Coating of Cubic Boron Nitride Powder with TiN in a Rotating Drum via Gas Phase Processes
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Particle Surface Characterization
3.2. Particle Cross-Section Analysis
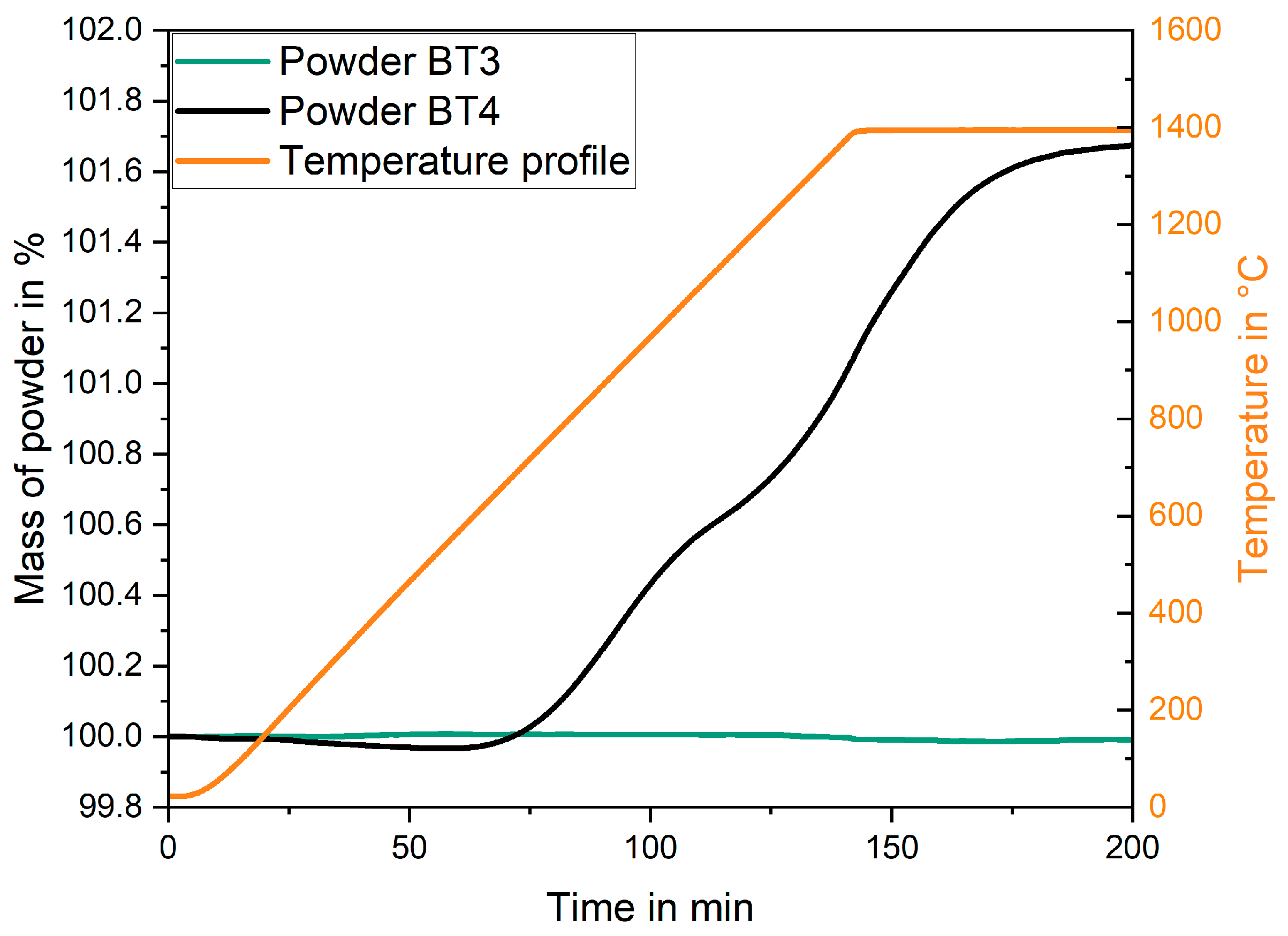
3.3. Phase Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALD | Atomic layer deposition |
| BSEs | Backscattered electrons |
| CVD | Chemical vapor deposition |
| FESEM | Field emission scanning electron microscope |
| GPC | Growth per cycle |
| hBN | Hexagonal boron nitride |
| HGE | Hot gas extraction |
| PcBN | Polycrystalline cubic boron nitride |
| Powder Diffraction File | |
| SE | Secondary electron |
| SPS | Spark plasma sintering |
| TG(A) | Thermogravimetric analysis/Thermogravimetry |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence analysis |
References
- Mills, B. Recent developments in cutting tool materials. J. Mater. Process. Technol. 1996, 56, 16–23. [Google Scholar] [CrossRef]
- Klimenko, S.A.; Mukovoz, Y.A.; Polonsky, L.G. Cutting Tools of Superhard Materials. In Advanced Ceramic Tools for Machining Application—II; Trans Tech Publications Ltd.: Baech, Switzerland, 1995; pp. 1–66. [Google Scholar]
- Haines, J.; Léger, J.M.; Bocquillon, G. Synthesis and Design of Superhard Materials. Annu. Rev. Mater. Res. 2001, 31, 1–23. [Google Scholar] [CrossRef]
