1. Introduction
New spacecraft are gradually becoming smaller, more complex and more versatile with the rapid development of space technology, and the thermal environment faced by spacecraft is increasingly complex and severe. The infrared emission characteristics of traditional thermal control coatings are fixed and cannot change with the changes in the external environment, which makes it difficult to meet the requirements of adaptive temperature control of spacecraft in the front and back of the sun. In recent years, intelligent thermal control materials have attracted much attention because of their ability to autonomously adjust the thermal radiation characteristics according to the ambient temperature [
1]. When the ambient temperature of the spacecraft is higher than that required to ensure the normal operation of the spacecraft, intelligent thermal control materials can improve the emissivity and expel excess heat. Conversely, intelligent thermal control materials can also reduce the emissivity and effectively reduce the heat loss of the spacecraft [
2,
3,
4,
5,
6,
7,
8].
As a typical perovskite structural material, lanthanum strontium manganese (La
1−xSr
xMnO
3) has excellent electrical, magnetic, and optical properties, which could exhibit a phase transition between ferromagnetic metal and paramagnetic insulator around room temperature when the Sr doping level is within a specific range [
9,
10,
11,
12,
13,
14,
15]. The magnetic, transport, and magnetoresistance properties of these materials strongly depend on the quantity of the dopant Sr atom. Shimazaki et al. have performed a lot of experimental research on the influence of material composition formulas on the infrared emissivity properties of the intelligent control material. Experimental results demonstrate that the La
0.8Sr
0.2MnO
3 system exhibits excellent emissivity modulation capability, indicating significant potential for smart thermal control applications [
16]. Shimazaki et al. also measured the spectral reflectance of La
1−xSr
xMnO
3 to demonstrate the role of Sr doping in driving the metal-insulator transition [
17]. However, the practical application of LSMO coatings faces significant challenges, particularly in achieving precise control over phase transition temperatures and emissivity switching efficiency—properties that are intrinsically linked to the material’s composition and microstructure.
The infrared emissivity of La1−xSrxMnO3 material is not only related to the material formula but also closely related to the crystal structure, composition distribution uniformity, and microstructure morphology of the material, and these factors largely depend on the preparation process of La1−xSrxMnO3 powder material. At present, the research on La1−xSrxMnO3 intelligent thermal control materials mainly focuses on the optimization of material composition and performance characterization. In terms of material synthesis techniques, current research on La1−xSrxMnO3 materials has predominantly focused on conventional solid-state reaction methods. There has been a limited amount of research investigating the influence of the chemical precipitation process on the properties of the synthesized La1−xSrxMnO3 material. Compared with conventional solid-state synthesis, chemical precipitation demonstrates enhanced compositional uniformity and relatively mild processing conditions. However, the fundamental relationships between precipitation process parameters and LSMO’s functional properties remain inadequately explored. Therefore, it is important to systematically study the influence of different preparation processes of La1−xSrxMnO3 powder on the infrared emission rate of the material, which will help optimize and improve the thermal control performance of La1−xSrxMnO3 intelligent thermal control materials.
In this paper, La0.8Sr0.2MnO3 intelligent thermal control powder material with variable emissivity was prepared using the chemical precipitation method. This study investigates the effects of precipitant concentration on the material characteristics and emissivity modulation performance of La0.8Sr0.2MnO3 smart thermal control materials. It provides a theoretical basis and technical guidance for the preparation process optimization and performance improvement of La1−xSrxMnO3 intelligent thermal control materials.
2. Materials and Methods
La(NO3)3·6H2O (≥99.9 wt.%, Qixin Chemical Co., Ltd., Jining, China) and Mn(NO3)2·4H2O (≥99 wt.%, Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China) were used as raw material sources for lanthanum and manganese, respectively. Strontium nitrate (Sr(NO3)2) solution provided the strontium source for precipitation. Strontium nitrate (Sr(NO3)2) solution was obtained through the dissolution of SrCO3 (≥99.9 wt.%, Qinhuangdao Yinuo Advanced Materials Development Co., Ltd., Qinhuangdao, China) in nitric acid. The molar ratio of La(NO3)3·6H2O:SrCO3:Mn(NO3)2·4H2O was maintained at 0.8:0.2:1 in the experimental procedure. The mixed metal nitrate solutions were prepared in deionized water with a total metal ion concentration of 1 mol/L. The mixture was stirred at room temperature for 2 h to achieve complete dissolution and even mixing. Ammonia solutions at different concentrations, including 6 wt.%, 9 wt.%, 12 wt.%, and 15 wt.%, were employed as chemical precipitants. The stirring rate of the solution was 400 r/min during the precipitation process. The stirring operation was continued for 2 h after the complete addition of the precipitant to allow the reaction to reach equilibrium. The precipitate was aged in the mother liquor for 24 h at room temperature to improve crystallinity and particle size distribution.
