Novel Features, Applications, and Recent Developments of High-Entropy Ceramic Coatings: A State-of-the-Art Review
Abstract
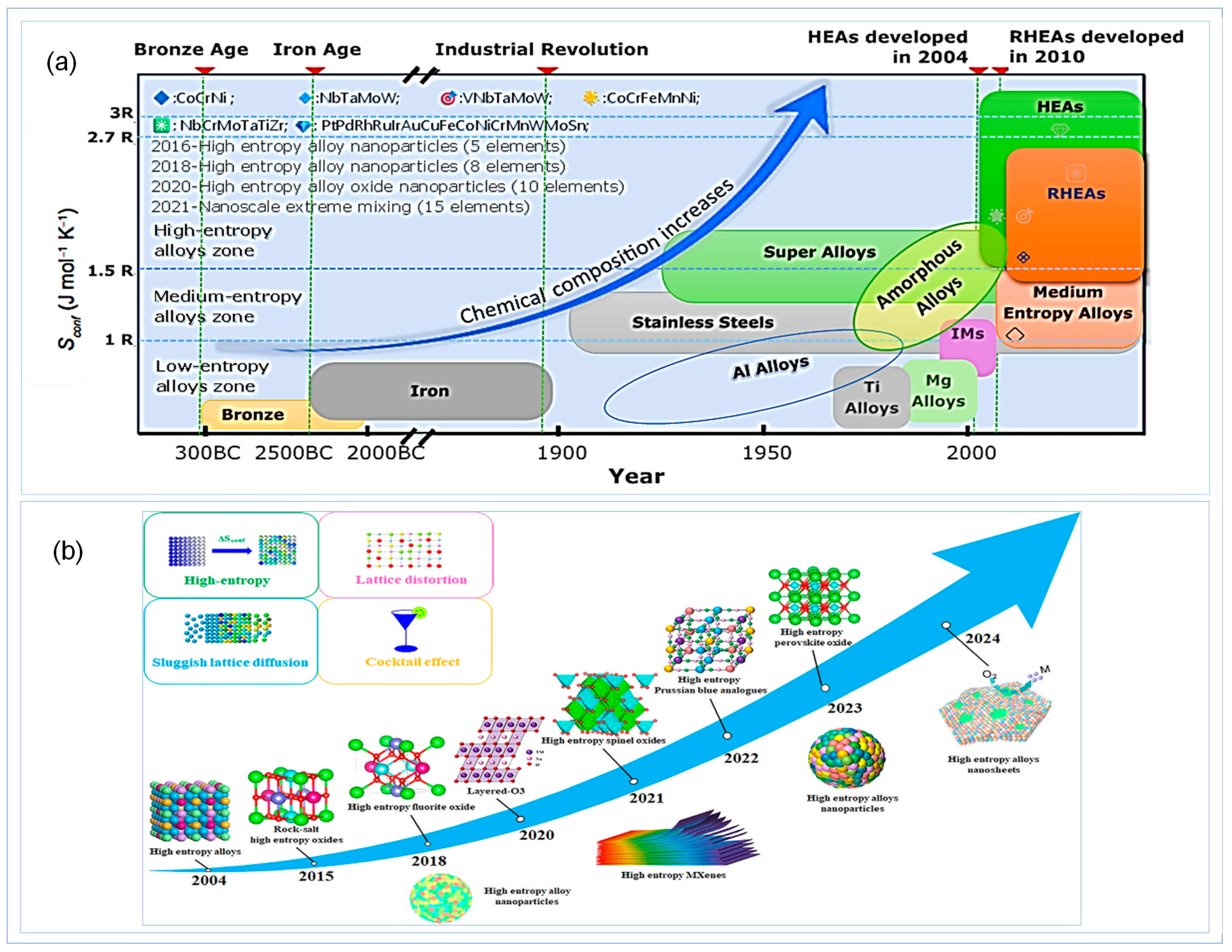
1. Introduction
1.1. High-Entropy Ceramics Coating
1.2. High-Entropy Carbide Coating
1.3. High-Entropy Nitride Coating
1.4. High-Entropy Boride Coatings (HEBC)
1.5. High-Entropy Oxide Coatings (HEOC)
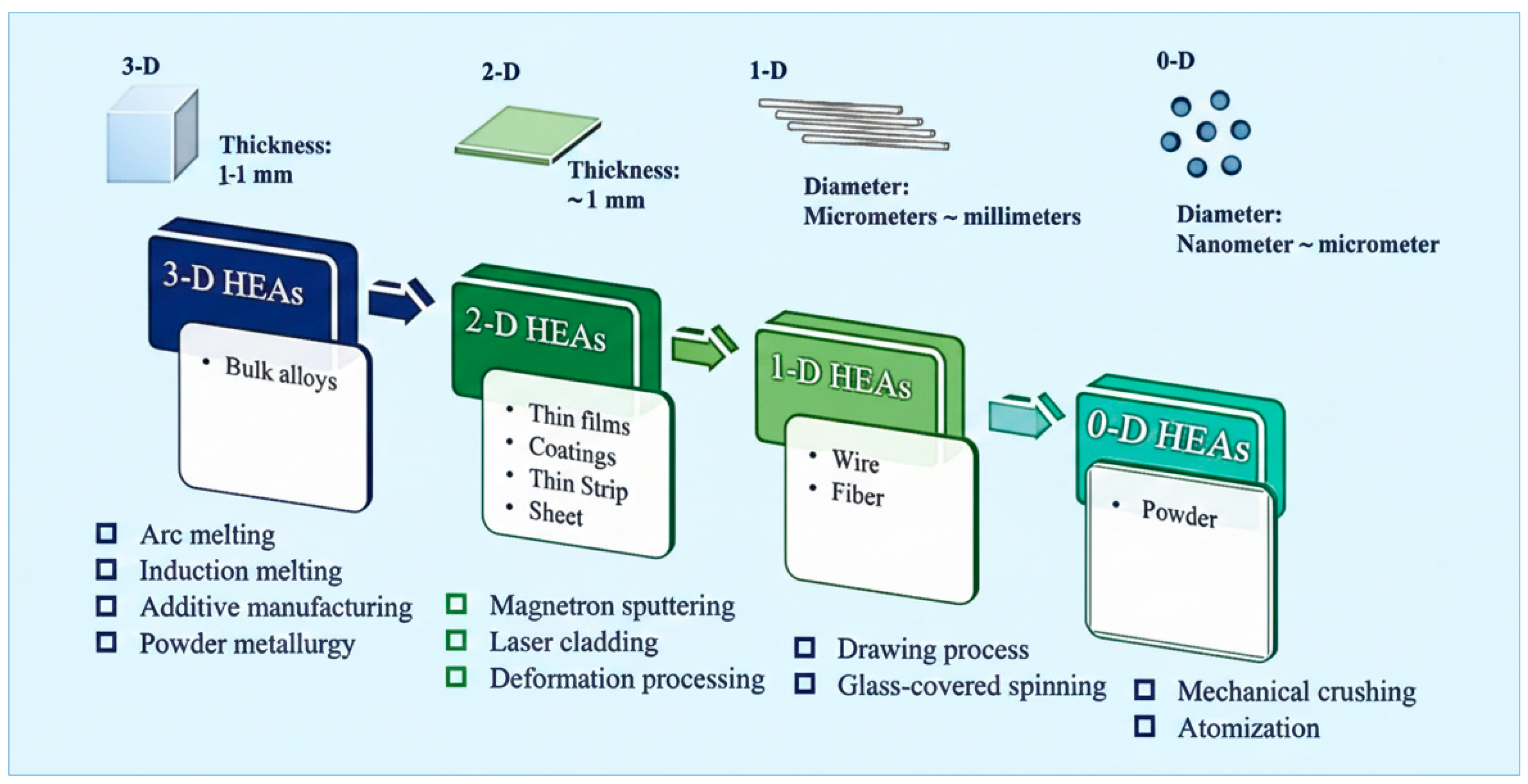
2. Synthesis and Fabrication
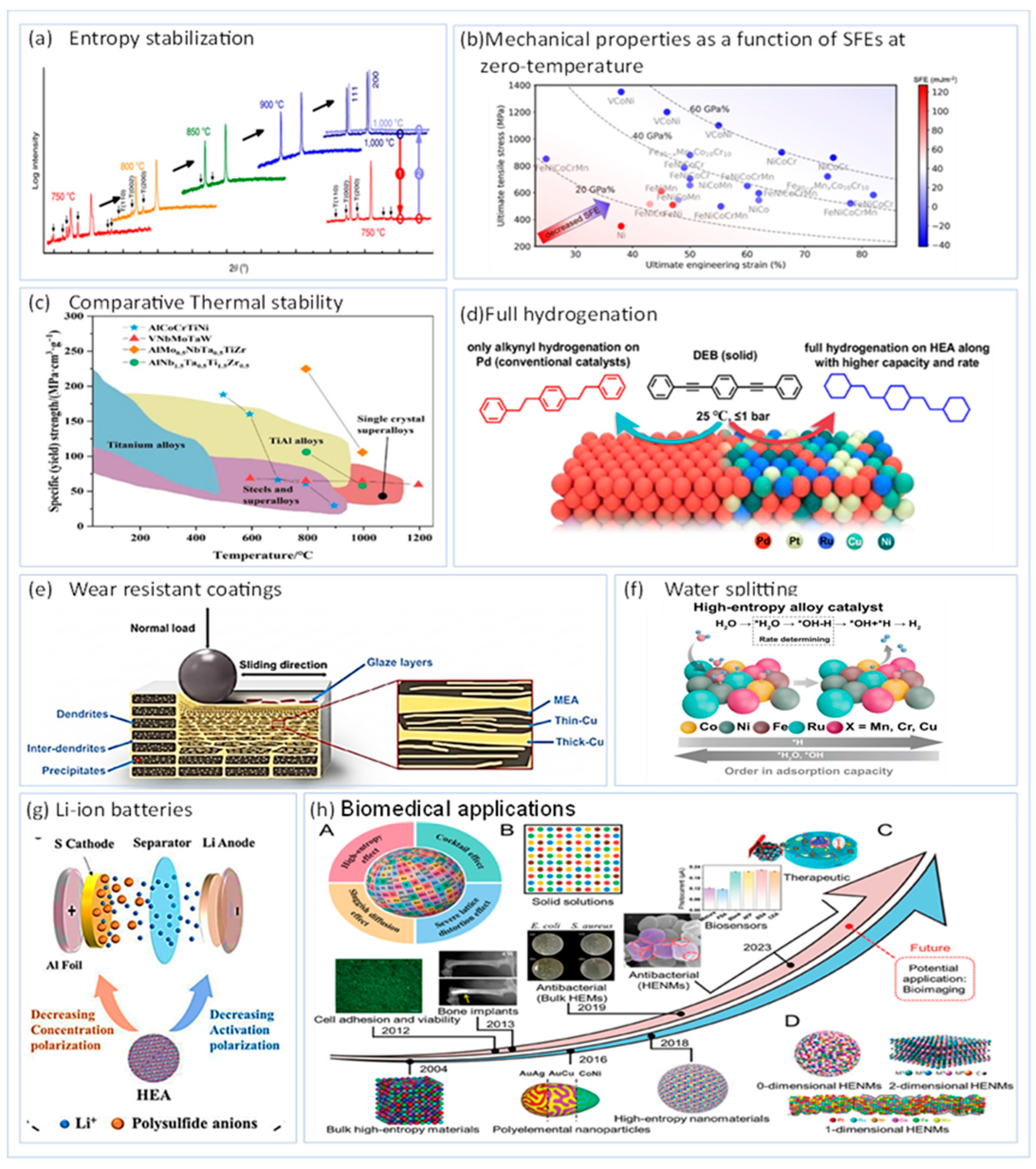
3. Properties
3.1. Thermal Conductivity and Wettability
3.2. Transition from Elastic to Plastic Contact (H3/E2)
3.3. Hardness and Elastic Modulus
3.4. Tribological Characteristic (Tool Flank Wear, Average Friction Coefficient, Wear Rate)
4. Influencing Parameters
4.1. Crystal Structure and Grain Size
4.2. Substrate Temperature and Bias
4.3. Densification and Compressive Residual Stress
5. Recent Developments in High-Entropy Ceramic Coatings
5.1. Improved Processing Techniques
5.2. Material Combinations
5.3. Application in Extreme Environments
6. Future Prospects and Applications
6.1. Advanced Thermal Barrier Coatings
6.2. Enhanced Wear Resistance
6.3. Corrosion Resistance
6.4. Biomedical Applications
6.5. Energy Storage and Conversion
6.6. Advancements in Material Design
6.7. Environmental Considerations
7. Conclusions
7.1. Phase/Property Prediction
7.2. Fabrication
7.3. Microstructure Property Relation
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xin, Y.; Li, S.; Qian, Y.; Zhu, W.; Yuan, H.; Jiang, P.; Guo, R.; Wang, L. High-entropy alloys as a platform for catalysis: Progress, challenges, and opportunities. ACS Catal. 2020, 10, 11280–11306. [Google Scholar] [CrossRef]
- Rajendrachari, S. An overview of high-entropy alloys prepared by mechanical alloying followed by the characterization of their microstructure and various properties. Alloys 2022, 1, 116–132. [Google Scholar] [CrossRef]
- Hummel, R.E.; Hummel, R.E. Optical Properties of Materials. In Understanding Materials Science: History, Properties, Applications, 2nd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004; pp. 245–270. ISBN 0387209395, 9780387209395. Available online: https://books.google.co.in/books/about/Understanding_Materials_Science.html?id=DaAmwiJ4rnEC&redir_esc=y (accessed on 1 December 2025).
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.-H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.T.; Liu, C.T.; Yang, Y.C. Design of high entropy alloys: A single-parameter thermodynamic rule. Scr. Mater. 2015, 104, 53–55. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, M.A.; Yuan, T.; Wang, G.Y.; Yeh, J.W.; Tsai, C.W.; Chuang, A.; Liaw, P.K. Fatigue behavior of Al0. 5CoCrCuFeNi high entropy alloys. Acta Mater. 2012, 60, 5723–5734. [Google Scholar] [CrossRef]
- Xia, S.Q.; Yang, X.; Yang, T.F.; Liu, S.; Zhang, Y. Irradiation resistance in Al x CoCrFeNi high entropy alloys. Jom 2015, 67, 2340–2344. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Swalin, R.A. Thermodynamics of Solids; John Wiley and Sons: New York, NY, USA, 1962. [Google Scholar]
- Ke, G.Y.; Chen, S.K.; Hsu, T.; Yeh, J.W. FCC and BCC equivalents in as-cast solid solutions of AlxCoyCrzCu0. 5FevNiw high-entropy alloys. Eur. J. Control. 2006, 31, 669–683. [Google Scholar]
- Liang, X.; Zhu, X.; Li, X.; Mo, R.; Liu, Y.; Wu, K.; Ma, J. High-entropy alloy and amorphous alloy composites fabricated by ultrasonic vibrations. Sci. China Phys. Mech. Astron. 2020, 63, 116111. [Google Scholar] [CrossRef]
- He, J.Y.; Liu, W.H.; Wang, H.; Wu, Y.; Liu, X.J.; Nieh, T.G.; Lu, Z.P. Effects of Al addition on structural evolution and tensile properties of the FeCoNiCrMn high-entropy alloy system. Acta Mater. 2014, 62, 105–113. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miller, J.D.; Miracle, D.B.; Woodward, C. Accelerated exploration of multi-principal element alloys with solid solution phases. Nat. Commun. 2015, 6, 6529. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Takeuchi, A.; Amiya, K.; Wada, T.; Yubuta, K.; Zhang, W. High-entropy alloys with a hexagonal close-packed structure designed by equi-atomic alloy strategy and binary phase diagrams. Jom 2014, 66, 1984–1992. [Google Scholar] [CrossRef]
- Oriani, A.E. Multimodal and Ultra High-Q Superconducting Niobium Cavities for Circuit Quantum Electrodynamics. Ph.D. Thesis, The University of Chicago, Chicago, IL, USA, 2022. [Google Scholar]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. The generalized thermodynamic rule for phase selection in multicomponent alloys. Intermetallics 2015, 59, 75–80. [Google Scholar] [CrossRef]
- Takeuchi, A.; Amiya, K.; Wada, T.; Yubuta, K.; Zhang, W.; Makino, A. Entropies in alloy design for high-entropy and bulk glassy alloys. Entropy 2013, 15, 3810–3821. [Google Scholar] [CrossRef]
- Inoue, A.; Gook, J.S. Multicomponent Fe-based glassy alloys with wide supercooled liquid region before crystallization. Mater. Trans. JIM 1995, 36, 1282–1285. [Google Scholar] [CrossRef]
- Hua, X.-J.; Hu, P.; Xing, H.-R.; Han, J.-Y.; Ge, S.-W.; Li, S.-L.; He, C.-J.; Wang, K.-S.; Cui, C.-J. Development and Property Tuning of Refractory High-Entropy Alloys: A Review. Acta Metall. Sin.-Engl. Lett. 2022, 35, 1231–1265. [Google Scholar] [CrossRef]
- Krishna, S.A.; Noble, N.; Radhika, N.; Saleh, B. A comprehensive review on advances in high entropy alloys: Fabrication and surface modification methods, properties, applications, and future prospects. J. Manuf. Process. 2024, 109, 583–606. [Google Scholar] [CrossRef]
- Xin, Y.; Zhu, M.; Zhang, H.; Wang, X. High-Entropy Materials: A New Paradigm in the Design of Advanced Batteries. Nano-Micro Lett. 2026, 18, 1. [Google Scholar] [CrossRef]
