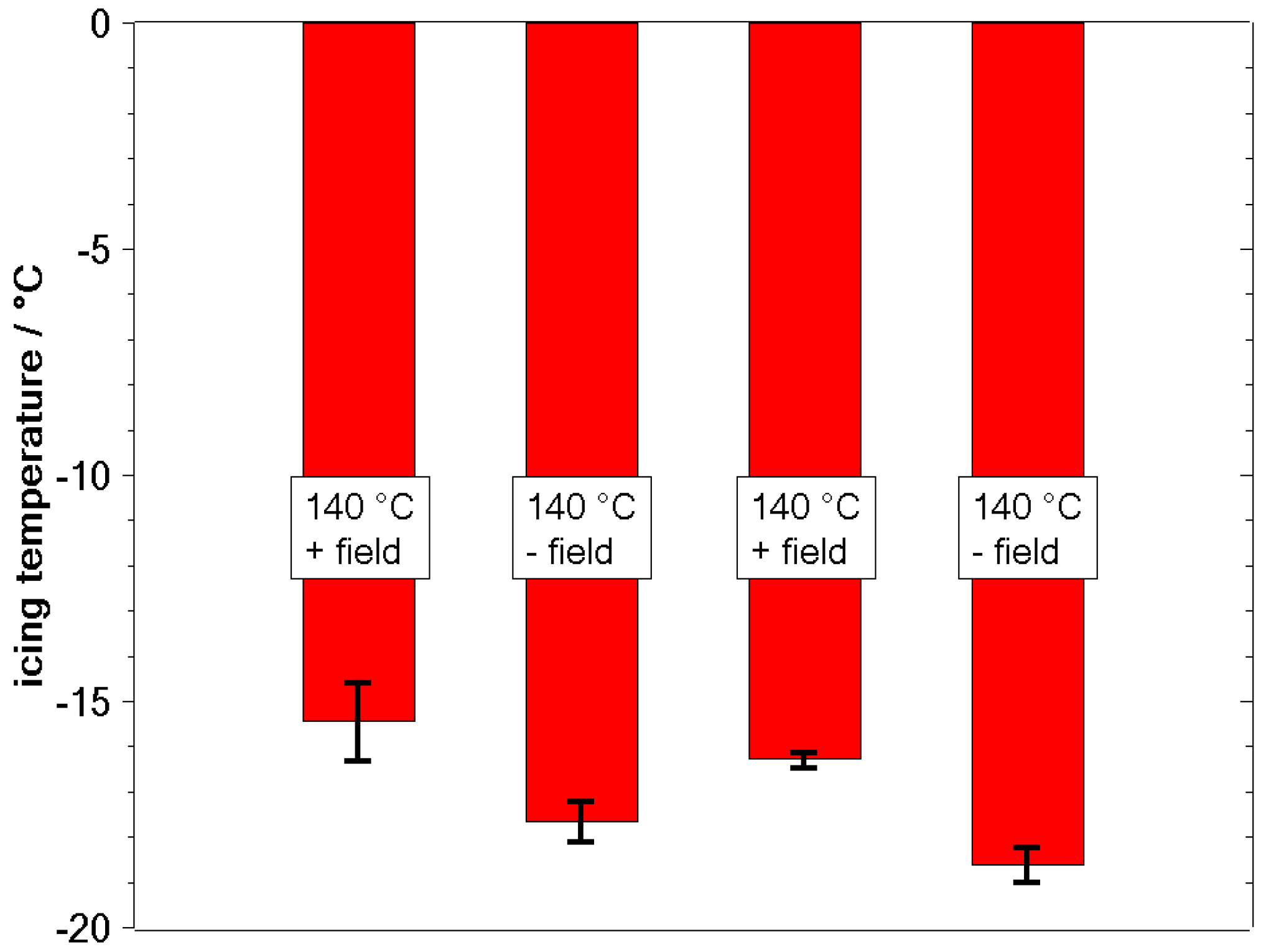
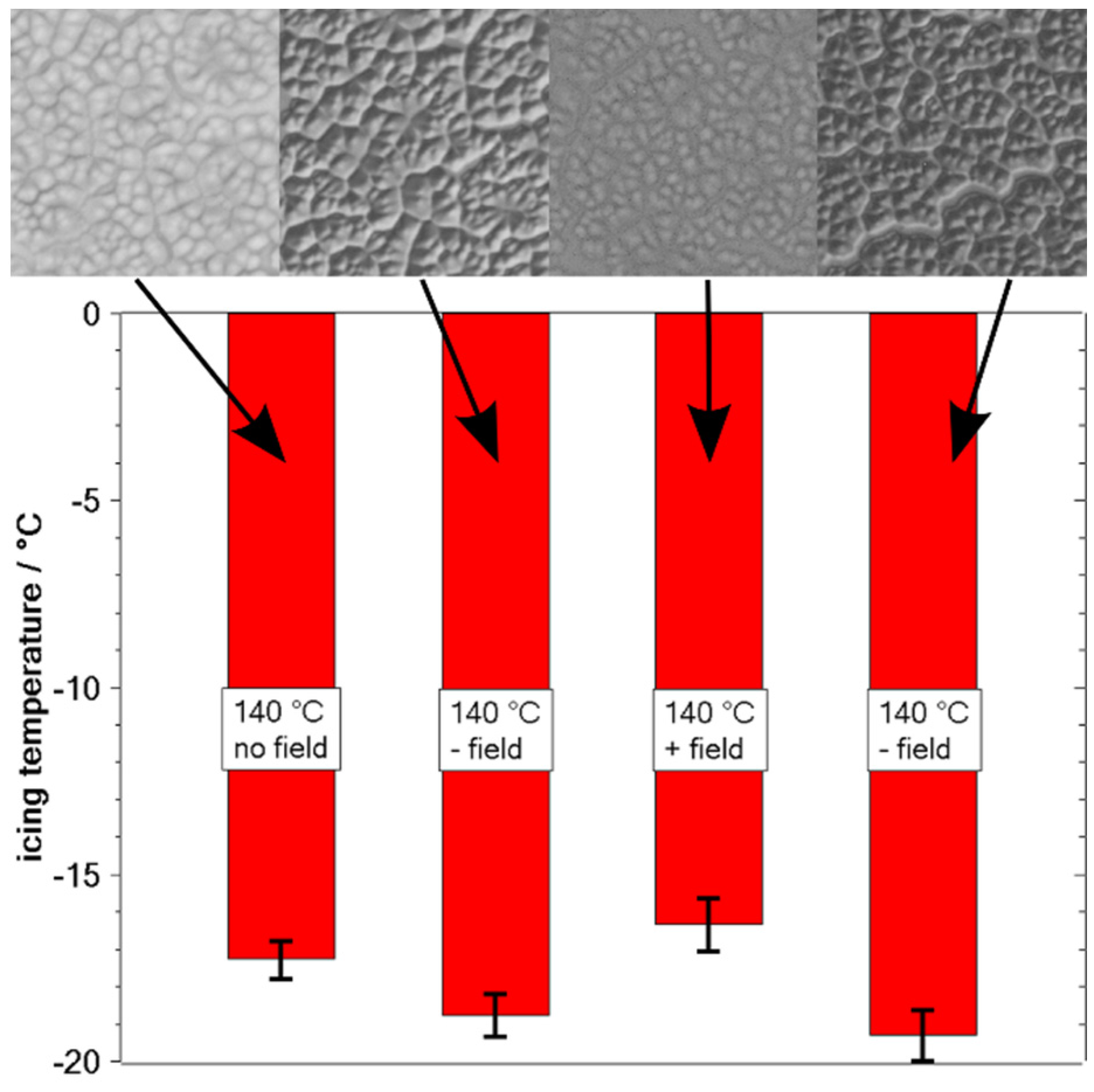
4. Discussion
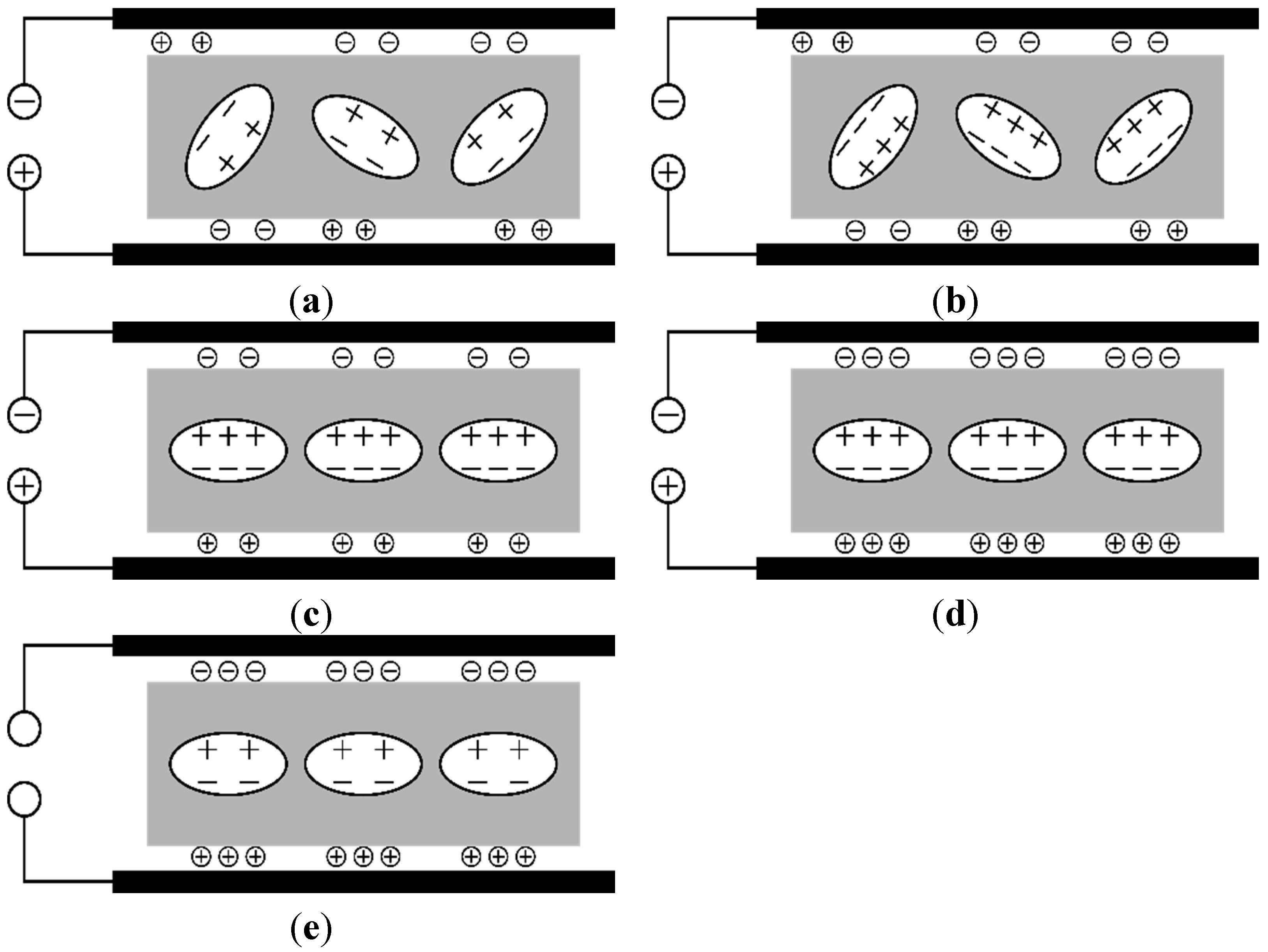
5. Summary/Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fillion, R.M.; Riahi, A.R.; Edrisy, A. A review of icing prevention in photovoltaic devices by surface engineering. Renew. Sustain. Energy Rev. 2014, 32, 797–809. [Google Scholar] [CrossRef]
- Croutch, C.K.; Hartley, R.A. Adhesion of ice to coatings and the performance of ice release coatings. J. Coat. Technol. 1992, 64, 41–53. [Google Scholar]
- Petrenko, V.F.; Peng, S. Reduction of ice adhesion to metal by using self-assembling monolayers (SAMs). Can. J. Phys. 2003, 278, 387–393. [Google Scholar] [CrossRef]
- Suh, H.S.; Jang, A.R.; Suh, Y.-H.; Suslick, K.S. Porous, hollow, and ball-in-ball metal oxide microspheres: Preparation, endocytosis, cytotoxicity. Adv. Mater. 2006, 18, 1832–1837. [Google Scholar] [CrossRef]
- Chatterji, S. Aspects of the freezing process in a porous material-water system: Part 1. Freezing and the properties of water and ice. Cem. Concr. Res. 1999, 29, 627–630. [Google Scholar] [CrossRef]
- Mishchenko, L.; Hatton, B.; Bahadur, V.; Tylor, J.A.; Krupenkin, T.; Aizenberg, J. Design of ice-free nanostructured surfaces based on repulsion of impacting water droplets. ACS Nano 2010, 4, 7699–7707. [Google Scholar] [CrossRef] [PubMed]
