1. Introduction
Due to the unevenness of solar and wind power generation in terms of time and space, efficient and convenient energy storage methods have attracted extensive attention from researchers. Energy storage methods include electrochemical energy storage, gravitational energy storage, molten salt energy storage, etc. Among them, electrochemical energy storage, especially lithium-ion battery (LIB) energy storage, due to its flexibility, convenience, and independence from terrain factors, can meet the energy storage needs of households and those of the power grid, occupying a vast market in the energy storage field [
1,
2,
3]. Lithium iron phosphate (LFP) batteries are considered as one of the most suitable cathode materials for the energy storage field due to their high energy density, long cycle life, non-toxicity, and low price. However, due to the limitations of its own working mechanism, capacity decay inevitably occurs during the cycling process [
4,
5]. Therefore, exploring the failure mechanism of LFP and conducting targeted detection of its failure mechanism are necessary means to improve the safety and stability of LFP in the energy storage field.
The LFP electrode presents a three-level structure of nano-lattice–carbon coating–microporous aggregates [
6]. The inner layer of phosphorus iron lithium exhibits an olivine-type crystal structure and belongs to the orthorhombic crystal system, with the space group being
Pnma. Here, FeO
6 octahedra and PO
4 tetrahedra are connected through common vertices or common edges to form a stable three-dimensional framework, while Li
+ occupies the octahedral gaps (4a positions) and forms a one-dimensional transport channel along the b-axis. The outer layer is wrapped by 2–3 nm amorphous carbon coating layers, and the carbon network provides electronic pathways [
7]. The grain boundaries and surface defects jointly constitute the initial active interface. During the cycling process, the repeated insertion and extraction of Li
+ cause the LiFePO
4/FePO
4 two-phase interface to migrate back and forth within the one-dimensional channel, accompanied by minor changes in lattice parameters, a volume change of approximately 6.8%, and microcracks in the carbon layer due to volume contraction [
8]. The internal crystal boundaries gradually shift and the pores collapse, while the surface non-stoichiometric layer thickens and Fe
3+ dissolves, resulting in a local interruption of the electron/ion dual continuous network. Therefore, the failure manifestations of the LFP electrode include active lithium deficiency, microcracks in the carbon coating layer, grain boundary misalignment, etc. When the one-dimensional Li
+ path is blocked by lattice distortion or Fe deposition, or the carbon network infiltration threshold is exceeded, the internal resistance of the electrode increases sharply. Moreover, the active lithium is irreversibly depleted and the battery capacity drops sharply, which indicate that although the olivine has high intrinsic stability, it still inevitably leads to the degradation of cycle life [
9].
Efficient failure detection of LFP batteries can help researchers understand the state of health (SOH) of the batteries and prevent their failure due to environmental issues or unexpected situations. Safety is the most important evaluation indicator for batteries, especially in the field of large-scale grid energy storage [
10]. Therefore, the safety performance and stability performance of batteries are given more attention. Moreover, the system’s analysis of failed batteries is the key path to reveal the cycling degradation mechanism and provide feedback for preparation optimization. Through multi-scale characterization coupled with electrochemical assessment, the consequence of active lithium loss, active material degradation, and interface impedance growth to capacity decay can be quantified to some extent [
11]. In this regard, target doping, gradient design of particle size, and interface passivation coating can be implemented at the material synthesis stage for directional optimization, achieving a closed loop of diagnosis to promote structural optimization and significantly improving the electrochemical stability of next-generation energy storage batteries.