- Ng, C.; Guo, S.; Luan, J.; Wang, Q.; Lu, J.; Shi, S.; Liu, C.T. Phase stability and tensile properties of Co-free Al0. 5CrCuFeNi2 high-entropy alloys. J. Alloys Compd. 2014, 584, 530–537. [Google Scholar] [CrossRef]
- Mansoori, G.A.; Carnahan, N.F.; Starling, K.E.; Leland, T.W., Jr. Equilibrium thermodynamic properties of the mixture of hard spheres. J. Chem. Phys. 1971, 54, 1523–1525. [Google Scholar] [CrossRef]
- Angelani, L.; Foffi, G. Configurational entropy of hard spheres. J. Phys. Condens. Matter 2007, 19, 256207. [Google Scholar] [CrossRef]
- Singh, S.; Wanderka, N.; Murty, B.S.; Glatzel, U.; Banhart, J. Decomposition in multi-component AlCoCrCuFeNi high-entropy alloy. Acta Mater. 2011, 59, 182–190. [Google Scholar] [CrossRef]
- Takeuchi, A.; Chen, N.; Wada, T.; Yokoyama, Y.; Kato, H.; Inoue, A.; Yeh, J.W. Pd20Pt20Cu20Ni20P20 high-entropy alloy as a bulk metallic glass in the centimeter. Intermetallics 2011, 19, 1546–1554. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Rost, C.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.; Hou, D.; Jones, J.; Curtarolo, S.; Maria, J. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485–8489. [Google Scholar] [CrossRef]
- Pei, Z.; Zhao, S.; Detrois, M.; Jablonski, P.D.; Hawk, J.A.; Alman, D.E.; Asta, M.; Minor, A.M.; Gao, M.C. Theory-guided design of high-entropy alloys with enhanced strength-ductility synergy. Nat. Commun. 2023, 14, 2519. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Huang, D.; Wang, W.; Dou, G.; Lyu, P. Recent Advances in High-Temperature Properties of High-Entropy Alloys. High-Temp. Mat. 2025, 2, 10011. [Google Scholar] [CrossRef]
- Jing, Z.; Guo, Y.; Wang, Q.; Yan, X.; Yue, G.; Li, Z.; Liu, H.; Qin, R.; Zhong, C.; Li, M.; et al. Ambient hydrogenation of solid aromatics enabled by a high entropy alloy nanocatalyst. Nat. Commun. 2024, 15, 5806. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Jia, Q.; Du, Y.; Zhou, Q.; Greiner, C.; Hua, K.; Wang, H.; Wang, J. A wear-resistant metastable CoCrNiCu high-entropy alloy with modulated surface and subsurface structures. Friction 2022, 10, 1722–1738. [Google Scholar] [CrossRef]
- Chen, J.; Wu, A.; Zhang, Y.; Xie, Y.; Zheng, H.; Teng, W. A mini-review on high-entropy alloy nanomaterials for electrocatalysis: Advances and prospects. Front. Mater. 2025, 12, 1613997. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, J.; Niu, R.; Zhao, Z.; Wang, X. High-Entropy Materials: Features for Lithium–Sulfur Battery Applications. Metals 2023, 13, 833. [Google Scholar] [CrossRef]
- Chang, L.; Jing, H.; Liu, C.; Qiu, C.; Ling, X. High-Entropy Materials for Prospective Biomedical Applications: Challenges and Opportunities. Adv. Sci. 2024, 11, 2406521. [Google Scholar] [CrossRef]
- Hsu, W.L.; Murakami, H.; Araki, H.; Watanabe, M.; Kuroda, S.; Yeh, A.C.; Yeh, J.W. A study of NiCo0. 6Fe0. 2CrxSiAlTiy high-entropy alloys for applications as a high-temperature protective coating and a bond coat in thermal barrier coating systems. J. Electrochem. Soc. 2018, 165, C524. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chang, S.Y.; Hong, Y.D.; Chen, S.K.; Lin, S.J. Anomalous decrease in X-ray diffraction intensities of Cu–Ni–Al–Co–Cr–Fe–Si alloy systems with multi-principal elements. Mater. Chem. Phys. 2007, 103, 41–46. [Google Scholar] [CrossRef]
- Yeh, J.W.; Lin, S.J.; Chin, T.S.; Gan, J.Y.; Chen, S.K.; Shun, T.T.; Tsau, C.-H.; Chou, S.Y. Formation of simple crystal structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V alloys with multiprincipal metallic elements. Metall. Mater. Trans. A 2004, 35, 2533–2536. [Google Scholar] [CrossRef]
- Tong, C.J.; Chen, M.R.; Yeh, J.W.; Lin, S.J.; Chen, S.K.; Shun, T.T.; Chang, S.Y. Mechanical performance of the Al x CoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36, 1263–1271. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, J.B.; Li, L.; Li, J.C.; Jiang, Q. Microstructure and tensile properties of FeMnNiCuCoSnx high entropy alloys. Mater. Des. 2013, 44, 223–227. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Z.P.; Ma, S.G.; Liaw, P.K.; Tang, Z.; Cheng, Y.Q.; Gao, M.C. Guidelines in predicting phase formation of high-entropy alloys. Mrs Commun. 2014, 4, 57–62. [Google Scholar] [CrossRef]
- Troparevsky, M.C.; Morris, J.R.; Kent, P.R.; Lupini, A.R.; Stocks, G.M. Criteria for predicting the formation of single-phase high-entropy alloys. Phys. Rev. X 2015, 5, 011041. [Google Scholar] [CrossRef]
- Mizutani, U. Hume-Rothery rules for structurally complex alloy phases. Mrs Bull. 2012, 37, 169. [Google Scholar] [CrossRef]
- Poletti, M.G.; Battezzati, L.J.A.M. Electronic and thermodynamic criteria for the occurrence of high entropy alloys in metallic systems. Acta Mater. 2014, 75, 297–306. [Google Scholar] [CrossRef]
- Oses, C.; Toher, C.; Curtarolo, S. High-entropy ceramics. Nat. Rev. Mater. 2020, 5, 295–309. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Miracle, D.B. High-entropy alloys: A current evaluation of founding ideas and core effects and exploring “nonlinear alloys”. Jom 2017, 69, 2130–2136. [Google Scholar] [CrossRef]
- Grzesik, Z.; Smoła, G.; Stygar, M.; Dąbrowa, J.; Zajusz, M.; Mroczka, K.; Danielewski, M. Defect structure and transport properties in (Co, Cu, Mg, Ni, Zn) O high entropy oxide. J. Eur. Ceram. Soc. 2019, 39, 4292–4298. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Sarkar, A.; Djenadic, R.; Wang, D.; Hein, C.; Kautenburger, R.; Clemens, O.; Hahn, H. Rare earth and transition metal based entropy stabilised perovskite type oxides. J. Eur. Ceram. Soc. 2018, 38, 2318–2327. [Google Scholar] [CrossRef]