- Rizzo, A.; Goel, S.; Luisa Grilli, M.; Iglesias, R.; Jaworska, L.; Lapkovskis, V.; Novak, P.; Postolnyi, B.O.; Valerini, D. The Critical Raw Materials in Cutting Tools for Machining Applications: A Review. Materials 2020, 13, 1377. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Tian, Y. Superhard materials: Recent research progress and prospects. Sci. China Mater. 2015, 58, 132–142. [Google Scholar] [CrossRef]
- Riedel, R. Novel Ultrahard Materials. Adv. Mater. 1994, 6, 549–560. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Carbon, Graphite, Diamond, and Fullerenes: Properties, Processing, and Applications; Noyes Publications: Park Ridge, NJ, USA, 1993. [Google Scholar]
- Zou, L.; Yin, J.; Huang, Y.; Zhou, M. Essential causes for tool wear of single crystal diamond in ultra-precision cutting of ferrous metals. Diam. Relat. Mater. 2018, 86, 29–40. [Google Scholar] [CrossRef]
- Monteiro, S.N.; Skury, A.L.D.; de Azevedo, M.G.; Bobrovnitchii, G.S. Cubic boron nitride competing with diamond as a superhard engineering material—An overview. J. Mater. Res. Technol. 2013, 2, 68–74. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Zhang, Z.; Hou, Z.; Wu, D.; Ouyang, X. Analysis of mechanical properties of Al2O3-cBN-hBN composites and identification of main influencing factors. Int. J. Appl. Ceram. Technol. 2022, 19, 3255–3266. [Google Scholar] [CrossRef]
- Hotta, M.; Goto, T. Effects of cubic BN addition and phase transformation on hardness of Al2O3–cubic BN composites. Ceram. Int. 2011, 37, 1453–1457. [Google Scholar] [CrossRef]