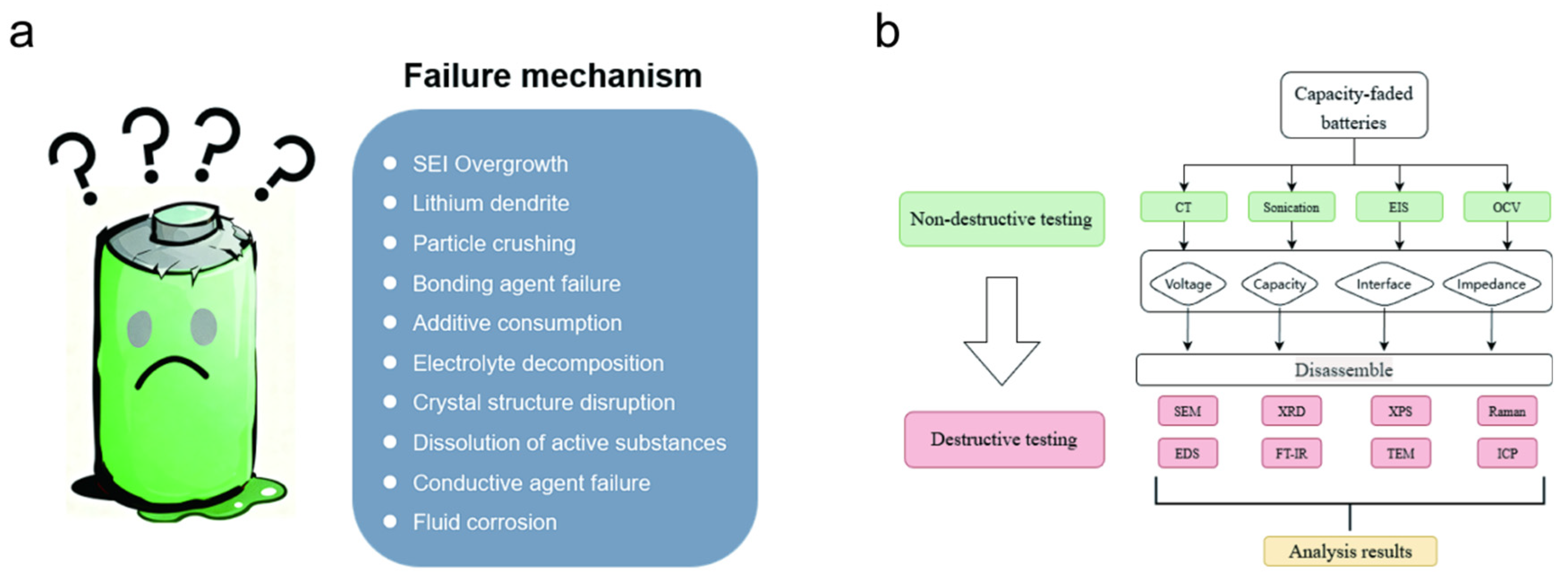
LFP batteries have become the mainstream technology route for large-scale energy storage stations and electric vehicles due to their high thermal stability, long cycle life, and abundant raw material resources. However, the LFP still exhibits non-linear degradation behaviors such as capacity decline and impedance increase under long-term deep charging and discharging, low temperature, or high rate operating conditions [
12]. To clarify the main factors causing degradation and implement targeted improvements in the development of the next generation of batteries, academics generally adopt the research paradigm of diagnosis of failure batteries, mechanism quantification, and feedback. Among these, the accuracy and dimension of the testing methods directly determine the completeness and reliability of the failure mechanism. Based on the degree of damage to the batteries, the testing methods can be divided into two major systems: non-destructive and destructive. They complement each other in terms of information depth, spatial resolution, and statistical ability, providing a methodological basis for the full life cycle analysis of LFP batteries [
13].
Non-destructive testing is most commonly carried out by using electrochemical impedance spectroscopy (EIS), which can obtain the evolution patterns of charge transfer impedance (Rct), solid-state diffusion impedance (Warburg), and ohmic impedance (Ro). This enables early failure warning without disassembling the battery. In recent years, the distribution time relaxation (DRT) algorithm based on multi-frequency EIS has further successfully identified the three-stage degradation sequence of anode lithium exfoliation, separator pore closure, and cathode CEI thickening in the LFP/graphite system [
14]. Additionally, micro computed tomography (μ-CT) can visualize the wrinkles, particle cracks, and lithium metal exfoliation morphology of the electrode sheet at sub-micron resolution, providing spatial evidence for EIS [
15]. Additionally, ultrasonic scanning and IR thermal imaging can rapidly screen for the distribution of the electrolyte and local hotspots, which are suitable for large-scale online monitoring in energy storage systems.
In contrast, non-destructive testing obtains information about interface components and microstructure through disassembly of batteries and chemical analysis, which can achieve verification at the mechanism level. The combination of focused ion beam-scanning electron microscopy (FIB-SEM) and transmission electron microscopy (TEM) with electron energy loss spectroscopy (EELS) can reveal the nucleation mechanism of the Fe
3(PO
4)
2·8H
2O inclusions on the surface of LFP particles at the nanoscale and quantify the thickness of the carbon coating layer [
16]. Additionally, X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (TOF-SIMS) further analyze the depth distribution of LiF, Li
2CO
3, and Fe–F bonds in CEI. For graphite anodes, low-temperature Raman mapping provides quantitative boundary conditions for graphite modification through the calculation of I
D/I
G ratio [
17].
In summary, the design of the failure analysis process is crucial for the validity, repeatability and economy of failure analysis. The capacity decay of LIB is often caused by multiple factors such as loss of active lithium, particle crack, and interface side reactions, which interact and affect each other. If the test process is improper, the dynamic information is easily masked, leading to incorrect identification of the dominant mechanism and waste of advanced characterization. Therefore, it is necessary to follow the progressive logic of preliminary screening in-depth validation. In this way, precise and advanced characterization is firstly achieved by combining electrochemical tests (capacity, EIS, cyclic voltammetry (CV)) with materials characterization (XRD, SEM, TEM, inductively coupled plasma (ICP), etc.). Then, a second-round validation tier deploys mechanism-matched in situ/ex situ tools (operando XRD, TEM, XPS, ToF-SIMS, etc.) to build a comprehensive evidence chain. In our work, a unified failure analysis system that combines scientific rigor, repeatability, and cost-effectiveness is achieved.