- Dong, Y.; Ren, K.; Lu, Y.; Wang, Q.; Liu, J.; Wang, Y. High-entropy environmental barrier coating for the ceramic matrix composites. J. Eur. Ceram. Soc. 2019, 39, 2574–2579. [Google Scholar] [CrossRef]
- Ren, X.; Tian, Z.; Zhang, J.; Wang, J. Equiatomic quaternary (Y1/4Ho1/4Er1/4Yb1/4) 2SiO5 silicate: A perspective multifunctional thermal and environmental barrier coating material. Scr. Mater. 2019, 168, 47–50. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Liu, J.; Zhang, X.; Yuan, M.; Zhao, Y.; Yan, J.; Hou, M.; Yan, J.; Kunz, M.; et al. Stability and compressibility of cation-doped high-entropy oxide MgCoNiCuZnO5. J. Phys. Chem. C 2019, 123, 17735–17744. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiang, H.; Dai, F.Z.; Peng, Z.; Zhou, Y. (TiZrHf) P2O7: An equimolar multicomponent or high entropy ceramic with good thermal stability and low thermal conductivity. J. Mater. Sci. Technol. 2019, 35, 2227–2231. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, H.; Dai, F.Z.; Liu, J.; Lei, Y.; Zhang, J.; Zhou, Y. High porosity and low thermal conductivity high entropy (Zr0. 2Hf0. 2Ti0. 2Nb0. 2Ta0. 2) C. J. Mater. Sci. Technol. 2019, 35, 1700–1705. [Google Scholar] [CrossRef]
- Tsai, D.C.; Deng, M.J.; Chang, Z.C.; Kuo, B.H.; Chen, E.C.; Chang, S.Y.; Shieu, F.S. Oxidation resistance and characterization of (AlCrMoTaTi)-Six-N coating deposited via magnetron sputtering. J. Alloys Compd. 2015, 647, 179–188. [Google Scholar] [CrossRef]
- Vladescu, A.; Titorencu, I.; Dekhtyar, Y.; Jinga, V.; Pruna, V.; Balaceanu, M.; Dinu, M.; Pana, I.; Vendina, V.; Braic, M. In vitro biocompatibility of Si alloyed multi-principal element carbide coatings. PLoS ONE 2016, 11, e0161151. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, J.; Zhang, F.; Niu, B.; Lei, L.; Wang, W. High-entropy carbide: A novel class of multicomponent ceramics. Ceram. Int. 2018, 44, 22014–22018. [Google Scholar] [CrossRef]
- Sarkar, A.; Velasco, L.; Wang, D.I.; Wang, Q.; Talasila, G.; de Biasi, L.; Kübel, C.; Brezesinski, T.; Bhattacharya, S.S.; Hahn, H.; et al. High entropy oxides for reversible energy storage. Nat. Commun. 2018, 9, 3400. [Google Scholar] [CrossRef]
- Zheng, Y.; Yi, Y.; Fan, M.; Liu, H.; Li, X.; Zhang, R.; Li, M.; Qiao, Z.A. A high-entropy metal oxide as chemical anchor of polysulfide for lithium-sulfur batteries. Energy Storage Mater. 2019, 23, 678–683. [Google Scholar] [CrossRef]
- Wang, Q.; Sarkar, A.; Wang, D.; Velasco, L.; Azmi, R.; Bhattacharya, S.S.; Bergfeldt, T.; Düvel, A.; Heitjans, P.; Brezesinski, T.; et al. Multi-anionic and -cationic compounds: New high entropy materials for advanced Li-ion batteries. Energy Environ. Sci. 2019, 12, 2433–2442. [Google Scholar] [CrossRef]
- Cheng, K.-H.; Tsai, C.-W.; Lin, S.-J.; Yeh, J.-W. Effects of silicon content on the structure and mechanical properties of (AlCrTaTiZr)–Six–N coatings by reactive RF magnetron sputtering. J. Phys. D 2011, 44, 205405. [Google Scholar] [CrossRef]
- Hsieh, M.-H.; Tsai, M.-H.; Shen, W.-J.; Yeh, J.-W. Structure and properties of two Al–Cr–Nb–Si–Ti high-entropy nitride coatings. Surf. Coat. Technol. 2013, 221, 118–123. [Google Scholar] [CrossRef]
- Braun, J.L.; Rost, C.M.; Lim, M.; Giri, A.; Olson, D.H.; Kotsonis, G.N.; Stan, G.; Brenner, D.W.; Maria, J.; Hopkins, P.E. Charge-induced disorder controls the thermal conductivity of entropy-stabilized oxides. Adv. Mater. 2006, 201, 3275–3280. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-H.; Lin, S.-J.; Yeh, J.-W.; Chang, S.-Y. Preparation and characterization of AlCrTaTiZr multi-element nitride coatings. Surf. Coat. Technol. 2006, 201, 3275–3280. [Google Scholar] [CrossRef]
- Huang, P.-K.; Yeh, J.-W. Effects of substrate bias on structure and mechanical properties of (AlCrNbSiTiV)N coatings. J. Phys. D 2009, 42, 115401. [Google Scholar] [CrossRef]
- Huang, P.-K.; Yeh, J.-W. Inhibition of grain coarsening up to 1000 °C in (AlCrNbSiTiV)N superhard coatings. Scr. Mater. 2010, 62, 105–108. [Google Scholar] [CrossRef]
- Harrington, T.J.; Gild, J.; Sarker, P.; Toher, C.; Rost, C.M.; Dippo, O.F.; McElfresh, C.; Kaufmann, K.; Marin, E.; Borowski, L.; et al. Phase stability and mechanical properties of novel high entropy transition metal carbides. Acta Mater. 2019, 166, 271–280. [Google Scholar] [CrossRef]
- Stevanović, V.; Lany, S.; Zhang, X.; Zunger, A. Correcting density functional theory for accurate predictions of compound enthalpies of formation: Fitted elemental-phase reference energies. Phys. Rev. B 2012, 85, 115104. [Google Scholar] [CrossRef]
- Friedrich, R.; Usanmaz, D.; Oses, C.; Supka, A.; Fornari, M.; Nardelli, M.B.; Toher, C.; Curtarolo, S. Coordination corrected ab initio formation enthalpies. npj Comput. Mater. 2019, 5, 59. [Google Scholar] [CrossRef]
- Toher, C.; Oses, C.; Hicks, D.; Curtarolo, S. Unavoidable disorder and entropy in multi-component systems. npj Comput. Mater. 2019, 5, 69. [Google Scholar] [CrossRef]
- Van de Walle, A. Multicomponent multisublattice alloys, nonconfigurational entropy and other additions to the alloy theoretic automated toolkit. Calphad 2009, 33, 266–278. [Google Scholar] [CrossRef]
- Lederer, Y.; Toher, C.; Vecchio, K.S.; Curtarolo, S. The search for high entropy alloys: A high-throughput ab-initio approach. Acta Mater. 2018, 159, 364–383. [Google Scholar] [CrossRef]
- Chen, T.-K.; Shun, T.T.; Yeh, J.-W.; Wong, M.S. Nanostructured nitride films of multi-element high-entropy alloys by reactive DC sputtering. Surf. Coat. Technol. 2004, 188–189, 193–200. [Google Scholar] [CrossRef]