The desired perovskite phase powder was obtained after drying and calcination. Precipitation was first dried at 100 °C for 12–16 h, followed by calcination at 1200 °C for 12 h in an air atmosphere. The drying process parameter was selected to thoroughly dry the water in the precipitate. Insufficient drying time prevents complete water removal from the precipitate, while prolonged drying beyond this duration results in unnecessary energy expenditure. Oxygen in the calcination atmosphere ensures the preservation of oxygen stoichiometry and helps maintain the desired oxidation state of metal cations. It is essential to consider achieving full perovskite phase crystallization of the resulting product and the appropriate powder density when determining calcination temperature and duration. Excessively high calcination temperatures and long times will induce the over-densification of the powder, significantly increasing the difficulty in preparing homogeneous slurries during subsequent ball milling for spray granulation processes. The powder with good fluidity was prepared by spray granulation. Spray granulation is a process that transforms liquid slurry into fine droplets through an atomizer and then exposes these droplets to hot air, causing rapid evaporation of moisture to produce dry and spherical powder. The spray granulation process operates with an inlet temperature of 300 °C and an outlet temperature of 120 °C. The obtained spherical powder was sieved using a 325-mesh sieve, and the powder that passed through the sieve was used for the subsequent preparation of bulk materials.
The weighed powder was loaded into the mold and pressed to form a compacted bulk material. Then the pressed bulk material was placed in the heat treatment furnace and sintered according to the set sintering process. The main process parameters of pressing sintering are shown in
Table 1, including pressure, holding time, sintering temperature, and sintering time. La
0.8Sr
0.2MnO
3 ceramic bulk material was obtained after surface grinding and polishing.
Micrographs of the synthesized La0.8Sr0.2MnO3 powders and press-sintered bulk materials were acquired using a scanning electron microscope (SU5000, Hitachi High-Technologies Corporation, Hitachinaka, Japan). The strontium content in the powders was quantified by inductively coupled plasma atomic emission spectrometry (PEAvio500, PerkinElmer, Waltham, MA, USA). The surface roughness of the ceramics was characterized using atomic force microscopy (Dimension Icon, Bruker, Karlsruhe, Germany). The phase composition of the bulk materials was analyzed using an X-ray diffractometer (D8 Advancd, Bruker, Karlsruhe, Germany). Spectral emissivity was measured with a variable temperature spectral emissivity measurement system under controlled environmental conditions free from strong magnetic fields and electromagnetic interference, with relative humidity at 20%–60%. The chamber vacuum was maintained at 1 Pa during testing.
3. Results and Discussion
The microstructures of powders prepared with precipitants of different concentrations are shown in
Figure 1. After high-temperature sintering, the powders formed agglomerates with particle sizes ranging from approximately 5 to 150 μm. When the precipitant concentration is 12 wt.% or 15 wt.%, the synthesized powder agglomerates predominantly form three-dimensional blocky structures after sintering. At lower concentrations such as 6 wt.% and 9 wt.%, the powder agglomerates predominantly form two-dimensional sheet-like structures after sintering. When the concentration of precipitator is relatively high, the concentration of OH
− ions in the solution is high, and the nucleation rate is much higher than the crystal growth rate. Then the particles synthesized under this condition are fine and uniform in size. During the sintering process, fine particles are easy to form massive structures through grain boundary diffusion and particle fusion. For low concentrations of precipitator, the concentration of OH
− ions in the solution is relatively low, which leads to a lower nucleation rate but a higher crystal growth rate. Combined with the influence of surface energy on specific crystal facets, the powder develops a characteristic two-dimensional sheet-like structure.
