Abstract
The dynamic response in propane atmospheres at different voltages was investigated for samples made from powders of the semiconductor oxide CoSb2O6 synthesized using the microwave-assisted colloidal method. Powders of the compound calcined at 700 °C were studied with X-ray diffraction, confirming the CoSb2O6 crystalline phase. The microstructural characteristics of the oxide were analyzed using scanning and transmission electron microscopy (SEM/TEM), revealing a high abundance of nanorods, nanoplates, and irregular nanoparticles. These nanoparticles have an average size of ~21 nm. Using UV-Vis, absorption bands associated with the electronic transitions of the CoSb2O6’s characteristic bonds were identified, which yielded a bandgap value of ~1.8 eV. Raman spectroscopy identified vibrational bands corresponding to the oxide’s Sb–O and Co–O bonds. Dynamic sensing tests at 300 °C confirmed the material’s p-type semiconductor behavior, showing an increase in resistance upon exposure to propane. Critically, these tests revealed that the sensor’s baseline resistance and overall response are tunable by the applied voltage (1–12 V), with the highest sensitivity observed at the lowest voltages. This establishes a clear relationship between the electrical operating parameters and the sensing performance. The samples exhibited good operational stability, capacity, and efficiency, along with short response and recovery times. Extra-dry air (1500 cm3/min) was used as the carrier gas to stabilize the films’ surfaces during propane detection. These findings lead us to conclude that the CoSb2O6 could serve as an excellent gas detector.
1. Introduction
In recent years, the development of propane (C3H8) gas sensors has been extensively pursued to create detection systems aimed at preventing gas leak-related accidents in both domestic and industrial environments [1]. Beyond the toxicity of its combustion byproducts, propane itself poses a significant hazard due to its explosive nature. Even minor leaks can be dangerous, as concentrations above its Lower Explosive Limit (LEL) of approximately 2.1% (21,000 ppm) create an immediate risk of explosion. Notably, C3H8 is a key precursor for liquefied petroleum gas (LPG), a versatile fuel for heating, cooking, welding, industrial processes, and as an automotive alternative [2]; however, the combustion of C3H8 yields carbon dioxide (CO2) and water. Critically, under oxygen-deficient conditions, such as those in enclosed spaces, its incomplete combustion can produce carbon monoxide (CO), a highly toxic gas [2,3]. Given its broad spectrum of applications, intensive research into reliable and highly sensitive sensor materials is indispensable [3,4,5].
Therefore, the study and development of C3H8 sensors are crucial for enhancing key performance metrics, including sensitivity, selectivity, response and recovery times, and stability under various operating conditions [1,2,3]. The literature indicates that trirutile-type nanostructured materials exhibit superior performance as gas sensors, effectively addressing challenges such as poor selectivity and the need for high operating temperatures [5].
The literature reports several types of synthesis processes [6], including chemical reduction, polyol synthesis, hot injection, non-injection or heating-up approach, sol–gel synthesis, solvothermal decomposition, microwave synthesis, and the microemulsion/colloidal, sonochemical, and solv or hydrothermal methods [6,7]. This reduction in particle size (less than 100 nm), along with enhanced physical and chemical properties, promotes the application of nanomaterials in various fields, including medical applications and those such as photocatalysis, optoelectronics, and biosensors [8,9]. Currently, there is considerable interest in applying wet chemistry processes, particularly those by Singh [10,11], which enable the synthesis of various morphologies (nanorods, nanowires, nanoparticles, bar-shaped granules, and microplates) with particle sizes below 100 nm [5,11,12]. These materials have already been investigated for applications such as photocatalytic materials and toxic gas sensors [13,14]. Michel et al. [15] reported the synthesis of CoSb2O6 with a trirutile-type structure using the colloidal route, successfully obtaining porous microspheres with particle sizes suitable for gas sensor applications. Recently, our group employed the colloidal method to synthesize nanoparticles with bar-type and polyhedron-type geometries of MgSb2O6 for application as a gas sensor, specifically propane [16].
CoSb2O6 belongs to the family of antimonates with a trirutile-type structure [17], which can be represented by the formula ASb2O6, where A is a divalent ion (e.g., Zn, Cu, Ni, Fe, or Co) [18]; with cobalt, it adopts the P42/mnm space group [18,19,20]. Traditionally, cobalt antimonate is synthesized using the ceramic method (solid-state reaction) [20]. However, this compound has also been prepared using wet chemistry methods, yielding different morphologies for various technological applications [15,18,21]. These applications include lithium-ion batteries [20], photocatalytic materials [18], and gas sensors (for O2, CO2, CO, C3H8, and LPG) [15,22]. The use of this material in such areas is based on its nature as a p-type semiconductor, where the charge carrier mobility is attributed to holes (h+) [22].
While CoSb2O6 has been identified as a promising p-type semiconductor for various gas sensing applications [15,22], including propane, previous research has primarily focused on material synthesis or performance evaluation at fixed operating conditions. A critical gap persists in the systematic exploration of operational parameters to actively tune sensor performance. The applied voltage represents a powerful yet underexplored tool for modulating the sensitivity and dynamic response of a sensor electronically, without requiring chemical modification or changes in operating temperature.
Addressing this gap, the present study investigates the voltage-dependent sensing properties of nanostructured CoSb2O6. We utilized a simple, microwave-assisted colloidal synthesis to produce the material and performed a comprehensive structural and optical characterization. The central aim of this work is to demonstrate that the applied voltage (from 1 to 12 V) is not merely a background condition but a key, tunable parameter that directly governs the sensor’s performance in propane atmospheres, thereby offering a new dimension for the optimization of trirutile-based gas sensors.
2. Materials and Methods
2.1. Synthesis of CoSb2O6 Powders
The synthesis of CoSb2O6 was carried out following the method described in references [10,12]. A 1:2 ratio of cobalt nitrate hexahydrate and antimony trichloride was used to form the trirutile-type cobalt antimonate. Initially, three solutions were prepared using 1.45 g of cobalt nitrate hexahydrate (Co(NO3)2·6H2O, Mallinckrodt, 99.9%), 2.28 g of antimony trichloride (SbCl3, Sigma-Aldrich, Jalmek, Guadalajara, Mexico, 98%), and 1 mL of ethylenediamine (C2H8N2, Sigma-Aldrich, Jalmek, Guadalajara, Mexico, 99%). Each solution was diluted in 5 mL of ethanol (C2H6O, Jalmek, Guadalajara, Mexico, 98%) and stirred at 25 rpm for 15 min at room temperature (30 °C) until homogeneous. Subsequently, the cobalt nitrate solution was added dropwise to the ethylenediamine solution, followed by the addition of the antimony trichloride solution, resulting in a turquoise-colored mixture. This solution was stirred at 360 rpm for 24 h. After stirring, the solvent was evaporated by subjecting the mixture to 10 microwave radiation exposures (General Electric, model JES769WK, Guadalajara, Mexico) at intervals of 50 s per exposure at a power of 180 W. Finally, the precursor material was dried for 8 h at 200 °C and subsequently calcined for 5 h at 700 °C. The thermal treatment was conducted using a programmable muffle furnace (Novatech, Guadalajara, Mexico).