- Klimczyk, P.; Wyżga, P.; Cyboroń, J.; Laszkiewicz-Łukasik, J.; Podsiadło, M.; Cygan, S.; Jaworska, L. Phase stability and mechanical properties of Al2O3-cBN composites prepared via spark plasma sintering. Diam. Relat. Mater. 2020, 104, 107762. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, R.; Goto, T. Cubic boron nitride-containing ceramic matrix composites for cutting tools. Adv. Ceram. Matrix Compos. 2014, 570–586. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, R.; Goto, T. Spark plasma sintering of Al2O3–cBN composites facilitated by Ni nanoparticle precipitation on cBN powder by rotary chemical vapor deposition. J. Eur. Ceram. Soc. 2011, 31, 2083–2087. [Google Scholar] [CrossRef]
- Yaman Islak, B.; Mandal, H. Friction and wear behavior of spark plasma-sintered cBN-added Al2O3–PSZ-based composites. J. Aust. Ceram. Soc. 2017, 53, 163–172. [Google Scholar] [CrossRef]
- Hotta, M.; Goto, T. Densification and microstructure of Al2O3-cBN composites prepared by spark plasma sintering. J. Ceram. Soc. Jpn. 2008, 116, 744–748. [Google Scholar] [CrossRef]
- Garrett, J.C.; Sigalas, I.; Herrmann, M.; Olivier, E.J.; O’Connell, J.H. cBN reinforced Y-α-SiAlON composites. J. Eur. Ceram. Soc. 2013, 33, 2191–2198. [Google Scholar] [CrossRef]
- Garrett, J.C.; Sigalas, I.; Herrmann, M. TEM investigation of the interface formation in cubic boron nitride containing α-SiAlON composites. Ceram. Int. 2014, 40, 16169–16175. [Google Scholar] [CrossRef]
- Hotta, M.; Goto, T. Densification and Phase Transformation of β-SiAlON–Cubic Boron Nitride Composites Prepared by Spark Plasma Sintering. J. Am. Ceram. Soc. 2009, 92, 1684–1690. [Google Scholar] [CrossRef]