The doping amount of Sr has an important influence on the metal insulation transition and emissivity modulation performance of l La
1−xSr
xMnO
3 materials. Therefore, the Sr contents in the powders synthesized by four kinds of precipitants were quantified using chemical methods, and the results are presented in
Figure 2. The precipitant concentration significantly influences the chemical composition of the synthesized powder. When the precipitant concentration is 15 wt.% and 12 wt.%, the Sr content of the synthesized powder is in good agreement with the theoretical value. When the precipitant concentration is 9 wt.% and 6 wt.%, the Sr content of the synthesized powder is low and has deviated from the designed theoretical value. The precipitant concentration is a critical parameter that significantly influences the Sr content through the solubility product (Ksp) mechanism. The solubility product of strontium hydroxide is relatively large, requiring a higher concentration of hydroxide ions to completely precipitate strontium ions. La
1−xSr
xMnO
3 has a perovskite ABO
3 structure in which La and Sr occupy the A-sites. The electrical and magnetic properties of La
1−xSr
xMnO
3 strongly depend on the ratio of Sr in the A-sites. When the strontium doping level is significantly lower than the designed value, the temperature-dependent emissivity performance of the material will deteriorate considerably [
7].
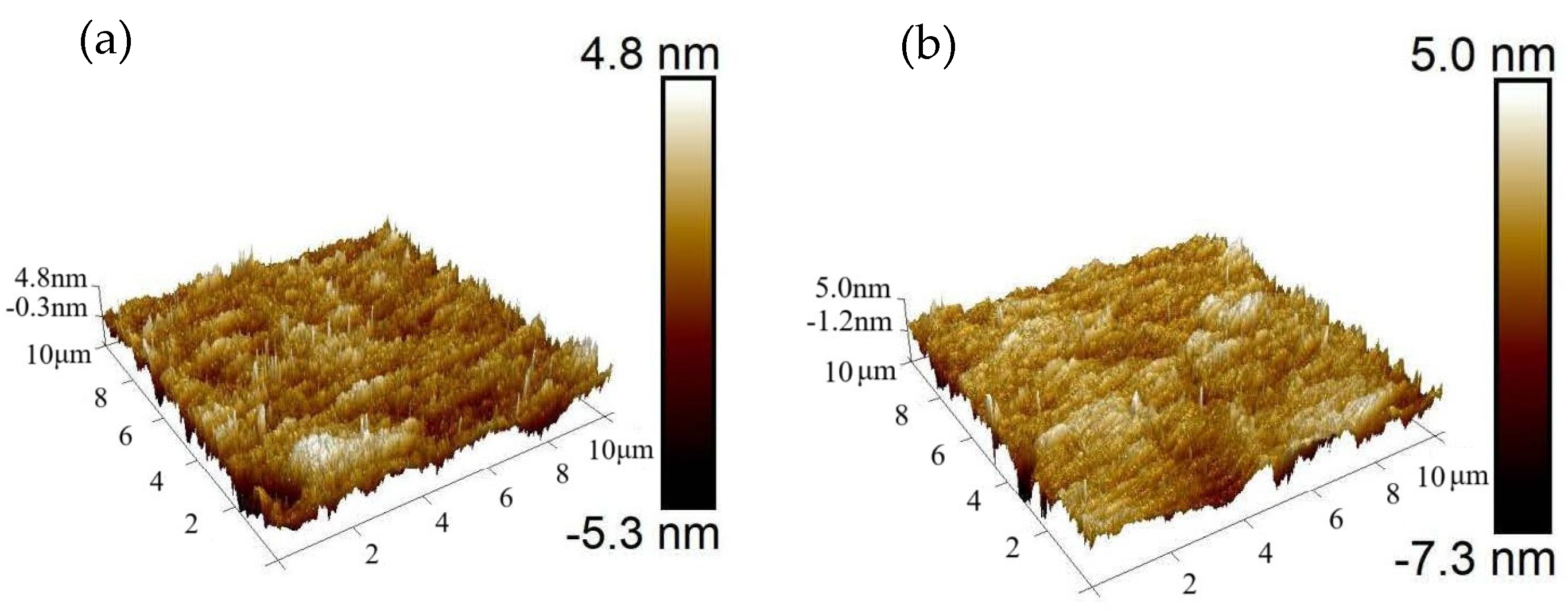
The surface condition of a material has a certain influence on the variation range of emissivity, with relatively smooth surfaces exhibiting larger variation in emissivity values [
17]. When the surface exhibits significant optical roughness, the presence of multiple reflections within the cavities formed between rough surface elements leads to enhanced trapping of incident radiation. As a result, the surface emissivity is altered by this phenomenon. In this experiment, the surfaces of the two bulk samples synthesized with 15 wt.% and 12 wt.% ammonia solutions were ground and polished to reduce surface roughness, and the roughness of the polished sample surfaces was subsequently evaluated and characterized. The corresponding surface morphology images for each tested region are presented in
Figure 3. The surface roughness of two samples synthesized with 15 wt.% and 12 wt.% ammonia solutions, respectively, was measured at both the center and edge regions after grinding and polishing. The surface roughness of two samples is less than 20 nm, demonstrating excellent surface smoothness. The reduced surface roughness establishes an optimal foundation for conducting subsequent temperature-dependent emissivity measurements.