2.2. Microstructural Characterization
The crystalline evolution of CoSb2O6 calcined at 700 °C was analyzed using X-ray diffraction (XRD, Guadalajara, Mexico) at room temperature with a Panalytical Empyrean system equipped with CuKα radiation (λ = 1.546 Å). Continuous 2θ scanning was performed in the range of 10° to 90° with 0.026° steps at a rate of 1 s per step. The microstructural characteristics of the oxide (particle size, porosity, and morphology) were examined using field-emission scanning electron microscopy (FE-SEM) with a Tescan MIRA 3 LMU (Mexico City, Mexico) system operated at an acceleration voltage of 10 kV in high vacuum. The analysis of CoSb2O6 nanostructures was conducted using transmission electron microscopy (TEM) with a JEOL JEM-ARM200F (Mexico City, Mexico) instrument in image mode. An amount of 0.01 g of oxide powders was dispersed in a plastic vial containing 1.5 mL of ethanol to separate the particles for individual nanostructure examination. After dispersion, a few drops of the suspension were deposited onto a copper grid with a 300-mesh Formvar/carbon membrane (Tedpella, Mexico City, Mexico) instrument in image mode. An amount of 0.01 g of oxide powders was dispersed in a).
2.3. Optical Characterization
To determine the bandgap of the CoSb2O6 calcined at 700 °C, a UV-Vis-NIR spectrophotometer (UV-3600 Plus, Mexico City, Mexico) was used to measure absorbance in the 200–800 nm range. The powders were also analyzed using a Thermo Scientific DXR Raman microscope with an excitation source of 633 nm. Raman spectra were recorded in the 200–800 cm−1 range with an exposure time of 60 s.
2.4. Fabrication of the CoSb2O6 Sensor
Dynamic tests were performed on ~500 µm thick films (~300 µm in diameter) derived from CoSb2O6 powders calcined at 700 °C, using the setup shown in Figure 1a. Measurements were conducted in atmospheres of extra-dry air (1500 cm3/min) and propane (3582, 3701, and 3184 ppm) at an operating temperature of 300 °C. This temperature was selected after preliminary tests indicated that, while the sensor was responsive at higher temperatures such as 400 °C, it exhibited significant thermal instability during prolonged measurements. Therefore, 300 °C was chosen as the optimal temperature to ensure both a strong signal and excellent operational stability for the voltage-dependent study. Voltages of 1, 3, 6, 9, and 12 V were applied to study their effect on the material’s dynamic properties and response. The films were placed inside a quartz tube housed in a tubular furnace (Lindberg/Blue, Mexico City, Mexico) instrument in image mode. An amount of 0.01 g of oxide powders was dispersed in a) with programmable temperature control (Figure 1b). Electrical connections were made once the films were positioned in the quartz tube. Gas concentrations were controlled using Brooks Instruments mass flow controllers, models GF100CXXC–SH452.6L (2600 cm3/min) and GF100CXXC–SH40010C (10 cm3/min) (Figure 1). The film’s electrical resistance variation was recorded with a digital multimeter. The measurement system was controlled using National Instruments LabVIEW 8.6 software.

Figure 1.
(a) Schematic representation of the measurement system for dynamic response tests in air–propane flows, and (b) thick films placed inside the quartz tube for measurements at 300 °C.
The response of the CoSb2O6 sensor to propane atmospheres was quantified using the following equation, as reported in reference [13]:
where Ra is its resistance in air, and Rg is its resistance in the test gas.
3. Results
3.1. XRD Analysis
Figure 2 shows the X-ray diffraction pattern of calcined CoSb2O6. The diffraction planes correspond to the standard crystalline phase of cobalt antimonate [17,18,19,23]. Using PDF file No. 18-0403, the peaks of the main CoSb2O6 phase correspond to a tetragonal structure with cell parameters a = 4.653 and c = 9.283, and a P42/mnm space group [15,20,22]. Based on these parameters, CoSb2O6 belongs to the trirutile-type family of crystalline structures [7,15,18,20,21,24,25]. The peak width indicates a very small particle size (on the nanometer scale), while the peak height suggests high material purity. However, a secondary phase of Co2.33Sb0.67O4 with low intensity was observed at 2θ = 42.29°. This minor inorganic phase was identified using PDF file No. 15-0517 and has been previously reported in other studies. For example, Michel et al. synthesized CoSb2O6 using the colloidal method and observed the formation of the secondary Co2.33Sb0.67O4 phase at 700 °C [15] and 600 °C [26]. The results shown in Figure 2 are consistent with those in references [15,17,18,19,20,22,23,26,27] for the same compound synthesized using wet chemistry and solid-state reaction methods. The presence of this minor Co2.33Sb0.67O4 phase could positively influence the material’s gas-sensing performance. The literature suggests that such secondary phases facilitate the formation of heterointerfaces. These interfaces can create regions of varying charge carrier density, thus enhancing charge mobility. Furthermore, they can increase the number of active sites for oxygen adsorption and alter the sensor’s surface kinetics [28,29].

Figure 2.
X-ray diffraction (XRD) pattern of CoSb2O6 powders calcined at 700 °C.
Compared to the results reported for CoSb2O6 or similar oxides (NiSb2O6 or CuSb2O6) synthesized using the ceramic method or wet chemistry [15,20,30], our method successfully reduced the calcination temperature and residence times in the muffle. For instance, using the solid-state reaction method, Larcher et al. [20] obtained the crystalline phase of CoSb2O6 at 800 °C. Michel et al. [31] reported the crystalline phase of the same oxide at 800 °C using the polymerization-solution method. In reference [32], the oxide was synthesized via gel combustion, achieving the crystalline phase at 800 and 1000 °C.
3.2. SEM Analysis
The microstructural features of CoSb2O6 were observed using scanning electron microscopy (SEM) at various magnifications. Figure 3 presents typical micrographs of the material’s surface.

Figure 3.
SEM micrographs showing the microstructural features of CoSb2O6 calcined at 700 °C: (a) 185x, (b) 2.08 kx, (c) 2.68 kx, (d) 15.9 kx, and (e) 30.0 kx.
At magnifications of 185x and 2.08 kx (Figure 3a,b), irregular particle formation and microrod growth across the material’s surface were observed. At 2.68 kx (Figure 3c), it was confirmed that the material’s surface consists of microrods and irregular microparticles that agglomerate because of temperature. Additionally, spherical morphologies (~90 μm in diameter) were observed, and microplates formed by the agglomeration of microrods and microparticles, due to the temperature and the influence of ethylenediamine (Figure 3b,c), were also observed. At 15.9 kx and 30.0 kx (Figure 3d,e), it was observed that the microrods consisted of agglomerated ultrafine particles (~0.1 μm) with no defined shape. The microrods grew individually, pointing in various directions. The granular surface of the microrods was attributed to the removal of organic material present during the synthesis process. The growth of these microstructures was attributed to the combined effects of temperature, ethylenediamine, and the material’s residence time in the muffle. The material’s surface exhibited more individual microrods and other irregular particles. The microrods’ lengths were estimated to range from 4 to 20 µm, with an average of ~10.8 µm and a standard deviation of 3.8 µm (Figure 4a). Their diameters were calculated to range from 1.2 to 2.6 µm, with an average of ~1.7 µm and a standard deviation of 0.33 µm (Figure 4b). Meanwhile, the particle sizes on the material’s surface were determined to range from 0.2 to 0.8 µm, with an average of ~0.368 µm and a standard deviation of 0.13 µm (Figure 4c).

Figure 4.
Particle size distribution of CoSb2O6 calcined at 700 °C: (a) microrod length, (b) microrod diameter, and (c) size of irregular particles on the material’s surface.
