Effect of B4C Content on the Oxidation Resistance of a B4C-SiO2–Albite/Al2O3 Coating at 900 °C
Abstract
1. Introduction
2. Experimental
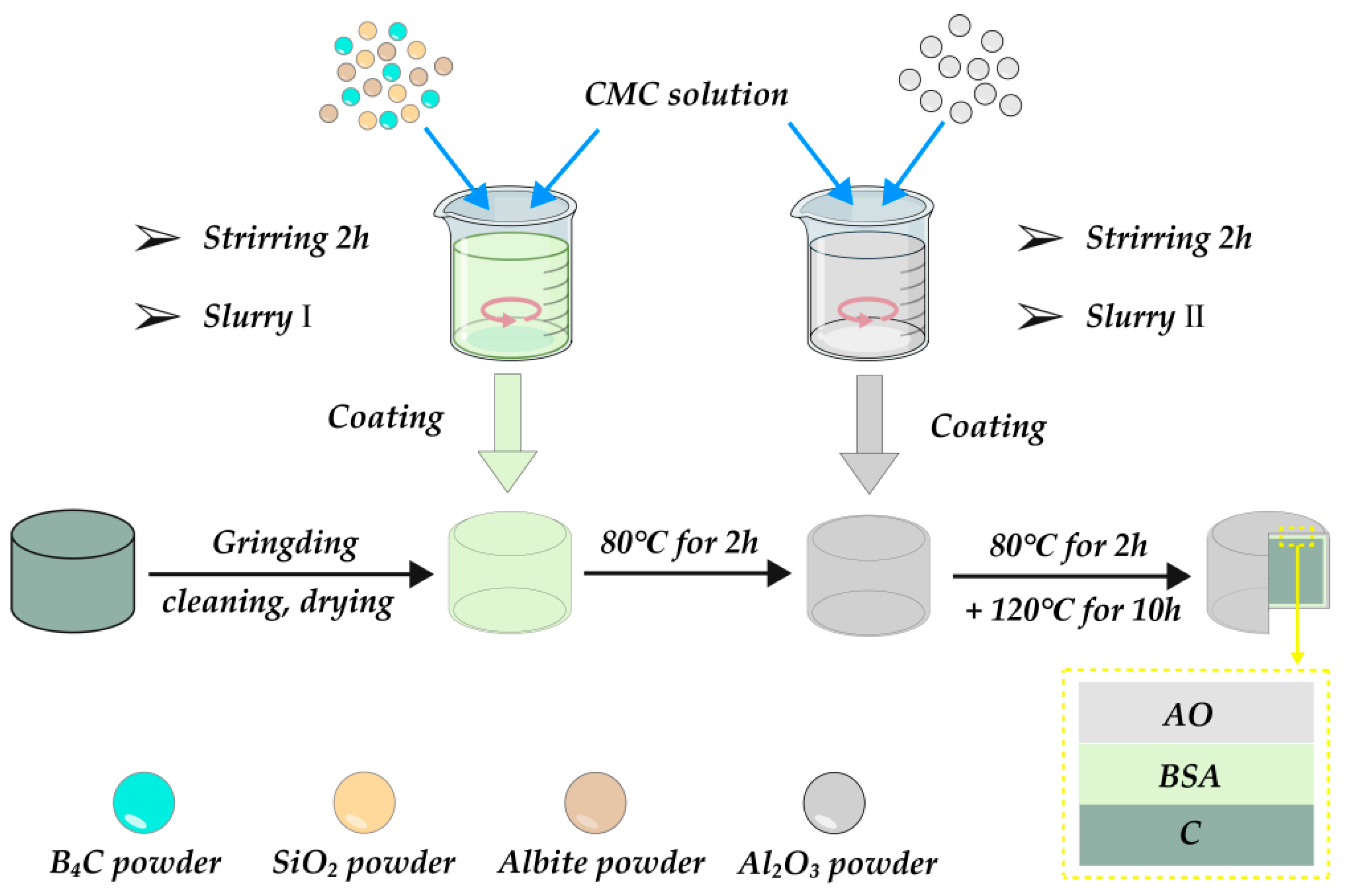