- Chen, T.-K.; Wong, M.-S.; Shun, T.-T.; Yeh, J.-W. Nanostructured nitride films of multi-element high-entropy alloys by reactive DC sputtering. Surf. Coat. Technol. 2005, 200, 1361–1365. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Chen, L.; Lui, H.-W.; Cai, M.-H.; Yeh, J.-W. Microstructure, hardness, resistivity and thermal stability of sputtered oxide films of AlCoCrCu0.5NiFe high-entropy alloy. Mater. Sci. Eng. A 2007, 457, 77–83. [Google Scholar] [CrossRef]
- Chen, T.-K.; Wong, M.-S. Structure and properties of reactively-sputtered AlxCoCrCuFeNi oxide films. Thin Solid Film. 2007, 516, 141–146. [Google Scholar] [CrossRef]
- Padture, N. Advanced structural ceramics in aerospace propulsion. Nat. Mater. 2016, 15, 804. [Google Scholar] [CrossRef]
- Fahrenholtz, W.; Hilmas, G. Ultra-high temperature ceramics: Materials for extreme environments. Scr. Mater. 2017, 129, 94. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, J.; Wang, L.; Zhong, F.; Huang, K.; Tian, Y. Electromagnetic coupling field strengthening of WC-TiC-Co cermet tools. Ceram. Int. 2021, 47, 3747. [Google Scholar] [CrossRef]
- Zeng, Y.; Xiong, X.; Li, G.; Chen, Z.; Sun, W.; Wang, D.; Wang, Y. Effect of fiber architecture and density on the ablation behavior of carbon/carbon composites modified by Zr–Ti–C. Carbon 2013, 63, 92. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, D.; Xiong, X.; Zhang, X.; Withers, P.; Sun, W.; Smith, M.; Bai, M.; Xiao, P. Ablation-resistant carbide Zr0.8Ti0.2C0.74B0.26 for oxidizing environments up to 3,000 °C. Nat. Commun. 2017, 8, 15836. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Bei, H.; Otto, F.; Pharr, G.; George, E. Recovery, recrystallization, grain growth and phase stability of a family of FCCstructured multi-component equiatomic solid solution alloys. Intermetallics 2014, 46, 131. [Google Scholar] [CrossRef]
- Wu, Z.; Bei, H.; Pharr, G.; George, E. Temperature dependence of the mechanical properties of equiatomic solid solution alloys with face-centered cubic crystal structures. Acta Mater. 2014, 81, 428. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Guo, S.; Jiang, L.; Kang, H.; Wang, T.; Wen, B.; Wang, Z.; Jie, J.; Cao, Z.; et al. A promising new class of high temperature alloys: Eutectic high-entropy alloys. Sci. Rep. 2014, 4, 6200. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Gao, X.; Jiang, L.; Chen, Z.; Wang, T.; Jie, J.; Kang, H.; Zhang, Y.; Guo, S.; Ruan, H.; et al. Directly cast bulk eutectic and near-eutectic high entropy alloys with balanced strength and ductility in a wide temperature range. Acta Mater. 2017, 124, 143. [Google Scholar] [CrossRef]
- Jia, N.; Li, Y.; Liu, X.; Zheng, Y.; Wang, B.; Wang, J.; Xue, Y.; Jin, K. Thermal stability and mechanical properties of low-activation single phase Ti–V–Ta medium entropy alloys. JOM 2019, 71, 3490. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Z.; Tong, Y.; Zhang, W.; Wu, Z. Microstructural and mechanical behavior of a CoCrFeNiCu4 non-equiatomic high entropy alloy. J. Mater. Sci. Technol. 2021, 60, 35. [Google Scholar] [CrossRef]
- Ding, Q.; Fu, X.; Chen, D.; Bei, H.; Gludovatz, B.; Li, J.; Zhang, Z.; George, E.; Yu, Q.; Zhu, T.; et al. Real-time nanoscale observation of deformation mechanisms in CrCoNi-based medium to high-entropy alloys at cryogenic temperatures. Mater. Today 2019, 25, 21. [Google Scholar] [CrossRef]
- Li, Q.; Sheng, H.; Ma, E. Strengthening in multi-principal element alloys with local-chemical-order roughened dislocation pathways. Nat. Commun. 2019, 10, 3563. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, Y.; Lou, H.; Zeng, Z.; Prakapenka, V.; Greenberg, E.; Ren, Y.; Yan, J.; Okasinski, J.; Liu, X.; et al. Polymorphism in a high-entropy alloy. Nat. Commun. 2017, 8, 15687. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Z.; Zhu, C.; Yang, W.; Meyers, M. Amorphization in extreme deformation of the CrMnFeCoNi high-entropy alloy. Sci. Adv. 2021, 7, 3108. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, X.; Wu, Y.; Wang, H.; Jiang, S.; Wang, S.; Hui, X.; Wu, Y.; Gault, B.; Kontis, P.; et al. Enhanced strength and ductility in a high-entropy alloy via ordered oxygen complexes. Nature 2018, 563, 546. [Google Scholar] [CrossRef]
- Zheng, Y.; Jia, N.; Qian, F.; Wang, J.; Xue, Y.; Jin, K. Thermal stability of (CoCrFeNi)94Ti2Al4 alloy containing coherent nanoprecipitates at intermediate temperatures. Materialia 2020, 12, 100775. [Google Scholar] [CrossRef]
- Jia, N.; Li, Y.; Huang, H.; Chen, S.; Li, D.; Dou, Y.; He, X.; Yang, W.; Xue, Y.; Jin, K. Helium bubble formation in refractory single-phase concentrated solid solution alloys under MeV He ion irradiation. J. Nucl. Mater. 2021, 550, 152937. [Google Scholar] [CrossRef]
- Gao, K.; Zhang, Y.; Yi, J.; Dong, F.; Chen, P. Overview of surface modification techniques for titanium alloys in modern material science: A comprehensive analysis. Coatings 2024, 14, 148. [Google Scholar] [CrossRef]
- Cheng, K.H.; Lai, C.H.; Lin, S.J.; Yeh, J.W. Structural and mechanical properties of multi-element (AlCrMoTaTiZr) Nx coatings by reactive magnetron sputtering. Thin Solid Film. 2011, 519, 3185–3190. [Google Scholar] [CrossRef]
- Lai, C.H.; Cheng, K.H.; Lin, S.J.; Yeh, J.W. Mechanical and tribological properties of multi-element (AlCrTaTiZr) N coatings. Surf. Coat. Technol. 2008, 202, 3732–3738. [Google Scholar] [CrossRef]
- Hsueh, H.T.; Shen, W.J.; Tsai, M.H.; Yeh, J.W. Effect of nitrogen content and substrate bias on mechanical and corrosion properties of high-entropy films (AlCrSiTiZr) 100− xNx. Surf. Coat. Technol. 2012, 206, 4106–4112. [Google Scholar] [CrossRef]