It has been reported in the literature that wet chemistry processes combined with ethylenediamine for the synthesis of trirutile- and spinel-type oxides result in the growth of microrods and nanoparticles, which can be utilized as gas sensors [33,34]. For instance, Michel et al. [26] synthesized CoSb2O6 microrods of various sizes using the colloidal method with a low concentration of ethylenediamine (0.5 mL). Similarly, Ramírez-Ortega et al. [5] synthesized spinel-type NiSb2O6 using the colloidal method with 2 mL of ethylenediamine, resulting in nanoparticles with a size of ~23 nm. Recently, we reported the synthesis of trirutile-type MgSb2O6 in the presence of 5 mL of ethylenediamine [16], achieving the nucleation and growth of filament- and microneedle-like particles, as well as polyhedron- and bar-shaped geometries composed of ultrafine particles (~0.1 µm). In this study, 1 mL of ethylenediamine was used, resulting in irregular particles and microrods composed of ultrafine particles (~0.1 µm). As demonstrated in all these studies, these types of microstructures enhance gas sensing.
3.3. TEM Analysis
To further analyze the morphology and particle size of the CoSb2O6 powders calcined at 700 °C, transmission electron microscopy (TEM) in image mode was used. The results are presented in Figure 5. The dark regions observed in the micrographs result from the agglomeration of nanostructures. These images corroborate the SEM results, showing the presence of nanorods and nanoparticles of various sizes (Figure 5a). The nanorods, ranging in size from 21 to 182 nm, were observed to agglomerate at a common point due to neck formation caused by temperature and ethylenediamine, as shown in Figure 5b. The average size of these necks was calculated to be ~86 nm. As seen in Figure 5c, nanoparticles of various sizes were identified on the material’s surface. These nanoparticles also agglomerated due to the calcination temperature. Their size was estimated to range from 8 to 49 nm, with an average of ~21 nm and a standard deviation of 13 nm.
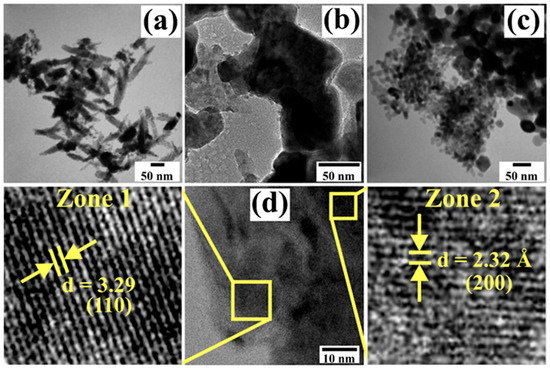
Figure 5.
TEM micrographs of nanostructures of CoSb2O6 calcined at 700 °C: (a) nanorod formation, (b) neck growth, (c) nanoparticle production, and (d) crystal planes analyzed by HRTEM.
Figure 5d shows the crystal planes of the CoSb2O6 structure using high-resolution transmission electron microscopy (HRTEM). A single nanorod was targeted, and two zones with clearly visible crystal planes were selected for analysis. The crystal planes were oriented in different directions. The interplanar distance was estimated at 3.29 Å in Zone I, corresponding to the (110) plane and a diffraction angle of 2θ = 27.06°. In Zone II, the interplanar distance was calculated to be 2.32 Å, associated with the (200) plane and a diffraction angle of 2θ = 38.64°. These results confirm the crystallinity of the CoSb2O6 and validate the X-ray diffraction pattern shown in Figure 2.
3.4. UV-Vis Analysis
Figure 6 presents the UV-Vis spectrum of CoSb2O6 calcined at 700 °C. This technique was used to identify characteristic absorption bands and determine the material’s bandgap. The analysis covered wavelengths ranging from 200 to 800 nm. In the range between 200 and 500 nm, absorption bands associated with the electronic transitions of the trirutile family’s characteristic bonds were identified (Figure 6a). To estimate the bandgap value, Tauc’s equation was applied by plotting (αℎυ)2 against E (see Figure 6b) [35].
where E is the energy of the incident photon, α is the optical absorption coefficient, Eg is the forbidden bandwidth, and A is the proportionality constant [35]. In this case, an indirect transition (n = 2) was considered for CoSb2O6. The intercept of the linear fit yielded a bandgap value of approximately 1.84 eV. Common and effective bandgap values for gas sensors typically range from 1.0 to 3.5 eV, indicating that the material studied has potential for application as a gas sensor [36,37,38].

Figure 6.
(a,b) UV-Vis spectroscopy results used to determine the bandgap of CoSb2O6 calcined at 700 °C.
3.5. Raman Analysis
Figure 7 shows the Raman spectrum of the CoSb2O6 powders. Previous studies have reported that semiconductor oxides crystallizing in a trirutile-type structure with space group P42/mnm () exhibit Raman vibrations in the energy range of 200–800 cm−1 [21].

Figure 7.
Raman spectrum of CoSb2O6 with a trirutile-type structure calcined at 700 °C.
The vibrational modes identified in this study are A1g, B1g, B2g, and Eg by group theory for compounds that maintain this symmetry [19]. The bands appearing in the range of 500–800 cm−1 are characteristic of vibrational modes associated with the Sb2O10 bonds (A1g and Eg) [18,19]. Vibrational bands associated with Sb-O bonds were observed in the 600–800 cm−1 range, while bands in the 200–400 cm−1 range correspond to vibrational modes of Co-O bonds [18,38]. Several authors have mentioned that vibrations caused by metal atoms are minimal; therefore, only three bands are characteristic of the MII-O6 octahedron, located at 521, 617, and 727 cm−1 [18,19,21,38]. Additionally, the band at 194 cm−1 may indicate the presence of a Co2.33Sb0.67O4 spinel-type phase and the translational movements of the cation Co2+ within the crystal lattice [18,38]. The spectrum also shows peak broadening, indicating the formation of nanoparticles [18]. An intense band at 681 cm−1 was identified, associated with the symmetric stretching of SbO6. Conversely, the band at 617 cm−1 is correlated with the symmetric stretching mode of Sb2–O10 [18], and the vibrational mode found at 521 cm−1 is associated with a symmetric bending of the O–Sb–O bond [18]. Table 1 presents a comparative analysis of the identified experimental frequencies and the values reported in the literature. Where variation in vibrational positions was observed, such discrepancies may be attributed to several factors, including the synthesis route, crystallite size, lattice strain, and any doping effects [18,19,21,38].

Table 1.
Experimental and theoretical frequency values of the Raman vibrational modes for the CoSb2O6.
The experimental frequencies observed are in good agreement with the theoretical values reported in the literature [18,19,21,38]. These results confirm that CoSb2O6 with a trirutile-type structure was successfully obtained [38].
3.6. Gas Sensing Properties in Propane
To evaluate the capability and efficiency of CoSb2O6 in detecting explosive atmospheres, such as propane (C3H8), tests were conducted on thick films derived from CoSb2O6 powders calcined at 700 °C. Measurements were performed at 300 °C, with readings taken every 5 min, using a flow of 1500 cm3/min of extra-dry air (comprising 21% O2 and 79% N2) as a stabilizer, mixed with varying propane concentrations. The tests involved changing the voltage across the films to 1, 3, 6, 9, and 12 V, while the propane concentrations were 3532, 3701, 3701, and 3184 ppm for respective voltages. We recorded the variation in the electrical resistance of the thick films throughout the tests. This process was repeated several times to ensure reproducibility of the results. The consistent cycles observed indicated that the material had an excellent dynamic response at the operating temperature, as illustrated in Figure 8 and Figure 9. The material also exhibited good repeatability and operational stability. In fact, the dynamic response corresponded to the typical p-type semiconductor oxides, where holes () are the primary charge carriers responsible for gas detection [17,22].