- Hotta, M.; Goto, T. Effect of time on microstructure and hardness of βSiAlON–cubic boron nitride composites during spark plasma sintering. Ceram. Int. 2011, 37, 521–524. [Google Scholar] [CrossRef]
- Ye, F.; Hou, Z.; Zhang, H.; Liu, L.; Zhou, Y. Spark plasma sintering of cBN/β-SiAlON composites. Mater. Sci. Eng. A 2010, 527, 4723–4726. [Google Scholar] [CrossRef]
- Wolfrum, A.-K. Verdichtung und Eigenschaften von hartstoffverstärkten Siliciumnitridwerkstoffen. Ph.D. Dissertation, Technical University of Dresden, Dresden, Germany, 2019. [Google Scholar]
- Yi, M.; Jing, G.; Li, D.; Xiao, G.; Chen, Z.; Zhang, J.; Wang, L.; Xu, C. Strengthening and toughening mechanism of (W,Ti,Ta)C based cermet with the addition of c-BN@Al2O3. Ceram. Int. 2021, 47, 32075–32085. [Google Scholar] [CrossRef]
- Zhao, M.; Kou, Z.; Zhang, Y.; Peng, B.; Wang, Y.; Wang, Z.; Yin, X.; Jiang, M.; Guan, S.; Zhang, J.; et al. Superhard transparent polycrystalline cubic boron nitride. Appl. Phys. Lett. 2021, 118, 151901. [Google Scholar] [CrossRef]
- Angseryd, J.; Elfwing, M.; Olsson, E.; Andrén, H.-O. Detailed microstructure of a cBN based cutting tool material. Int. J. Refract. Met. Hard Mater. 2009, 27, 249–255. [Google Scholar] [CrossRef]
- Hotta, M.; Goto, T. Spark plasma sintering of TiN-cubic BN composites. J. Ceram. Soc. Jpn. 2010, 118, 137–140. [Google Scholar] [CrossRef]