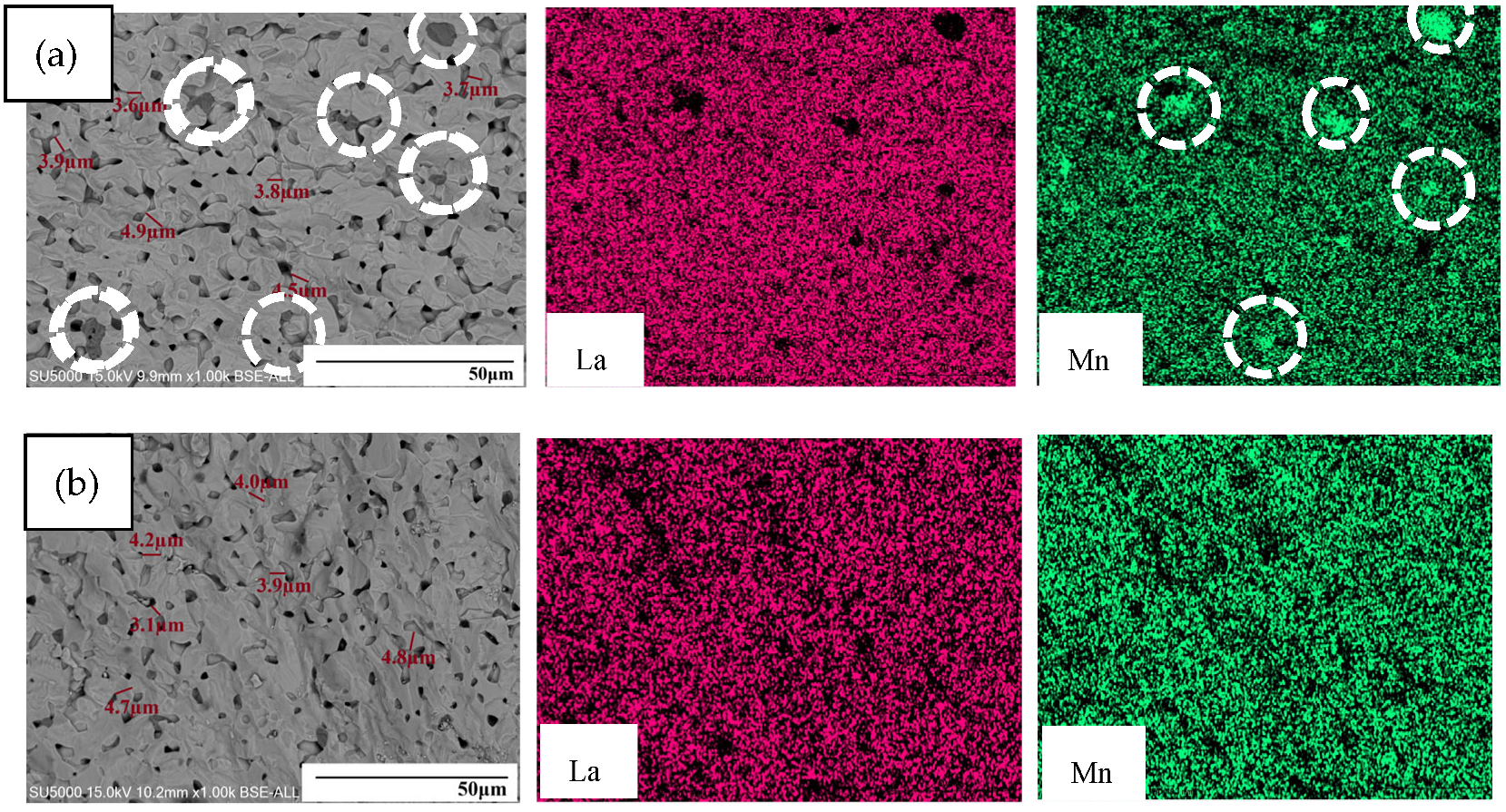
Figure 4 presents the fracture morphology and energy-dispersive spectroscopy mapping results of the bulk material obtained by pressing and sintering the powder. As shown in the figure, the pressed bulk material exhibits a relatively dense structure overall. The average particle size is approximately 2 to 5 μm. The red and green colors in
Figure 4 represent the distribution of La and Mn elements, respectively, within the analyzed region. However, the energy-dispersive spectroscopy mapping results reveal that the lanthanum strontium manganite material synthesized with 15 wt.% ammonia solution shows localized manganese enrichment in specific regions. The lanthanum strontium manganite material synthesized with 12 wt.% ammonia solution demonstrates a homogeneous distribution of elements, showing no evidence of manganese elemental segregation. The occurrence of this phenomenon may be attributed to the reaction mechanism because of which, during material synthesis using a 15 wt.% ammonia solution, partial manganese (II) hydroxide precipitates can be converted into soluble complexes and subsequently be dissolved in localized regions with relatively high ammonia concentrations [
18]. At elevated ammonia concentrations, localized regions with relatively high NH
3 levels facilitate the formation of soluble [Mn(NH
3)
4]