3. Failure Mechanism of Lithium Iron Phosphate
In our work, the batteries are discharged before disassembly. The final SOC of the battery and the cathode material is 0%. The sampling process was carried out strictly in accordance with statistical methods. The sampling method was random sampling while ensuring an adequate sample size at the same time and maintaining an inert environment during sampling to avoid introducing interfering factors during the sample preparation process. During the Rietveld process, the FullProf Suite software was used. In terms of parameter selection, the target material was Cu-Kα1 (λ = 1.5406 Å), with a 2θ range of 10–80° and a step size of 0.02°.
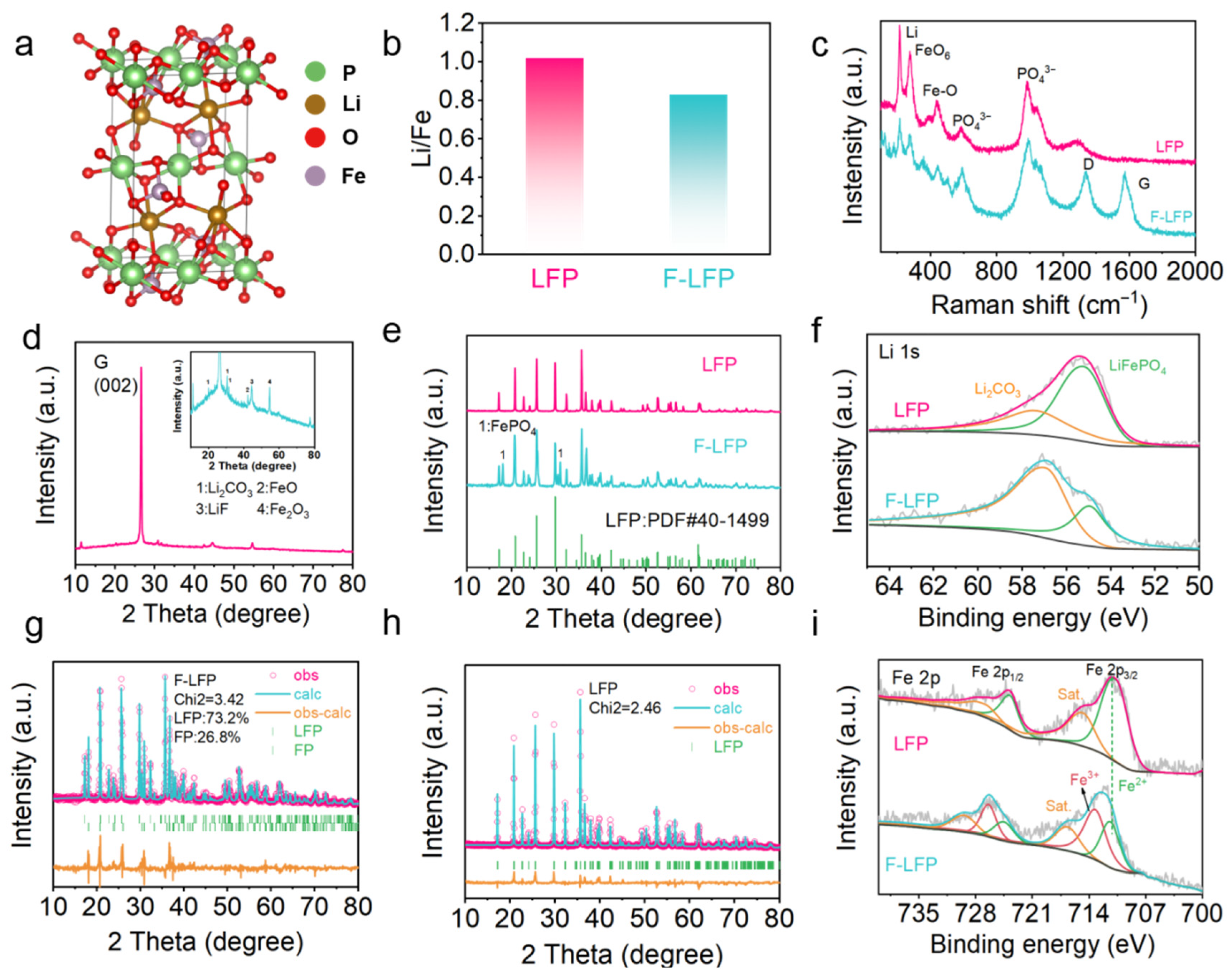
At the atomic/ion scale, lithium ions act as the medium for energy transmission in LIB, directly influencing the capacity of the batteries. LFP belongs to the olivine crystal structure, as shown in
Figure 2a. The proportion was quantitatively detected for iron and lithium in LFP before and after cycling using ICP to verify the active lithium content after cycling. The experimental results are shown in
Figure 2b. Before cycling, the Li/Fe ratio was 1.02, and after cycling in the failed batteries (F-LFP), it dropped to 0.83, confirming the reduction of active lithium. Similar characterization was also confirmed in the Raman spectrum. In
Figure 2c, the peak at 218 cm
−1 is related to the vibration of Li
+ in the lattice, and its peak intensity is related to the content of Li in the crystal cell. The peak value in the F-LFP decreases, indicating that the active lithium in the crystal cell has decreased. Additionally, the peaks at 272, 440, 590, and 980 cm
−1 represent the distorted vibration of FeO
6 octahedra, Fe-O bending vibration, v
4 bending vibration of PO
43−, and v
1 symmetric stretching vibration of PO
43−. These two peaks correspond to the ν
1 (A
1) symmetric stretching mode of PO
43− in LFP. The Fe
2+—O bond in LiFePO
4 is longer (~2.15 Å), and the P—O bond is weakened, resulting in a frequency of 943 cm
−1. In FP, the Fe
3+—O bond is shortened (~2.01 Å), and the P—O bond is strengthened, resulting in a frequency of 963 cm
−1. Additionally, in the F-LFP, a large number of impurity peaks were found, which may be due to the introduction of binders or electrolytes [
18].
To further explore the causes of reduced active lithium, XRD was used to characterize the structure of the material. In
Figure 2d, Li signals are found in the XRD spectrum of the anode graphite, which can be attributed to LiF and Li
2CO
3 phases. This indicates that some Li that was partially embedded in graphite cannot be returned to the cathode and is encapsulated between the graphite layers or on the surface, forming dead lithium. In
Figure 2e, the LFP before cycling was an olivine-type crystal structure, corresponding to the standard card pdf#40-1499. The diffraction peaks at 17.1, 20.7, 22.6, 25.5, 29.7, and 35.5 2-theta correspond to the (020), (011), (120), (111), (121), and (131) crystal planes of LFP. In the F-LFP, new diffraction peaks that do not belong to LFP appeared at 18.0, 23.7, 30.2, 30.7, and 37.5 2-theta, which can be attributed to the diffraction peaks of FePO