Figure 8.
Dynamic response (electrical resistance variation) of CoSb2O6 thick films at 300 °C under different voltages: 1 V, 3 V, 6 V, 9 V, and 12 V.

Figure 9.
Variation in response percentage of CoSb2O6 thick films at 300 °C under different voltages: 1 V, 3 V, 6 V, 9 V, and 12 V.
The propane concentrations evaluated in this study were chosen for their relevance in detecting severe leaks in industrial and safety scenarios. These concentrations, ranging from 3184 to 3701 ppm, reflect severe conditions where fast and reliable detection is critical for safety. Such high concentrations are typical in major leak situations. Emergency procedures related to propane leak scenarios, as outlined by the National Oceanic and Atmospheric Administration (NOAA) [39] and the National Academy of Sciences’ Board on Environmental Studies and Toxicology [40], indicate that propane concentrations in emergency scenarios can range from 5500 ppm to 33,000 ppm. Evaluating our sensor at high concentrations aligns with the demands of these environments, where sensor reliability at elevated levels is crucial for prompt response.
Additionally, these concentrations complement previous studies conducted by our group in lower ranges (0–300 ppm) [12], which demonstrated the sensor’s sensitivity across different propane concentration ranges. At 150 °C, CoSb2O6 showed a response of 0.12 at a concentration of 5 ppm of C3H8, and the maximum response was 1.04 in the presence of 300 ppm. By increasing the sensor’s operating temperature to 250 °C and 350 °C, maximum responses of 2.7 and 4.8 were recorded, respectively, in the presence of 300 ppm of C3H8 [12]. Thus, the CoSb2O6 demonstrates the significant advantage of detecting a broad C3H8 concentration range (5 to 3700 ppm). This versatility is a key property, making it suitable for applications ranging from the early detection of low-level gas leaks to the monitoring and control of severe and hostile industrial scenarios.
The material exhibited a distinct p-type semiconductor behavior at all tested voltages; upon exposure to propane, its electrical resistance consistently increased relative to its baseline in air (Figure 8). However, a crucial second trend was observed: the sensor’s baseline resistance in air systematically decreased as the applied voltage was raised from 1 V to 12 V (see Figure 10b). This confirms that the applied voltage directly modulates the sensor’s fundamental electrical properties and, consequently, its calculated response (Figure 9). The estimated changes in electrical resistance (ΔR) at 300 °C were 11.0, 9.9, 7.9, 6.1, and 5.2 MΩ for the respective voltages of 1, 3, 6, 9, and 12 V. The response and recovery times for each voltage were estimated based on the results shown in Figure 8. For these calculations, the criteria outlined in references [41,42] were applied, considering 10% of the response in air and 90% of the response at each propane concentration. The estimated response times were 196.5, 201.1, 213.4, 200.8, and 206.5 s, while the recovery times were 304.1, 336.2, 316.8, 383.1, and 318.4 s, respectively, for the corresponding voltages. Furthermore, based on the results from Figure 9, the response percentage was determined, yielding values of 17.3%, 16.5%, 14.9%, 14.0%, and 13.4%, respectively. A summary of these results is provided in Table 2.

Figure 10.
Variation in the response percentage and electrical resistance of CoSb2O6 thick films as a function of (a) propane concentration, (b) activation voltage, and (c) response fitting as a function of voltage.

Table 2.
Dynamic response of the CoSb2O6 in propane at 300 °C.
The results indicate that the initial trend showed an increase in electrical resistance. However, as the voltage surpassed a certain threshold, both resistance and sensitivity percentage decreased, regardless of the test gas concentration (Figure 10a,b). This behavior can be attributed to the effect of voltage on oxygen adsorption on the films’ surface. It is important to note that the sensor’s response remained unaffected by the use of extra dry air (21% O2 and 79% N2), indicating a certain level of robustness under low humidity conditions. Figure 8 and Figure 9 illustrate that our sensor maintained stable responses under these measurement conditions.
Due to the temperature, there was a gradual diffusion and adsorption of oxygen on the surface of the films. This process ultimately led to a reduction in both electrical resistance and response percentage (Figure 10b). According to the literature, for a p-type semiconductor like the one used in this study, the decreased dynamic response does not depend on the formation of mixed-valence states of, in this case, the cobalt (), but instead on the adsorption of oxygen on the semiconductor’s surface [22,26], which aligns with our findings. Additionally, as depicted in Figure 8, Figure 9 and Figure 10, a clear correlation exists between voltage and test gas concentration. The highest response percentages were recorded at 1 V and 3 V, with values of 17.3% and 16.5%, respectively, for propane concentrations of 3582 ppm and 3701 ppm. The maximum response at these voltages is attributed to oxygen adsorption on the surface of the thick films. This process increased the holes in the valence band, which enhanced resistance and sensitivity [22,26,43,44,45]. This behavior is typically observed in p-type semiconductors. At the operating temperature, the dynamic response involved not only efficient oxygen adsorption and desorption but also a strong reaction between the test gas and ionic oxygen species, leading to significant surface energy generation on the films. This process caused a displacement of charge carriers (holes, in the case of CoSb2O6), leading to a decrease in the material’s conductivity (Figure 10b), thereby enhancing the sensor’s dynamic response.
It is important to note that, within the concentration range of 3184 to 3701 ppm, no sensor saturation was observed, as the electrical resistance and response continued to vary with the presence of propane. This indicates that the sensor operated within the studied propane range and did not reach its saturation limit at these concentrations.
In conjunction with the activation voltage, this improvement in dynamic response enhances key material properties, including stability, selectivity, efficiency, and response and recovery times. This was further validated through the calibration curve (Figure 10c), which was fitted to the equation reported in reference [46]:
where S(V) represents the response as a function of the applied voltage, m is the response coefficient for propane, and b is the power law constant. The correlation coefficient indicates a good fit, as evidenced by the observed reduction in electrical resistance and response percentage with increasing voltage.
The changes in the response to propane as a function of voltage variations are presented in Figure 11.

Figure 11.
Voltage-dependent response of the CoSb2O6 sensor at 300 °C.
As expected for a p-type semiconductor like the CoSb2O6, the thick films exhibit enhanced response towards propane at 1 V and 3 V, with the peak response at 1 V. This is evidenced by the high response values of 17.36% and 16.55% at these respective voltages. These findings are consistent with Figure 10.
The enhanced capability to detect propane at these voltages is attributed to the strong oxidation of propane molecules by reactive oxygen species (). It is known that these species become highly reactive at temperatures above 150 °C, which is consistent with the operating temperature (300 °C) used in this work. This oxidation process on the material’s surface enhances the mobility of charge carriers, leading to significant changes in electrical resistance and, consequently, greater response towards propane at 1 V and 3 V. The literature supports these observations, reporting that the propane detection capability of trirutile-type oxides is increased by a strong chemical interaction between the target gas, the adsorbed oxygen species (), the applied voltage, and the operating temperature [16,46]. Furthermore, reference [45] indicates that the kinetic energy of charge carriers, influenced by the applied electrical potential, significantly improves propane detection. This aligns with our findings.