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussion
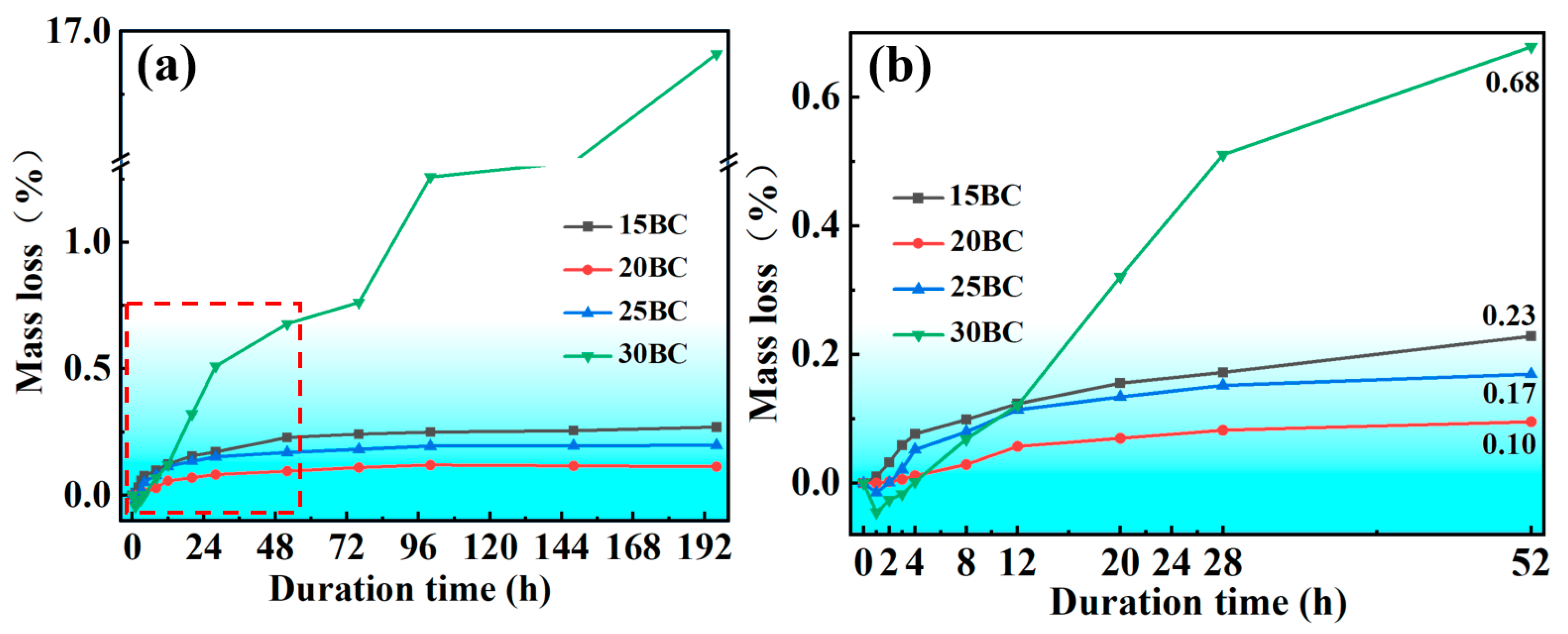
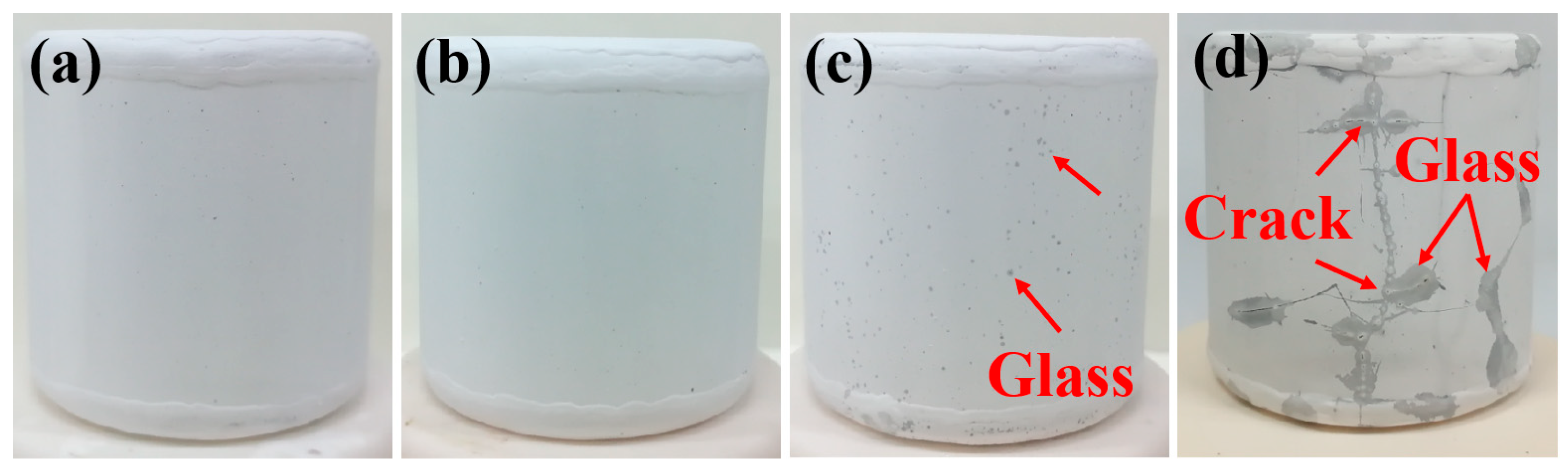
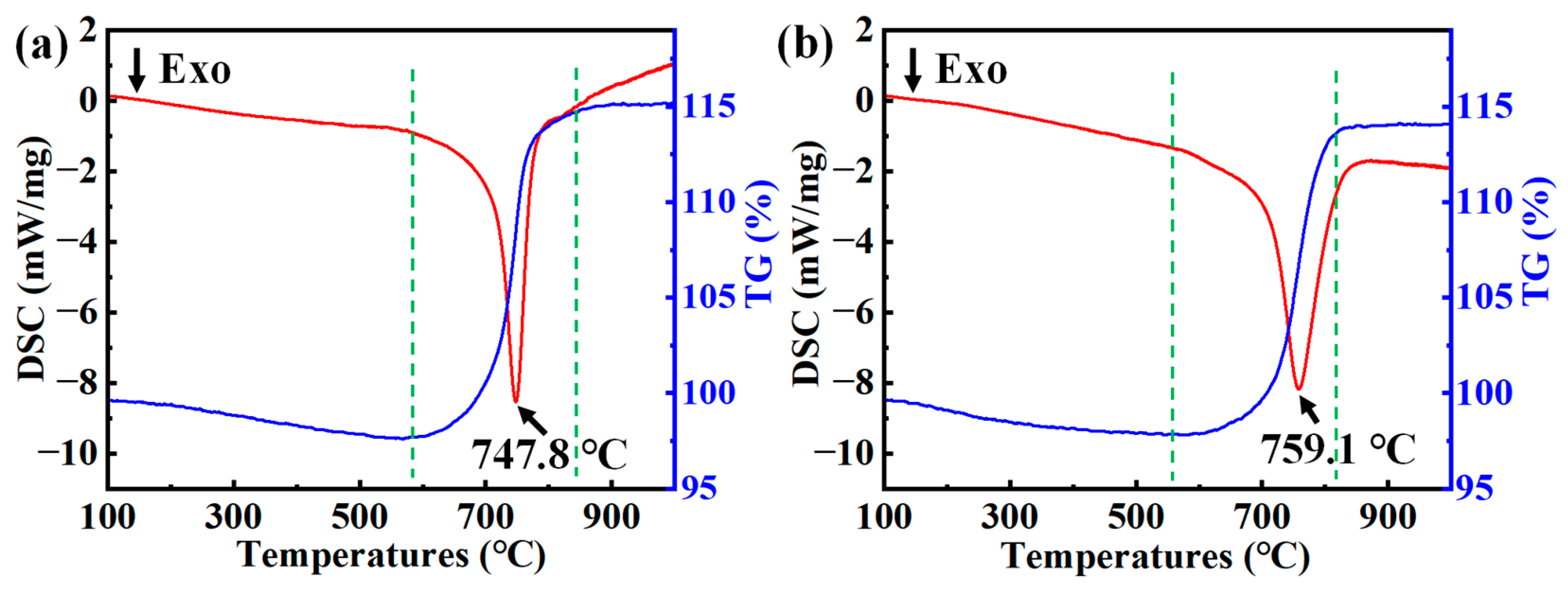
