1. Introduction
The high-pressure acid leaching (HPAL) hydrometallurgical process involves the operation of autoclaves, pipelines, valves, and other equipment under extremely aggressive conditions. HPAL autoclaves used to process nickel–calcium ores typically operate at temperatures around 260 °C, with process engineers aiming to increase these operating temperatures further. The process medium contains concentrated sulfuric acid (>95%), is subject to high pressure (up to 5500 kPa), and also contains a significant content of solids (>20 wt%) [
1]. These factors lead to the severe degradation of components due to corrosion, thermal stress, erosion, and abrasion, making the durability of materials crucial for cost-effective operation.
The growing demand for nickel and cobalt, driven by their use in energy storage systems such as lithium-ion batteries, has made laterite ores more attractive for industrial processing. Over the past two decades, metal-seated ball valves (MSBVs) have become the industry standard in hydrometallurgy due to their ability to provide a tight and reliable shut-off, ensuring operational safety and simplifying maintenance. A typical MSBV design features a floating ball in contact with a stationary seat, both made of titanium or duplex stainless steel, and protected by a ceramic coating. This coating primarily serves to improve the wear resistance and extend the service life, particularly during ball movement [
2].
Initially, air plasma-sprayed (APS) Cr
2O
3 coatings were widely used in gold processing POx systems. Later, SiO
2 and TiO
2 were added to improve the ductility and resistance to cracking. Velan’s Cr
2O
3-based coatings have proven effective in gold mining operations in Canada, the United States, and Papua New Guinea [
3].
However, HPAL presents a more aggressive environment than POx due to its higher temperatures, pressures, and chloride content. As a result, coatings optimised for POx tend to degrade rapidly under HPAL conditions [
4]. TiO
2, while offering excellent corrosion resistance, has an inferior wear resistance compared to that of Cr
2O
3, as field data from the Cawse and Murrin nickel mines in Australia confirm [
5]. Therefore, combined coatings that integrate the advantages of both materials are highly relevant for HPAL operations.
The balls used in HPAL plants are commonly made from titanium alloys with ceramic coatings to protect against abrasive and chemical wear [
4]. Cr
2O
3 ensures superior wear protection, whereas TiO
2 exhibits excellent chemical resistance. To combine these properties, engineers have developed composite coatings. Nanostructured TiO
2 and Cr
2O
3 coatings have shown promising results at sites like Ravensthorpe, Ramu, and Coral Bay [
3,
4,
5].
Thermal spray technologies, including plasma spraying and HVOF, are the most common techniques for applying such coatings. However, these methods typically exhibit a low powder utilisation efficiency, making them less suitable for expensive materials. In such cases, detonation spraying offers an effective alternative by providing a high coating density and strong adhesion with minimal material loss. For instance, tantalum—a highly corrosion-resistant metal—is proposed as an intermediate bond layer applied using the detonation gun method [
1,
6,
7,
8,
9,
10].
While the use of intermediate metallic layers between the substrate and the ceramic top coat is uncommon in hydrometallurgical applications [
11], incorporating a tantalum sublayer beneath TiO
2 and Cr
2O
3 may improve the coating adhesion and overall durability. To our knowledge, no prior studies have investigated the performance of a tantalum + nanostructured Cr
2O
3/TiO
2 coating system specifically for HPAL conditions.
Therefore, the goal of this study is to develop a method for applying wear- and corrosion-resistant coatings based on TiO2/Ta and TiO2 + Cr2O3/Ta onto titanium alloy substrates using air plasma spraying. Additionally, this study aims to investigate the effects of the spraying parameters on the microstructure, phase composition, and tribological performance of the resulting coatings.
2. Materials and Methods
TiO2 powder (Sulzer Metco, New York, NY, USA, Amdry 6505) with a dispersity of 45 ± 5 μm and Cr2O3 powder (Sulzer Metco, IKH 6003) with a particle size of 45 ± 5 μm were used for spraying.
The surface morphology was investigated through scanning electron microscopy (SEM) using backscattered electrons on a TESCAN VEGA scanning electron microscope (SEM, Tescan Vega 4, Brno, Czech Republic) equipped with an energy-dispersive X-ray spectrometer (EDS). The microhardness of the samples was measured through the Vickers method on an HVM Corporation instrument (HVM Corporation, Tokyo, Japan) at a 100 g load and with a 10 s exposure time. The phase composition of the samples was studied through a quantitative X-ray phase analysis on a RİGAKU D/MAX 2200 PC (Rigaku Corporation, Tokyo, Japan) diffractometer using CuKα radiation (λ = 1.5406 Å). The analysis was performed using Rigaku PDXL2 software, version 2.8.4.0, with the ICDD PDF-4+ database (2024 release). The specific JCPDS card numbers for the identified phases are provided in the Results section. Friction and wear tests of the materials were carried out on a CSM Instruments Tribometer (SM Instruments SA, Peseux, Switzerland) at a load of 3 N, at a speed of 25 cm/s, and at a run length of 500 m. The porosity of the coating surface and the data obtained after friction testing were measured using a KLA Tencor P6 profilometer (KLA-Tencor Corporation, Milpitas, CA, USA). The corrosion resistance was investigated using a CS300 galvanostat–potentiostat (CorrTest Instruments Corp., Wuhan, China). The tests were conducted at room temperature (25 °C) in a 3.5% aqueous NaCl solution. A three-electrode cell was used for the experiment, in which an Ag/AgCl electrode was used as the reference electrode, and a platinum electrode served as the auxiliary electrode. The corrosion potential and the corrosion current density were determined from the polarisation curves using the Tafel extrapolation method for the four samples under investigation. Potential scanning was performed in the range from −0.1 to +0.1 V relative to the OCP (open circuit potential) at a rate of 0.5 mV/s. To ensure reproducibility, each measurement was repeated three times, and data processing was performed using CS Studio 6 software.
