Enhanced Photocathodic Protection Performance of TiO2/NiCo2S4 Composites for 304 Stainless Steel
Abstract
1. Introduction
2. Experimental
2.1. Raw Materials
2.2. Preparation of Photoelectrodes
2.3. Characterization
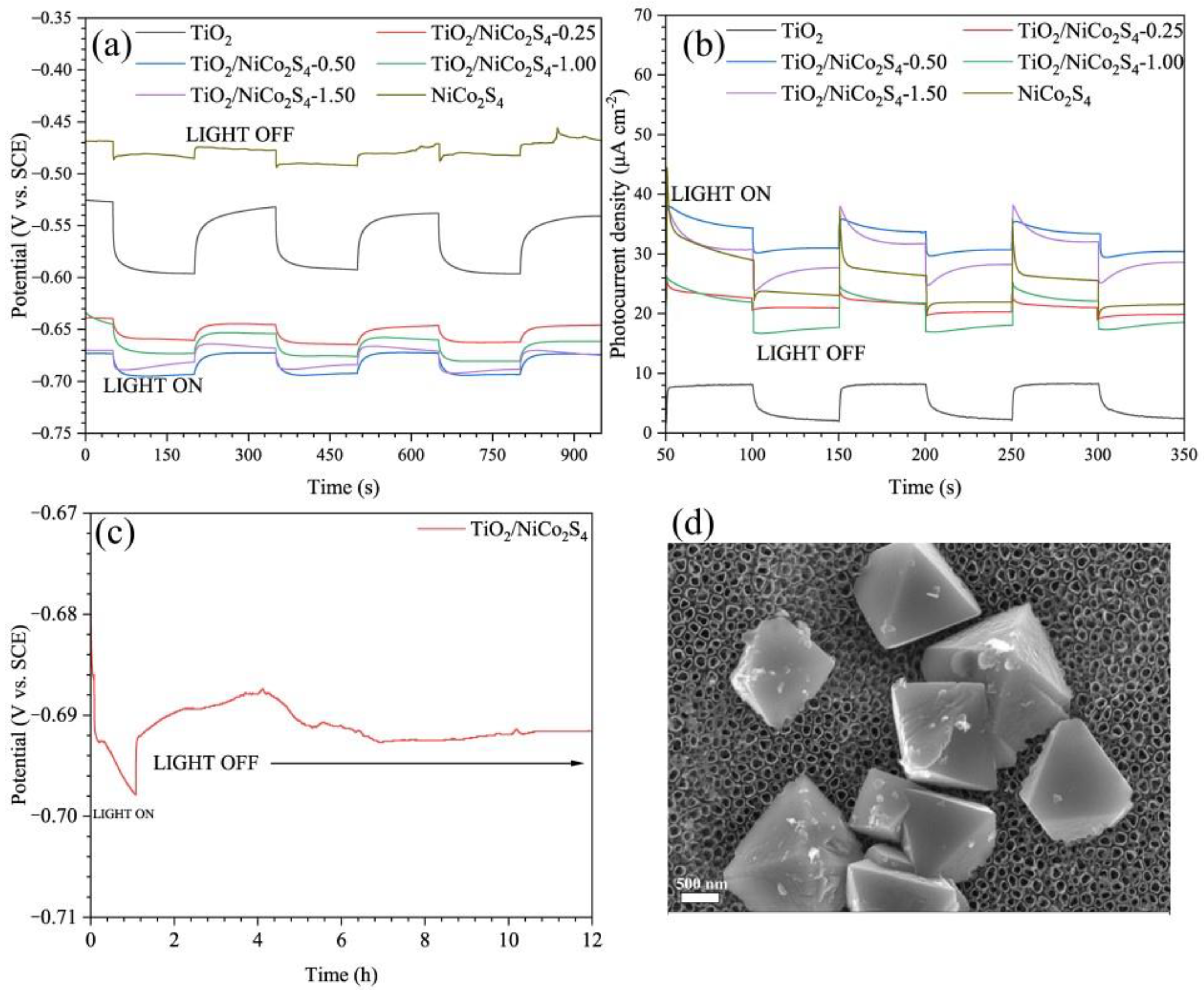
2.4. Photoelectrochemical Tests
3. Results and Discussion
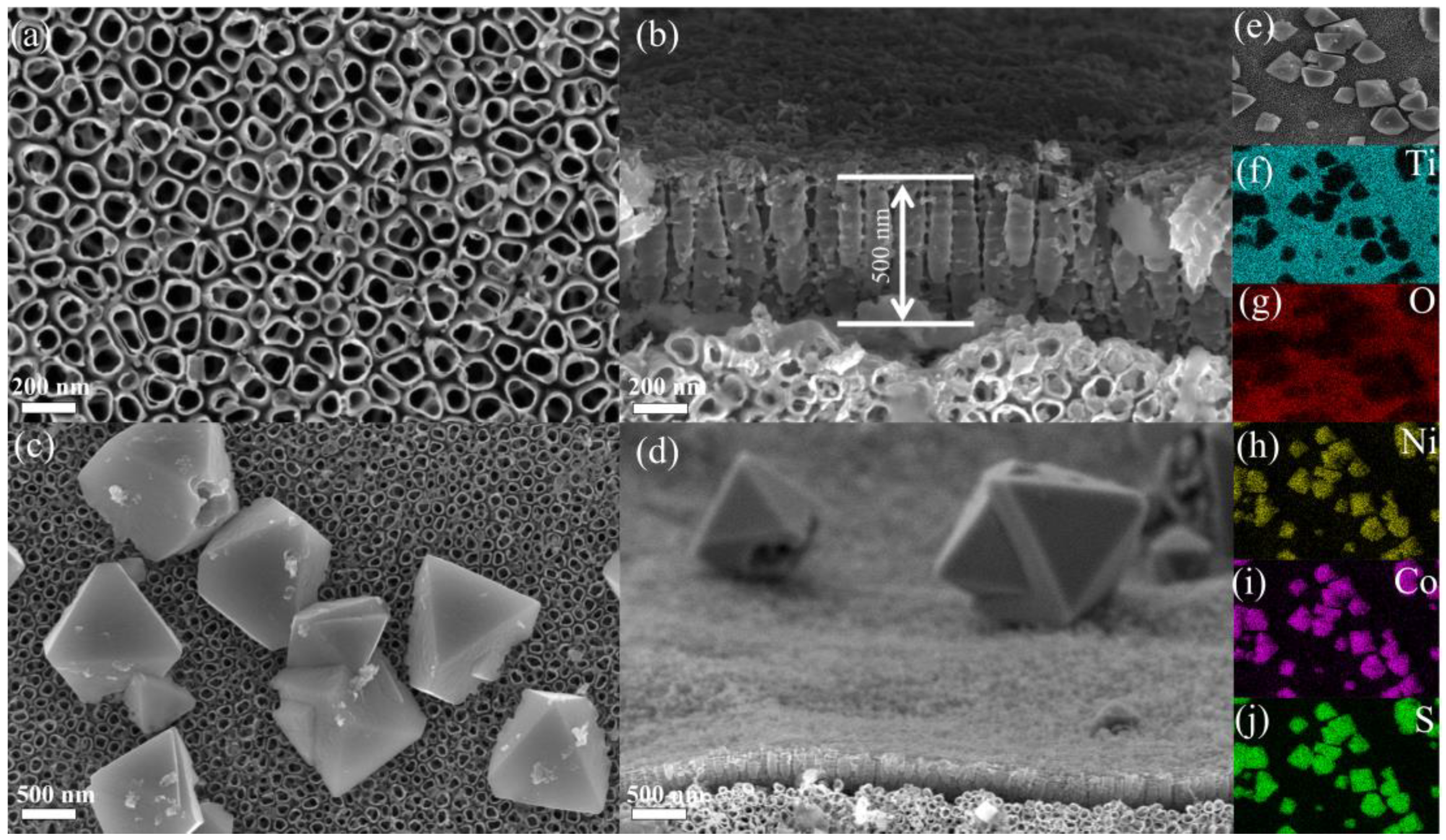
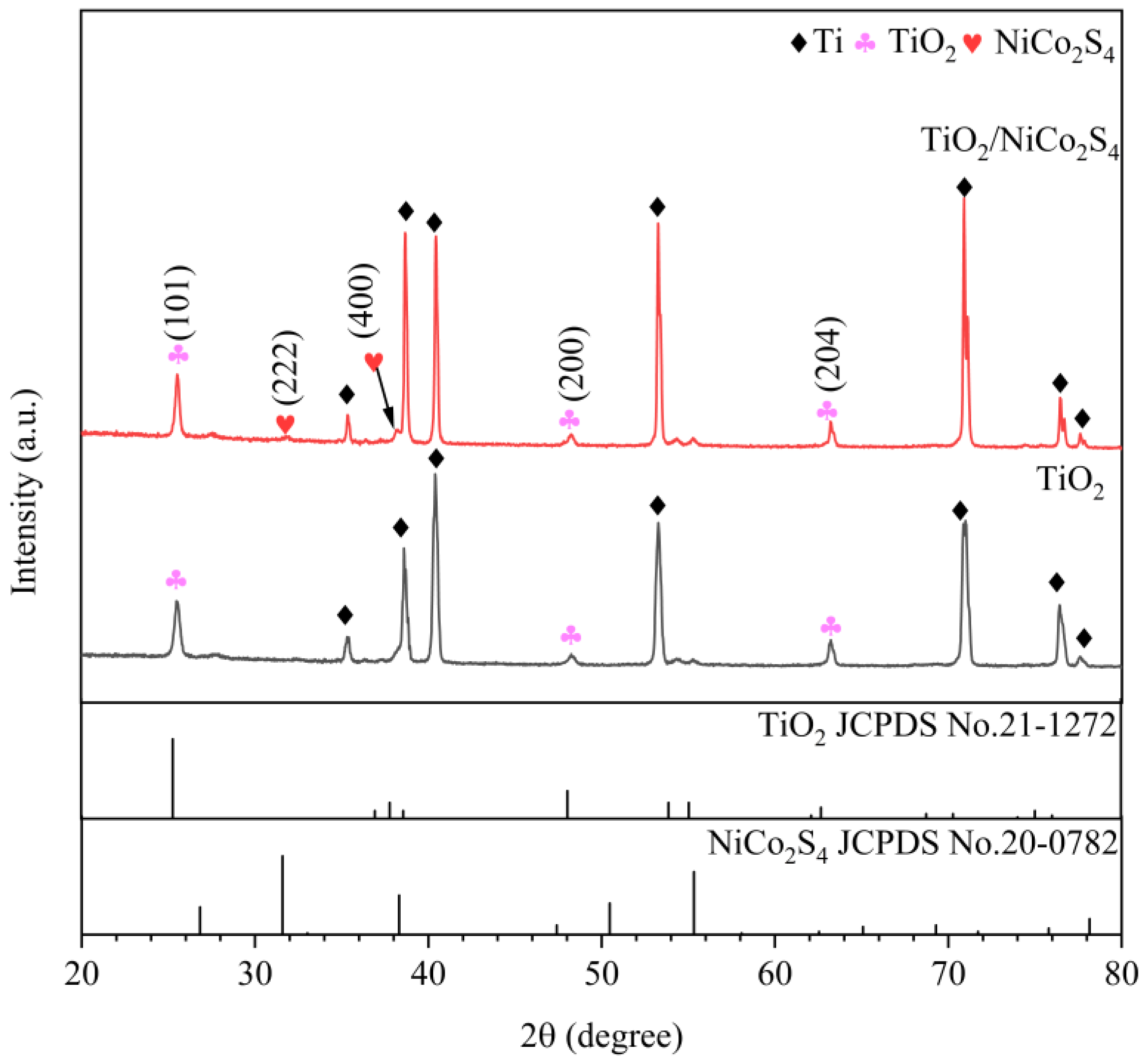
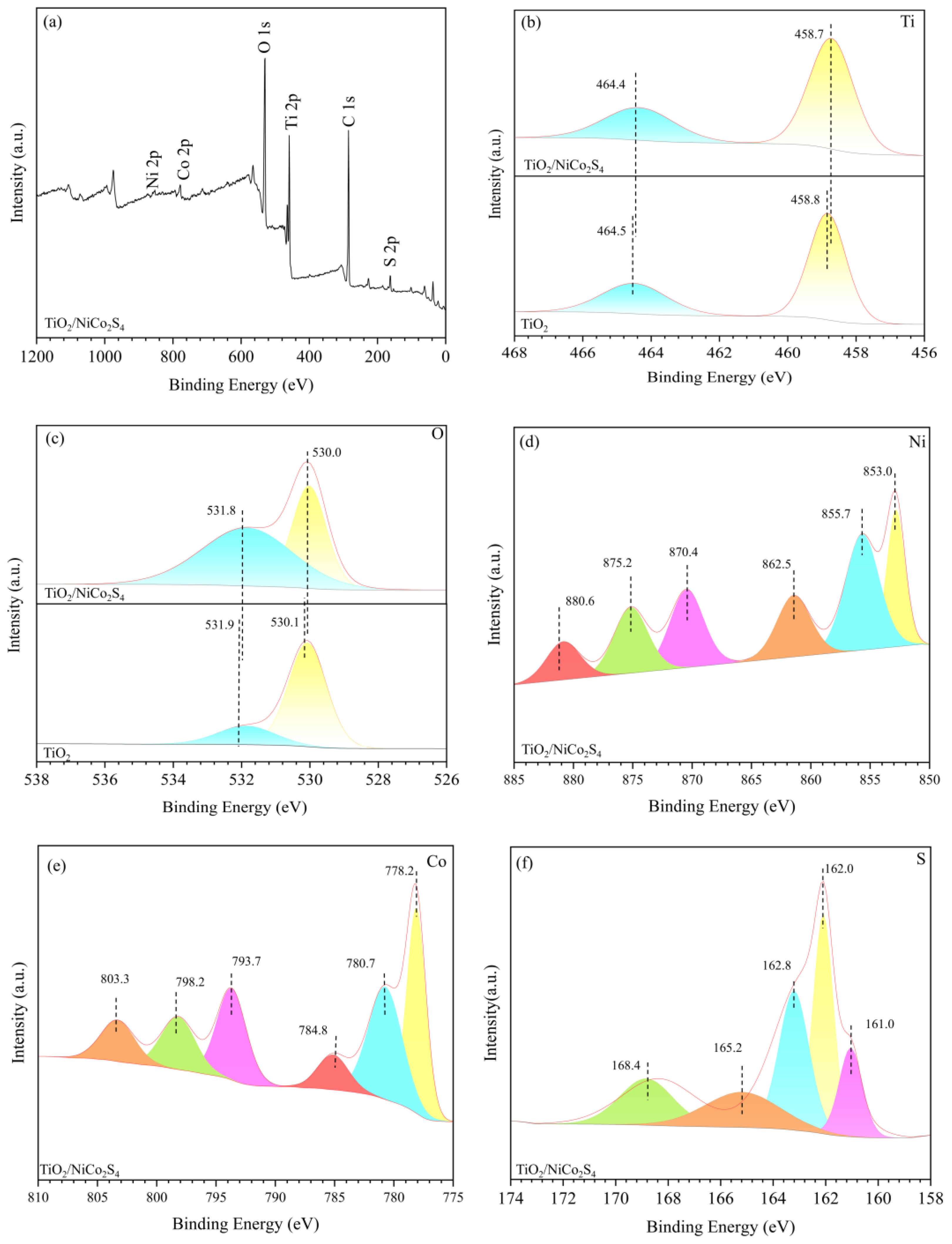
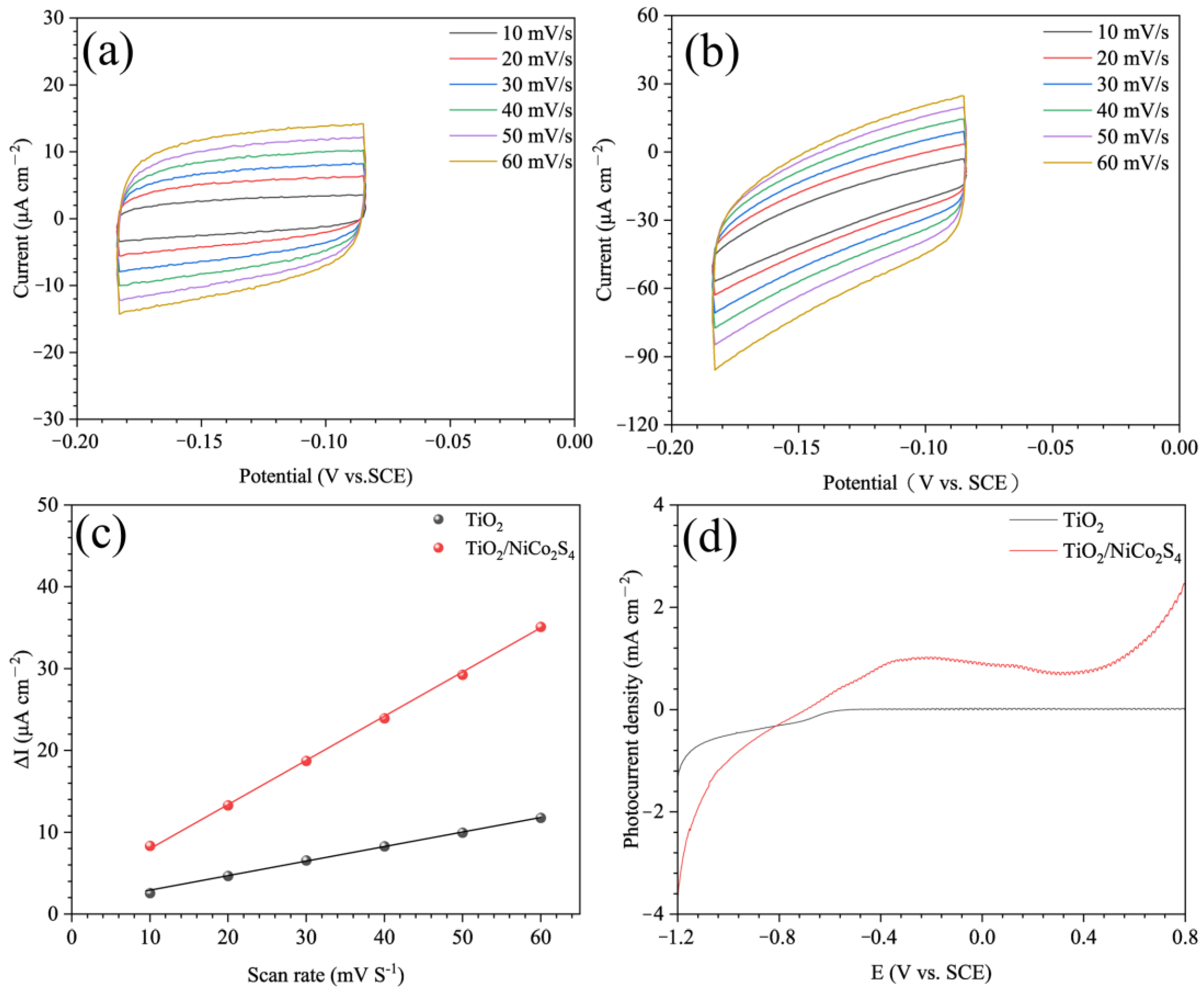
3.1. Morphology and Characterization
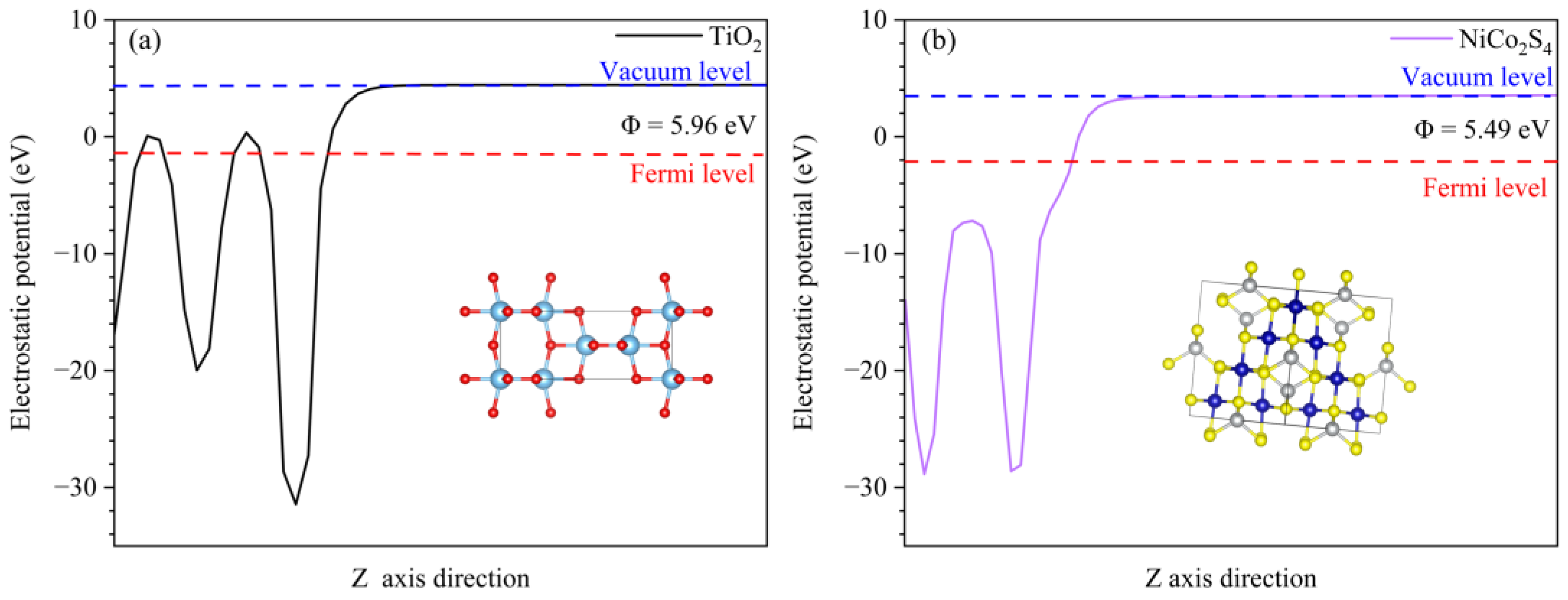
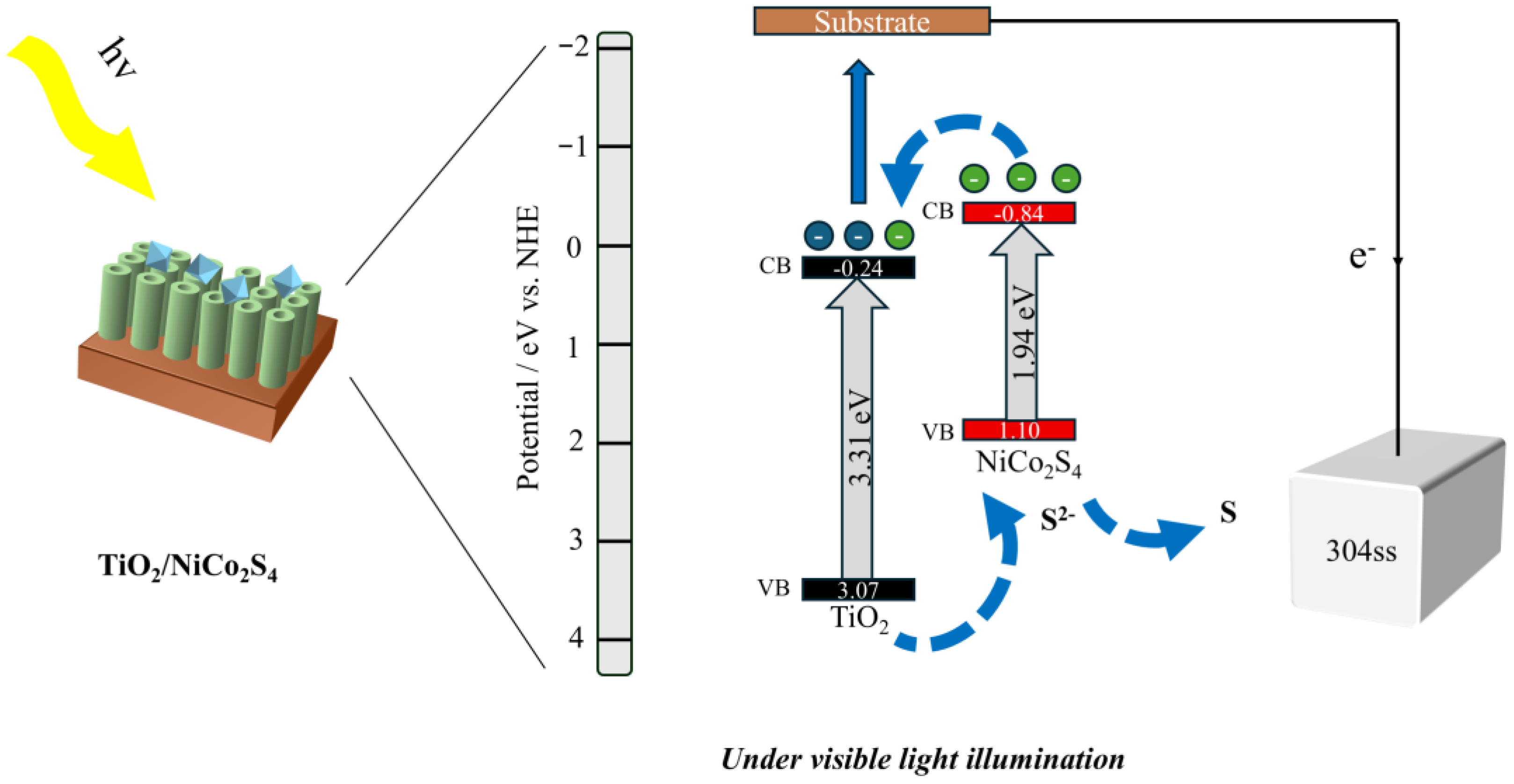
3.2. Mechanism of the TiO2/NiCo2S4 Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Xu, H.; Nan, Y.; Sun, X.; Duan, J.; Huang, Y.; Hou, B. Research progress of TiO2 photocathodic protection to metals in marine environment. J. Oceanol. Limnol. 2020, 38, 1018. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, Z.; Zhang, H.; Li, Z.; Peng, H.; Kawi, S.; Xie, A.; Qiu, P. In-situ fabrication of TiO2/ZIF-67 composite film with electron storage characteristics for significantly enhanced photoelectrochemical cathodic protection of 304 stainless steel. Chem. Eng. J. 2025, 503, 158693. [Google Scholar] [CrossRef]
- Pang, S.; Zhou, C.; Sun, Y.; Zhang, K.; Ye, W.; Zhao, X.; Cai, L.; Hui, B. Natural wood-derived charcoal embedded with bimetallic iron/cobalt sites to promote ciprofloxacin degradation. J. Clean. Prod. 2023, 414, 137569. [Google Scholar] [CrossRef]
- Ye, M.; Yu, J.; Wang, T.; Gao, R. Fabrication and Photocathodic Protection Performance of Bi2S3/CdS/TiO2 Nanocomposites for 304 Stainless Steel. J. Chin. Soc. Corros. Prot. 2024, 44, 372. [Google Scholar]
- An, H.; Jiang, C.; Yin, X.; Liu, K.; Liang, S.; Wang, X.; Xiao, J.; Zhao, X.; Sun, Z. Polyaniline/TiO2/MXene Ternary Composites for Enhancing Corrosion Resistance of Waterborne Epoxy Coatings. ACS Appl. Nano Mater. 2024, 8, 340. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, G.; Wang, X.; Xu, H.; Wang, C.; Yao, Q.; Chi, J.; Fu, X.; Wang, Y.; Yin, X.; et al. Progress in semiconductor materials for photocathodic protection: Design strategies and applications in marine corrosion protection. Chemosphere 2023, 323, 138194. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gou, G.; Qiu, X.; Feng, Q.; Zhang, K.; Qin, S.; Gao, W. Research on Regulation Mechanism of TiO2 Photocathodic Protection Based on Cohesive Energy, Defect Patterns, and Nanofilm Size. J. Mater. Eng. Perform. 2025, 34, 2398. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Zhang, H.; Deng, Y.; Tan, B.; Wang, J.; Yang, T.; Wang, W. Construction of S-doped cellulose nanocrystals with edge sulfur vacancies to enhance the generation of 1O2 and promote peroxymonosulfate (PMS) activation. Appl. Surf. Sci. 2024, 671, 160717. [Google Scholar] [CrossRef]
- Xu, S.; Li, Z.; Wen, J.; Qiu, P.; Xie, A.; Peng, H. Review of TiO2-Based Heterojunction Coatings in Photocathodic Protection. ACS Appl. Nano Mater. 2024, 7, 8464. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Shen, S. Rational engineering of semiconductor-based photoanodes for photoelectrochemical cathodic protection. Chem. Phys. Rev. 2024, 5, 11302. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.; Li, W.; Li, G.; Liu, H.; Yang, Z.; Li, Y.; Li, H. Enhanced visible light-driven photocathodic protection performance of CuBi2O4 modified TiO2 nanotube array S-type nano-heterojunction photoanodes for 316 stainless steel. Appl. Surf. Sci. 2025, 681, 161503. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, K.; Zhang, H.; Tan, J.; Gong, D.; Lu, G.; Qiu, P. Fabrication of Z-Scheme TiO2/Au/CdS Nanostructured Coating with Enhanced Photocathodic Protection Performance for Carbon Steel. Acs Appl. Nano Mater. 2023, 6, 2385–2393. [Google Scholar] [CrossRef]
- Ma, Z.; Ma, X.; Liu, N.; Wang, X.; Wang, L.; Hou, B. Study on the photocathodic protection of 304 stainless steel by Ag and In2S3 co-sensitized TiO2 composite. Appl. Surf. Sci. 2020, 507, 145088. [Google Scholar] [CrossRef]
- Qiao, L.-y.; Xie, F.-y.; Xie, M.-h.; Gong, C.-h.; Wang, W.-l.; Gao, J.-c. Characterization and photoelectrochemical performance of Zn-doped TiO2 films by sol-gel method. Trans. Nonferrous Met. Soc. China 2016, 26, 2109. [Google Scholar] [CrossRef]