- Braic, V.; Vladescu, A.; Balaceanu, M.; Luculescu, C.R.; Braic, M. Nanostructured multi-element (TiZrNbHfTa) N and (TiZrNbHfTa) C hard coatings. Surf. Coat. Technol. 2012, 211, 117–121. [Google Scholar] [CrossRef]
- Sanyal, S.; Park, S.; Chelliah, R.; Yeon, S.-J.; Barathikannan, K.; Vijayalakshmi, S.; Jeong, Y.-J.; Rubab, M.; Oh, D.H. Emerging Trends in Smart Self-Healing Coatings: A Focus on Micro/Nanocontainer Technologies for Enhanced Corrosion Protection. Coatings 2024, 14, 324. [Google Scholar] [CrossRef]
- Barrino, F. Hybrid Organic–Inorganic Materials Prepared by Sol–Gel and Sol–Gel-Coating Method for Biomedical Use: Study and Synthetic Review of Synthesis and Properties. Coatings 2024, 14, 425. [Google Scholar] [CrossRef]
- Ujah, C.O.; Kunar, S.; Olubambi, P. Composite High-Entropy Alloy Coatings. In High-Entropy Alloy Coatings: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2025; pp. 223–250. [Google Scholar] [CrossRef]
- Prasad, C.; Kumar, R.S.; Kumaraswamy, G.N.; Kumar, M.P.; Yogesha, K.B.; Kumar, H.A.; Prasad, C.D.; Ahobal, N.; Acharya, S. Impact of Waveform Shape on the Properties of Ni Coatings in Pulsed Electro-Deposition for Durability of Industrial Surfaces: A Comparative Study of Rectangular and Triangular Pulses. Results Surf. Interfaces 2025, 21, 100680. [Google Scholar] [CrossRef]
- Meghwal, A.; Singh, S.; Sridar, S.; Xiong, W.; Hall, C.; Munroe, P.; Ang, A.S.M. Development of composite high entropy-medium entropy alloy coating. Scr. Mater. 2023, 222, 115044. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.-T.; Zhao, S.-J.; Wu, Z.-G. High-entropy carbide ceramics: A perspective review. Tungsten 2021, 3, 131–142. [Google Scholar] [CrossRef]
- Ye, B.; Wen, T.; Liu, D.; Chu, Y. Oxidation behavior of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high-entropy ceramics at 1073–1473 K in air. Corros Sci. 2020, 153, 327. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Wu, Z.; Xu, B.; Zhao, S.; Zhang, W.; Lin, N. Phase, microstructure and related mechanical properties of a series of (NbTaZr)C-Based high entropy ceramics. Ceram. Int. 2021, 47, 14341. [Google Scholar] [CrossRef]
- Ye, B.; Wen, T.; Huang, K.; Wang, C.; Chu, Y. First-principles study, fabrication, and characterization of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high-entropy ceramic. J. Am. Ceram. Soc. 2019, 102, 4344. [Google Scholar] [CrossRef]
- Yan, X.; Constantin, L.; Lu, Y.; Silvain, J.; Michael, N.; Bai, C. (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high-entropy ceramics with low thermal conductivity. J. Am. Ceram. Soc. 2018, 101, 4486. [Google Scholar] [CrossRef]
- Yan, X.; Zou, Y.; Zhang, Y. Properties and processing technologies of high-entropy alloys. Mater. Futures 2022, 1, 022002. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, H.; Dai, F.; Liu, J.; Zhou, Y. Porous high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)B2: A novel strategy towards making ultrahigh temperature ceramics thermal insulating. J. Mater. Sci. Technol. 2019, 35, 2404. [Google Scholar] [CrossRef]
- Feng, L.; Chen, W.; Fahrenholtz, W.; YLu Michael, N.; Yan, C.; Bai, C. Strength of single-phase high-entropy carbide ceramics up to 2300 °C. J Am Ceram Soc. 2021, 104, 419. [Google Scholar] [CrossRef]
- Wen, T.; Ye, B.; Nguyen, M.; Ma, M.; Chu, Y. Thermophysical and mechanical properties of novel high-entropy metal nitride-carbides. J. Am. Ceram. Soc. 2020, 103, 1. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, A.; Jia, J.; Meng, J.; Su, B. Phase evolution and properties of (VNbTaMoW)C high entropy carbide prepared by reaction synthesis. J. Eur. Ceram. Soc. 2020, 40, 2746. [Google Scholar] [CrossRef]
- Wang, K.; Chen, L.; Xu, C.; Zhang, W.; Liu, Z.; Wang, Y.; Ouyang, J.; Zhang, X.; Fu, Y.; Zhou, Y. Microstructure and mechanical properties of (TiZrNbTaMo)C high-entropy ceramic. J. Mater. Sci. Technol. 2020, 39, 99. [Google Scholar] [CrossRef]
- Balko, J.; Csanádi, T.; Sedlák, R.; Vojtko, M.; KovalĿíková, A.; Koval, K.; Wyzga, P.; Naughton-Duszová, A. Nanoindentation and tribology of VC, NbC and ZrC refractory carbides. J. Eur. Ceram. Soc. 2017, 37, 4371. [Google Scholar] [CrossRef]
- Lu, K.; Liu, J.; Wei, X.; Bao, W.; Wu, Y.; Li, F.; Xu, F.; Zhang, G. Microstructures and mechanical properties of high-entropy (Ti0.2Zr00.2Hf0.2Nb0.2Ta0.2)C ceramics with the addition of SiC secondary phase. J. Eur. Ceram. Soc. 2020, 40, 1839. [Google Scholar] [CrossRef]
- Castle, E.; Csanádi, T.; Grasso, S.; Dusza, J.; Reece, M. Processing and properties of high-entropy ultra-high temperature carbides. Sci. Rep. 2018, 8, 8609. [Google Scholar] [CrossRef]
- Sarker, P.; Harrington, T.; Toher, C.; Oses, C.; Samiee, M.; Maria, J.; Brenner, D.; Vecchio, K.; Curtarolo, S. High-entropy high-hardness metal carbides discovered by entropy descriptors. Nat. Commun. 2018, 9, 4980. [Google Scholar] [CrossRef]
- Kaufmann, K.; Maryanovsky, D.; Mellor, W.; Zhu, C.; Rosengarten, A.; Harrington, T.; Oses, C.; Toher, C.; Curtarolo, S.; Vecchio, K. Discovery of high-entropy ceramics via machine learning. NPJ Comput. Mater. 2020, 42, 1. [Google Scholar] [CrossRef]
- Pierson, H. Handbook of Refractory Carbides and Nitrides: Properties, Characteristics, Processing, and Applications; Noyes Publications: Westwood, NJ, USA, 1996. [Google Scholar]
- Gild, J.; Kaufmann, K.; Vecchio, K.; Luo, J. Reactive flash spark plasma sintering of high-entropy ultrahigh temperature ceramics. Scr. Mater. 2019, 170, 106. [Google Scholar] [CrossRef]
- Ye, B.; Wen, T.; Chu, Y. High-temperature oxidation behavior of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high entropy ceramics in air. J. Am. Ceram. Soc. 2019, 103, 1. [Google Scholar] [CrossRef]