3.1. Analysis of Oxidation Performance
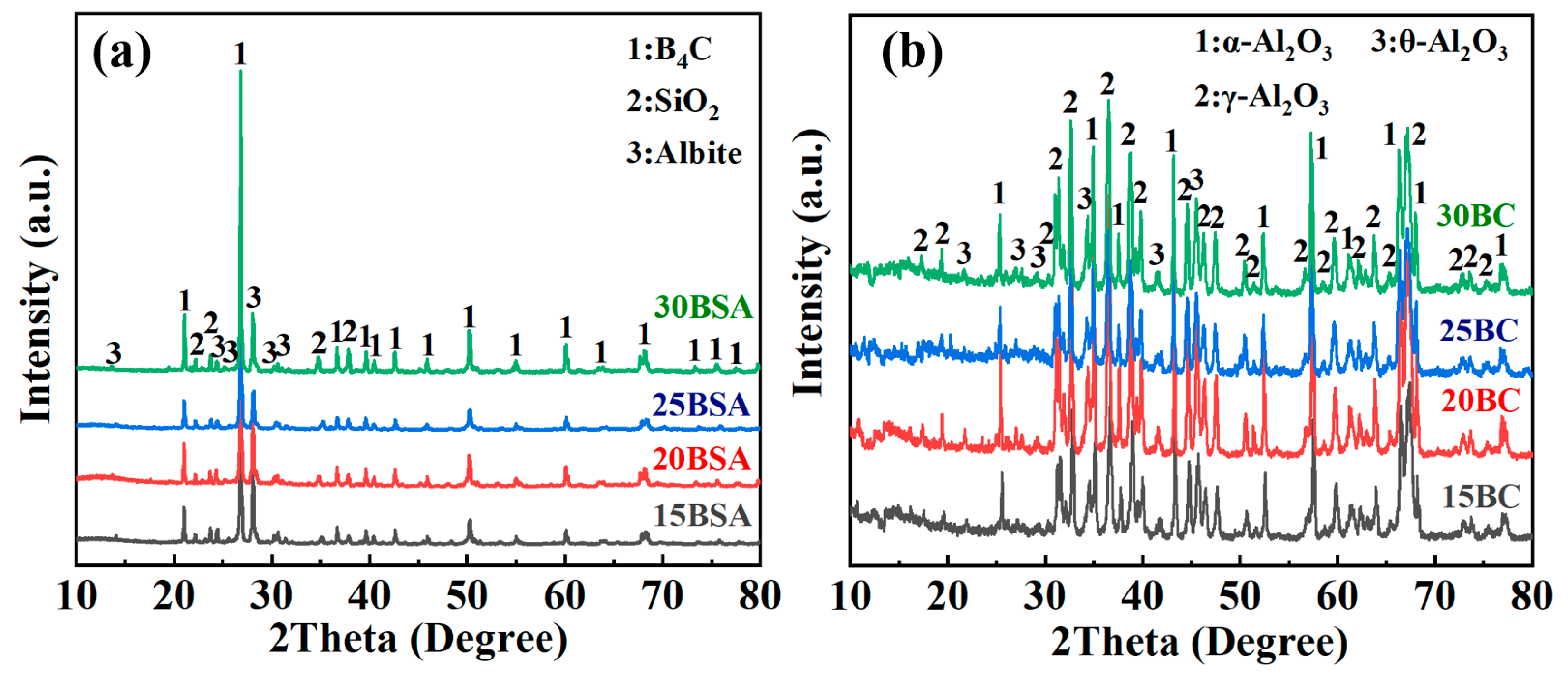
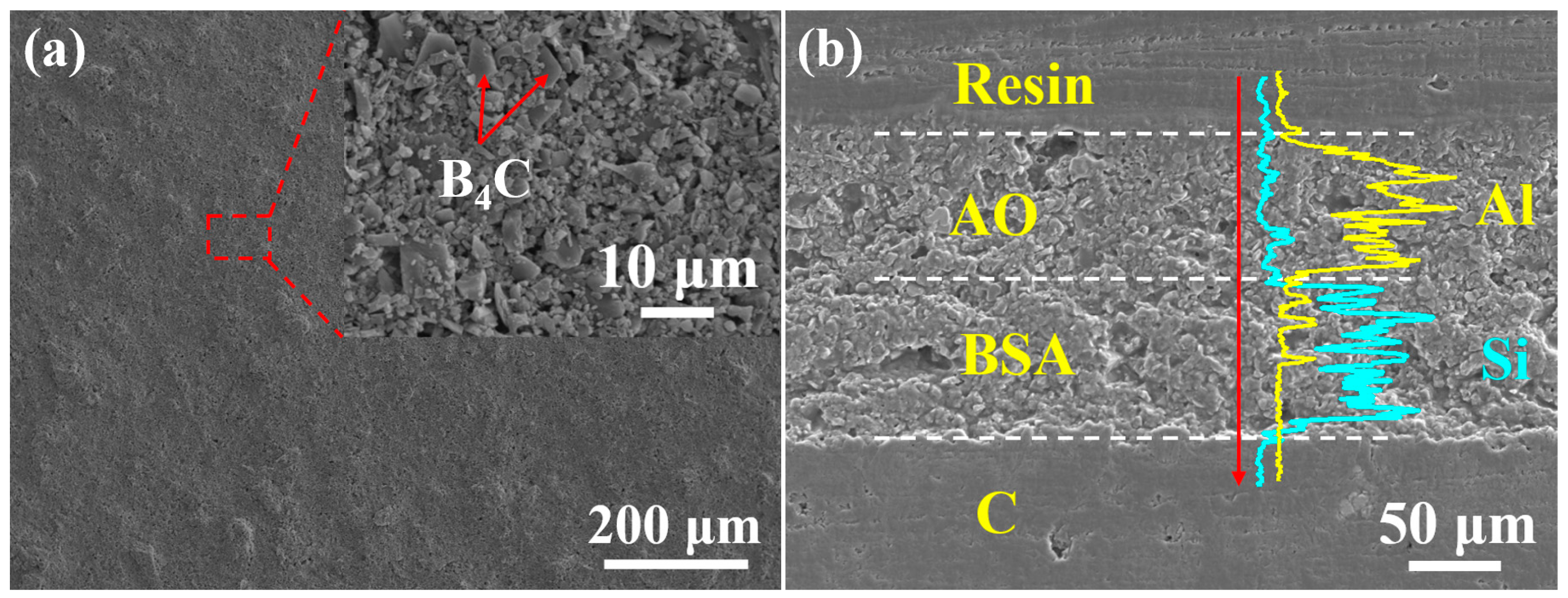
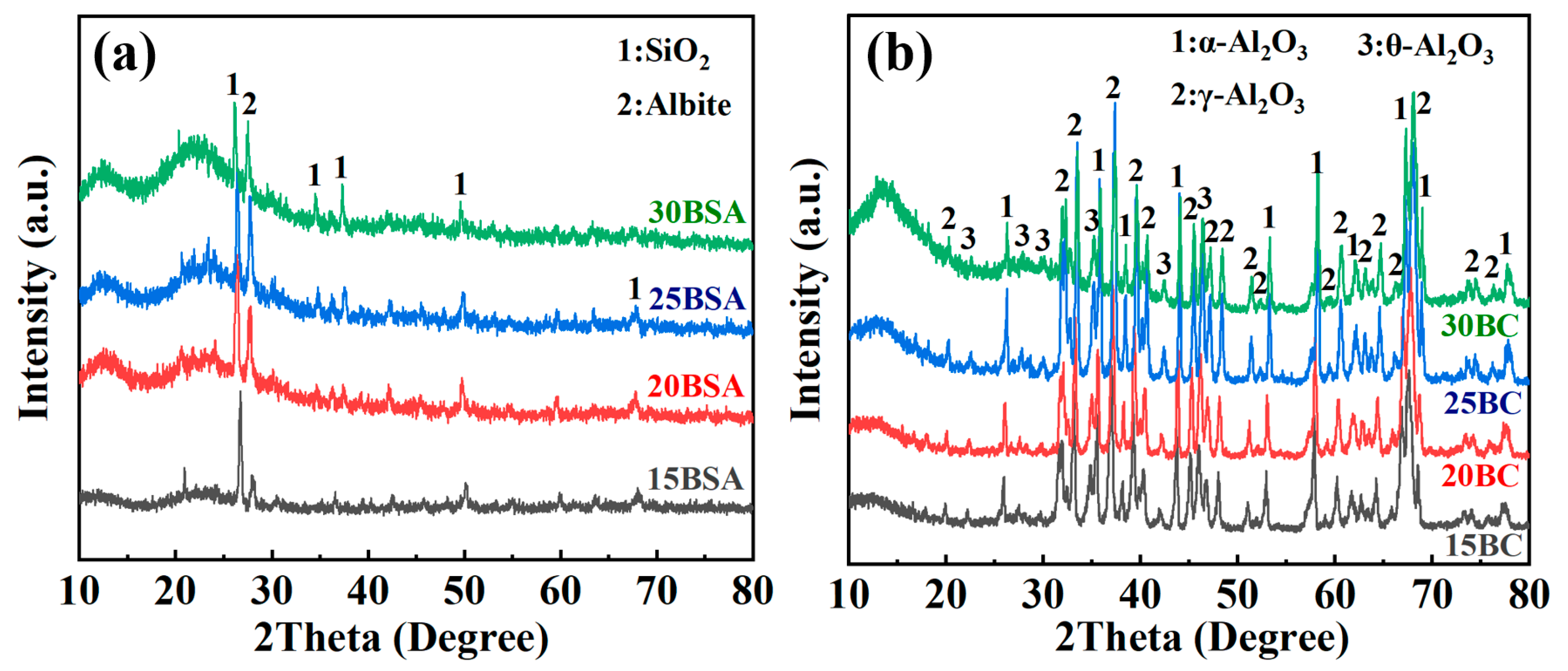
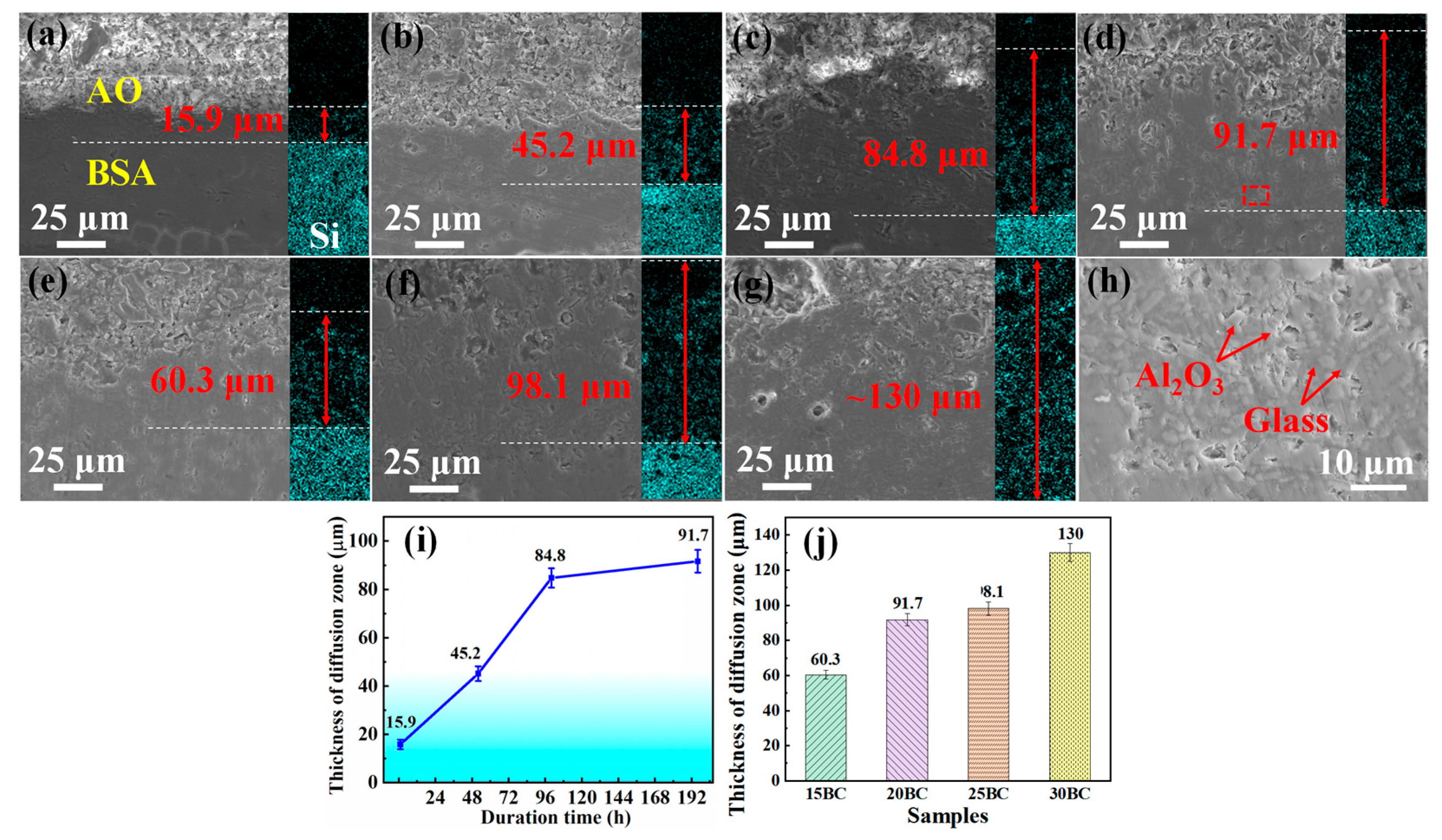