2+ complexes through coordination reactions. The dissolved manganese ions may subsequently re-precipitate in other regions of the solution, forming fresh manganese (II) hydroxide. This dissolution-reprecipitation process facilitates the redistribution of manganese within the precipitate, leading to localized manganese enrichment in specific areas. The localized enrichment of manganese results in compositional inhomogeneity within the material, which may have a certain impact on the temperature-dependent emissivity properties of the material.
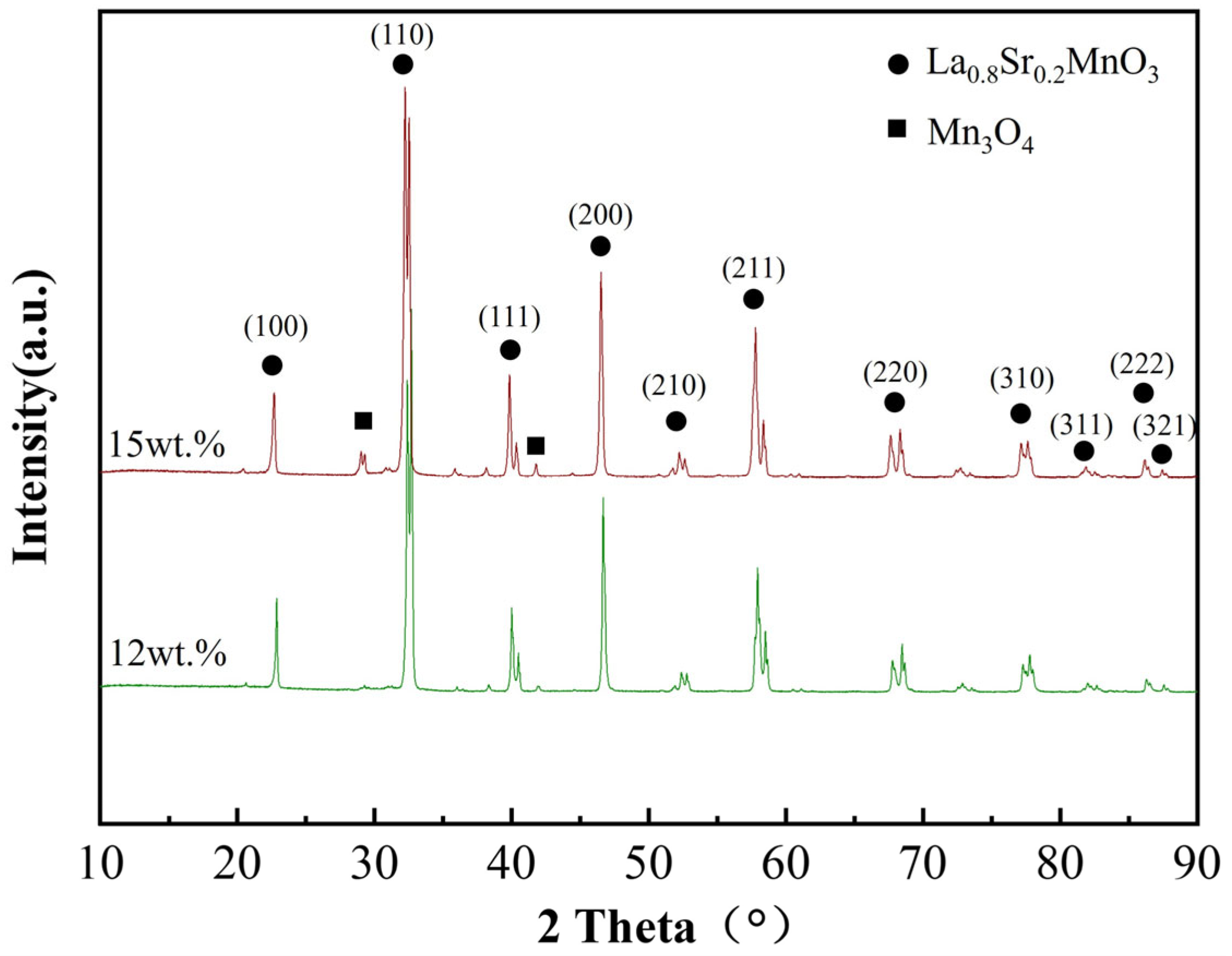
The phase composition of bulk materials fabricated from two types of lanthanum strontium manganite powders was analyzed, and the XRD patterns are shown in
Figure 5. As evidenced by the XRD patterns, the lanthanum strontium manganite material synthesized using 15 wt.% ammonia solution primarily consists of La
0.8Sr
0.2MnO
3 with a minor presence of manganese oxide phases, and the content of the La
0.8Sr
0.2MnO
3 phase is 96%. The corresponding index of reflection has been marked in
Figure 5 [
2]. The lanthanum strontium manganite material synthesized with 12 wt.% ammonia solution exhibits a single phase of La
0.8Sr
0.2MnO
3 with no detectable secondary phases. A detailed analysis of the diffraction peaks reveals distinct splitting phenomena in several characteristic peaks; the primary diffraction peaks between 2θ = 30–35° exhibit a doublet structure, accompanied by similar peak splitting at approximately 2θ = 43°, 58°, and 68°. This peak splitting demonstrates the structural modification induced by Sr doping, specifically indicating a distortion of the perovskite framework. Considering the ionic radius difference between La
3+ (0.106 nm) and Sr
2+ (0.112 nm), the incorporation of larger Sr