- Jafari, R.; Menini, R.; Farzaneh, M. Superhydrophobic and icephobic surface structures prepared by RF-sputtered polytetrafluoroethylene coatings. Appl. Surf. Sci. 2010, 257, 1540–1543. [Google Scholar] [CrossRef]
- Auer, F.; Harenburg, J.; Roth, C. Funktionelle schichten auf metallen: Maßgeschneiderte eigenschaften durch sol-gel-technologie/functional layers on metals: Tailored properties by sol-gel-technology. Materialwiss. Werkstofftech. 2001, 32, 767–773. (In Germany) [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farhadi, S.; Nose, K.; Du, X.W. Superhydrophobic surfaces: Are they really ice-repellent? Langmuir 2011, 27, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.B.; Emelyanenko, A.M.; Ivanov, V.K.; Pashinin, A.S. Durable icephobic coating for stainless steel. ACS Appl. Mater. Interfaces 2013, 5, 2549–2554. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Jones, A.K.; Sikka, V.K.; Wu, J.; Gao, D. Anti-icing superhydrophobic coatings. Langmuir 2009, 25, 12444–12448. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Tiwari, M.K.; Doan, N.V.; Poulikakos, D. Mechanism of supercooled droplet freezing on surfaces. Nat. Commun. 2012, 3, 615. [Google Scholar] [CrossRef] [PubMed]
- Kreider, A.; Weber, B.; Stenzel, V.; Tornow, C.; Grunwald, I. Natural frost protection—Coupling antifreeze proteins to a PU coating inhibits ice deposition. Eur. Coat. J. 2011, 5, 34–39. [Google Scholar]
- Gibson, M.I. Slowing the growth of ice with synthetic macromolecules: Beyond antifreeze(glyco) proteins. Polym. Chem. 2010, 1, 1141–1152. [Google Scholar] [CrossRef]