- Irshad, H.M.; Ahmed, B.A.; Ehsan, M.A.; Khan, T.I.; Laoui, T.; Yousaf, M.R.; Ibrahim, A.; Hakeem, A.S. Investigation of the structural and mechanical properties of micro-/nano-sized Al2O3 and cBN composites prepared by spark plasma sintering. Ceram. Int. 2017, 43, 10645–10653. [Google Scholar] [CrossRef]
- Azam, M.U.; Ahmed, B.A.; Hakeem, A.S.; Irshad, H.M.; Laoui, T.; Ehsan, M.A.; Patel, F.; Khalid, F.A. Tribological behaviour of alumina-based nanocomposites reinforced with uncoated and Ni-coated cubic boron nitride. J. Mater. Res. Technol. 2019, 8, 5066–5079. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, R.; Goto, T. Evaluation of CVD-Deposited SiO2 as a Sintering Aid for Cubic Boron Nitride Consolidated with Alumina by Spark Plasma Sintering. J. Am. Ceram. Soc. 2012, 95, 2827–2832. [Google Scholar] [CrossRef]
- Chu, D.; Ma, H.; Zhang, Z.; Peng, F.; Jia, X. Synthesis and characterization of cubic boron nitride (Al)-Al2O3 composites under high pressure and high temperature conditions. Int. J. Refract. Met. Hard Mater. 2022, 106, 105876. [Google Scholar] [CrossRef]
- Hering, B.; Wolfrum, A.-K.; Gestrich, T.; Herrmann, M. Thermal Stability of TiN Coated Cubic Boron Nitride Powder. Materials 2021, 14, 1642. [Google Scholar] [CrossRef]
- Umer, M.A.; Sub, P.H.; Lee, D.J.; Ryu, H.J.; Hong, S.H. Polycrystalline cubic boron nitride sintered compacts prepared from nanocrystalline TiN coated cBN powder. Mater. Sci. Eng. A 2012, 552, 151–156. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Z.; Fan, B.; Li, J.; Cao, W.; Wang, H.; Lu, H.; Xu, H.; Zhang, R. Synthesis and Characterization of TiN-coated Cubic Boron Nitride Powders. Int. J. Appl. Ceram. Technol. 2014, 11, 946–953. [Google Scholar] [CrossRef]