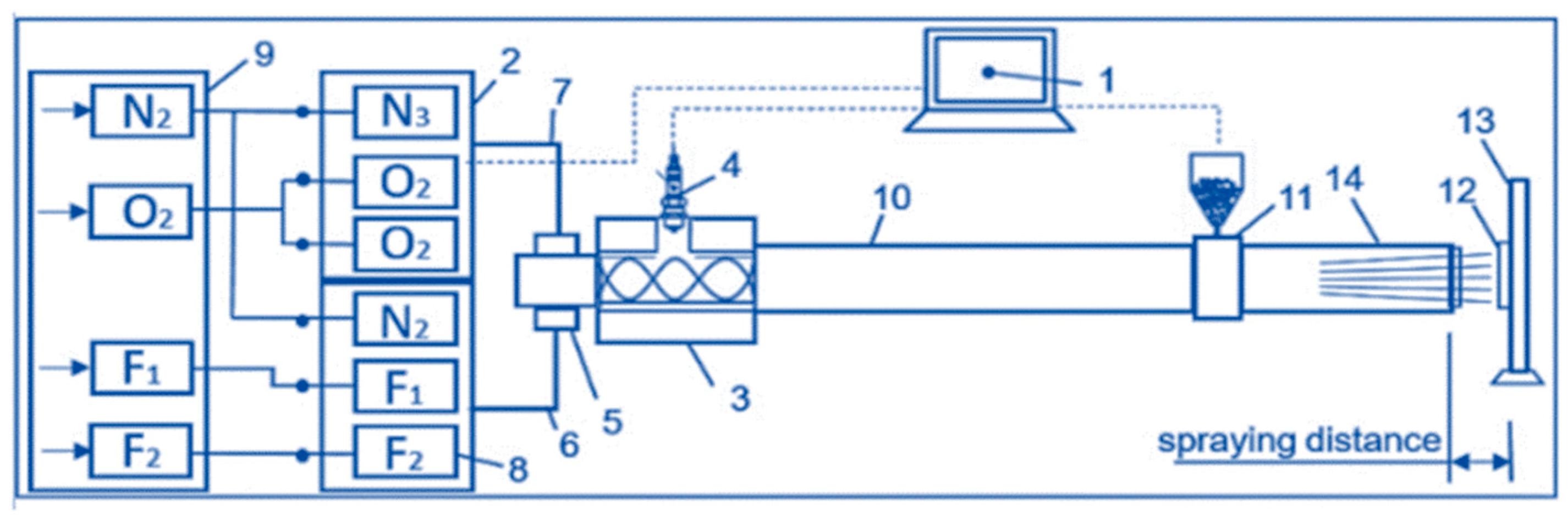
In the first stage of our work, we obtained the tantalum coating using the CCDS2000 detonation sputtering unit developed at LiH SB RAS (Siberian Technologies of Protective Coatings LLC, Novosibirsk, Russia) [
12], in which detonation is carried out inside the barrel in an explosive mixture generated by flowing gas components through a specialised mixing device [
13,
14], as can be seen in
Figure 1. The parameters of detonation spraying are shown in
Table 1.
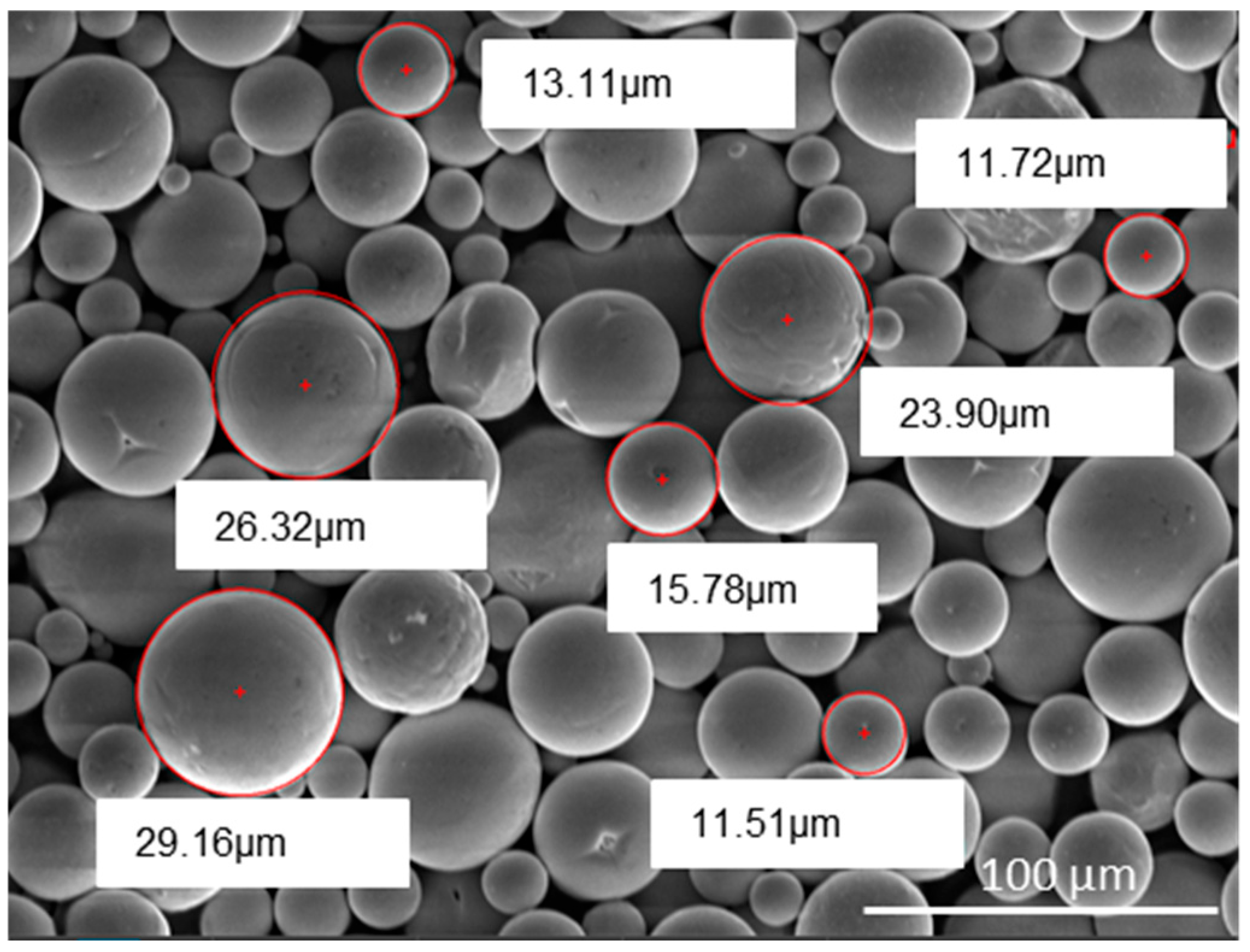
The SEM image (1000× magnification) shows tantalum powder particles. The particle sizes range from 30 ± 5 µm, which is confirmed by measurements of the image. Ta has a