- Dusza, J.; Svec, P.; Girman, V.; Sedlák, R.; Castle, E.; Csanádi, T.; Kovalčíková, A.; Reece, M. Microstructure of (Hf-Ta-Zr-Nb)C high entropy carbide at micro and nano/atomic level. J. Eur. Ceram. Soc. 2018, 38, 4303. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Zhang, B.; Skurikhina, O.; Balaz, P.; Araullo-Peters, V.; Reece, M. The role of multi-elements and interplayer on the oxidation behaviour of (Hf-Ta-Zr-Nb)C high entropy ceramics. Corros. Sci. 2020, 176, 109019. [Google Scholar] [CrossRef]
- Han, X.; Girman, V.; Sedlak, R.; Dusza, J.; Castle, E.; Wang, Y.; Reece, M.; Zhang, C. Improved creep resistance of high entropy transition metal carbides. J. Eur. Ceram. Soc. 2019, 40, 2709. [Google Scholar] [CrossRef]
- Csanádi, T.; Castle, E.; Reece, M.; Dusza, J. Strength enhancement and slip behaviour of high-entropy carbide grains during microcompression. Sci. Rep. 2019, 9, 10200. [Google Scholar] [CrossRef] [PubMed]
- Demirskyi, D.; Borodianska, H.; Suzuki, T.; Sakkaa, Y.; Yoshimic, K.; Vasylkiv, O. High-temperature flexural strength performance of ternary high-entropy carbide consolidated via spark plasma sintering of TaC, ZrC, and NbC. Scr. Mater. 2019, 164, 12. [Google Scholar] [CrossRef]
- Wang, F.; Yan, X.; Wang, T.; Wu, Y.; Shao, L.; Nastasi, M.; Lu, Y.; Cui, B. Irradiation damage in (Zr0.25Ta0.25Nb0.25Ti0.25)C high-entropy carbide ceramics. Acta Mater. 2020, 195, 739. [Google Scholar] [CrossRef]
- Wei, X.; Liu, J.; Li, F.; Qin, Y.; Liang, Y.; Zhang, G. High entropy carbide ceramics from different starting materials. J. Eur. Ceram. Soc. 2019, 39, 2989. [Google Scholar] [CrossRef]
- Wei, X.; Qin, Y.; Liu, J.; Li, F.; Liang, Y.; Zhang, G. Gradient microstructure development and grain growth inhibition in high-entropy carbide ceramics prepared by reactive spark plasma sintering. J. Eur. Ceram. Soc. 2020, 40, 935. [Google Scholar] [CrossRef]
- Sedegov, A.; Vorotilo, S.; Tsybulin, V.; Kuskov, K.; Moscovskikh, D. Synthesis and study of high-entropy ceramics based on the carbides of refractory metals. IOP Conf. Ser. Mater. Sci. Eng. 2019, 558, 012043. [Google Scholar] [CrossRef]
- Feng, L.; Fahrenholtz, W.; Hilmas, G. Low-temperature sintering of single-phase, high-entropy carbide ceramics. J. Am. Ceram. Soc. 2019, 102, 7217. [Google Scholar] [CrossRef]
- Feng, L.; Fahrenholtz, W.; Hilmas, G.; Zhou, Y. Synthesis of single-phase high-entropy carbide powders. Scr. Mater. 2019, 162, 90. [Google Scholar] [CrossRef]
- Tsai, K.; Tsai, M.; Yeh, J. Sluggish diffusion in Co–Cr–Fe–Mn–Ni high-entropy alloys. Acta Mater. 2013, 61, 4887. [Google Scholar] [CrossRef]
- Jin, K.; Zhang, C.; Zhang, F.; Bei, H. Influence of compositional complexity on interdiffusion in Ni-containing concentrated solid-solution alloys. Mater. Res. Lett. 2018, 6, 293. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, Y.; Bei, H. Thermal activation mechanisms and Labusch-type strengthening analysis for a family of high entropy and equiatomic solid-solution alloys. Acta Mater. 2016, 120, 108. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, S.; Ding, J.; Chong, Y.; Jia, T.; Ophus, C.; Asta, M.; Ritchie, R.; Minor, A. Short-range order and its impact on the CrCoNi medium-entropy alloy. Nature 2020, 581, 283. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Katiyar, J.K.; Bhaumik, S.; Roy, S. Influence of alumina/MWCNT hybrid nanoparticle additives on tribological properties of lubricants in turning operations. Friction 2019, 7, 153–168. [Google Scholar] [CrossRef]
- Tanshen, M.R.; Lee, S.; Kim, J.; Kang, D.; Noh, J.; Chung, H.; Jeong, H.; Huh, S. Pressure distribution inside oscillating heat pipe charged with aqueous Al2O3 nanoparticles, MWCNTs and their hybrid. J. Cent. South Univ. 2014, 21, 2341–2348. [Google Scholar] [CrossRef]
- Nine, M.J.; Batmunkh, M.; Kim, J.H.; Chung, H.S.; Jeong, H.M. Investigation of Al2O3-MWCNTs hybrid dispersion in water and their thermal characterization. J. Nanosci. Nanotechnol. 2012, 12, 4553–4559. [Google Scholar] [CrossRef]
- Ahammed, N.; Asirvatham, L.G.; Wongwises, S. Entropy generation analysis of graphene-alumina hybrid nanofluid in multiport mini channel heat exchanger coupled with thermoelectric. Int. J. Heat. Mass. Transf. 2016, 103, 1084–1097. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Jia, D.; Li, B.; Wang, Y.; Yang, M.; Hou, Y.; Zhang, X. Experimental study on the effect of nanoparticle concentration on the lubricating property of nanofluids for MQL grinding of Ni-based alloy. J. Mater. Process. Technol. 2016, 232, 100–115. [Google Scholar] [CrossRef]
- Abbasi, S.M.; Rashidi, A.; Nemati, A.; Arzani, K. The effect of functionalization method on the stability and the thermal conductivity of nanofluid hybrids of carbon nanotubes/gamma alumina. Ceram. Int. 2013, 39, 3885–3891. [Google Scholar] [CrossRef]
- Kanthavel, K.; Sumesh, K.; Saravanakumar, P. Study of tribological properties on Al/Al2O3/MoS2 hybrid composite processed by powder metallurgy. Alex. Eng. J. 2016, 55, 13–17. [Google Scholar] [CrossRef]
- Khandekar, S.; Sankar, M.R.; Agnihotri, V.; Ramkumar, J. Nano-cutting fluid for enhancement of metal cutting performance. Mater. Manuf. Process. 2012, 27, 963–967. [Google Scholar] [CrossRef]
- Wasan, D.; Nikolov, A.; Kondiparty, K. The wetting and spreading of nanofluids on solids: Role of the structural disjoining pressure. Curr. Opin. Colloid Interface Sci. 2011, 16, 344–349. [Google Scholar] [CrossRef]