- Umer, M.A.; Park, H.S.; Lee, D.J.; Ryu, H.J.; Hong, S.H. A sol–gel route to nanocrystalline TiN coated cubic boron nitride particles. J. Alloys Compd. 2011, 509, 9764–9769. [Google Scholar] [CrossRef]
- Daoush, W.M.; Park, H.S.; Hong, S.H. Fabrication of TiN/cBN and TiC/diamond coated particles by titanium deposition process. Trans. Nonferrous Met. Soc. China 2014, 24, 3562–3570. [Google Scholar] [CrossRef]
- Wang, S.; Guo, W.; Ma, H.-A.; Jia, X. Direct coating of cubic boron nitride with titanium powder under high pressure and high temperature. Mater. Lett. 2014, 123, 210–213. [Google Scholar] [CrossRef]
- Hoehn, S.; Sempf, K.; Herrmann, M. Artefact-free Preparation and Characterisation of Ceramic Materials and Interfaces. Ceram. Forum Int. 2011, 88, E16–E20. [Google Scholar]
- Aigner, K.; Lengauer, W.; Rafaja, D.; Ettmayer, P. Lattice parameters and thermal expansion of Ti(CxN1−x), Zr(CxN1−x), Hf(CxN1−x) and TiN1−x from 298 to 1473 K as investigated by high-temperature X-ray diffraction. J. Alloys Compd. 1994, 215, 121–126. [Google Scholar] [CrossRef]
- Lengauer, W. Properties of bulk δ-TiN1−x prepared by nitrogen diffusion into titanium metal. J. Alloys Compd. 1992, 186, 293–307. [Google Scholar] [CrossRef]
- Wriedt, H.A.; Murray, J.L. The N-Ti (Nitrogen-Titanium) system. Bull. Alloy Phase Diagr. 1987, 8, 378–388. [Google Scholar] [CrossRef]