major role in improving the mechanical and tribological properties of the resulting coating and reducing the probability of cracking (
Figure 2).
After obtaining our tantalum coating on titanium, in the second stage, we applied agglomerated TiO2 and TiO2 + Cr2O3 nanopowders through the air plasma spraying method.
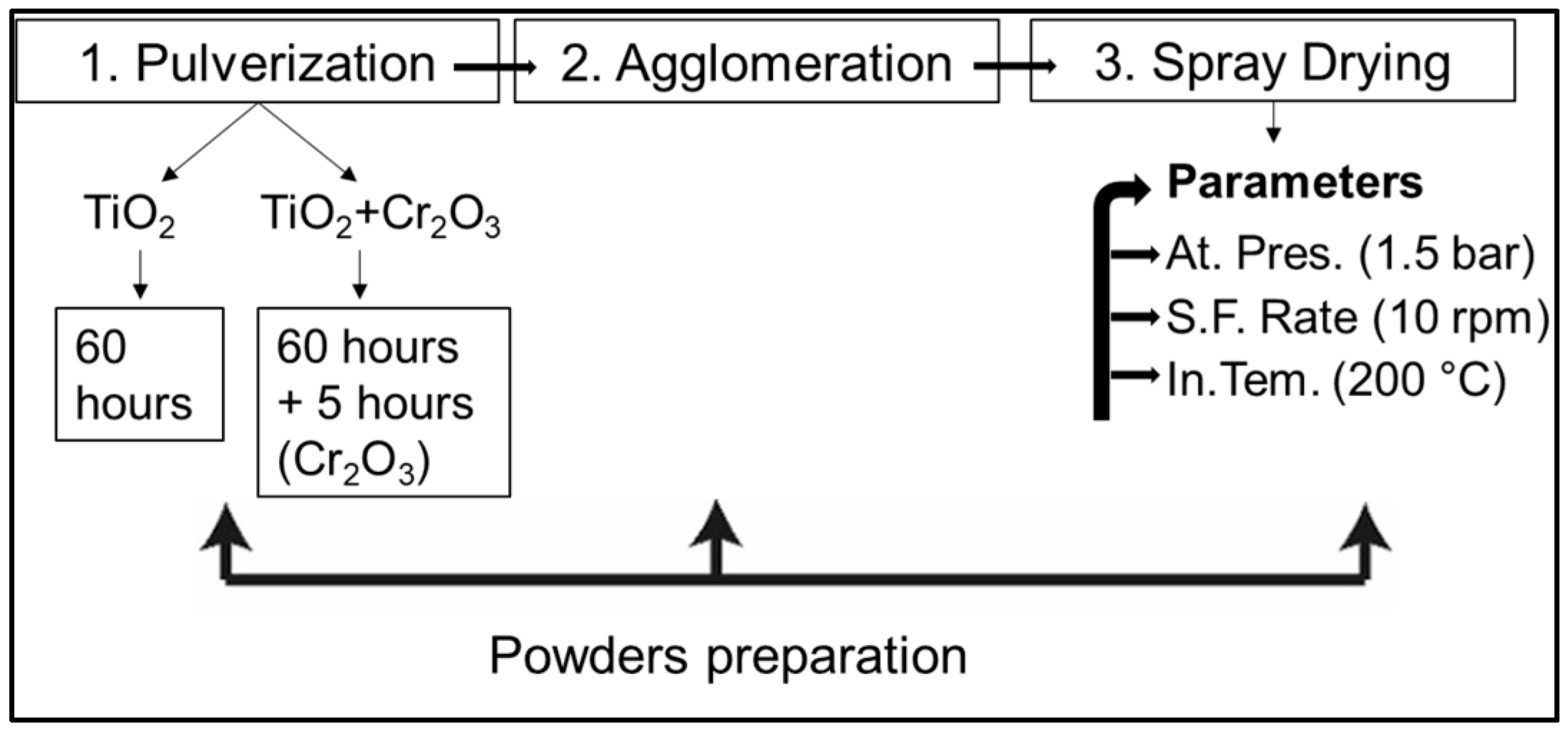
The experimental part of the preparation of the agglomerated nanopowder is divided into three steps, as shown in
Figure 3. Briefly, firstly, the micro powders were milled to a nanometer size. Then, polyvinyl alcohol mixed with purified water was added to the nanopowder and mixed for a certain time, followed by spray drying using the selected parameters.