- Momeni, M.M.; Motalebian, M.; Ghayeb, Y.; Atapour, M. Photoelectrochemical Cathodic Protection of Stainless Steel using W- and Cr-Doped/Codoped TiO2 Nanotube Thin Film Photoanodes. J. Electrochem. Soc. 2021, 168, 81504. [Google Scholar] [CrossRef]
- Podelinska, A.; Neilande, E.; Pankratova, V.; Serga, V.; Bandarenka, H.; Burko, A.; Piskunov, S.; Pankratov, V.A.; Sarakovskis, A.; Popov, A.I.; et al. Structural and Spectroscopic Characterization of TiO2 Nanocrystalline Materials Synthesized by Different Methods. Nanomaterials 2025, 15, 498. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xu, H.; Zhao, Q.; Zeng, H.; Lin, B.; Xiao, Y.; Tang, J.; Nie, Z.; Yan, Y.; Di, Z.; et al. Research of two kinds of PANI@semiconductor based photocathodic coating corrosion protection effect and mechanism. Anti-Corros. Methods Mater. 2024, 71, 650. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, H.; Zhang, Z.; Li, Z.; Xie, A.; Qiu, P.; Peng, H. High-efficiency photocathodic protection performance of novel TiO2/ZnCdS heterojunction films for Q235 carbon steel. Mater. Today Commun. 2024, 40, 110125. [Google Scholar] [CrossRef]
- Zu, W.; Pan, G.; Li, J.; Wang, S.; Zhang, G.; Liu, Y.; Liu, Y. Carbon nitride interlayer-enhanced TiO2/WO3 nanorods with surface oxygen vacancies for durable photocathodic performance on 304 stainless steel in simulated seawater. Chem. Eng. J. 2024, 490, 151533. [Google Scholar] [CrossRef]
- Rempel, A.A.; Valeeva, A.A.; Vokhmintsev, A.S.; Weinstein, I.A. Titanium dioxide nanotubes: Synthesis, structure, properties and applications. Russian Chem. Rev. 2021, 90, 1397. [Google Scholar] [CrossRef]
- Guo, Y.; Mao, L.; Tang, Y.; Shang, Q.; Cai, X.; Zhang, J.; Hu, H.; Tan, X.; Liu, L.; Wang, H.; et al. Concentrating electron and activating H-OH bond of absorbed water on metallic NiCo2S4 boosting photocatalytic hydrogen evolution. Nano Energy 2022, 95, 107028. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, Z.; Hu, C.; Wen, W.; Zhang, X.; Wang, S. NiCo2S4@ZnIn2S4-Assisted All-in-One Self-Powered electrochemical device for ultrasensitive detection and degradation of AFB1. Chem. Eng. J. 2023, 469, 143830. [Google Scholar] [CrossRef]