- Li, B.; Bai, J.; He, J.; Ding, C.; Dai, X.; Ci, W.; Zhu, T.; Liao, R.; Yuan, Y. A Review on Superhydrophobic Surface with Anti-Icing Properties in Overhead Transmission Lines. Coatings 2023, 13, 301. [Google Scholar] [CrossRef]
- Fang, Z.; Ma, B.; Liang, E.; Jia, Y.; Guan, S. Interaction Regularity of Biomolecules on Mg and Mg-Based Alloy Surfaces: A First-Principles Study. Coatings 2024, 14, 25. [Google Scholar] [CrossRef]
- Tung, H.M.; Huang, J.H.; Tsai, D.G.; Ai, C.F.; Yu, G.P. Hardness and residual stress in nanocrystalline ZrN films: Effect of bias voltage and heat treatment. Mater. Sci. Eng. A 2009, 500, 104–108. [Google Scholar] [CrossRef]
- Patsalas, P.; Charitidis, C.; Logothetidis, S. The effect of substrate temperature and biasing on the mechanical properties and structure of sputtered titanium nitride thin films. Surf. Coat. Technol. 2000, 125, 335–340. [Google Scholar] [CrossRef]
- Lin, Y.C.; Hsu, S.Y.; Song, R.W.; Lo, W.L.; Lai, Y.T.; Tsai, S.Y.; Duh, J.G. Improving the hardness of high entropy nitride (Cr0. 35Al0. 25Nb0. 12Si0. 08V0. 20) N coatings via tuning substrate temperature and bias for anti-wear applications. Surf. Coat. Technol. 2020, 403, 126417. [Google Scholar] [CrossRef]
- Pogrebnjak, I.; Yakushchenko, G.; Abadias, P.; Chartier, O.; Bondar, V.; Beresnev, Y.; Takeda, K.; Oyoshi, A.; Andreyev, B. Mukushev. J. Superhard Mater. 2013, 35, 356–368. [Google Scholar] [CrossRef]
- Yang, C.; Chen, P.; Wu, W.; Sheng, L.; Zheng, Y.; Chu, P.K. A Review of Corrosion-Resistant PEO Coating on Mg Alloy. Coatings 2024, 14, 451. [Google Scholar] [CrossRef]
- Wood, R.J.K.; Lu, P. Coatings and Surface Modification of Alloys for Tribo-Corrosion Applications. Coatings 2024, 14, 99. [Google Scholar] [CrossRef]
- Milojević, S.; Savić, S.; Mitrović, S.; Marić, D.; Krstić, B.; Stojanović, B.; Popović, V. Solving the problem of friction and wear in auxiliary devices of internal combustion engines on the example of reciprocating air compressor for vehicles. Teh. Vjesn. 2023, 30, 122–130. [Google Scholar]
- Ahlgren, M.; Blomqvist, H. Influence of bias variation on residual stress and texture in TiAlN PVD coatings. Surf. Coat. Technol. 2005, 200, 157–160. [Google Scholar] [CrossRef]
- Lomello, F.; Sanchette, F.; Schuster, F.; Tabarant, M.; Billard, A. Influence of bias voltage on properties of AlCrN coatings prepared by cathodic arc deposition. Surf. Coat. Technol. 2013, 224, 77–81. [Google Scholar] [CrossRef]
- Huang, J.H.; Yu, K.J.; Sit, P.; Yu, G.P. Heat treatment of nanocrystalline TiN films deposited by unbalanced magnetron sputtering. Surf. Coat. Technol. 2006, 200, 4291–4299. [Google Scholar] [CrossRef]
- Sharma, A.K.; Tiwari, A.K.; Dixit, A.R. Improved machining performance with nanoparticle enriched cutting fluids under minimum quantity lubrication (MQL) technique: A review. Mater. Today Proc. 2015, 2, 3545–3551. [Google Scholar] [CrossRef]
- Dai, W.; Kheireddin, B.; Gao, H.; Liang, H. Roles of nanoparticles in oil lubrication. Tribol. Int. 2016, 102, 88–98. [Google Scholar] [CrossRef]
- Bolbasov, E.N.; Maryin, P.V.; Stankevich, K.S.; Kozelskaya, A.I.; Shesterikov, E.V.; Khodyrevskaya, Y.I.; Nasonova, M.; Shishkova, D.; Kudryavtseva, Y.A.; Anissimov, Y.; et al. Surface modification of electrospun poly-(l-lactic) acid scaffolds by reactive magnetron sputtering. Colloids Surf. B Biointerfaces 2018, 162, 43–51. [Google Scholar] [CrossRef]
- Stoney, G.G. Proceedings of the Royal Society of London. Ser. A Contain. Pap. Math. Phys. Character 1909, 82, 172–175. [Google Scholar]
- Escalona, M.; Bhuyan, H.; Ibacache, S.; Retamal, M.J.; Saikia, P.; Borgohain, C.; Valenzuela, J.; Veloso, F.; Favre, M.; Wyndham, E. Study of titanium nitride film growth by plasma enhanced pulsed laser deposition at different experimental conditions. Surf. Coat. Technol. 2021, 405, 126492. [Google Scholar] [CrossRef]
- Roy, M.; Mucha, N.R.; Ponnam, R.G.; Jaipan, P.; Scott-Emuakpor, O.; Yarmolenko, S.; Majumdar, A.K.; Kumar, D. Quantum interference effects in titanium nitride films at low temperatures. Thin Solid Film. 2019, 681, 1–5. [Google Scholar] [CrossRef]
- Elmkhah, H.; Zhang, T.F.; Abdollah-Zadeh, A.; Kim, K.H.; Mahboubi, F. Surface characteristics for the TiAlN coatings deposited by high power impulse magnetron sputtering technique at the different bias voltages. J. Alloys Compd. 2016, 688, 820–827. [Google Scholar] [CrossRef]
- Sekar, B.K.; Pradeep, G.V.K.; Silambarasan, R.; Dhairiyasamy, R. Microstructural and mechanical characterization of AA2124 aluminum alloy matrix composites reinforced with Si3 N4 particulates fabricated by powder metallurgy and high-energy ball milling. Matéria 2024, 29, e20240196. [Google Scholar] [CrossRef]
- Sobol, O.V.; Andreev, A.A.; Postelnyk, H.O.; Meylekhov, A.A.; Sagaidashnikov, Y.Y.; Stolbovoy, V.A.; Yevtushenko, N.S.; Syrenko, T.O.; Kraievska, Z.V.; Zvyagolskiy, A.V. Structural Engineering and Mechanical Properties of (Ti-V-Zr-Nb-Hf-Ta)N Coatings Obtained at Different Pressures. Nano-Electron. Phys. 2019, 11, 03013-1–03013-6. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, X.; Yan, X.; Lu, Y.; Nastasi, M.; Chen, Y.; Cui, B. The effect of submicron grain size on thermal stability and mechanical properties of high-entropy carbide ceramics. J. Am. Ceram. Soc. 2020, 103, 4463–4472. [Google Scholar] [CrossRef]
- Qi, Z.B.; Sun, P.; Zhu, F.P.; Wang, Z.C.; Peng, D.L.; Wu, C.H. The inverse Hall–Petch effect in nanocrystalline ZrN coatings. Surf. Coat. Technol. 2011, 205, 3692–3697. [Google Scholar] [CrossRef]
- Oettel, H.; Wiedemann, R. Residual stresses in PVD hard coatings. Surf. Coat. Technol. 1995, 76, 265–273. [Google Scholar] [CrossRef]