Powder granulation was carried out in an aqueous medium with the addition of polyvinyl alcohol and mixed in a 1 S Attritor ball mill for 24 h (ball-to-powder mass ratio: 1:1; drum speed: 150 rpm) using zirconia balls of two sizes: 3 mm and 5 mm in diameter (
Figure 4).
Then, it was placed in a special container and dried in a spray dryer [
15]. In the process of granule powder production through spray drying, the prepared slurry of fine particles enters the spray tower. Under the pressure created in the atomiser, the slurry is atomised into droplets and dried using hot air. By changing the process parameters, it is possible to obtain spherical powder granules of the required size. This stage can be regarded as granulation, which represents the final step of the overall agglomeration process, ensuring the formation of flowable spherical granules suitable for plasma spraying.
The quality of coatings in air plasma spraying depends on several factors: the current, voltage, and plasma gas flow rates. Of particular importance among these factors is the current strength and the flow rate of working gas (Ar) and (H2) [
15,
16]. In this regard, the parameters shown in
Table 2 were selected for spraying.
3. Results and Discussion
Figure 5 presents SEM micrographs of TiO
2 and TiO
2 + Cr
2O
3 powders in their initial, milled, and agglomerated states.
The morphology of the pristine TiO
2 powder (
Figure 5a) is characterised by angular and irregularly shaped particles, indicative of mechanical fragmentation. Upon milling (
Figure 5b), the powder becomes finer and more compact, with evidence of particle refinement and potential agglomeration at the nanoscale. The agglomerated TiO
2 powder (
Figure 5c), likely obtained via spray drying or granulation, consists of predominantly spherical clusters ranging in size from approximately 15 to 30 µm.
For the TiO
2 + Cr
2O
3 composite powder, a similar progression in morphology is observed. In the unmilled state (
Figure 5d), the mixture retains a sharp-edged morphology comparable to that of the pure TiO
2, though with minor changes in the surface texture due to the addition of Cr
2O
3. After milling (
Figure 5e), the composite exhibits a more heterogeneous structure with fragments and partial spherical formations, suggesting the initial stages of agglomeration. The agglomerated TiO
2 + Cr
2O
3 powder (
Figure 5f) shows well-developed, spherical agglomerates with diameters ranging from 15 to 45 µm. These structures are more uniform and densely packed compared to the TiO
2-only agglomerates, indicating that the presence of Cr
2O
3 may enhance the spheroidisation and compaction behaviour during agglomeration.
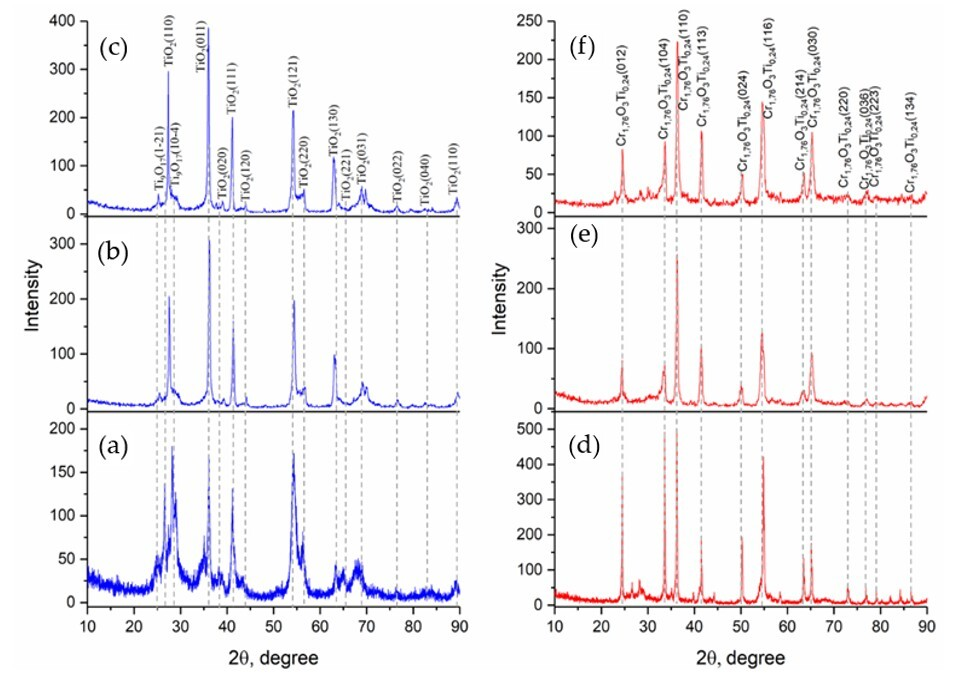
Figure 6 illustrates the XRD analysis results for the powders and coatings based on TiO
2 and TiO
2 + Cr
2O
3 compositions, obtained at different processing stages. A comparison of the diffraction patterns reveals evolution of the crystalline phases, influenced by milling, agglomeration, and thermal spraying.