- Farhadizadeh, A.R.; Amadeh, A.A.; Ghomi, H. Structural and Mechanical Properties of TiN-TiC-TiO System: First Principle Study*. Commun. Theor. Phys. 2017, 68, 678. [Google Scholar] [CrossRef]
- Chen, H.T.; Yan, M.F. Population analysis solution to hardness enhancement in TiCxN1−x. Phys. B Condens. Matter 2012, 407, 1183–1185. [Google Scholar] [CrossRef]
- Vegard, L. Die Konstitution der Mischkristalle und die Raumfüllung der Atome. Z. Für Phys. 1921, 5, 17–26. [Google Scholar] [CrossRef]

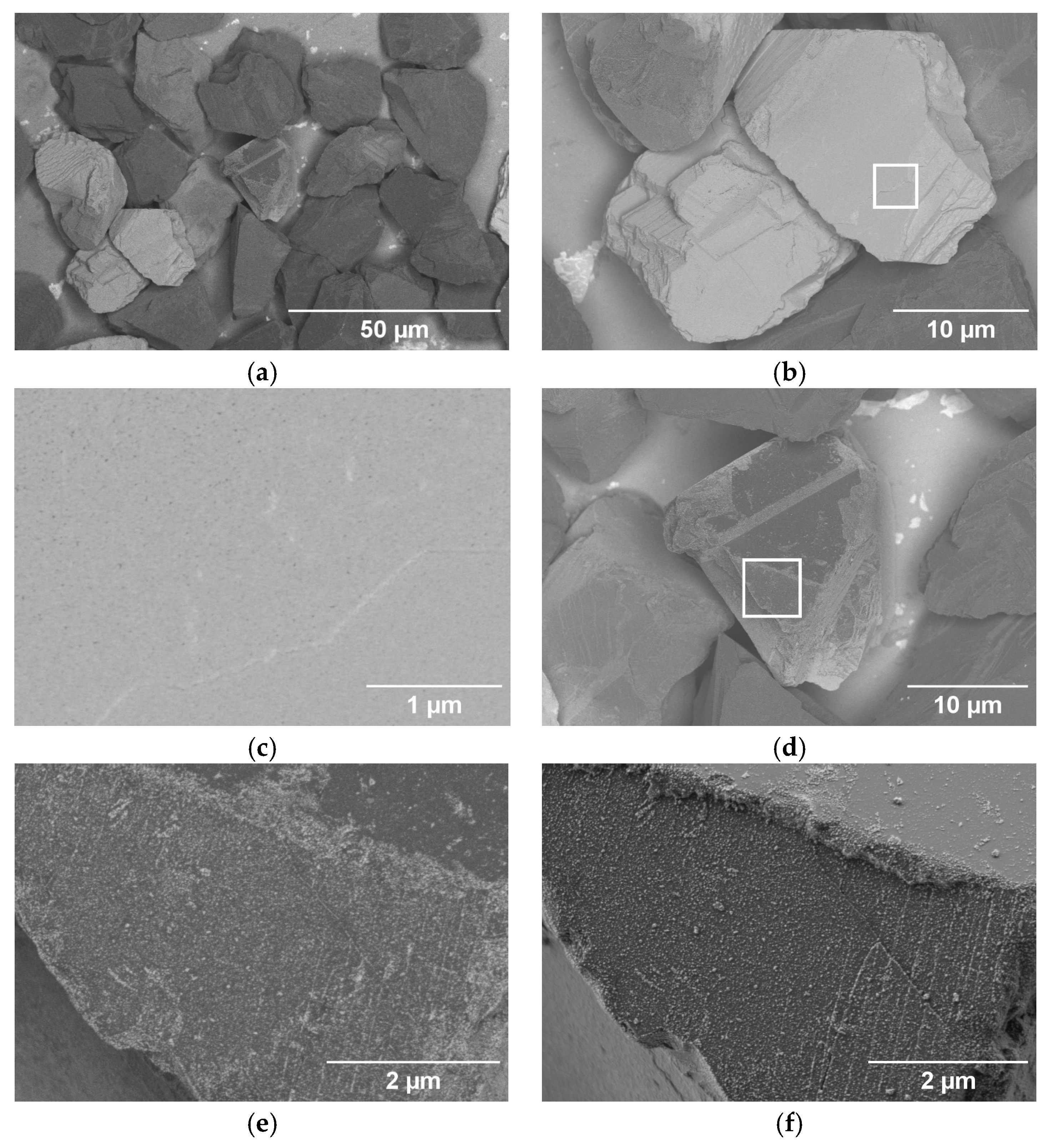
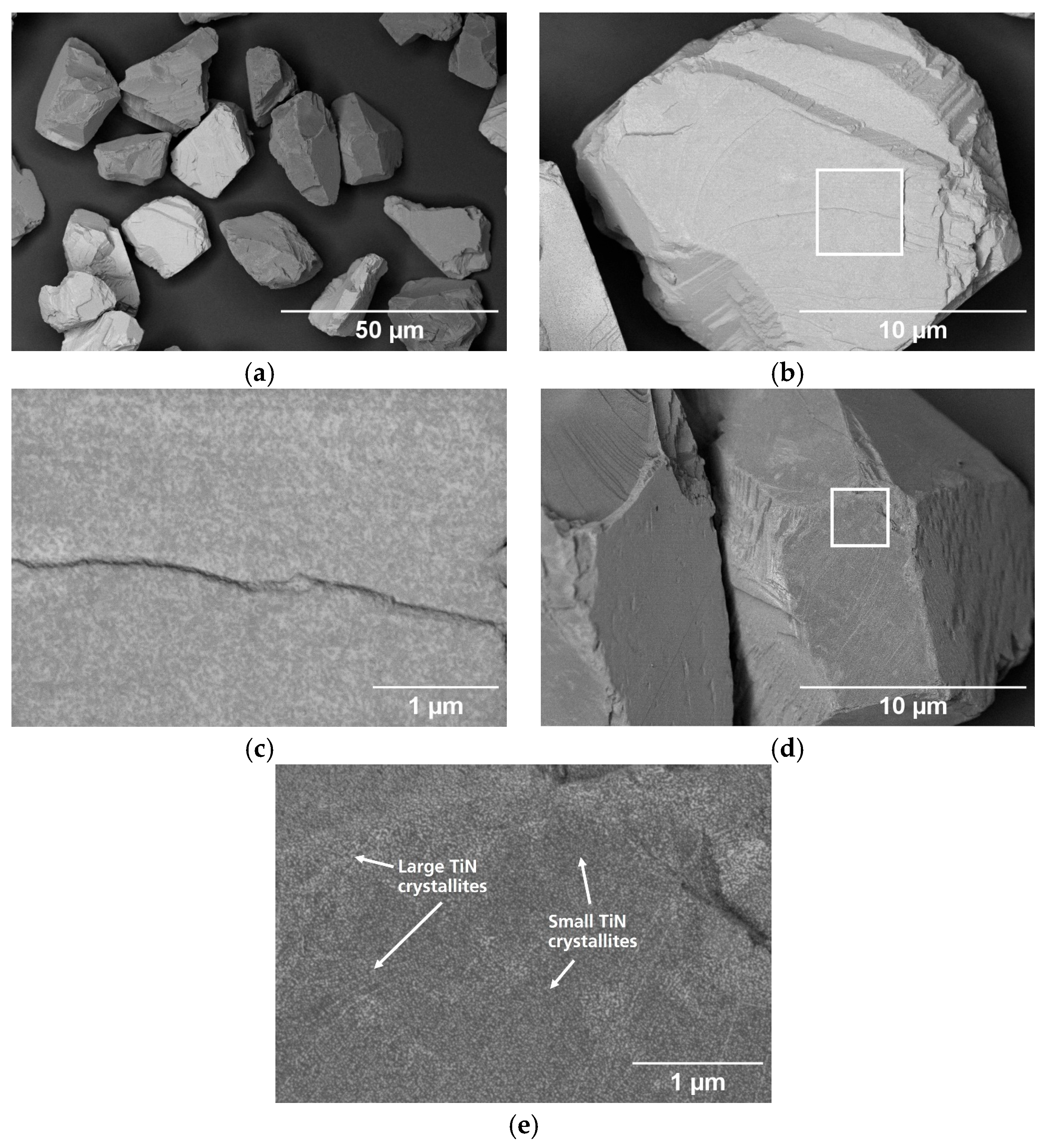
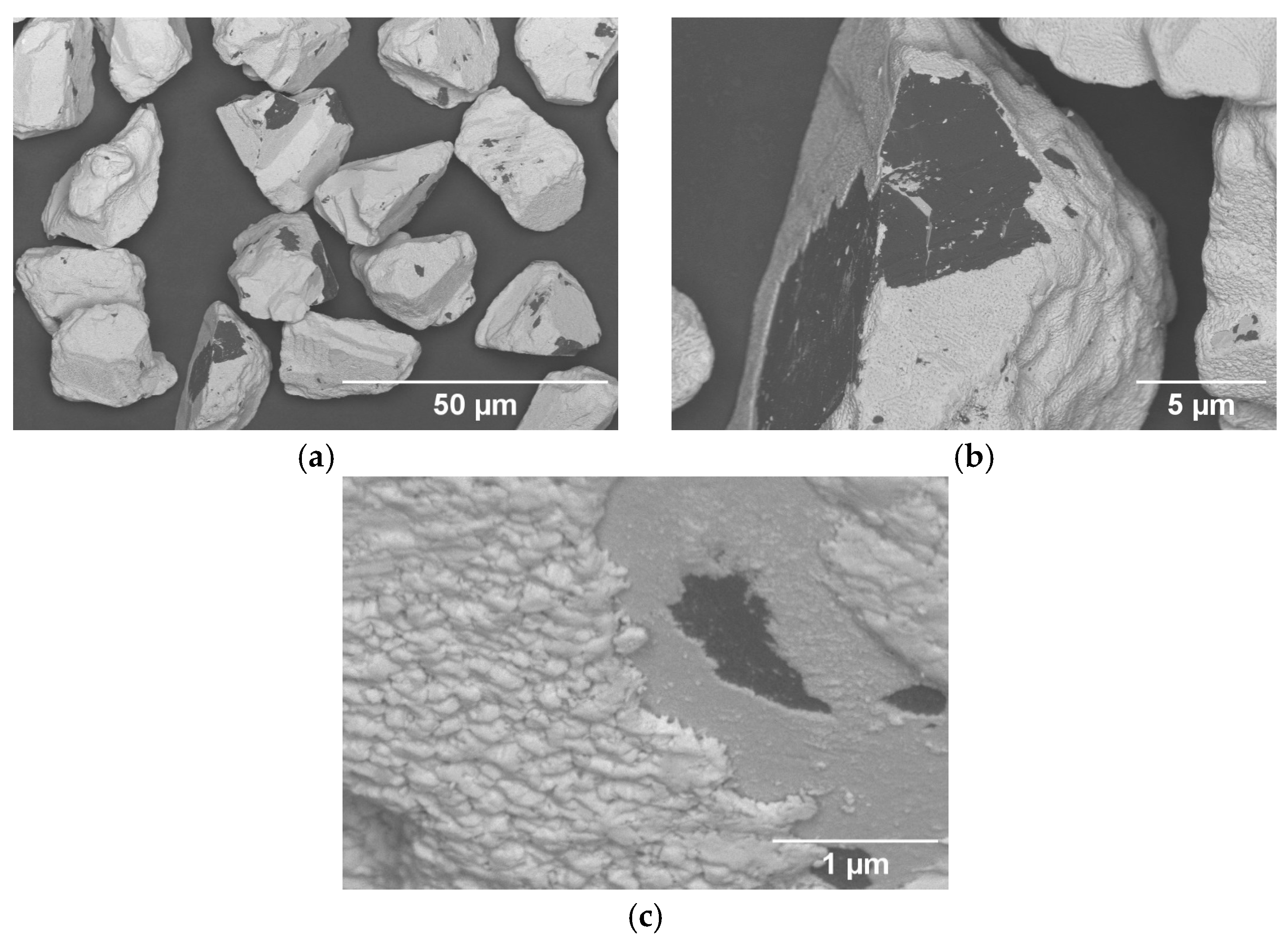
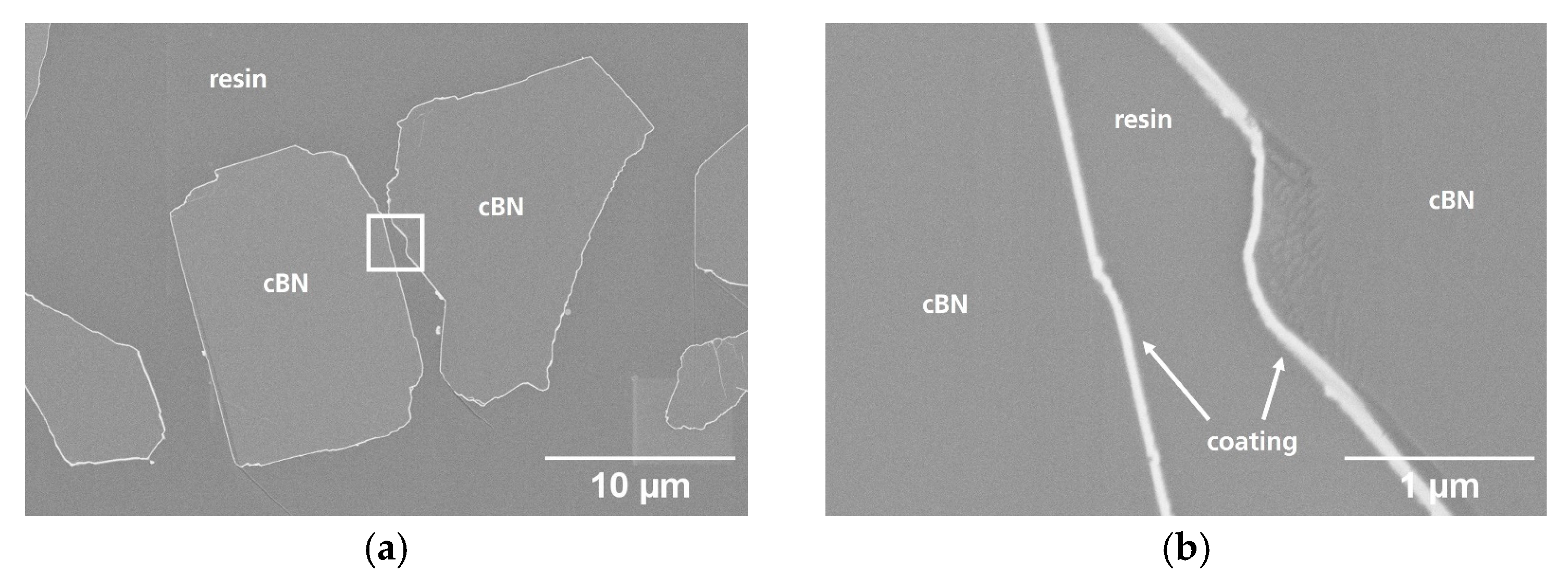
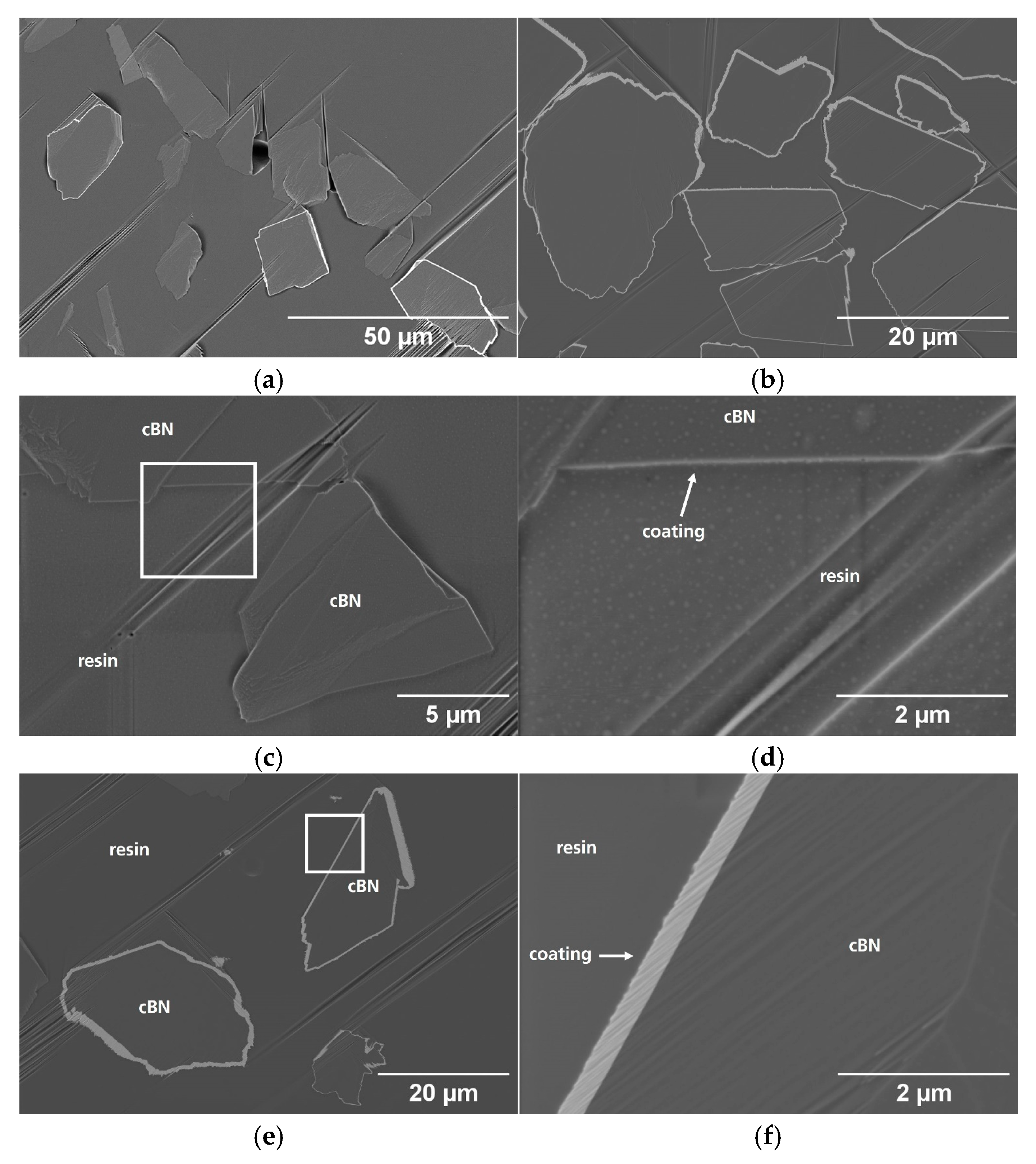
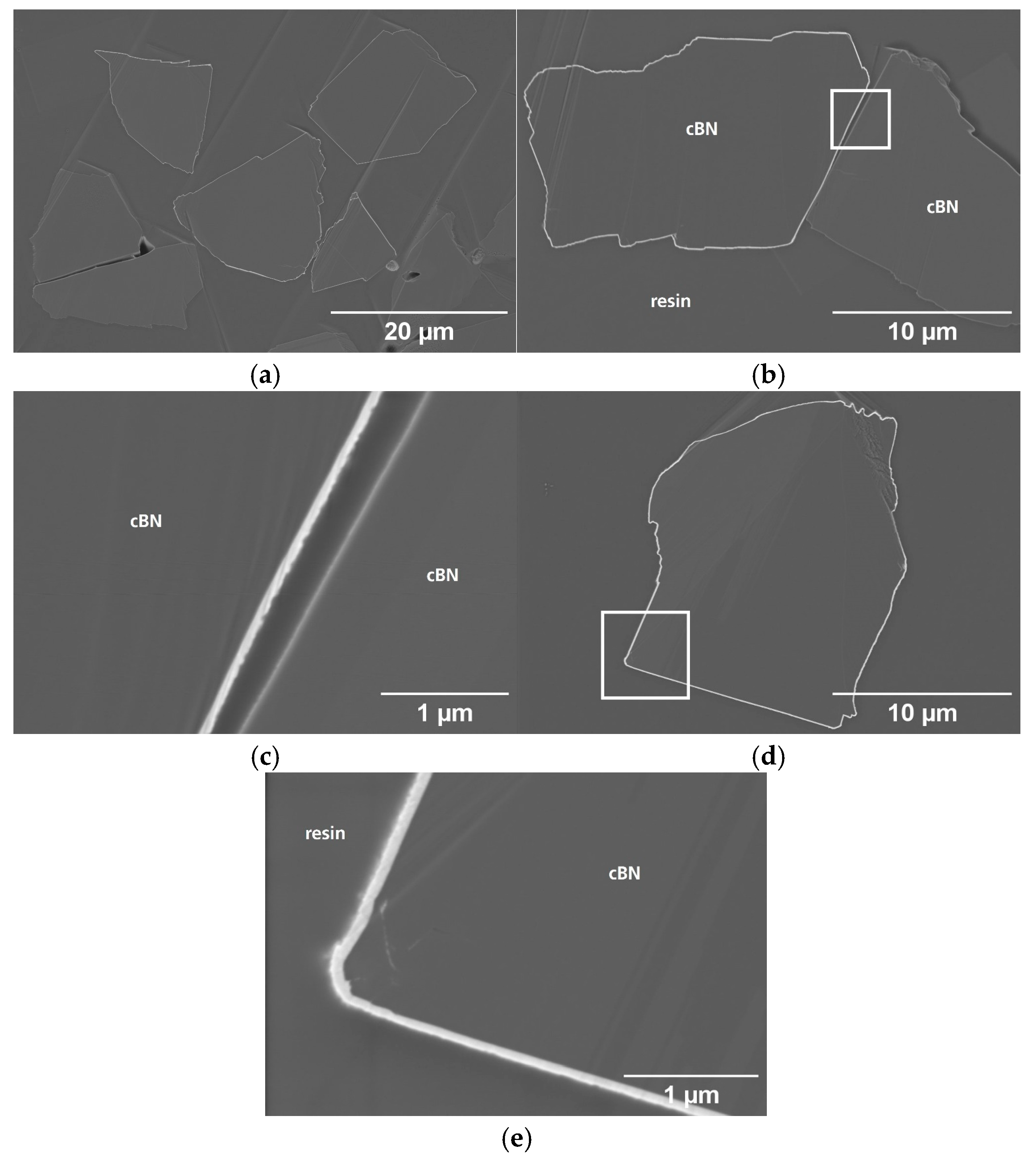
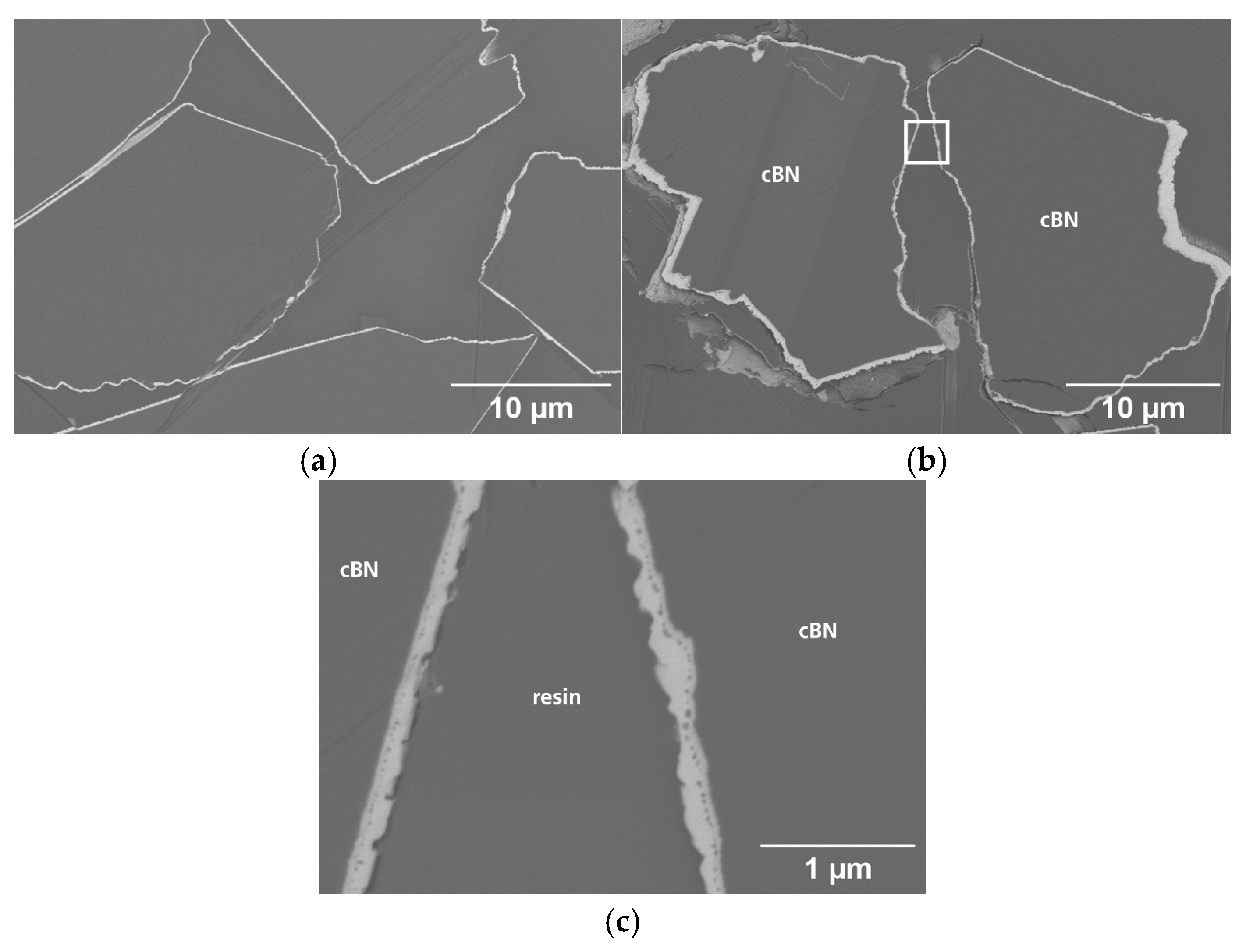

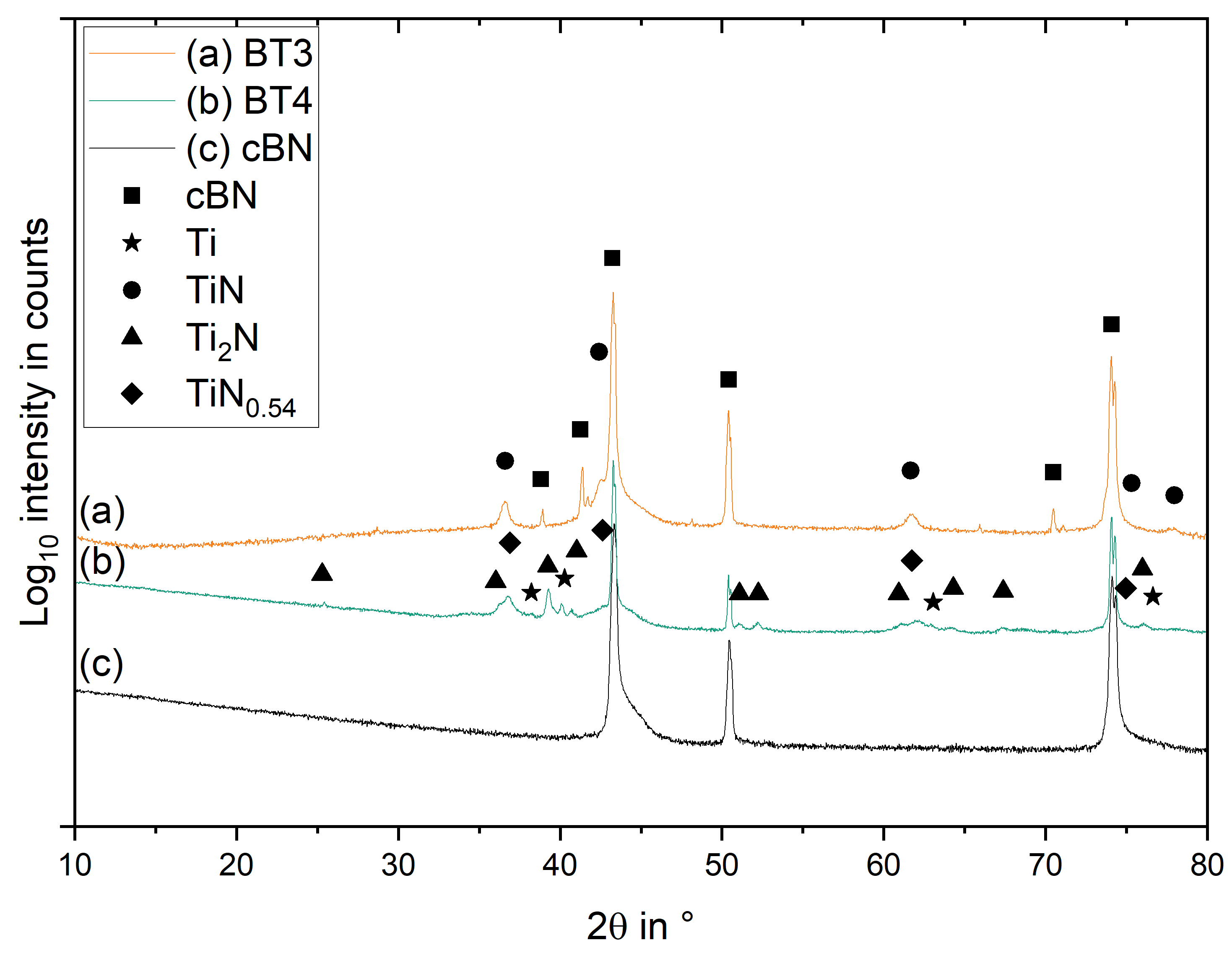



| Name | Process | Number of Cycles (ALD) | Temperature in °C | Coating Time in min (CVD) | Utilized Nitrogen Source (ALD/CVD) | Pressure in mbar | cBN Grade | Range of Coating Thickness in nm |
|---|---|---|---|---|---|---|---|---|
| BT1 | ALD | 1500 | 420 | / | NH3 | 3.7 | B20 | 40–50 |
| BT2 | ALD | 30 | 420 | / | NH3 | 2.4–3.3 | 20–500 | |
| CVD | / | 950 | 90 | N2 | 82 | |||
| BT3 | ALD | 100 | 420 | / | NH3 | 12.5–16 | C41 | 20–100 |
| CVD | / | 950 | 90 (and reduced gas flow) | N2 | 38–45 | |||
| BT4 | unknown | / | / | / | / | / | unknown | 100–1000 |
| Powder | α-Ti, wt% | Ti2N, wt% | TiN1−x, wt% | X in TiN1−x * | Lattice Parameter of Titanium Nitride in nm |
|---|---|---|---|---|---|