2+ ions effectively reduces the crystal symmetry and induces lattice distortion.
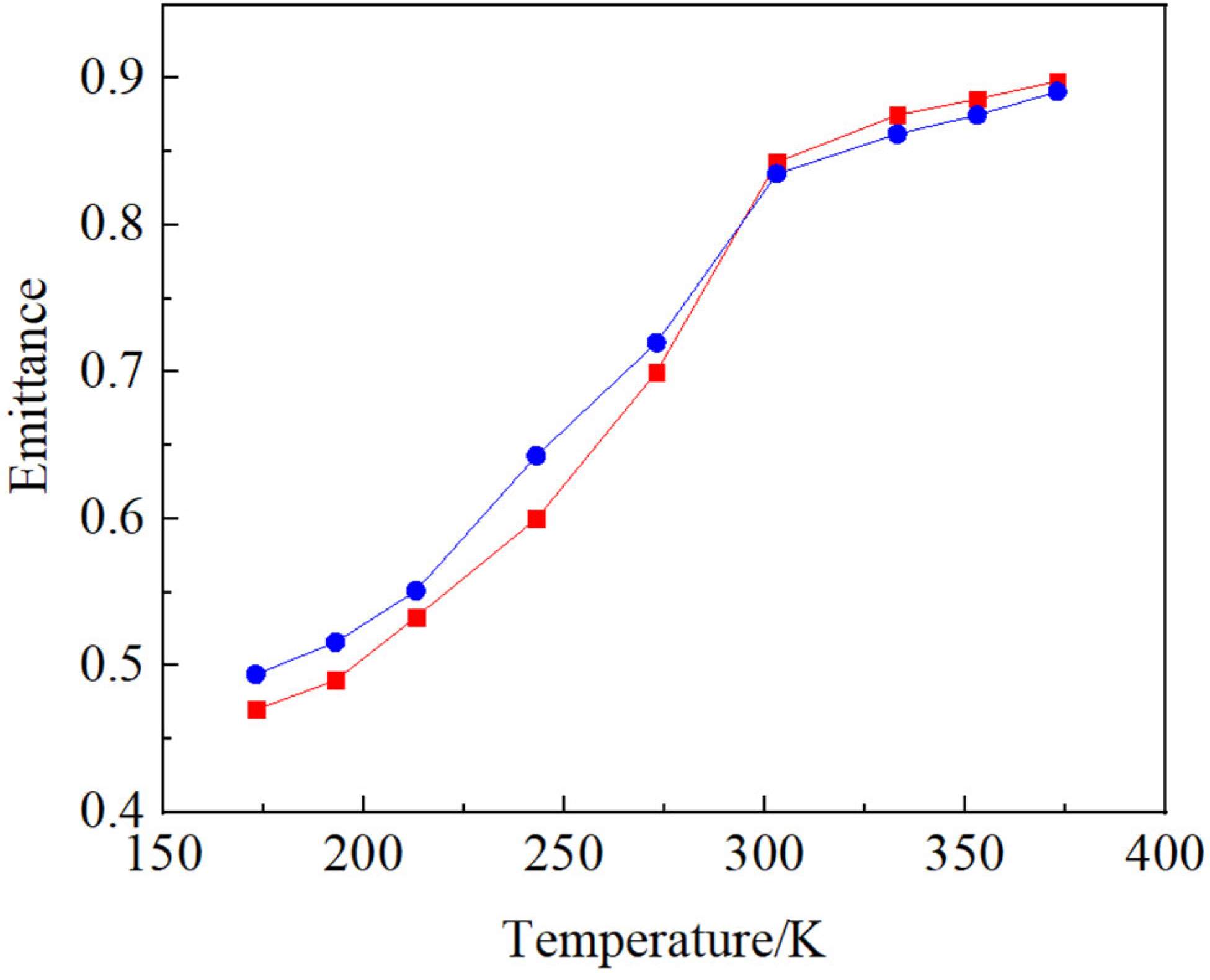
Figure 6 illustrates the temperature dependence of the emittance of the lanthanum strontium manganite material. Emissivity was measured with a variable temperature spectral emissivity measurement system with a temperature controller under controlled environmental conditions free from strong magnetic fields and electromagnetic interference. The low-temperature conditions of the samples were maintained through liquid nitrogen cooling. The sample temperature was regulated to the desired measurement temperature prior to experimental data acquisition. The lanthanum strontium manganite material prepared with 12 wt.% ammonia solution exhibited an emissivity variation range of 0.428 within the temperature interval of 173 K to 373 K, while the material synthesized using 15 wt.% ammonia solution showed an emissivity variation of 0.397 over the same temperature range. The lanthanum strontium manganite material fabricated using 12 wt.% ammonia solution demonstrates a broader range of emissivity variation in the temperature range from 173 K to 373 K than that prepared with 15 wt.% ammonia solution. The emissivity of a material strongly depends on its metallic or insulating state. When a material exhibits strong conductivity and enters a metallic state, it typically has lower emissivity, reducing radiative heat loss. Materials with low electrical conductivity in their insulating state generally demonstrate higher emissivity. The lanthanum strontium manganite material with