- Wang, X.; Yao, B.; Li, Y.; Xu, Y.; Wu, Y.; Zhu, Q.; Zhao, S.; Li, M.; Zheng, R.; Yan, K.; et al. Microstructure and mechanical properties of Hf-Nb-Ta-Ti-Zr refractory high-entropy alloys fabricated by laser directed energy deposition. J. Mater. Res. Technol. 2025, 36, 8136–8145. [Google Scholar] [CrossRef]
- Huang, P.K.; Yeh, J.W. Effects of substrate temperature and post-annealing on microstructure and properties of (AlCrNbSiTiV) N coatings. Thin Solid Film. 2009, 518, 180–184. [Google Scholar] [CrossRef]
- Durusoy, H.Z.; Duyar, Ö.; Aydınlı, A.; Ay, F. Influence of substrate temperature and bias voltage on the optical transmittance of TiN films. Vacuum 2003, 70, 21–28. [Google Scholar] [CrossRef]
- Tang, D.; Chen, H.; Xiao, W.; Deng, H.; Zou, S.; Ren, Y.; Lei, M.; Zhou, X. Substrate bias effects on mechanical properties and high temperature oxidation performance of sputtered TiN-coated Zr-4. J. Nucl. Mater. 2019, 524, 330–339. [Google Scholar] [CrossRef]
- Akhter, R.; Bendavid, A.; Munroe, P. The influence of substrate bias on the surface morphology, microstructure and mechanical behaviour of TiNiN coatings. Appl. Surf. Sci. 2022, 590, 153107. [Google Scholar] [CrossRef]
- Mirzaei, S.; Alishahi, M.; Souček, P.; Buršíková, V.; Zábranský, L.; Gröner, L.; Burmeister, F.; Blug, B.; Daum, P.; Mikšová, R.; et al. Effect of substrate bias voltage on the composition, microstructure and mechanical properties of WBC coatings. Appl. Surf. Sci. 2020, 528, 146966. [Google Scholar] [CrossRef]
- Kumar, M.; Mitra, R. Effect of substrate temperature and annealing on structure, stress and properties of reactively co-sputtered Ni-TiN nanocomposite thin films. Thin Solid Film. 2017, 624, 70–82. [Google Scholar] [CrossRef]
- Hibbs, M.K.; Johansson, B.O.; Sundgren, J.E.; Helmersson, U. Effects of substrate temperature and substrate material on the structure of reactively sputtered TiN films. Thin Solid Film. 1984, 122, 115–129. [Google Scholar] [CrossRef]
- Bajpai, S.; Kundu, R.; Balani, K. Effect of B4C reinforcement on microstructure, residual stress, toughening and scratch resistance of (Hf, Zr) B2 ceramics. Mater. Sci. Eng. A 2020, 796, 140022. [Google Scholar] [CrossRef]
- Cui, Y.; Guo, M.; Wang, C.; Tang, Z.; Cheng, L. Evolution of the residual stress in porous ceramic abradable coatings under thermal exposure. Surf. Coat. Technol. 2020, 394, 125915. [Google Scholar] [CrossRef]
- Chen, W.; Hu, T.; Wang, C.; Xiao, H.; Meng, X. The effect of microstructure on corrosion behavior of a novel AlCrTiSiN ceramic coating. Ceram. Int. 2020, 46, 12584–12592. [Google Scholar] [CrossRef]
- Lesyk, D.A.; Martinez, S.; Mordyuk, B.N.; Dzhemelinskyi, V.V.; Lamikiz, A.; Prokopenko, G.I. Post-processing of the Inconel 718 alloy parts fabricated by selective laser melting: Effects of mechanical surface treatments on surface topography, porosity, hardness and residual stress. Surf. Coat. Technol. 2020, 381, 125136. [Google Scholar] [CrossRef]
- Ye, K.; Wang, Z. Residual stress effects on toughening of ultrafine-grained B4C-SiC ceramics. Mater. Today Commun. 2023, 36, 106649. [Google Scholar] [CrossRef]
- Abdelkawi, A.; Slim, A.; Zinoune, Z.; Pathak, Y. Surface Modification of Metallic Nanoparticles for Targeting Drugs. Coatings 2023, 13, 1660. [Google Scholar] [CrossRef]
- Hao, X.N.; Liu, X. Molecular dynamics study on microscale residual stress of graphene/aluminum nanocomposites by selective laser sintering. Rare Met. 2022, 41, 3677–3683. [Google Scholar] [CrossRef]
- Yan, M.; Hu, C.; Li, J.; Pang, S.; Zhao, R.; Luo, R.; Sun, B.; Liang, B.; Tang, S. Construction of a ceramic coating with low residual stress on C/CA composites for thermal protection at ultra-high temperatures. Compos. Part B Eng. 2023, 266, 110970. [Google Scholar] [CrossRef]
- Laera, A.M.; Massaro, M.; Dimaio, D.; Vencl, A.; Rizzo, A. Residual Stress and Tribological Performance of ZrN Coatings Produced by Reactive Bipolar Pulsed Magnetron Sputtering. Materials 2021, 14, 6462. [Google Scholar] [CrossRef]
- Li, M.; Mo, R.; Xu, Z.; Zhou, J.; Zhang, C.; Cui, X.; Riedel, R. Residual stress and interface debonding behavior in Si3N4w reinforced SiCN composites prepared by the PIP process: A case study. J. Eur. Ceram. Soc. 2023, 44, 1505–1510. [Google Scholar] [CrossRef]
- Grigoriev, O.N.; Stepanenko, A.V.; Vinokurov, V.B.; Neshpor, I.P.; Mosina, T.V.; Silvestroni, L. ZrB2–SiC ceramics: Residual stresses and mechanical properties. J. Eur. Ceram. Soc. 2021, 41, 4720–4727. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Mandal, G.; Haldar, B.; Samanta, R.; Ma, G.; Kunar, S.; Ataya, S.; Nath, M.; Ghosh, S.K. Novel Features, Applications, and Recent Developments of High-Entropy Ceramic Coatings: A State-of-the-Art Review. Coatings 2026, 16, 48. https://doi.org/10.3390/coatings16010048
Mandal G, Haldar B, Samanta R, Ma G, Kunar S, Ataya S, Nath M, Ghosh SK. Novel Features, Applications, and Recent Developments of High-Entropy Ceramic Coatings: A State-of-the-Art Review. Coatings. 2026; 16(1):48. https://doi.org/10.3390/coatings16010048
Chicago/Turabian StyleMandal, Gurudas, Barun Haldar, Rahul Samanta, Guojun Ma, Sandip Kunar, Sabbah Ataya, Mithun Nath, and Swarup Kumar Ghosh. 2026. "Novel Features, Applications, and Recent Developments of High-Entropy Ceramic Coatings: A State-of-the-Art Review" Coatings 16, no. 1: 48. https://doi.org/10.3390/coatings16010048
APA StyleMandal, G., Haldar, B., Samanta, R., Ma, G., Kunar, S., Ataya, S., Nath, M., & Ghosh, S. K. (2026). Novel Features, Applications, and Recent Developments of High-Entropy Ceramic Coatings: A State-of-the-Art Review. Coatings, 16(1), 48. https://doi.org/10.3390/coatings16010048