In the TiO
2-based series (
Figure 6a–c), the initial milled and agglomerated powder (
Figure 6a) exhibits broadened peaks, typical of partially amorphous or nanocrystalline materials. As the coating is formed (
Figure 6b,c), the peak intensities increase significantly, indicating enhanced crystallinity. The dominant reflections belong to the rutile phase (R-TiO
2), while the anatase phase is either suppressed or absent, likely due to the high temperatures and rapid solidification involved in thermal spraying. This trend is consistent with prior reports, where the metastable anatase structure typically transforms into rutile during coating formation.
In the TiO
2 + Cr
2O
3-based group (
Figure 6d–f), the presence of composite oxide phases, such as Cr
1.379Ti
0.621O
3, is evident. These peaks are well resolved in both the powder (
Figure 6d) and coating samples (
Figure 6f), indicating that the formation of complex oxides occurs during high-energy milling and remains stable during subsequent thermal processing. The addition of the Ta interlayer (
Figure 6e) does not result in new detectable phases but may influence the coating’s microstructure or adhesion.
Additionally, minor reflections attributed to ZrO2 could appear due to mechanical interactions with zirconia-based milling components, particularly in Cr-containing mixtures, where the abrasive action is intensified.
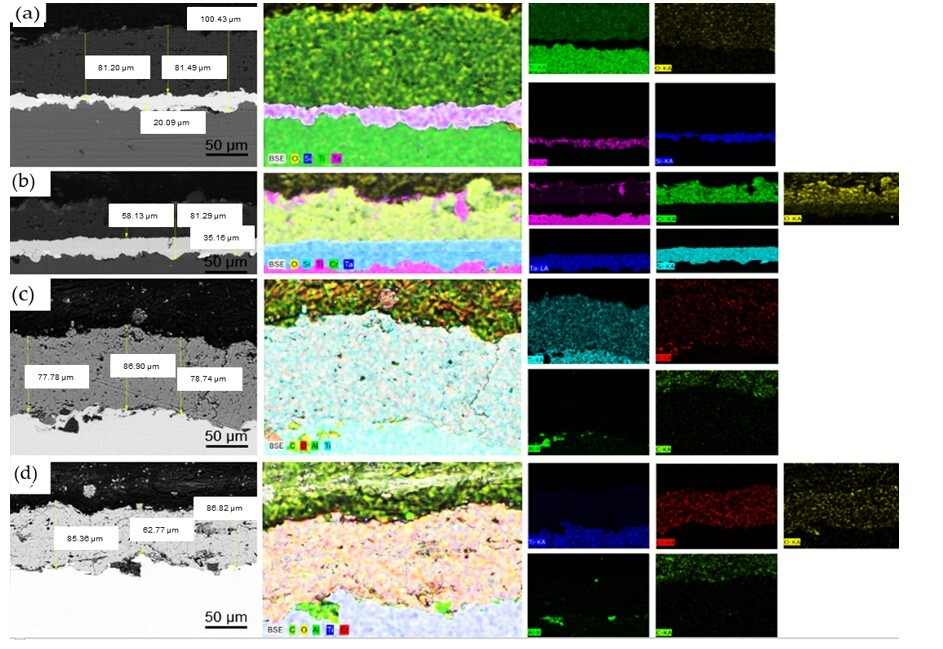
The cross-sectional microstructure of the coatings with (
Figure 7a,b) and without (
Figure 7c,d) a Ta sublayer and the feature mapping are shown in
Figure 7. The SEM images show that the coatings with a Ta sublayer have a dense structure and low porosity. In the case of the TiO
2 + Cr
2O
3 coatings (
Figure 7b), it is clearly seen that TiO
2 solid particles formed in one place without Cr
2O
3 during sputtering. It can be argued that some particles were not completely mixed during granulation in the attritor mill. Cracks and inhomogeneous pores are clearly visible in the Ta-free coatings. Cracks may form during sputtering and may be caused by poor adhesion to the substrate.
The thicknesses of all coatings, including differences in their structure and morphology, are shown in the SEM images.
Table 3 shows the correlation data for the coating properties.