In comparison with other similar semiconducting oxides (Table 3), the dynamic response of CoSb2O6 at a constant temperature of 300 °C exhibited greater thermal stability as both the applied voltage and propane concentration increased. Additionally, improved reversibility of the sensing process and good reproducibility of the dynamic response were confirmed, as evidenced by the uniform response cycles observed during the tests. Notably, responses of 17.3% and 16.5% were recorded at 3582 and 3701 ppm of propane, respectively. These values are higher than those reported for other trirutile-type oxides, whose sensitivities range between 1.49% and 13.3% (see Table 3). Overall, the favorable dynamic response and high response observed in Figure 8, Figure 9 and Figure 10 are attributed to the nanostructural characteristics achieved during synthesis, suggesting that CoSb2O6 is a promising candidate for propane sensing under various applied voltages.

Table 3.
Comparative gas sensing performance of metal oxide nanostructures.
3.7. Detection Mechanism
The dynamic response of CoSb2O6 is associated with the reaction mechanism involving oxygen species () [53,54,55], the propane concentration, the effect of voltage, and the operating temperature (Figure 12a). At temperatures above 150 °C, the predominant oxygen species are ionic and [11,55], which are highly reactive. In contrast, at temperatures below 150 °C, the predominant species is [11,53,54,55].

Figure 12.
Schematic of the sensing mechanism. (a) Oxygen from the atmosphere is adsorbed onto the material’s surface, forming reactive species that interact with C3H8. (b) Subsequent oxidation of C3H8 yields CO2 and H2O as products.
These highly reactive oxygen species facilitate the oxidation of the test gas, promoting increased mobility of charge carriers (holes) [54,55], thereby enhancing the sensor’s dynamic response [22,44]. Additionally, the adsorption and desorption processes on the thick films’ surface improve significantly at elevated temperatures. It has been reported that propane oxidation generates , , and free electrons, which induce a stronger dynamic response; see Figure 12b [55,56,57].
The chemical reactions occurring on the film surface are as proposed in reference [53]:
where corresponds to propane () [5,57]. According to this reaction mechanism, removing oxygen species from the CoSb2O6 thick film surface releases electrons, leading to variations in electrical resistance and response [57].
In a p-type semiconductor such as CoSb2O6, where holes are the predominant charge carriers, the released electrons recombine with these holes, decreasing their concentration and leading to an increase in electrical resistance or a reduction in conductivity [57], as shown in Figure 8, Figure 9 and Figure 10. This electron–hole recombination forms a depletion layer, creating a potential barrier that hinders the movement of charge carriers, leading to the observed phenomena. Additionally, the sensor’s ability to detect propane molecules is closely related to its morphology and nanoscale particle size [56,58].
4. Discussion
The dynamic response of the CoSb2O6 thick films is intrinsically linked to the material’s combined microstructural and phase characteristics. Firstly, the nanoscale particle size of ~21 nm, observed via TEM, is a key factor [59]. This small dimension drastically increased the surface-area-to-volume ratio, which in turn enhanced the number of catalytic (active) sites on the surface available for oxygen adsorption and interaction with propane molecules [58,59,60,61,62,63]. Secondly, beyond particle size, the phase composition revealed by XRD provides further insight into the material’s effectiveness. The presence of a minor Co2.33Sb0.67O4 secondary phase alongside the main trirutile structure is significant. It has been reported that such secondary phases can foster the formation of p-p heterointerfaces, which are known to increase active sites for oxygen adsorption and modify the sensor’s surface kinetics by creating regions of varying charge carrier density. Therefore, the combination of a high surface area from the nanoparticles and the beneficial electronic effects of these heterointerfaces likely establishes the material’s strong baseline sensitivity.
To contextualize the performance of our sensor, a direct comparison with other oxide-based sensors from the literature is essential. As detailed in Table 3, our CoSb2O6 sensor exhibits a highly competitive response. Specifically, its response of 17.3% to propane at 300 °C surpasses that of other trirutile-type structures reported for propane detection, such as NiSb2O6 (6.69%) [5] and MgSb2O6 (13.3%) [44], our sensor operates effectively at a moderate temperature and, crucially, offers the unique advantage of voltage tunability as a method to optimize its performance, which was the central investigation of this work. This performance, achieved via a facile and cost-effective synthesis method, positions our CoSb2O6 nanostructures as a highly promising candidate for propane sensing applications.
Additionally, the features of the thick films, particularly their thickness and effective surface area [56,62,63], contributed to optimizing the contact area where propane molecules reacted [57,63]. Another factor affecting the dynamic response was voltage [45,46]. Specifically, the sensor’s electrical resistance consistently decreased as the applied voltage was increased from 1 V to 12 V (Figure 8 and Figure 10). This behavior, which demonstrates that the applied field can modulate the material’s conductivity, directly influences the calculated sensor response.
The observed reduction in electrical resistance can be interpreted as a classic phenomenon in semiconductor physics [64,65]. This behavior is strongly related to the modulation of potential barriers at grain boundaries. In a nanostructured material like this, an increasing electric field provides sufficient energy for charge carriers (holes) to more easily overcome these barriers, leading to higher conductivity [65,66]. Of course, the specific voltage-dependent behavior ultimately depends on the material’s intrinsic characteristics, including its p-type nature, doping level, and the thick-film geometry [67,68].
Additionally, the literature reports that self-heating due to the Joule effect can occur, wherein energy dissipation from the applied voltage raises the semiconductor’s local temperature [66,67]. This heating further enhances the mobility of charge carriers, contributing to the reduction in resistance. The kinetic energy imparted to these carriers by the electrical power can be described by the expression [45], , where m is the mass of the charge carriers (kg), v is their velocity (m/s), Isen is the current in the sensor (A), and k is a constant. This equation relates the velocity of charge carriers to the applied voltage and sensor current. Applying Ohm’s law, it can be rewritten as [45] Based on this formula, the charge carrier velocity can be optimized, improving the semiconductor’s response.
In a previous study [46], we reported that an activation voltage of 7–25 V in a semiconductor with a trirutile-type crystalline structure, such as MgSb2O6, enhanced the dynamic response (i.e., operational stability, capacity, efficiency, and reproducibility) at 400 °C [16,46]. We also observed that increasing the voltage and the operating temperature led to higher energy at the sensor’s surface, triggering a strong reaction between oxygen ions and the test gas (also, ), thereby improving the oxide’s electrical response [45,46]. The similar effect observed here with CoSb2O6 (using 1–12 V at 300 °C) reinforces the conclusion that applied voltage is a key and predictable tuning parameter for this family of materials.
5. Conclusions
A simple and cost-effective microwave-assisted colloidal method was successfully employed to synthesize CoSb2O6 powders with a trirutile-type crystalline structure at a relatively low temperature (700 °C). The comprehensive characterization (SEM, TEM, UV-Vis, Raman) confirmed the formation of nanostructured material with features ideal for gas sensing, including a high density of nanoparticles (~21 nm) and a suitable optical bandgap of ~1.84 eV.
The central finding of this study is that the propane response of the CoSb2O6 sensor is tunable by the applied voltage. Dynamic tests at 300 °C demonstrated that systematically varying the voltage from 1 V to 12 V directly modulates the sensor’s electrical properties and response. The mechanism is attributed to the increased energy facilitating the reaction between propane and adsorbed oxygen species on the nanostructured surface. This resulted in excellent stability and efficiency, with the highest sensitivity observed at low voltages (1, 3, and 6 V).