4 [
19].
Figure 2f shows the Li 1s spectra of LFP and F-LFP. It is found that the lithium in F-LFP exists not only in the form of LFP but also in some organic lithium or LiF, Li
2O, Li
2CO
3 forms. This is due to the irreversible polarization at the solid–liquid interface between LFP particles and the electrolyte during the first cycle, forming an SEI composed of various lithium compounds such as organic lithium and LiF, Li
2O, Li
2CO
3. This consumed some active lithium. Similar phenomena also occur at the SEI between the anode graphite particles and the electrolyte. This also causes the loss of active lithium.
Based on the above characterizations, the reduction of active lithium is due to the repeated shuttle of lithium ions between LFP and graphite. When lithium ions migrate to graphite, Li vacancies will form in LFP. Fe ions will diffuse into these vacancies under the effect of potential difference, and the Li ions returned from graphite will occupy the vacant Fe sites, forming Li-Fe inversion defects. Some of the migrated lithium in graphite is restricted in the graphite and cannot return to LFP, forming dead lithium. Some participate in the construction of the SEI. These reasons lead to the absence of active lithium in LFP, directly causing a decrease in battery capacity.
At the molecular scale, the stable crystal structure of LFP is the prerequisite for its stable energy storage. However, in the XRD spectra, an impurity phase of FePO
4 (FP) was observed, indicating that the failure of LFP is related to the formation of the FP impurity phase. To confirm the accurate content of FP, we calculated the content of the LFP/FP phases through Rietveld refinements of XRD. In the calculations shown in
Figure 2g,h, it was found that the content of FP in the F-LFP after cycling was 26.8%. Similar conclusions can also be confirmed by the Fe 2p and O 1s spectra.
Figure 2i shows the XPS spectra of Fe 2p before and after failure. To verify the change in the oxidation state of Fe in LFP, we conducted XPS testing on LFP before and after failure. In the Fe 2p spectrum, Fe 2p is split into Fe 2p
3/2 and Fe 2p
1/2. In the LFP spectrum, Fe exists in the Fe(II) form, and the corresponding peaks are 710.7 eV (Fe 2p
3/2) and 723.7 eV (Fe 2p
1/2). However, in the XPS spectrum of F-LFP, new peaks at 713.2 eV and 726.4 eV appeared, which can be attributed to Fe(III) 2p
3/2 and Fe(III) 2p
1/2. This confirms the presence of Fe(III) in F-LFP. This is because during cycling, when the charging voltage increases, Li
+ is released and migrates to the anode, Fe
2+ loses electrons and becomes Fe
3+, and the original LiFePO
4 framework shrinks in situ and gradually forms the same-structure FP impurity phase. During discharge, Li
+ flows back, Fe
3+ gains electrons and reverts to Fe
2+, and the LFP phase can be reversibly restored [
20]. However, inevitably, a small amount of Fe
3+ migrates to lithium vacancies, forming defective FePO
4 phases, resulting in some lithium iron phosphate not being able to reinsert lithium and causing capacity degradation.