3.2. Analysis of Microstructure and Performance
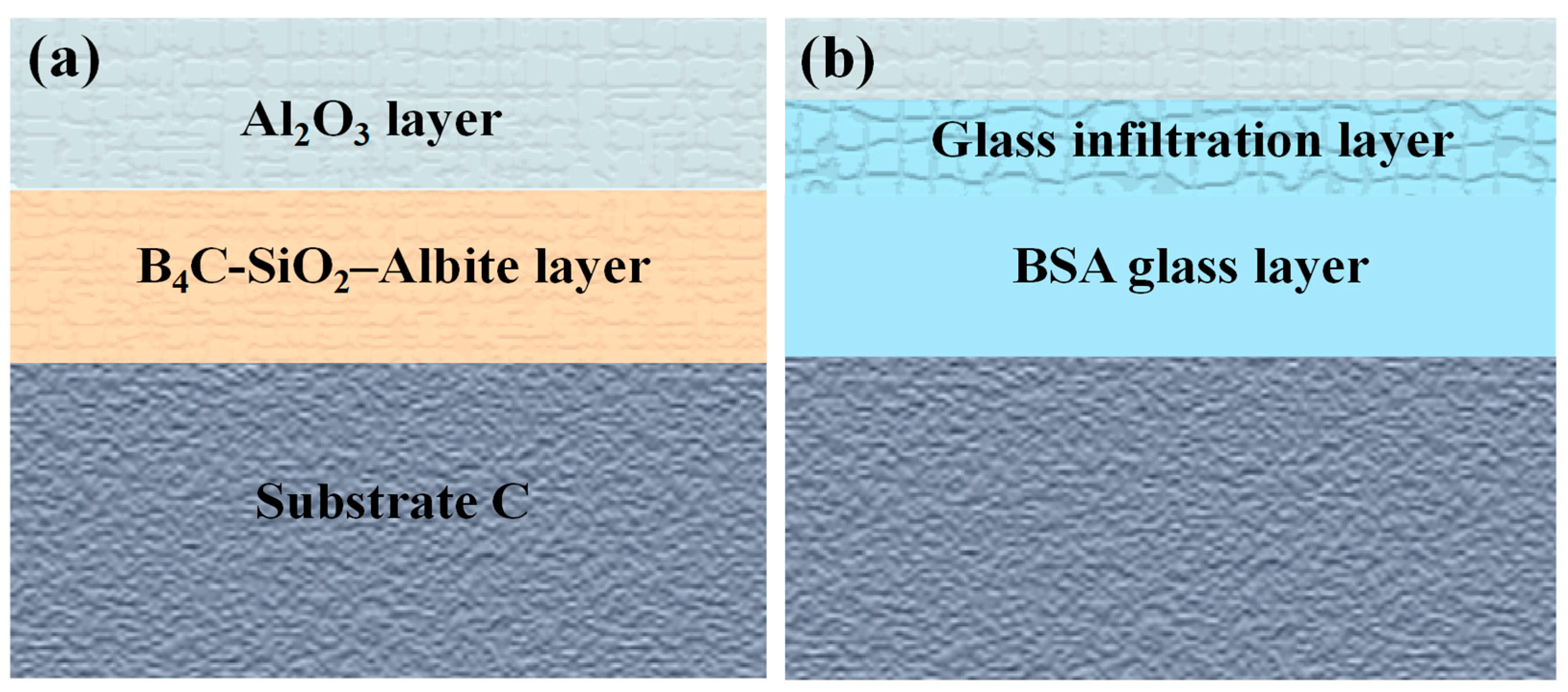
3.3. Analysis of Mechanism
4. Conclusions
- (1)
- The B4C addition promotes the formation of the amorphous component in the composite coating, which is helpful to improve the oxidation resistance of the composite coating due to the excellent oxygen diffusion barrier.