These findings not only position nanostructured CoSb2O6 as a highly promising candidate for propane detection but also validate voltage modulation as a viable and simple electronic strategy for optimizing the performance of trirutile-based sensors. This offers a new dimension for the design of adaptable gas detection systems where performance can be fine-tuned without requiring chemical or thermal adjustments.
Author Contributions
Research Supervisors: H.G.B., L.I.J.A., A.G.B., J.T.G.B. and J.A.R.O. Research: H.G.B., L.I.J.A., A.G.B., J.T.G.B., A.C.Z. and E.H.P. Formal Analysis: H.G.B., L.I.J.A., V.M.R.B., A.G.B., J.T.G.B., A.C.Z. and E.H.P. Methodology: L.I.J.A., E.H.P., A.G.B., V.M.R.B., H.G.B., A.C.Z. and J.A.R.O. Writing—Review and Editing: L.I.J.A., H.G.B., A.G.B., J.T.G.B. and J.A.R.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The results of this work are available upon request to the corresponding author via e-mail. The data that support the findings of this study are available from the corresponding authors upon request.
Acknowledgments
The authors express their gratitude to Mexico’s Secretariat of Science, Humanities, Technology, and Innovation (SECIHTI) and the University of Guadalajara for their support. Lucía Ivonne Juárez Amador thanks SECIHTI for a postdoctoral fellowship. Likewise, we thank Víctor Manuel Soto García, Jacob Morales Bautista, Darío Pozas Zepeda, Juan Pablo Parada Vázquez, and M. de la Luz Olvera-Amador for their technical assistance. This research was carried out following the line of research “Nanostructured Semiconductor Oxides” of the academic group UDG–CA–895 “Nanostructured Semiconductors” of CUCEI, University of Guadalajara.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jana, R.; Hajra, S.; Rajaitha, P.M.; Mistewicz, K.; Kim, H.J. Recent advances in multifunctional materials for gas sensing applications. J. Environ. Chem. Eng. 2022, 10, 108543. [Google Scholar] [CrossRef]
- Tladi, B.C.; Kroon, R.E.; Swart, H.C.; Motaung, D.E. A holistic review on the recent trends, advances, and challenges for high-precision room temperature liquefied petroleum gas sensors. Anal. Chim. Acta 2023, 1253, 341033. [Google Scholar] [CrossRef]
- Kowsuki, K.; Navamathavan, R. Facile synthesis and characterizations of Mn-doped nickel cobalt oxide nanoparticles with improved electrochemical performance for energy storage applications. Surf. Interfaces 2025, 56, 105641. [Google Scholar] [CrossRef]
- Fu, Y.; Nie, Y.; Zhao, Y.; Wang, P.; Xing, L.; Zhang, Y.; Xue, X. Detecting Liquefied Petroleum Gas (LPG) at Room Temperature Using ZnSnO3/ZnO Nanowire Piezo-Nanogenerator as Self-Powered Gas Sensor. ACS Appl. Mater. Interfaces 2015, 7, 10482–10490. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Ortega, J.A.; Guillén-Bonilla, H.; Guillén-Bonilla, A.; Rodríguez-Betancourt, V.M.; Sánchez-Martínez, A.; Guillén-Bonilla, J.-T.; Gildo-Ortiz, L.; Huízar-Padilla, E.; Reyes-Gómez, J. Synthesis of the oxide NiSb2O6 and its electrical characterization in toxic atmospheres for its application as a gas sensor. J. Mater. Sci. Mater. Electron. 2022, 33, 18268–18283. [Google Scholar] [CrossRef]
- Terna, A.D.; Elemike, E.E.; Mbonu, J.I.; Osafile, O.E.; Ezeani, R.O. The future of semiconductor nanoparticles: Synthesis, properties, and applications. Mater. Sci. Engl. B 2021, 272, 115363. [Google Scholar] [CrossRef]
- Huston, M.; DeBella, M.; DiBella, M.; Gupta, A. Green Synthesis of Nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef] [PubMed]
- Lite, M.C.; Constantinescu, R.; Tănăsescu, E.C.; Kuncser, A.; Romanițan, C.; Mihaiescu, D.E.; Lacatusu, I.; Badea, N. Phytochemical Synthesis of Silver Nanoparticles and Their Antimicrobial Investigation on Cotton and Wool Textiles. Materials 2023, 16, 3924. [Google Scholar] [CrossRef]
- Butt, M.A. Loop-Terminated Mach–Zehnder Interferometer Integrated with Functional Polymer for CO2 Gas Sensing. Appl. Sci. 2024, 14, 4714. [Google Scholar] [CrossRef]
- Michel, C.R.; López-Contreras, N.L.; López-Alvarez, M.A.; Martínez-Preciado, A.H. Gas selectivity of nanostructured ZnSb2O6 synthesized by a colloidal method. Sens. Actuators B Chem. 2012, 171–172, 686–690. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.; Sing, A.; Rathore, S.; Yadav, B.C.; Tandon, P. Nanostructured cobalt antimonate: A fast responsive and highly stable sensing material for liquefied petroleum gas detection at room temperature. RSC Adv. 2020, 10, 33770–33781. [Google Scholar] [CrossRef]
- Guillén-Bonilla, H.; Gildo-Ortiz, L.; Olvera-Amador, M.L.; Santoyo-Salazar, J.; Rodríguez-Betancourtt, V.M.; Guillen-Bonilla, A.; Reyes-Gómez, J. Response of Mesoporous CoSb2O6 Nanoparticles to Gaseous CO and C3H8 at Low Temperatures. J. Nanomater. 2015, 2015, 308465. [Google Scholar] [CrossRef]
- More, M.A.; More, S.A.; Femi, M.D.; Jain, G.H.; Shinde, S.D.; Patil, Y.D.; Kajale, D.D.; Patil, G.E. Hydrothermally synthesized nanostructured NiTiO3 thick films for H2S and room temperature CO2 gas sensing. J. Mater. Sci. Mater. Electron. 2024, 35, 1706. [Google Scholar] [CrossRef]
- Morán-Lázaro, J.P.; Courel-Piedrahita, M.; Guillén-Bonilla, A.; López-Urías, F.; Guillén-Bonilla, H.; Soto-García, V.M.; Palafox-Corona, A.; Hernández-Poot, D.A. A Novel Sensor for the Detection of n-Butanol Based on CoMn2O4 Nanoparticles. Electron. Mater. Lett. 2024, 20, 610–620. [Google Scholar] [CrossRef]