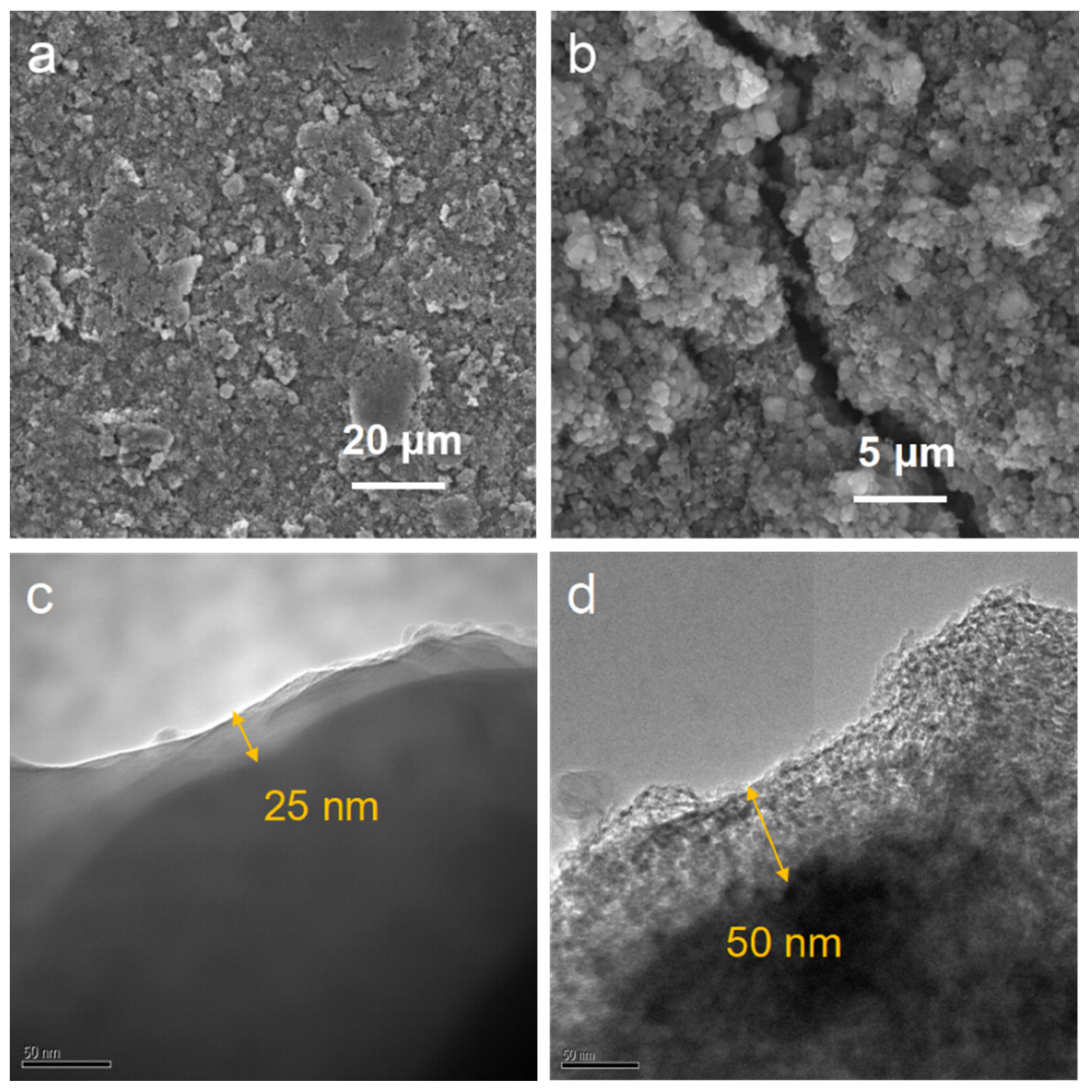
At the particle scale, the complete particle structure and conductive carbon layer enhance conductivity through a continuous electron network. In
Figure 3a, which is the SEM image of the LFP before failure, the surface of the LFP electrode sheet is smooth. This is because the fresh LFP is a complete and uniform spherical particle. The LFP electrode after failure is shown in
Figure 3b, and obvious cracks appear in the electrode. The sampling process was carried out strictly in accordance with statistical methods. The sampling method was random sampling while ensuring an adequate sample size at the same time and maintaining an inert environment during sampling to avoid introducing interfering factors during the sample preparation process. This is due to the lattice mismatch at the interface of the two phases within the particles, resulting in the accumulation of dislocations and the formation of cracks. The grain boundary hard phase and dislocation defects cause stress concentration, accelerating the initiation of microcracks.