- Li, W.; Ma, H.; Liu, Z.; Li, J.; Fang, P.; Xiong, R.; Pan, C.; Wei, J. In situ electronic redistribution tuning of ZnIn2S4 nanosheets on NiCo2S4 hollow tube for boosted photocatalytic hydrogen evolution. Appl. Surf. Sci. 2022, 598, 153801. [Google Scholar] [CrossRef]
- Amritha, V.K.; Badhulika, S. Fabrication of broadband MnS/NiCo2S4 heterojunction photodetector by successive ionic layer adsorption and reaction technique. J. Photochem. Photobiol. A-Chem. 2024, 453, 115627. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Zheng, L.; Bao, C.; Sun, X. Synthesis of NiCo2S4 and their effect as counter electrodes on performance of dye-sensitized solar cells. Electron. Compon. Mater. 2016, 35, 44. [Google Scholar]
- Dong, Z.; Zhang, Q.; Shu, X.; Hu, J.; Han, S. Photo-assisted charging of heterostructured NiCo2S4@NiCo-LDH composite electrode with remarkable photoelectronic memory effect for high-performance asymmetric supercapacitor. Energy Convers. Manag. 2024, 315, 118769. [Google Scholar] [CrossRef]
- Fan, L.; Guo, X.; Wang, L.; Jin, Z.; Tsubaki, N. Excellent charge separation over NiCo2S4/CoTiO3 nanocomposites improved photocatalytic hydrogen production. Front. Chem. Sci. Eng. 2025, 19, 7. [Google Scholar] [CrossRef]
- Fu, Z.; Shu, X.; Zhang, Q.; Qin, D.; Han, S.; Dong, Z. Solar-driven induced photoelectron remember effect involved in core-shell NiCo2S4@Ni3V2O8 composite electrode with superior electrochemical energy storage for asymmetric supercapacitor. Energy Convers. Manag. 2025, 323, 119190. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Sahoo, M.R.; Ray, A.; Singh, N.; Harish, S.; Kumar, E.S.; Navaneethan, M. NiCo2S4 cocatalyst supported Si nanowire heterostructure for improved solar-driven water reduction: Experimental and theoretical insights. Sustain. Energy Fuels 2023, 7, 1687. [Google Scholar] [CrossRef]
- Shandilya, M.; Rai, R.; Singh, J. Review: Hydrothermal technology for smart materials. Adv. Appl. Ceram. 2016, 115, 354. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.T.; Zhang, L.; Hou, B.R. Preparation and photocathodic protection performance of CdSe/reduced graphene oxide/TiO2 composite. Corros. Sci. 2015, 94, 342. [Google Scholar] [CrossRef]