- Michel, C.R.; Guillén-Bonilla, H.; Martínez-Preciado, A.H.; Morán-Lázaro, J.P. Synthesis and gas sensing properties of nanostructured CoSb2O6 microspheres. Sens. Actuators B Chem. 2009, 143, 278–285. [Google Scholar] [CrossRef]
- Guillén-Bonilla, H.; Guillén-Bonilla, J.T.; Rodríguez-Betancourtt, V.-M.; Ramírez-Ortega, J.A.; Morán Lázaro, J.P.; Guillén-Bonilla, A. Synthesis and Sensing Response of Magnesium Antimoniate Oxide (MgSb2O6) in the Presence of Propane Atmospheres at Different Operating Voltages. Sensors 2024, 24, 2147. [Google Scholar] [CrossRef]
- Jamal, A.; Rahman, M.M.; Khan, S.B.; Faisal, M.; Akhtar, K.; Rub, M.A.; Asiri, A.M.; Al-Youbi, A.O. Cobalt doped antimony oxide nano-particles based chemical sensor and photo-catalyst for environmental pollutants. Appl. Surf. Sci. 2012, 261, 52–58. [Google Scholar] [CrossRef]
- Arunkumar, N.; Naraginti, S. Facile synthesis of nanostructured trirutile antimonates M(II)Sb2O6 (M = Co, Cu, Ni, Fe) and its visible photocatalytic studies. Inorg. Nano-Met. Chem. 2022, 52, 151–160. [Google Scholar] [CrossRef]
- Haeuseler, H. Infrared and Raman spectra and normal coordinate calculations on trirutile-type compounds. Spectrochim. Acta Part A Mol. Spectrosc. 1981, 37, 487–495. [Google Scholar] [CrossRef]
- Larcher, D.; Prakash, A.S.; Laffont, L.; Womes, M.; Jumas, J.C.; Olivier-Fourcade, J.; Hedge, M.S.; Tarascon, J.M. Reactivity of antimony oxides and MSb2O6 M = (Cu, Ni, Co), trirutile-type phases with metallic lithium. J. Electrochem. Soc. 2006, 153, A1778–A1787. [Google Scholar] [CrossRef]
- Ham, K.; Hong, S.; Kang, S.; Cho, K.; Lee, J. Extensive Active-Site Formation in Trirutile CoSb2O6 by Oxygen Vacancy for Oxygen Evolution Reaction in Anion Exchange Membrane Water Splitting. ACS Energy Lett. 2021, 6, 364–370. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.; Singh, A.; Tandon, P. A stable and highly sensitive room-temperature liquefied petroleum gas sensor based on nanocubes/cuboids of zinc antimonate. RSC Adv. 2020, 10, 20349–20357. [Google Scholar] [CrossRef] [PubMed]
- Sunku, M.; Gundeboina, R.; Shilpa-Chakra, C.H.; Kaniki-Reddy, V.; Vithal, M. Preparation, characterization and photocatalytic activity studies of C- and N- doped CoSb2O6. Inorg. Chem. Commun. 2021, 134, 109064. [Google Scholar] [CrossRef]
- Vanzetti, F.; Guzmán, H.; Hernández, S. Solid-State Reaction Synthesis of CoSb2O6-Based Electrodes Towards Oxygen Evolution Reaction in Acidic Electrolytes: Effects of Calcination Time and Temperature. Catalysts 2025, 15, 68. [Google Scholar] [CrossRef]
- Nikulin, A.Y.; Zvereva, E.A.; Nalbandyan, V.B.; Shukaev, I.L.; Kurbakov, A.I.; Kuchugura, M.D.; Raganyan, G.V.; Popov, Y.V.; Ivanchenko, V.D.; Vasiliev, A.N. Preparation and characterization of metastable trigonal layered MSb2O6 phases (M = Co, Ni, Cu, Zn, and Mg) and considerations on FeSb2O6. Dalton Trans. 2017, 46, 6059–6068. [Google Scholar] [CrossRef]
- Michel, C.R.; Martínez-Preciado, A.H.; Morán-Lázaro, J.P. Effect of the frequency on the gas sensing response of CoSb2O6 prepared by a colloidal method. Sens. Actuators B Chem. 2009, 140, 149–154. [Google Scholar] [CrossRef]
- Chen, J.; Tang, M.; Wang, G.; Liu, L.; Hu, X.; Liao, H.; Hu, X. Facile preparation of CoSb2O6/rGO composite as the anode material of lithium-ion batteries, Materials Letters. Mater. Lett. 2024, 354, 135346. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxides gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Yang, S.; Lei, G.; Xu, H.; Lan, Z.; Wang, Z.; Gu, H. Metal Oxide Based Heterojunctions for Gas Sensors: A Review. Nanomaterials 2021, 11, 1026. [Google Scholar] [CrossRef]
- Mizoguchi, H.; Woodward, P.M. Electronic structure studies of main group oxides possessing edge-sharing octahedra: Implications for the design of transparent conducting oxides. Chem. Mat. 2004, 16, 5233–5248. [Google Scholar] [CrossRef]
- Michel, C.R.; Martínez, A.H.; Jiménez, S. Gas sensing response of nanostructured trirutile-type CoSb2O6 synthesized by solution-polymerization method. Sens. Actuators B Chem. 2008, 132, 45–51. [Google Scholar] [CrossRef]
- Jocić, M.; Dašić, M.; Holl, K.; Ilić, D.; Mentus, S. Gel-combustion synthesis of CoSb2O6 and its reduction to powdery Sb2Co alloy. J. Serb. Chem. Soc. 2009, 74, 53–60. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y. Solution-based synthetic strategies for 1-D. nanostructures. Inorg. Chem. 2006, 45, 7522–7534. [Google Scholar] [CrossRef]
- Gorbunov, V.V.; Shidlovskii, A.A.; Shmagin, L.F. Combustion of transition-metal ethylenediamine nitrates. Combust. Explos. Shock. Waves 1983, 19, 172–173. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, C.; Maheswari, A.R.; Lee, D.W. Structural, optical, and selective ethanol sensing properties of p-type semiconducting CoNb2O6 nanopowder. Sens. Actuators B Chem. 2014, 205, 289–297. [Google Scholar] [CrossRef]
- Shur, M. Wide band gap semiconductor technology: State-of-the-art. Solid-State Electron. 2019, 155, 65–75. [Google Scholar] [CrossRef]
- Atri, S.; Uma, S.; Nagarajan, R. Magnetic and photocatalytic properties of nano-sized sulfur-doped trirutile oxide, CuSb2O6. Mater. Sci. Semicond. Process. 2020, 119, 105226. [Google Scholar] [CrossRef]
- NOAA’s Office of Response and Restoration. CAMEO Chemicals. Available online: https://cameochemicals.noaa.gov/search/simple (accessed on 2 March 2025).