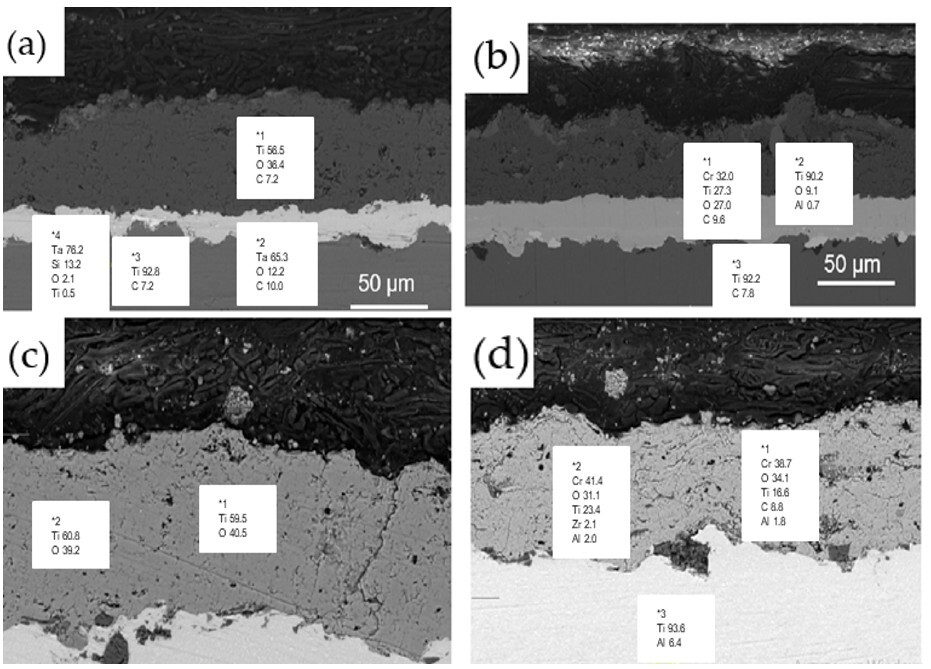
Figure 8 presents the cross-sectional analysis of coatings with the energy-dispersive X-ray spectroscopy (EDS) results. Panel (a) displays the TiO
2/Ta coating, while panel (b) shows the TiO
2 + Cr
2O
3/Ta composite coating. Panel (c) depicts the TiO
2 coating, and panel (d) illustrates the TiO
2 + Cr
2O
3 coating. The EDS results provide the elemental distribution, highlighting the composition and structure of each coating system, contributing to understanding their performance in terms of hardness and wear resistance. The structure of all of the coatings is homogeneous, with clear separation between the layers. The composite coating is more heterogeneous compared to pure TiO
2 (
Figure 8c), which is due to the distribution of Cr
2O
3 in the matrix (
Figure 8d). The structure of the coating with a tantalum sublayer appears to be denser, which provides improved adhesion and coating integrity (
Figure 8a,b).
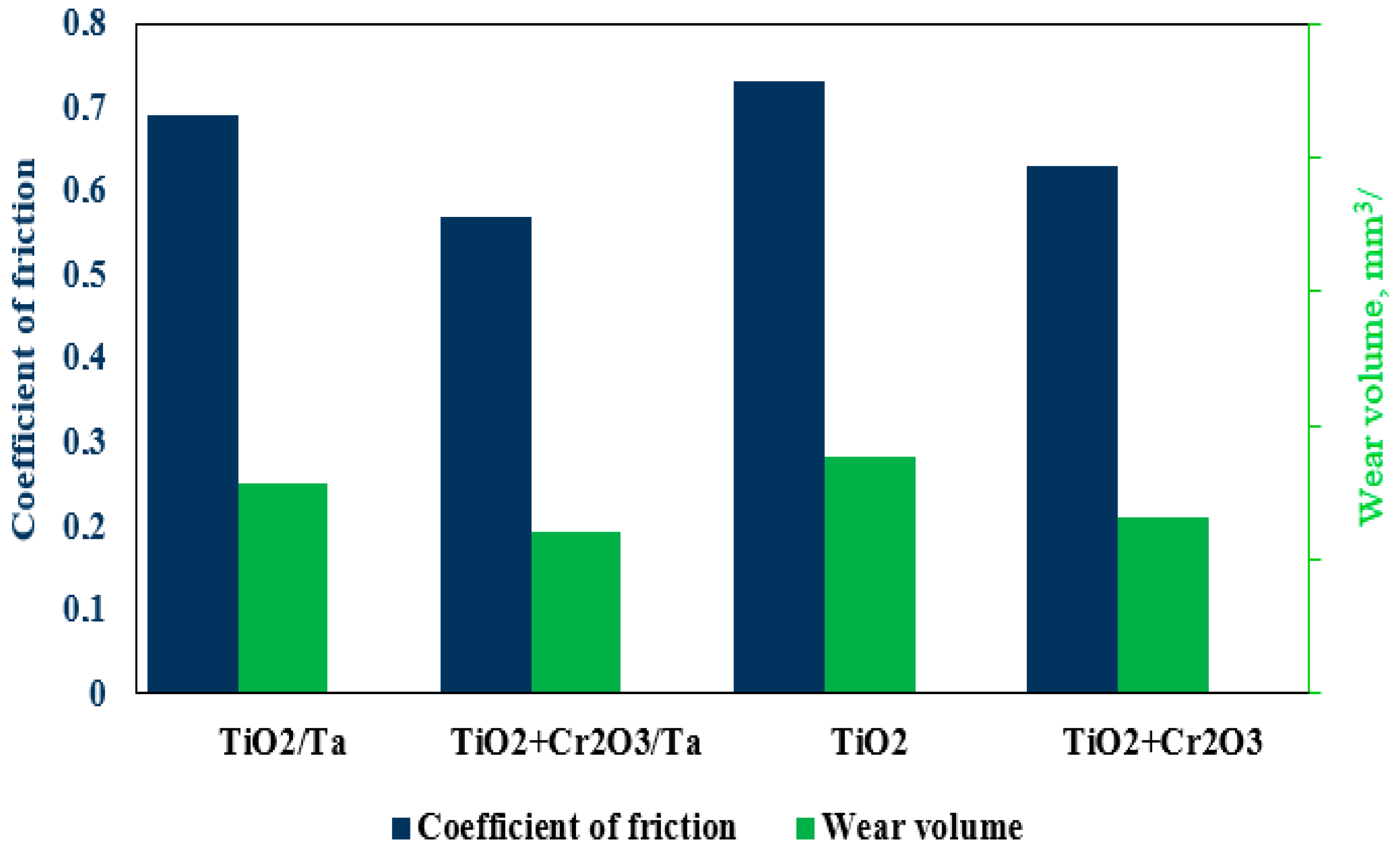
Figure 9 shows the results for the friction coefficient and the wear volume. The TiO
2 coating (without the Ta substrate) demonstrates the highest values for the friction coefficient and wear volume, indicating low wear resistance. This may be due to the brittle nature of the coating and its ineffective resistance to abrasive wear under friction conditions. The TiO
2 + Cr
2O
3 coating (without the Ta substrate) is characterised by a lower friction coefficient and wear volume values compared to those for TiO
2, confirming the positive effect of Cr
2O
3 on the tribological behaviour of the coating, even in the absence of a refractory metal substrate [
17,
18]. The TiO
2 + Cr
2O
3/Ta coating shows a significant reduction in both the coefficient of friction and the wear volume, which indicates a synergistic effect arising from the combined presence of TiO
2 and Cr
2O
3. The likely reason for the improvement in the performance is the increase in the hardness and chemical stability of the coating due to the introduction of Cr
2O
3, as well as the more uniform distribution of contact stresses.