- Ran, M.-J.; Wang, M.; Hu, Z.-Y.; Huang, Y.-F.; Wang, L.-D.; Wu, L.; Yuan, M.-M.; Zhang, J.; Li, B.; Van Tendeloo, G.; et al. A hollow core-shell TiO2/NiCo2S4 Z-Scheme heterojunction photocatalyst for efficient hydrogen evolution. J. Mater. Sci. Technol. 2025, 212, 182. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Zhou, G.; Sun, Y.; Nan, Y.; Xie, H.; Zhao, X. Facile preparation of ZIF-67 on TiO2 and enhancement photocathodic protection for 316L stainless steel. Electrochim. Acta 2024, 492, 144350. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, J.; Mao, M.; Li, L.; Li, X. NiCo2S4@Zn0.5Cd0.5S with direct Z-scheme heterojunction constructed by band structure adjustment of ZnxCd1-xS for efficient photocatalytic H2 evolution. Appl. Surf. Sci. 2020, 528, 147016. [Google Scholar] [CrossRef]
- Cai, J.; Li, Y.; Cai, Z.; Zhang, Z.; Zhang, X.; Wang, J.; Xie, Q. Urchin-like Bi2S3 modified TiO2 for improved photocathodic protection of 304SS. J. Alloys Compd. 2024, 1002, 175456. [Google Scholar] [CrossRef]
- Chi, L.; Wang, X.; Guo, S.; Ren, M.; Guo, H.; Wang, M.; Nan, Y.; Wang, J. Construction of NH2-MIL-101(Cr)/TiO2 heterojunction for enhancing visible light response in photocathodic protection. Mater. Today Commun. 2025, 44, 111947. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.K.; Yang, Z.Y.; Li, Y.H.; Zhang, P.F.; Li, H. Enhancing photocathodic protection with Bi quantum dots and ZIF-8 nanoparticle co-sensitized TiO2 nanotubes. Nanotechnology 2024, 35, 045701. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Bahrami, A.; Atapour, M.; Momeni, M.M. Chemical bath co-deposition of Co3O4 and In2O3 on TiO2 nanotubes, aiming for photoanodes with improved photoelectrochemical properties. J. Alloys Compd. 2024, 1002, 175359. [Google Scholar] [CrossRef]
- Selvaraj, S.; Moon, H.; Kim, D.-H. Combined effect of nano -structured NiCo2S4 coated hematite photoanodes for efficient photoelectrochemical water oxidation. Catal. Today 2020, 347, 63. [Google Scholar] [CrossRef]
- Zuo, S.; Qin, S.; Xue, B.; Xu, R.; Shi, H.; Lu, X.; Yao, C.; Gui, H.; Li, X. Development of Plasmonic Attapulgite/Co(Ti)Ox Nanocomposite Using Spent Batteries toward Photothermal Reduction of CO2. Molecules 2024, 29, 2865. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Wang, W.; Yang, Z.; Wang, W.; Chen, W.; Wu, K. Enhancing photocathodic protection performance by controlled synthesis of Bi/BiOBr/TiO2 NTAs Z-scheme heterojunction films. J. Alloys Compd. 2023, 960, 170675. [Google Scholar] [CrossRef]