- Chen, M.-L.; Chiou, T.-K.; Jiang, S.-T. Isolation of ice-nucleating active bacterium from mackerel and its properties. Fish. Sci. 2002, 68, 934–941. [Google Scholar] [CrossRef]
- Gwak, Y.; Park, J.; Kim, M.; Kim, H.S.; Kwon, J.K.; Oh, S.J.; Kim, Y.-P.; Jin, E. Creating anti-icing surfaces via the direct immobilization of antifreeze proteins on aluminium. Sci Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Wong, T.-S.; Alvarenga, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. Liquid-infused nanostructured surfaces with extreme anti-ice and anti-frost performance. ACS Nano 2012, 6, 6569–6577. [Google Scholar] [CrossRef] [PubMed]
- Sidorenko, A.; Krupenkin, T.; Aizenberg, J. Controlled switching of the wetting behavior of biomimetic surfaces with hydrogel-supported nanostructures. J. Mater. Chem. 2008, 18, 3841–3846. [Google Scholar] [CrossRef]
- Chen, J.; Dou, R.; Cui, D.; Zhang, Q.; Zhang, Y.; Xu, F.; Zhou, X.; Wang, J.; Song, Y.; Jiang, L. Robust prototypical anti-icing coatings with a self-lubricating liquid water layer between ice and substrate. ACS Appl. Mater. Interfaces 2013, 5, 4026–4030. [Google Scholar] [CrossRef] [PubMed]
- Rykaczewski, K.; Anand, S.; Subramanyam, S.B.; Varanasi, K.K. Mechanism of frost formation on lubricant-impregnated surfaces. Langmuir 2013, 29, 5230–5238. [Google Scholar] [CrossRef] [PubMed]