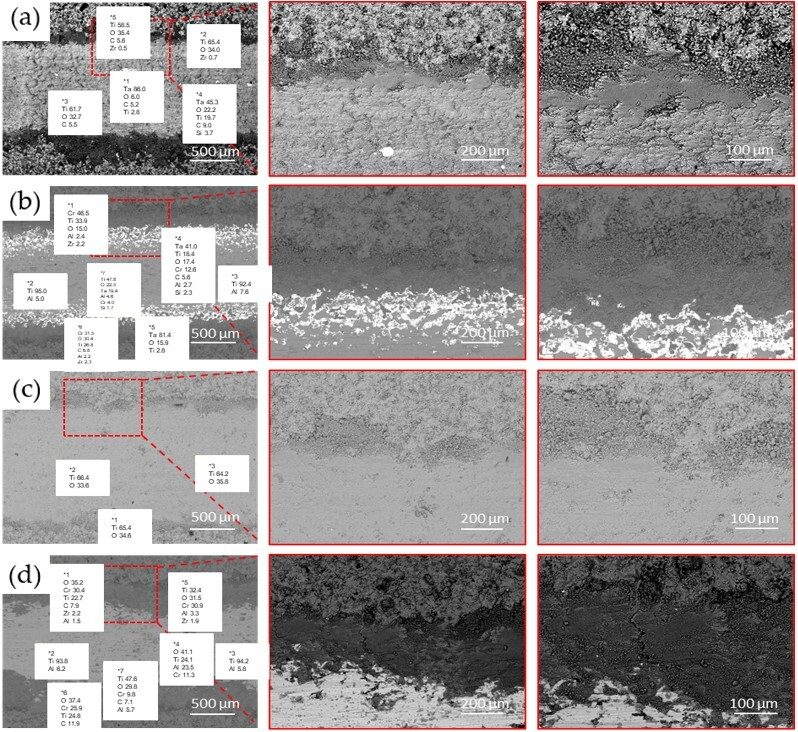
Figure 10 presents the morphology of the worn surfaces of various oxide coatings after tribological tests under reciprocating dry sliding conditions. The SEM images reveal typical signs of abrasive wear, including grooves, longitudinal scratches, and localised micro-cutting areas, which are especially pronounced on the pure TiO
2 coating (
Figure 10c). These features indicate the dominance of harsh contact interactions between the counterbody and the coating surface. In contrast, the coatings applied on the tantalum substrates—TiO
2/Ta (
Figure 10a) and particularly TiO
2 + Cr
2O
3/Ta (
Figure 10b)—exhibit significantly less damage: the wear tracks show a smoother topography and fewer cracks and grooves. This indicates that mechanical stability and resistance to abrasive wear are improved through the use of a tantalum substrate, as well as by the addition of Cr
2O
3, which likely enhances the hardness and reduces the brittleness of the coating. Therefore, the combination of TiO
2 + Cr
2O
3 with a tantalum substrate can be considered an effective approach to improving the wear resistance of oxide coatings under dry friction conditions.