- Jin, Z.; Liu, Y.; Jiang, H.; Zhang, X. Enhanced photocathodic protection on 304SS in marine environment by Z-Scheme polydopamine-modified CdS. Constr. Build. Mater. 2024, 416, 135216. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.; Li, W. Assembly of WO3-Nb2O5-ZnIn2S4 with embedded quasi-planar heterojunction characteristics and its efficient and stable dark-state photoelectrochemical cathodic protection performance. J. Mater. Sci. Technol. 2025, 221, 117. [Google Scholar] [CrossRef]
- Qian, F.; Tian, J.; Guo, C.; Liu, L.; Chen, S.; Li, J.; Wang, N.; Wang, L. Dual-function photoelectrode of TiO2 nanotube array/CdZnS/ZnS heterojunction for efficient photoelectrochemical cathodic protection and anti-biofouling. J. Mater. Sci. Technol. 2024, 189, 25. [Google Scholar] [CrossRef]
- Wang, W.; Yu, H.; Yang, Z.; Wang, W.; Zhang, Q.; Jiang, Y.; Wang, P.; Marano, G.C. Photocathodic protection performance of CuInSe2/TiO2 n-n heterojunction films for HRB400 steel rebar under visible light. Colloids Surf. A-Physicochem. Eng. Asp. 2025, 715, 136638. [Google Scholar] [CrossRef]
- Osório, W.R.; Cheung, N.; Peixoto, L.C.; Garcia, A. Corrosion Resistance and Mechanical Properties of an Al 9wt%Si Alloy Treated by Laser Surface Remelting. Int. J. Electrochem. Sci. 2009, 4, 820. [Google Scholar] [CrossRef]
- Wu, H.; Bai, Z.; Cheng, H.; Zhou, Z.; Zhang, Z. Application of NiS modified WS2/TiO2 heterostructure in photocathodic protection. Nanotechnology 2025, 36, 1361–6528. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Xu, J.; Jiang, H.; Gao, F.; Lu, Q. Thickness-control of ultrathin two-dimensional cobalt hydroxide nanosheets with enhanced oxygen evolution reaction performance. Chem. Eng. J. 2017, 316, 225. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Jin, Z.; Jiang, H.; Wang, X.; Chen, Y.; Zhang, Y.; Hou, B. Enhanced anticorrosion mechanism of photocathodic protection coatings with zinc indium sulfide/titanium oxide Z-scheme heterojunctions. Constr. Build. Mater. 2024, 453, 139109. [Google Scholar] [CrossRef]