- (2)
- The addition content of B4C greatly affects the oxidation resistance property of the coating due to the amount of formed amorphous glass, attributed to the fluidity and the diffusion of the glass. The optimal addition content is about 20 wt% in the inner BSA layer. The 20 wt% BSA/AO composite coating shows the best oxidation performance, owing to only about 0.11% mass loss after exposure for 196 h at 900 °C.
- (3)
- The formation of the infiltration layer effectively prevents the further loss of the B element, keeps the glass stable, and enhances the long-term oxidation resistance property of the composite coating. The average mass loss rate is about 0.14 mg/cm2•h for the coating with 20 wt% B4C after 196 h duration.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, W.; Li, M.; Liu, L.; Li, H. A new approach for improving the quality of the carbon anode for aluminum electrolysis—An impregnation-baking process. Alex. Eng. J. 2024, 96, 195–205. [Google Scholar] [CrossRef]
- Gao, B.; Niu, H.; Guan, Y.; Wang, Z.; Liu, J.; Taylor, M.P.; Chen, J.J.J. Visualization of Anode Effect in Aluminum Electrolysis. J. Electrochem. Soc. 2022, 169, 013505. [Google Scholar] [CrossRef]
- Cairo, C.A.A.; Florian, M.; Graça, M.L.A.; Bressiani, J.C. Kinetic study by TGA of the effect of oxidation inhibitors for carbon–carbon composite. Mater. Sci. Eng. A 2003, 358, 298–303. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, R.; Hou, C.; Shen, Q.; Zhao, M.; Wan, X. A PD-FEM coupling approach for the analysis of oxidation and out-of-plane compression failure of 2D braided C/C composites. Eng. Fract. Mech. 2025, 316, 110866. [Google Scholar] [CrossRef]
- Zhang, S.; Lee, W.E. Improving the water-wettability and oxidation resistance of graphite using Al2O3/SiO2 sol-gel coatings. J. Eur. Ceram. Soc. 2003, 23, 1215–1221. [Google Scholar] [CrossRef]
- Wang, L.; Fu, Q.; Zhao, F. Improving oxidation resistance of MoSi2 coating by reinforced with Al2O3 whiskers. Intermetallics 2018, 94, 106–113. [Google Scholar] [CrossRef]
- Cheng, C.; Li, H.; Fu, Q.; Guo, L. Effect of Al2O3 on the densification and oxidation behavior of SiC coating for carbon/carbon composites. Ceram. Int. 2018, 44, 12702–12708. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, J.; Cao, L.; Hao, W.; Wu, W. A novel design of oxidation protective β-Y2Si2O7 nanowire toughened Y2SiO5/Y2O3-Al2O3-SiO2 glass ceramic coating for SiC coated carbon/carbon composites. Corros. Sci. 2018, 135, 233–242. [Google Scholar] [CrossRef]
- Manolescu, P.; Duchesne, C.; Lauzon-Gauthier, J.; Saevarsdottir, G. Net Carbon Consumption in Aluminum Electrolysis: Impact of Anode Properties and Reduction Cell-Operation Variables. J. Sustain. Metall. 2022, 8, 1167–1179. [Google Scholar] [CrossRef]
- Hao, P.; Lv, X.; Han, Z.; Wu, Y.; Tan, X. The oxidation resistance study of a novel quasi-molten coating for the prebaked anode. Int. J. Appl. Ceram. Technol. 2024, 21, 4114–4126. [Google Scholar] [CrossRef]
- Huang, J.; Guo, L.; Li, K.; Yan, N.; Zhou, L.; Li, Y. Microstructures and oxidation behaviors of Al-modified and Al2O3-modified SiC coatings on carbon/carbon composites via pack cementation. Ceram. Int. 2021, 47, 8105–8112. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, Y.; Shen, Y.; Chen, W.; Cheng, H.; Wang, L. Room-temperature aqueous plasma electrolyzing Al2O3 nano-coating on carbon fiber. Appl. Surf. Sci. 2017, 419, 357–364. [Google Scholar] [CrossRef]
- Wang, P.; Ji, X.; Sun, W.; Luo, H.; Bai, Y.; Wu, Y.; Kiryukhantsev-Korneev, P.V.; Levashov, E.A.; Shi, J.; Ren, X. Oxidation protection of ZrB2-SiC-LaB6 ceramics coating in a wide temperature range. Ceram. Int. 2025, 51, 6916–6925. [Google Scholar] [CrossRef]
- Liu, B.; Guo, L.; Shi, H.; Zhang, Y.; Yin, X.; Sun, J.; Fu, Q. Improved oxidation resistance of CVD-SiC coating at 1300 °C by Ti addition. J. Eur. Ceram. Soc. 2025, 45, 117289. [Google Scholar] [CrossRef]
- He, Y.; Zhou, K.-C.; Zhang, Y.; Xiong, H.-W.; Zhang, L. Recent progress of inert anodes for carbon-free aluminium electrolysis: A review and outlook. J. Mater. Chem. A 2021, 9, 25272–25285. [Google Scholar] [CrossRef]