Figure 3c,d show the TEM images of the SEI films of LFP before and after failure. The thickness of the SEI film before failure is approximately 25 nm, and it is uniform and dense. However, the SEI film of the F-LFP is significantly thicker, with a thickness of approximately 50 nm. This is mainly due to lattice stress and interface chemical corrosion. The SEI film undergoes shear fatigue due to the anisotropic expansion of the elastic modulus of the particles, and interface slip leads to local peeling. In addition, during the cycling process, the SEI undergoes electrochemical oxidation, and the binder decomposes to release corrosive substances, destroying the SEI structure and reducing the electron conduction. Anions can become co-embedded, further destroying the graphite structure of the carbon layer, ultimately interrupting the electron pathway.
In summary, the main reasons for the failure of the LFP cathode include the following four mechanisms: (1) Loss of active lithium during charging and discharging. (2) Formation of Fe3+ and FePO4 phases. (3) Inevitable particle fragmentation under long-term cycling. (4) Breakage of the SEI.
4. Design and Optimize Failure Diagnosis Process
4.1. General Designed Failure Diagnosis Process
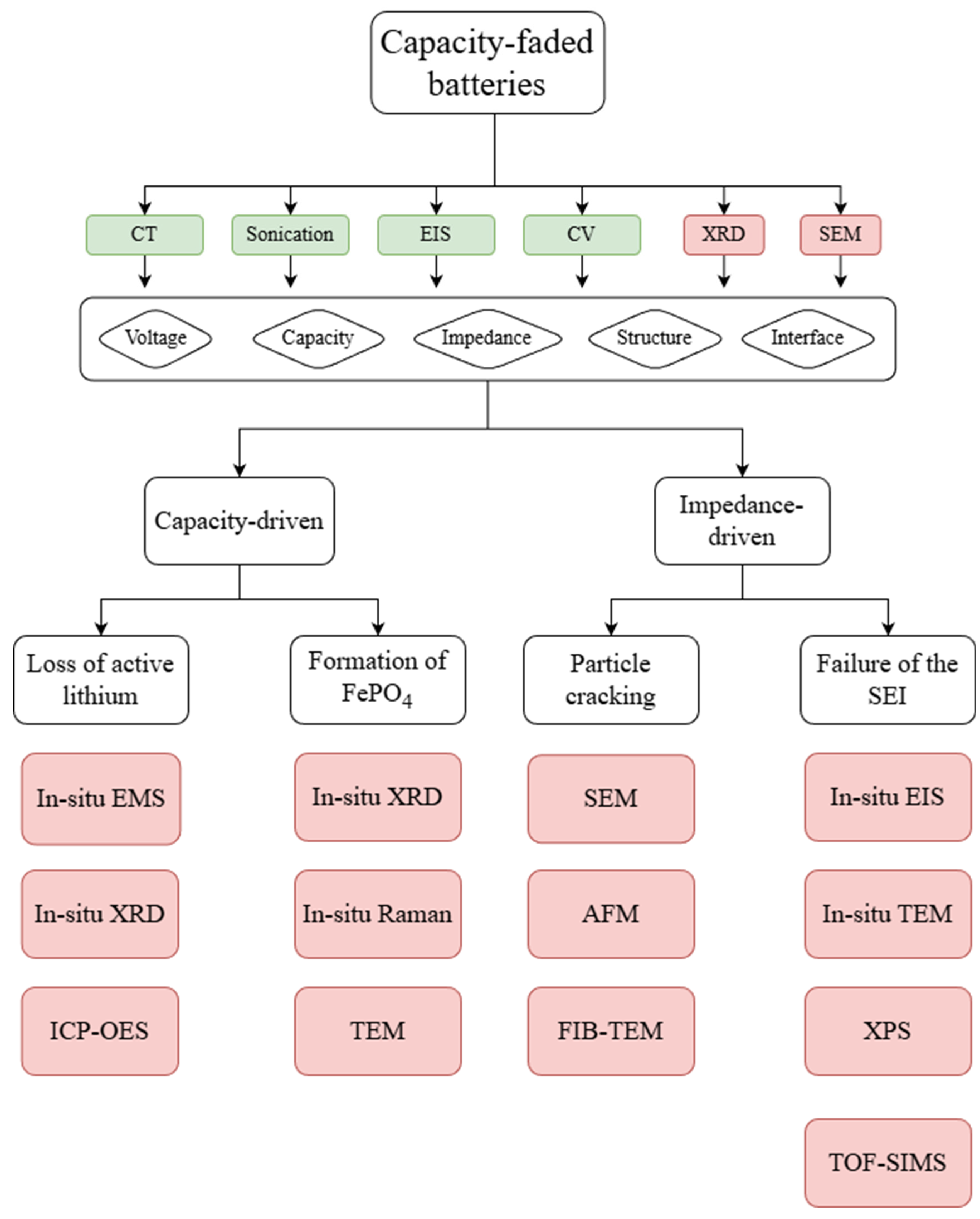
The above four mechanisms exhibit distinctly differentiated characteristics: Active lithium loss manifests primarily as a straightforward decrease in capacity without significant impedance changes, classifying it as a typical capacity-dominant mechanism. The formation of FP phases is accompanied by a reduction in the effective content of cathode active material, also demonstrating capacity-dominant characteristics, and it presents identifiable characteristic XRD signals within specific phase intervals (2θ = 18.0°, 23.7°). Additionally, SEI failure is primarily characterized by a sharp increase in charge transfer resistance (Rct), subsequently triggering a secondary capacity fade, thus categorizing it as an impedance-dominant mechanism. At last, particle cracking leads to the obstruction of lithium-ion diffusion pathways, exhibiting dual characteristics of microstructural damage and increased diffusion impedance. By selectively screening test items and scientifically planning the test sequence, efficient identification of degradation mechanisms can be achieved.