optimal Sr doping concentration exhibits a temperature-dependent metal-insulator transition, demonstrating metallic characteristics at low temperatures while transforming into an insulating phase at higher temperatures. Consequently, the emissivity gradually increases with rising temperature, demonstrating obvious tunable emissivity characteristics. The metal-insulator phase transition in lanthanum strontium manganite material primarily arises from valence state variations of manganese ions and the resulting Mn
3+-O
2−-Mn
4+ double exchange mechanism. The temperature dependence of emissivity of lanthanum strontium manganite materials is strongly influenced by the electronic structure and double-exchange interactions of manganese ions (Mn
3+ and Mn
4+) [
19,
20,
21,
22,
23]. The double-exchange interaction is strengthened under conditions of high free electron concentration and aligned spin orientations between Mn
4+ and Mn
3+, and as a result, the electrical resistivity is decreased. Below the metal-insulator transition temperature (TMI), the spin orientation of Mn
4+ and Mn
3+ is the same, which enhances the double-exchange interaction, and then the materials are in a metallic state. Above T
MI, the spin orientations of Mn
4+ and Mn
3+ are different, which resists the electron transition in Mn
3+-O
2−-Mn
4+ and makes the materials insulators [
24]. The localized segregation of manganese may exert a certain influence on the double exchange interaction, resulting in a constrained range of emissivity variation in the material synthesized with 15 wt.% ammonia solution.