- Péter, Z.; Farzaneh, M.; Kiss, L.I. Assessment of the current intensity for preventing ice accretion on overhead conductors. IEEE Trans. Power Deliv. 2007, 22, 565–574. [Google Scholar] [CrossRef]
- Venna, V.V.; Lin, Y.-J. Mechatronic development of self-actuating in-flight deicing structures. IEEE-ASME Trans. Mechatron. 2006, 11, 585–592. [Google Scholar] [CrossRef]
- Petrenko, V.F. The effect of static electric fields on ice friction. J. Appl. Phys. 1994, 76, 1216–1219. [Google Scholar] [CrossRef]
- Petersen, A.; Rau, G.; Glasmacher, B. Reduction of primary freeze-drying time by electric field influenced ice nucleus formation. Heat Mass Transf. 2006, 42, 929–938. [Google Scholar] [CrossRef]
- Ehre, D.; Lavert, E.; Lahav, M; Lubomirsky, I. Water freezes differently on positively and negatively charged surfaces of pyroelectric materials. Science 2010, 327, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.B. Pyroelectricity: From ancient curiosity to modern imaging tool. Phys. Today 2005, 58, 31–36. [Google Scholar] [CrossRef]
- Poulsen, M.; Ducharme, S. Why ferroelectric polyvinylidene fluoride is special. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 1028–1035. [Google Scholar] [CrossRef]
- Harb, A. Energy harvesting: State-of-the-art. Renew. Energy 2011, 36, 2641–2654. [Google Scholar] [CrossRef]
- Navid, A.; Pilon, L. Pyroelectric energy harvesting using Olsen cycles in purified and porous poly(vinylidene fluoride-trifluoroethylene) [P(VDF-TrFE)] thin films. Smart Mater. Struct. 2011, 20. [Google Scholar] [CrossRef]