- Yang, W.; Ren, J.; Tang, Y.; Wang, Y.; Yang, Y. Effect of heat treatment on microstructure and oxidation resistance of SiO2-B4C-glass coating on alumina-carbon refractories. Ceram. Int. 2025, 51, 7825–7839. [Google Scholar] [CrossRef]
- Deng, J.; Hu, K.; Lu, B.; Ma, X.; Li, H.; Wang, J.; Fan, S.; Zhang, L.; Cheng, L. Effect of B4C addition on the oxidation behavior of borosilicate glass repairing coating for C/C brake materials. Ceram. Int. 2020, 46, 14496–14504. [Google Scholar] [CrossRef]
- Fan, S.; Ma, X.; Li, Z.; Hu, J.; Xie, Z.; Deng, J.; Zhang, L.; Cheng, L. Design and optimization of oxidation resistant coating for C/C aircraft brake materials. Ceram. Int. 2018, 44, 175–182. [Google Scholar] [CrossRef]
- Cairo, C.A.A.; Graça, M.L.A.; Silva, C.R.M.; Bressiani, J.C. Functionally gradient ceramic coating for carbon–carbon antioxidation protection. J. Eur. Ceram. Soc. 2001, 21, 325–329. [Google Scholar] [CrossRef]
- Du, W.; Zeng, F.; Gao, Y.; Wang, Z.; Chen, M.; Li, Z. Oxidation mechanism of HfC-TaC-B4C-SiC/ZrSiO4-glass coating with largely enhanced oxidation inhibition for C/C composites. Surf. Interfaces 2024, 53, 105100. [Google Scholar] [CrossRef]
- Deng, J.; Hu, K.; Lu, B.; Zheng, B.; Fan, S.; Zhang, L.; Cheng, L. Influence of B4C on oxidation resistance of PSN/borosilicate glass-B4C field-based repair coating of C/C aircraft brake materials at 700–900 °C. Ceram. Int. 2019, 45, 20860–20872. [Google Scholar] [CrossRef]
- Lv, C.; Ren, J.; Duan, Y.; Wu, Y.; Li, X. Effect of SiO2 content on the oxidation resistance of SiO2–B4C-glass coating for alumina–carbon refractories. Ceram. Int. 2023, 49, 8554–8564. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, M.; Sun, W.; Ren, X. Oxidation protection of B4C modified HfB2-SiC coating for C/C composites at 1073–1473 K. Ceram. Int. 2022, 48, 3206–3215. [Google Scholar] [CrossRef]
- Li, Y.Q.; Qiu, T. Oxidation behaviour of boron carbide powder. Mater. Sci. Eng. A 2007, 444, 184–191. [Google Scholar] [CrossRef]
- Zhang, Q.; Zuo, X.; Liu, Y.; Zhang, L.; Cheng, L.; Liu, X. Oxidation behaviors and mechanisms of CVD Si-B-C ceramic in wet oxygen from 700 °C to 1400 °C. J. Eur. Ceram. Soc. 2016, 36, 3709–3715. [Google Scholar] [CrossRef]
- Fan, S.; Ma, X.; Ji, B.; Li, Z.; Xie, Z.; Deng, J.; Zhang, L.; Cheng, L. Oxidation resistance and thermal shock properties of self-healing SiCN/borosilicate glass-B4C-Al2O3 coatings for C/C aircraft brake materials. Ceram. Int. 2019, 45, 550–557. [Google Scholar] [CrossRef]
- Li, X.; Feng, J.; Jiang, Y.; Lin, H.; Feng, J. Preparation and anti-oxidation performance of Al2O3-containing TaSi2–MoSi2–borosilicate glass coating on porous SiCO ceramic composites for thermal protection. RSC Adv. 2018, 8, 13178–13185. [Google Scholar] [CrossRef]
- Gu, Y.; Li, R.; Zeng, F.; Jiang, Z.; Shi, X.; Wu, A.; Huang, L. Microstructure evolution and oxidation resistance of CaO-Al2O3-SiO2/ZrO2-borosilicate multilayer coating for C/C composites. Corros. Sci. 2021, 191, 109723. [Google Scholar] [CrossRef]
- Yu, Y.; Luo, R.; Shang, H.; Wang, T.; Wang, J.; Wang, L. Oxidation behavior of carbon/carbon composites coated with a Si-SiOx/BN-B2O3-SiO2-Al2O3 oxidation protection system at intermediate temperature. Vacuum 2016, 128, 9–16. [Google Scholar] [CrossRef]
- Hu, C.; Pang, S.; Tang, S.; Yang, Z.; Wang, S.; Cheng, H.-M. Long-term oxidation behavior of carbon/carbon composites with a SiC/B4C–B2O3–SiO2–Al2O3 coating at low and medium temperatures. Corros. Sci. 2015, 94, 452–458. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Li, H.; Zhang, J.; Fu, Y.; Su, Y. SiC/SiC-ZrSi2 coating with micro-pore to protect C/C composites against oxidation for long-life service at high temperatures. Corros. Sci. 2021, 191, 109780. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Zhang, J.; Su, Y.; Chen, R.; Zhang, P. SiC/HfB2-based ceramic/SiC multilayer coating to protect C/C composites against oxidation at medium and high temperatures for long-life service. Corros. Sci. 2022, 201, 110299. [Google Scholar] [CrossRef]
- Brosh, E.; Pelton, A.D.; Decterov, S.A. A model to calculate the viscosity of silicate melts. Part IV Alkali-Free. Borosilicate Melts 2012, 103, 494–501. [Google Scholar]