- Xu, S.; Li, Z.; Kawi, S.; Xie, A.; Qiu, P.; Yang, L.; Peng, H. Fabrication of multi-layered TiO2/CdS@ZnS nanocomposite film for highly stable and efficient photocathodic protection of carbon steel. Mater. Today Commun. 2025, 42, 111134. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, X.; Yang, Z.; Zhang, X.; Li, Y.; Li, D.; Li, H. Enhancing photocathodic protection of Q235 carbon steel by co-sensitizing TiO2 nanotubes with CdIn2S4 nanogranules and WO3 nanoplates. J. Alloys Compd. 2024, 976, 173184. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Lin, C.; Zhang, J.; Feng, J.; Hou, B.; Yan, W.; Li, M.; Ren, Z. Spontaneous polarization of ferroelectric heterostructured nanorod arrays for high-performance photoelectrochemical cathodic protection. Appl. Surf. Sci. 2023, 609, 155345. [Google Scholar] [CrossRef]
- Wang, L.; Xue, Y.; Yang, G.; Liu, X.; Zhang, M.; Liu, Z. Electronic structure regulation of an S-scheme CuBi2O4/Sr0.5NaTaO3 heterojunction with efficient carrier spatial transfer. Sep. Purif. Technol. 2023, 317, 123856. [Google Scholar] [CrossRef]
- Pan, G.; Li, J.; Zhang, G.; Zhan, Y.; Liu, Y. Binder-integrated Bi/BiOI/TiO2 as an anti-chloride corrosion coating for enhanced photocathodic protection of 304 stainless steel in simulated seawater. J. Alloys Compd. 2023, 938, 168469. [Google Scholar] [CrossRef]
- Wang, X.-T.; Pu, J.-Y.; Liu, J.-Q.; Ren, M.-P.; Nan, Y.-B.; Liu, M.-S.; Xu, H.; Yang, L.-H.; Huang, Y.-L.; Hou, B.-R. PDA decorated spaced TiO2 nanotube array photoanode material for photocathodic protection of 304 stainless steels. J. Electroanal. Chem. 2022, 914, 116319. [Google Scholar] [CrossRef]
- Xie, Z.-H.; Wen, Y.; Yao, H.; Liu, Y.; Yu, G.; Zhong, C.-J. A Z-scheme g-C3N4/TiO2 heterojunction for enhanced performance in protecting magnesium and nickel couple from galvanic corrosion. J. Alloys Compd. 2025, 1010, 177463. [Google Scholar] [CrossRef]



| Elements | Cr | Ni | Mn | Si | C | P | S | Fe |
|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 18.250 | 8.500 | 1.860 | 0.720 | 0.080 | 0.035 | 0.029 | 70.526 |