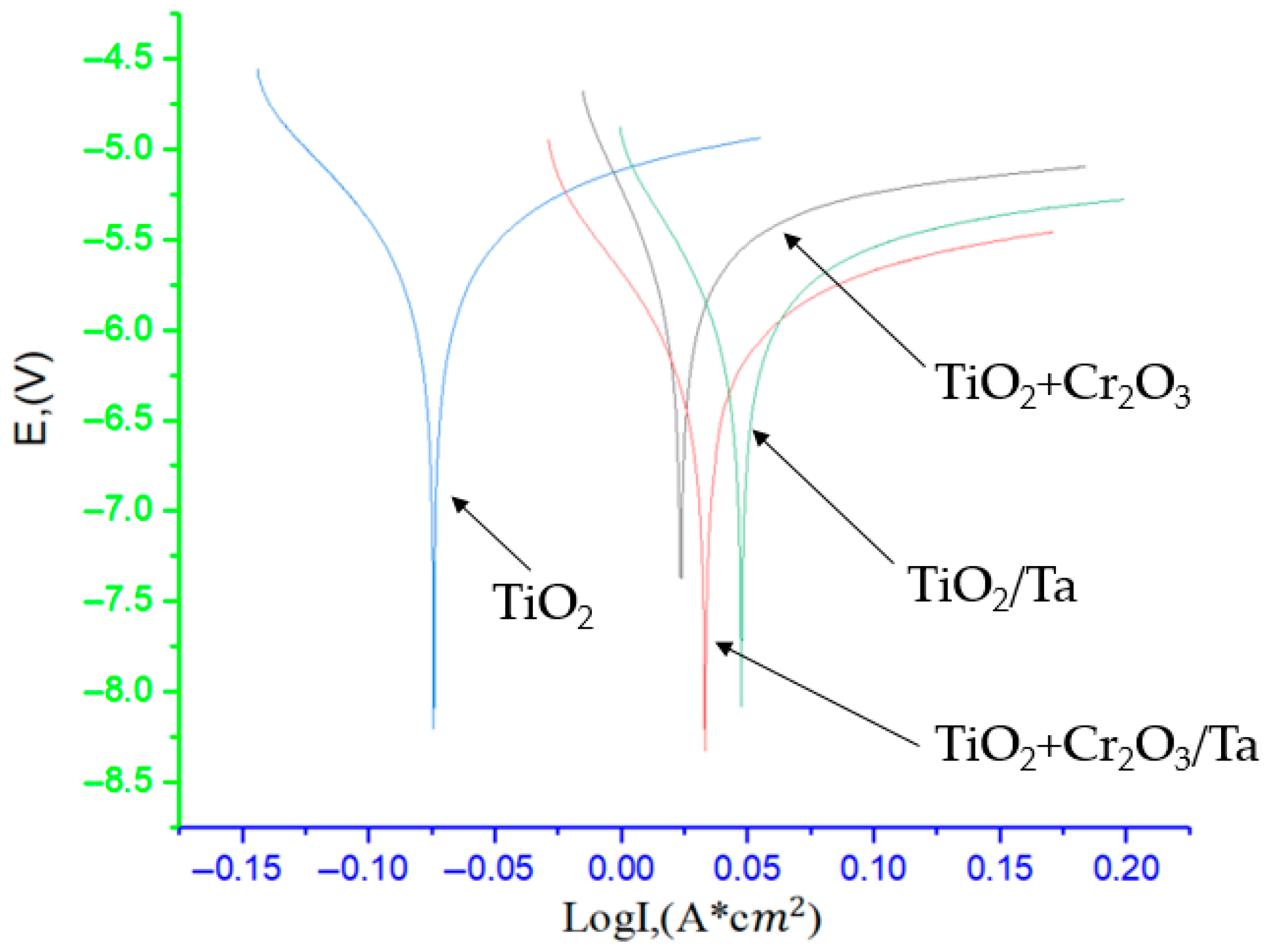
Figure 11 shows the anodic and cathodic potentiodynamic polarisation curves (Tafel plots) reflecting the corrosion behaviour of the coatings obtained. The TiO
2 + Cr
2O
3/Ta and TiO
2/Ta samples demonstrate more positive potential values, which indicate increased resistance to corrosion damage. The highest current density is observed for the TiO
2 coating, which indicates its poor protective ability. A comparison of the coatings with and without a tantalum substrate shows that the tantalum substrate improves the corrosion resistance of the system further. This is due to the high corrosion inertness of tantalum itself and the possible participation of tantalum oxides in the formation of the protective layer.
4. Conclusions
Based on the research conducted, the following conclusions can be drawn:
A three-stage scheme for the preparation of agglomerated nanopowder was implemented: (i) bringing the initial micropowders to the nanopowder range, (ii) the formation of an aqueous suspension with polyvinyl alcohol, and (iii) granulation through spray drying. Prolonged agitation in a ball mill ensured efficient dispersion and preparation of the homogeneous suspension. Spray drying produced spherical, flowable granules; by varying the spraying and drying parameters, controlled formation of granules of the required size was achieved. Overall, the incorporation of Cr2O3 leads to an increase in the size and uniformity of the agglomerates, which may significantly influence the flowability and sintering behaviour of the composite powders during thermal spraying or consolidation processes. The results confirm that both TiO2 and TiO2 + Cr2O3 coatings maintain their structural integrity through processing and that tailored phase composition can be preserved—a critical aspect in optimising the high-temperature performance of functional coatings. The microstructural analysis confirms that the incorporation of Cr2O3 into TiO2 coatings leads to the formation of denser, less defective structures, especially in the case of the coating on a tantalum substrate (b). The presence of tantalum (a, b) provides improved adhesion and coating integrity, whereas the coatings without the substrate (c, d) show more pronounced structural defects. Thus, the TiO2 + Cr2O3/Ta coating has the most stable and protected structure, confirming its promising potential for corrosion and wear-resistant applications. The results of this study confirm that the introduction of chromium oxide (Cr2O3) into the structure of titanium dioxide coatings contributes to a reduction in friction and wear by increasing the hardness, resistance to deformation, and possibly the formation of a favourable microstructure. The most promising variant from the point of view of increasing the operational reliability is the TiO2 + Cr2O3 system applied onto a tantalum substrate, combining high adhesion and improved antifriction characteristics. A comparison of coatings with and without a tantalum sublayer shows that the tantalum sublayer contributes to an improved corrosion performance. Tantalum is known for its high resistance to aggressive media, and its presence can favour the formation of protective oxide layers or reduce the interfacial corrosion.