- Zhang, X.; Liu, C.; Jiang, M. Effect of B2O3 on the Melt Structure and Viscosity of CaO–SiO2 System. Steel Res. Int. 2022, 93, 2100520. [Google Scholar] [CrossRef]
- Wang, C.; Lin, P.; Liu, X.; Li, G.; Lin, T.; He, P.; Long, W.; Liu, H. Microstructure evolution and cooperative reinforcement mechanisms of Al2O3/Al2O3 joints brazed by low-melting borosilicate glass. Ceram. Int. 2020, 46, 186–195. [Google Scholar] [CrossRef]
- Sun, T.; Xiao, H.; Guo, W.; Hong, X. Effect of Al2O3 content on BaO–Al2O3–B2O3–SiO2 glass sealant for solid oxide fuel cell. Ceram. Int. 2010, 36, 821–826. [Google Scholar] [CrossRef]
- Machowski, P.M.; Varsamis, C.P.E.; Kamitsos, E.I. Dependence of sodium borate glass structure on depth from the sample surface. J. Non-Cryst. Solids 2004, 345–346, 213–218. [Google Scholar] [CrossRef]
- Koroleva, O.N.; Shabunina, L.A.; Bykov, V.N. Structure of borosilicate glass according to raman spectroscopy data. Glass Ceram. 2011, 67, 340–342. [Google Scholar] [CrossRef]
- Hou, X.; Chou, K.-C. Quantitative investigation of oxidation behavior of boron carbide powders in air. J. Alloys Compd. 2013, 573, 182–186. [Google Scholar] [CrossRef]
- Yu, Y.; Stevensson, B.; Edén, M. A unified 23Na NMR chemical shift correlation with structural parameters in multicomponent silicate-based glasses. J. Am. Ceram. Soc. 2020, 103, 762–767. [Google Scholar] [CrossRef]
- Jia, Z.; Liu, C.; Niu, C.; Li, K.; Xu, K. Volatilization of sodium and boron from nuclear waste glass and associated effects on glass structure and thermal stability. J. Nucl. Mater. 2023, 587, 154712. [Google Scholar] [CrossRef]
- Kwinda, T.I.; Koppka, S.; Sander, S.A.H.; Kohns, R.; Enke, D. Effect of Al2O3 on phase separation and microstructure of R2O-B2O3-Al2O3-SiO2 glass system (R = Li, Na). J. Non-Cryst. Solids 2020, 531, 119849. [Google Scholar] [CrossRef]
- Stoch, L.; Środa, M. Infrared spectroscopy in the investigation of oxide glasses structure. J. Mol. Struct. 1999, 511–512, 77–84. [Google Scholar] [CrossRef]
- Stolyarova, V.L. Vaporization Processes and Thermodynamic Properties of Oxide Systems Studied by High Temperature Mass Spectrometry. ECS Trans. 2013, 46, 55. [Google Scholar] [CrossRef]
- Jiang, Z.-H.; Zhang, Q.-Y. The structure of glass: A phase equilibrium diagram approach. Prog. Mater. Sci. 2014, 61, 144–215. [Google Scholar] [CrossRef]
| Samples | Inner BSA Layer | Outer AO Layer | ||
|---|---|---|---|---|
| B4C | SiO2 | Albite | Al2O3 | |
| 15BC | 15 | 40 | 45 | 100 |
| 20BC | 20 | 40 | 40 | 100 |
| 25BC | 25 | 40 | 35 | 100 |
| 30BC | 30 | 40 | 30 | 100 |
| Exposure/h | O | Na | Al | Si | |
|---|---|---|---|---|---|
| 0 h | 15BC | 53.4 | 2.1 | 3.9 | 40.6 |
| 20BC | 53.9 | 1.8 | 3.8 | 40.4 | |
| 25BC | 56.3 | 1.0 | 3.3 | 39.4 | |
| 30BC | 58.9 | 0.7 | 2.4 | 38.0 | |
| 1 h | 15BC | 62.7 | 2.3 | 2.1 | 32.9 |
| 20BC | 64.1 | 1.6 | 2.0 | 32.4 | |
| 25BC | 65.3 | 1.3 | 1.8 | 31.6 | |
| 30BC | 66.7 | 1.1 | 1.7 | 30.5 | |
| 196 h | 15BC | 59.0 | 3.7 | 3.8 | 33.5 |
| 20BC | 60.1 | 3.3 | 4.6 | 32.0 | |
| 25BC | 59.5 | 3.1 | 5.8 | 31.7 | |
| 30BC | 60.8 | 1.8 | 6.3 | 31.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Luo, Q.; Wang, H.; He, H.; Liu, T.; Huang, Y.; Liang, T. Effect of B4C Content on the Oxidation Resistance of a B4C-SiO2–Albite/Al2O3 Coating at 900 °C. Coatings 2025, 15, 688. https://doi.org/10.3390/coatings15060688
Chen P, Luo Q, Wang H, He H, Liu T, Huang Y, Liang T. Effect of B4C Content on the Oxidation Resistance of a B4C-SiO2–Albite/Al2O3 Coating at 900 °C. Coatings. 2025; 15(6):688. https://doi.org/10.3390/coatings15060688
Chicago/Turabian StyleChen, Pengbin, Quanhao Luo, Haoze Wang, Huan He, Tao Liu, Yingheng Huang, and Tianquan Liang. 2025. "Effect of B4C Content on the Oxidation Resistance of a B4C-SiO2–Albite/Al2O3 Coating at 900 °C" Coatings 15, no. 6: 688. https://doi.org/10.3390/coatings15060688
APA StyleChen, P., Luo, Q., Wang, H., He, H., Liu, T., Huang, Y., & Liang, T. (2025). Effect of B4C Content on the Oxidation Resistance of a B4C-SiO2–Albite/Al2O3 Coating at 900 °C. Coatings, 15(6), 688. https://doi.org/10.3390/coatings15060688