- Gardner, D.E.; Bishop, E.C.; Chi-Chen, L.; Dixit, R.; Gabrielson, K.L.; Johason, G.; Kelly, D.P.; Macys, D.A.; Morandi, M.T.; Oesch, F.; et al. Acute Exposure Guideline Levels for Selected Airborne Chemicals; National Academy of Sciences: Washington, DC, USA, 2012; Volume 12, pp. 1–100. [Google Scholar]
- Arshak, K.; Moore, E.; Lyons, G.M.; Harris, J.; Clifford, S. A review of gas sensors employed in electronic nose applications. Sens. Rev. 2004, 24, 181–198. [Google Scholar] [CrossRef]
- Cavallari, M.R.; Pastrana, L.M.; Sosa, C.D.F.; Marquina, A.M.R.; Izquierdo, J.E.E.; Fonseca, F.J.; Amorim, C.A.d.; Paterno, L.G.; Kymissis, I. Organic Thin-Film Transistors as Gas Sensors: A Review. Materials 2021, 14, 3. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Wetchakun, K.; Samerjai, T.; Tamaekong, N.; Liewhiran, C.; Siriwong, C.; Kruefu, V.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Semiconducting metal oxides as sensors for environmentally hazardous gases. Sens. Actuators B Chem. 2011, 160, 580–591. [Google Scholar] [CrossRef]
- Guillén-Bonilla, A.; Guillén-Bonilla, J.T.; Guillén-Bonilla, H.; Huízar-Padilla, E.; Casillas-Zamora, A.; Olvera-Amador, M.L.; Rodríguez-Betancourtt, V.M. A theoretical and experimental study on the dynamic response in propane atmospheres of sensors made from trirutile magnesium antimonate powders. J. Mater. Sci. Mater. Electron. 2024, 35, 1849. [Google Scholar] [CrossRef]
- Juárez-Amador, L.I.; Guillén-Bonilla, H.; Guillén-Bonilla, A.; Guillén-Bonilla, J.T.; Morales-Bautista, J.; Casillas-Zamora, A.; Rodríguez-Betancourtt, V.M.; Olvera-Amador, M.L. Photocatalytic and sensing properties in propane atmospheres of MgSb2O6 nanoparticles synthesized by a chemical method. J. Mater. Sci. Mater. Electron. 2024, 35, 1857. [Google Scholar] [CrossRef]
- Panneer-Selvam, G.K.; Olvera Amador, M.L.; Maldonado-Álvarez, A. Gas sensing capabilities of sol-gel dip-coated pure SnO2 thin films for CO and C3H8 detection. J. Mater. Sci. Mater. Electron. 2024, 35, 1621. [Google Scholar] [CrossRef]
- Rydosz, A.; Szkudlarek, A. Gas-Sensing Performance of M-Doped CuO-Based Thin Films Working at Different Temperatures upon Exposure to Propane. Sensors 2015, 15, 20069–20085. [Google Scholar] [CrossRef]
- Gao, X.; Chen, Y.; Xu, P.; Zheng, D.; Li, X. γ-Fe2O3-Based MEMS Gas Sensor for Propane Detection. Electronics 2025, 14, 1050. [Google Scholar] [CrossRef]
- Shooshtari, M.; Vollebregt, S.; Vaseghi, Y.; Rajati, M.; Pahlavan, S. The sensitivity enhancement of TiO2-based VCOs Sensor decorated by gold at room temperature. Nanotecchnology 2023, 34, 255501. [Google Scholar] [CrossRef]
- Shooshtari, M. Gold-decorated vertically aligned carbon nanofibers for high-performance room-temperature ethanol sensing. Microchim. Acta 2025, 192, 517. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, Y.; Zhou, P.; Lu, R.; Li, A.; Zhao, S.; Liu, W.; Wei, D.; Wei, K. Fabrication, characterization and n-propanol sensing properties of perovskite-type ZnSnO3 nanospheres based gas sensor. Appl. Surf. Sci. 2020, 509, 146335. [Google Scholar] [CrossRef]
- Almaev, A.V.; Kopyev, V.V.; Novikov, V.A.; Chikiryaka, A.V.; Yakovlev, N.N.; Usseinov, A.B.; Karipbayev, Z.T.; Akilbekov, A.T.; Koishybayeva, Z.K.; Popov, A.I. ITO Thin Films for Low-Resistance Gas Sensors. Materials 2023, 16, 342. [Google Scholar] [CrossRef]
- Meena, D.; Jain, M.; Bhatnagar, M.C. Resistive gas sensors based on nanostructured ternary metal oxide: A review. J. Mater. Sci. 2024, 59, 12177–12218. [Google Scholar] [CrossRef]
- Ananya, D. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Engl. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Regmi, G.; Rohini, M.; Reyes-Figueroa, P.; Maldonado, A.; Olvera, M.L.; Velumani, S. Deposition and characterization of ultrathin intrinsic oxide (i-ZnO) films by radio frequency (RF) sputtering for propane gas sensing application. J. Mater. Sci: Mater. Electron. 2018, 29, 15682–15692. [Google Scholar] [CrossRef]
- Bharati, K.; Tiwari, P.R.; Singh, R.P.; Singh, A.; Yadav, B.C.; Singh, M.P.; Kumar, S. Synthesis of bismuth-doped praseodymium ortho ferrite nanomaterials for LPG sensing. Appl. Nanosci. 2024, 14, 277–289. [Google Scholar] [CrossRef]
- Aishwarya, K.; Nirmala, R.; Navamathavan, R. Recent advancements in liquefied petroleum gas sensors: A topical review. Sens. Int. 2021, 2, 100091. [Google Scholar] [CrossRef]
- Mostafa, S.; Alireza, S. An electronic nose based on carbon nanotube -titanium dioxide hybrid nanostructures for detection and discrimination of volatile organic compounds. Sens. Actuators B Chem. 2022, 357, 131418. [Google Scholar] [CrossRef]
- Karthik, T.V.K.; Olvera-Amador, M.L.; Maldonado, A.; Hernandez, A.G.; Gómes-Pozos, H. Propane gas-sensing properties of pure and Pd-doped tin oxide nanostructures. J. Mater. Sci. Mater. Electron. 2023, 34, 228. [Google Scholar] [CrossRef]
- Norizan, M.N.; Abdullah, N.; Halim, N.A.; Demon, S.Z.N.; Mohamad, I.S. Heterojunctions of rGO/Metal Oxide Nanocomposites as Promising Gas-Sensing Materials—A Review. Nanomater 2022, 12, 2278. [Google Scholar] [CrossRef] [PubMed]
- Nadargi, D.Y.; Umar, A.; Nadargi, J.D.; Lokare, S.A.; Mulla, I.S.; Suryavanshi, S.S.; Chaskar, M.G. Gas sensors and factors influencing sensing mechanism with a special focus on MOS sensors. J. Mater. Sci. 2023, 58, 559. [Google Scholar] [CrossRef]
- Pasupuleti, K.S.; Chougule, S.S.; Vidyasagar, D.; Bak, N.; Jung, N.; Kim, Y.H.; Lee, J.H.; Kim, S.G.; Kim, M.D. UV light driven high-performance room temperature surface acoustic wave NH3 gas sensor using sulfur-doped g-C3N4 quantum dots. Nano Res. 2023, 16, 7682–7695. [Google Scholar] [CrossRef]
- Senanayak, S.P.; Dey, K.; Shivanna, R.; Li, W.; Ghosh, D.; Zhang, Y.; Roose, B.; Zelewski, S.J.; Andaji-Garmaroudi, Z.; Wood, W.; et al. Charge transport in mixed metal halide perovskite semiconductors. Nat. Mater. 2023, 22, 216–224. [Google Scholar] [CrossRef]
- Bruevich, V.; Patel, Y.; Singer, J.P.; Podzorov, V. Significant Joule self-heating pervasive in the emergent thin-film transistor studies. J. Mater. Chem. C 2024, 12, 17802. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Yu, Z.; Lou, S.; Qu, Y.; Chang, Y. An Investigation into the Comprehensive Impact of Self-Heating and Hot Carrier Injection. Electronics 2022, 11, 2753. [Google Scholar] [CrossRef]
- Chakraborty, S.; Amir, W.; Shin, J.-W.; Shin, K.-Y.; Cho, C.-Y.; Kim, J.-M.; Hoshi, T.; Tsutsumi, T.; Sugiyama, H. Explicit Thermal Resistance Model of Self-Heating Effects of AlGaN/GaN HEMTs with Linear and Non-Linear Thermal Conductivity. Materials 2022, 15, 8415. [Google Scholar] [CrossRef] [PubMed]
- Pop, E. Energy dissipation and transport in nanoscale devices. Nano Res. 2010, 3, 147–169. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).