- Olsen, R.B.; Bruno, D.A.; Briscoe, J.M. Cascaded pyroelectric energy converter. Ferroelectrics 1984, 59, 205–209. [Google Scholar] [CrossRef]
- Nordheim, D.; Hahne, S.; Ploss, B. Nonlinear dielectric properties and polarization in thin ferroelectric P(VDF-TrFE) copolymer films. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1175–1180. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spitzner, D.; Bergmann, U.; Apelt, S.; Boucher, R.A.; Wiesmann, H.-P. Reversible Switching of Icing Properties on Pyroelectric Polyvenylidene Fluoride Thin Film Coatings. Coatings 2015, 5, 724-736. https://doi.org/10.3390/coatings5040724
Spitzner D, Bergmann U, Apelt S, Boucher RA, Wiesmann H-P. Reversible Switching of Icing Properties on Pyroelectric Polyvenylidene Fluoride Thin Film Coatings. Coatings. 2015; 5(4):724-736. https://doi.org/10.3390/coatings5040724
Chicago/Turabian StyleSpitzner, Dirk, Ute Bergmann, Sabine Apelt, Richard A. Boucher, and Hans-Peter Wiesmann. 2015. "Reversible Switching of Icing Properties on Pyroelectric Polyvenylidene Fluoride Thin Film Coatings" Coatings 5, no. 4: 724-736. https://doi.org/10.3390/coatings5040724
APA StyleSpitzner, D., Bergmann, U., Apelt, S., Boucher, R. A., & Wiesmann, H.-P. (2015). Reversible Switching of Icing Properties on Pyroelectric Polyvenylidene Fluoride Thin Film Coatings. Coatings, 5(4), 724-736. https://doi.org/10.3390/coatings5040724