| Ni (NO3)2·6H2O | Co (NO3)2·6H2O | C2H5NS | |
|---|---|---|---|
| TiO2/NiCo2S4-0.25 | 0.25 mmol | 0.50 mmol | 1.00 mmol |
| TiO2/NiCo2S4-0.50 | 0.50 mmol | 1.00 mmol | 2.00 mmol |
| TiO2/NiCo2S4-1.00 | 1.00 mmol | 2.00 mmol | 4.00 mmol |
| TiO2/NiCo2S4-1.50 | 1.50 mmol | 3.00 mmol | 6.00 mmol |
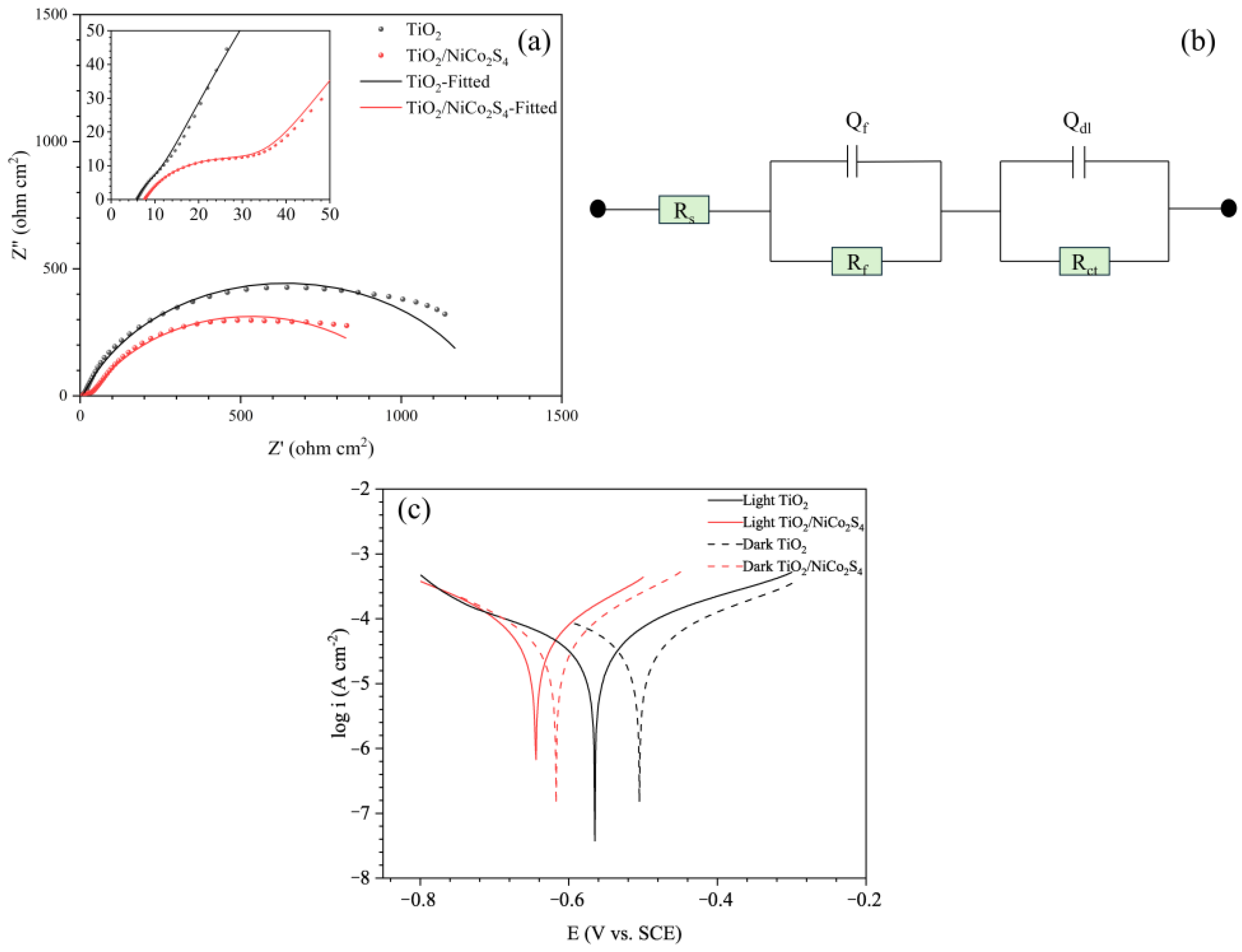
| Samples | Rs | Rf | Qf | Rct | Qdl | χ2 | ||
|---|---|---|---|---|---|---|---|---|
| (Ω·cm2) | (Ω·cm2) | Y01 (10−5·Ω−1·cm−2·sn) | n1 | (Ω·cm2) | Y02 (10−5·Ω−1·cm−2·sn) | n2 | 10−4 | |
| TiO2 | 5.914 | 3.255 | 92.073 | 0.90669 | 1263 ± 8 | 125.77 | 0.77939 | 52.8 |
| TiO2/NiCo2S4 | 7.705 | 25.08 | 23.100 | 0.76651 | 999 ± 5 | 266.68 | 0.71192 | 9.28 |
| Samples | Ecorr (V vs. SCE) | icorr (μA cm−2) | βc (mV dec−1) | βa (mV dec−1) |
|---|---|---|---|---|
| Light TiO2 | −0.565 | 41.05 | 456.8 | 569.1 |
| Light TiO2/NiCo2S4 | −0.642 | 50.40 | 649.1 | 696.0 |
| Dark TiO2 | −0.505 | 46.72 | 476.7 | 549.6 |
| Dark TiO2/NiCo2S4 | −0.617 | 48.27 | 605.4 | 684.3 |
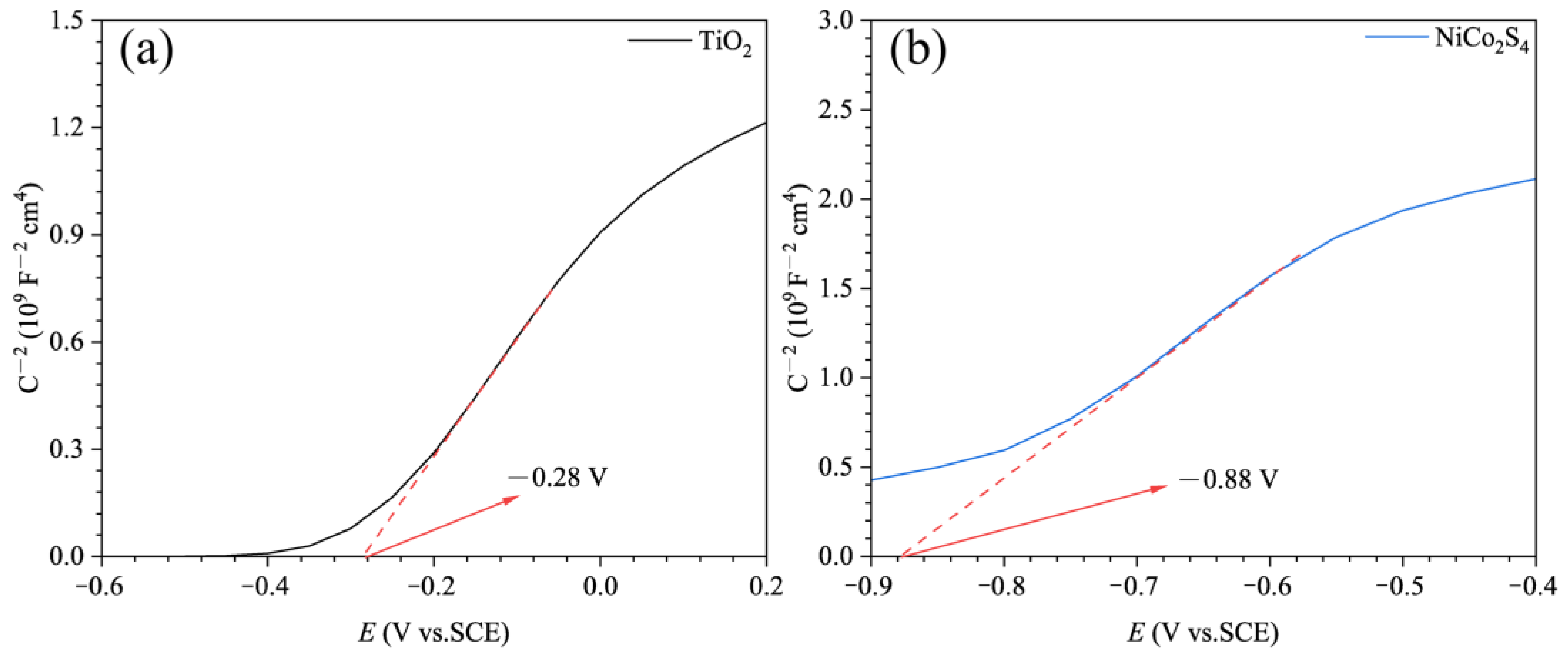
| TiO2 (V) | NiCo2S4 (V) | |
|---|---|---|
| Efb vs. SCE | −0.28 | −0.88 |
| Efb vs. NHE | −0.04 | −0.64 |
| ECB | −0.24 | −0.84 |
| EVB | 3.07 | 1.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Li, H.; Zhang, X.; Xing, B.; Sun, Z.; Li, Y. Enhanced Photocathodic Protection Performance of TiO2/NiCo2S4 Composites for 304 Stainless Steel. Coatings 2025, 15, 874. https://doi.org/10.3390/coatings15080874
Liu H, Li H, Zhang X, Xing B, Sun Z, Li Y. Enhanced Photocathodic Protection Performance of TiO2/NiCo2S4 Composites for 304 Stainless Steel. Coatings. 2025; 15(8):874. https://doi.org/10.3390/coatings15080874
Chicago/Turabian StyleLiu, Honggang, Hong Li, Xuan Zhang, Baizhao Xing, Zhuangzhuang Sun, and Yanhui Li. 2025. "Enhanced Photocathodic Protection Performance of TiO2/NiCo2S4 Composites for 304 Stainless Steel" Coatings 15, no. 8: 874. https://doi.org/10.3390/coatings15080874
APA StyleLiu, H., Li, H., Zhang, X., Xing, B., Sun, Z., & Li, Y. (2025). Enhanced Photocathodic Protection Performance of TiO2/NiCo2S4 Composites for 304 Stainless Steel. Coatings, 15(8), 874. https://doi.org/10.3390/coatings15080874