Therefore, a designed and optimized failure analysis diagnosis process is proposed, as shown in
Figure 4. This approach avoids resource waste associated with indiscriminate testing and provides targeted guidance for battery performance optimization. The preliminary screening phase centers on integrating results from capacity test, CV, EIS, and basic characterization (low-magnification SEM, XRD). This integration enables initial accurate identification of failure mechanisms through multi-dimensional signal correlation. A capacity-dominant failure can be characterized by a substantial capacity fade (≥20%) that coincides with a marked reduction in redox peak area in CV without a significant peak shift, and an absence of notable anomalies in EIS. Conversely, the observation of a sharp internal resistance increase leads to its classification as an impedance-dominant failure. For capacity-dominant cases, subsequent XRD analysis is employed for mechanistic differentiation: the detection of characteristic FP phase peaks (2θ = 18.0° and 23.7°) confirms FP phase formation, while the absence of these peaks identifies active lithium loss as the root cause. For impedance-dominant failures, a detailed analysis of EIS and SEM is conducted. The mechanism is identified as particle cracking if a significant rise in low-frequency diffusion impedance in EIS is coupled with observable particle cracks in SEM. Alternatively, a sharp increase in the mid-frequency Rct is diagnostically attributed to SEI failure. This streamlined methodology, centered on the core issue of capacity/impedance dominance and the recognition of key distinctive characterization signals, enables the efficient identification of a primary failure mechanism, thereby providing a precise target for subsequent in-depth validation. Conventional protocols require 7–8 days (168–192 h) due to full-scale testing (e.g., in situ characterization for all four mechanisms, redundant electrochemical tests like multi-rate CV). The optimized workflow shortens this to 1.5–2 days (36–48 h) by cutting non-essential items: preliminary screening locks the dominant mechanism (e.g., particle cracking), so only 1–2 targeted tests (e.g., in situ SEM and FIB-TEM) are conducted. The optimized workflow trims this to 5–6 core items, reducing test items by ~67%. This avoids the blind full-scale testing of traditional protocols, directly lowering equipment occupancy and consumable usage.
4.2. Specified Optimized Failure Diagnosis Process
The in-depth validation phase aims to precisely identify the essential characteristics of each failure mechanism, clarify their distinct analytical focuses, and strategically select appropriate in situ and ex situ advanced characterization techniques for accurate confirmation and quantification. Therefore, a precise target for a subsequent second-tier in-depth validation process is optimized as follows.
Firstly, active lithium loss is characterized by a dynamic consumption process coupled with a static reduction in total lithium inventory. The analytical focus lies in tracking the loss pathways and precisely quantifying the loss amount. Therefore, the following techniques can be employed: In situ electrochemical mass spectrometry (EMS) captures real-time CO2 and H2 release peaks during 1C cycling, directly linking side reactions to the dynamic consumption of active lithium. In situ XRD of the anode monitors the graphite (002) plane (2θ = 26° ± 0.5°), assessing whether the interlayer spacing recovers after delithiation to detect “dead lithium” formation. Ex situ ICP-OES quantifies the total loss ratio by measuring lithium concentration differences across the cathode, anode, and electrolyte. Together, these methods establish a comprehensive evidence chain from dynamic process to static quantification.