| BT1 | / | / | 1.77 (±0.05) | 0.16 | 0.42338 (±0.00009) |
| BT2 | / | / | 4.63 (±0.08) | 0 | 0.42503 (±0.00003) |
| BT3 | / | / | 0.85 (±0.08) | 0 | 0.42519 (±0.00017) |
| BT4 | 0.37 (±0.02) | 0.81 (±0.02) | 4.29 (±0.08) | 0.46 | 0.42168 (±0.00012) |
| BT3 (TGA) | / | / | 0.98 (±0.05) | 0. | 0. 42556 (±0.000011) |
| BT4 (TGA) | / | / | 6.32 (±0.09) | 0 | 0.42576 (±0.000004) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maier, L.; Krug, M.; Höhn, M.; Wolfrum, A.-K.; Matthey, B.; Herrmann, M.; Höhn, S.; Michaelis, A. Coating of Cubic Boron Nitride Powder with TiN in a Rotating Drum via Gas Phase Processes. Coatings 2025, 15, 711. https://doi.org/10.3390/coatings15060711
Maier L, Krug M, Höhn M, Wolfrum A-K, Matthey B, Herrmann M, Höhn S, Michaelis A. Coating of Cubic Boron Nitride Powder with TiN in a Rotating Drum via Gas Phase Processes. Coatings. 2025; 15(6):711. https://doi.org/10.3390/coatings15060711
Chicago/Turabian StyleMaier, Louis, Mario Krug, Mandy Höhn, Anne-Kathrin Wolfrum, Björn Matthey, Mathias Herrmann, Sören Höhn, and Alexander Michaelis. 2025. "Coating of Cubic Boron Nitride Powder with TiN in a Rotating Drum via Gas Phase Processes" Coatings 15, no. 6: 711. https://doi.org/10.3390/coatings15060711
APA StyleMaier, L., Krug, M., Höhn, M., Wolfrum, A.-K., Matthey, B., Herrmann, M., Höhn, S., & Michaelis, A. (2025). Coating of Cubic Boron Nitride Powder with TiN in a Rotating Drum via Gas Phase Processes. Coatings, 15(6), 711. https://doi.org/10.3390/coatings15060711