Secondly, FP phase formation is defined by the irreversible phase transition from LFP to FP. The analysis focuses on capturing this dynamic phase transformation and quantifying the generated FP. Accordingly, in situ XRD of the cathode scans the 20°–36° range to track the emergence and intensification of characteristic FP peaks during cycling. In situ Raman spectroscopy is expected to establish a quantitative correlation with capacity fade by monitoring the intensity ratio of the 943 cm−1 (LFP) and 963 cm−1 (FP) peaks. Ex situ high-resolution TEM would effectively confirm the phase identity by resolving the 0.35 nm lattice fringes characteristic of FP and statistically determining its distribution, thereby verifying the causality between phase transformation and capacity decay.
Then, SEI failure manifests as a sharp increase in interfacial impedance accompanied by structural and compositional degradation. The investigation targets pinpointing the failure timing, structural evolution, and compositional changes. The selected methodologies are as follows: In situ EIS is suggested to monitor real-time charge transfer resistance (Rct) evolution, identifying the critical cycle number of coating failure. In situ TEM can be used for direct visualization of the coating’s structural progression from intact to thickened and finally cracked/delaminated. In situ XPS is desired to analyze compositional changes in key components like LiF (685 eV) and Li2CO3 (289 eV), revealing the chemical basis of degradation. Ex situ TOF-SIMS maps the depth distribution of Li and F elements to assess coating thickness uniformity and locate failure points, providing a complete analysis of the interfacial degradation mechanism.
Lastly, particle cracking is identified based on microstructural damage (crack initiation and propagation) and consequent ionic diffusion blockage. The analysis focuses on tracking the dynamic crack evolution and assessing overall structural integrity. In situ AFM is suggested to correlate mechanical stress during cycling with particle fracture by measuring changes in surface roughness (Ra) and particle expansion. Ex situ FIB-TEM is also expected to examine cross-sectioned particles to observe the depth, density, and distribution of internal cracks, complementing surface observations and fully reconstructing the crack initiation and propagation pathway.
Through the battery level, electrode level, and material level, we conducted multi-level cross-scale research and discussion on the failure mechanism of failed batteries. The final version concludes that the failure causes of LFP mainly include four aspects: active-Li loss, FP formation, SEI degradation, and particle fracture. These four reasons contribute to battery failure due to different aspects, but they also influence each other. Among them, active-Li loss and FP formation mainly affect the capacity. SEI degradation and particle fracture mainly affect the cycling stability. Among them, the contribution of active-Li loss and FP formation to the battery capacity can be calculated. Specifically, by testing the lithium inventory of the full battery using ICP-OES, the total Li content and Fe content in LFP are obtained, and Li mol%/Fe mol% (N) is obtained. Based on the Rietveld refinement, the quality ratio of FP and LFP and the Li-Fe inversion situation (α) are given, and the residual active lithium content m is finally obtained.
According to the theoretical capacity calculation formula of LFP:
where C is the theoretical specific capacity; n is the number of electrons transferred per mole of material; F is the Faraday constant, 96,485 C/mol; M is the molar mass of LFP; and the number of electrons transferred is the active lithium. Therefore, the theoretical capacity (c) of the F-LFP battery is as follows:
SEI degradation and particle fracture mainly affect the cycle stability of the battery. The contribution of battery failure cannot be quantitatively calculated, but qualitative analysis and assessment can be conducted. Specifically, the SEI degradation and particle fracture of batteries with different SOH are detected. The proportion of SEI degradation is A, and its impact factor on cycle stability is x. The proportion of broken particles is B, and its impact factor on cycle stability is y. The contribution factor of battery cycle stability is η = Ax + By.
Finally, it is concluded that the comprehensive contribution of the four failure causes—active-Li loss, FP formation, SEI degradation, and particle fracture—to the battery capacity is c*η.
5. Conclusions
In summary, we have confirmed that the capacity fade of LFP batteries originates from the combined effect of four core mechanisms: active lithium loss, formation of LFP phase, SEI failure, and particle fracture by coupling multi-scale characterization with electrochemical testing. This approach has clarified the dominant mechanisms and differentiated characteristics of LFP battery capacity fade, breaking through the limitations of traditional detection processes. To address the drawbacks of traditional processes, which are disorderly, costly, and time-consuming, we have established a progressive analytical framework of preliminary screening to in-depth validation. In the preliminary screening stage, we integrate low-cost methods such as capacity-internal resistance retest, CV, EIS, low-magnification SEM, and simple XRD, based on the core classification of capacity-dominated/impedance-dominated, to quickly identify the suspected failure mechanisms. In the in-depth validation stage, we selectively employ in situ/ex situ advanced characterization techniques according to the essential characteristics of different mechanisms, forming a complete evidence chain of dynamic process capture–loss path tracking–quantitative result validation, thus avoiding resource waste caused by random testing. This optimized process achieves precise identification of failure mechanisms through the logic of core feature classification and key signal matching, balancing the scientific nature, reproducibility, and cost-effectiveness of the analysis.