Photocatalytic Properties of ZnO/WO3 Coatings Formed by Plasma Electrolytic Oxidation of a Zinc Substrate in a Tungsten-Containing Electrolyte
Abstract
1. Introduction
2. Materials and Methods
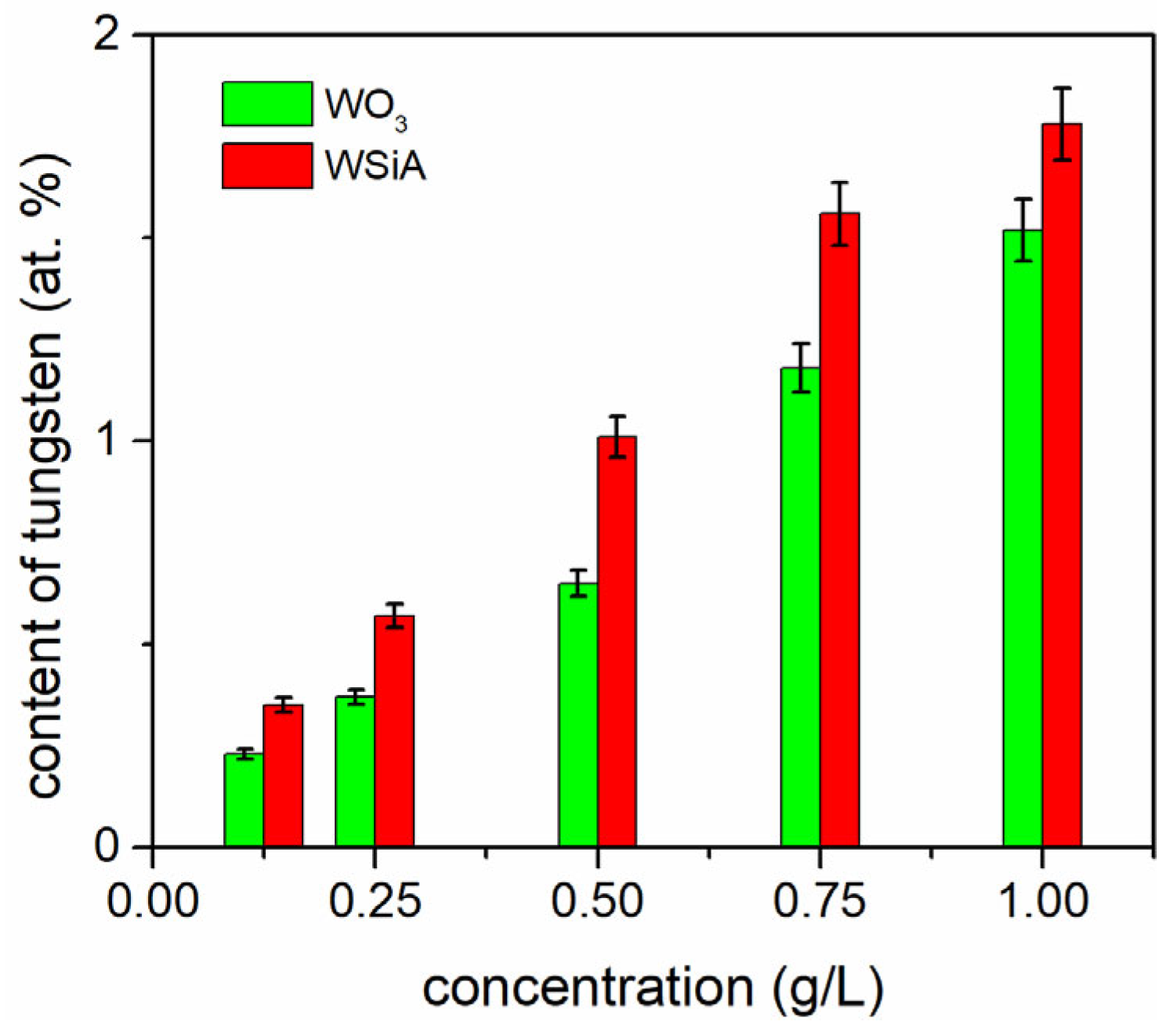
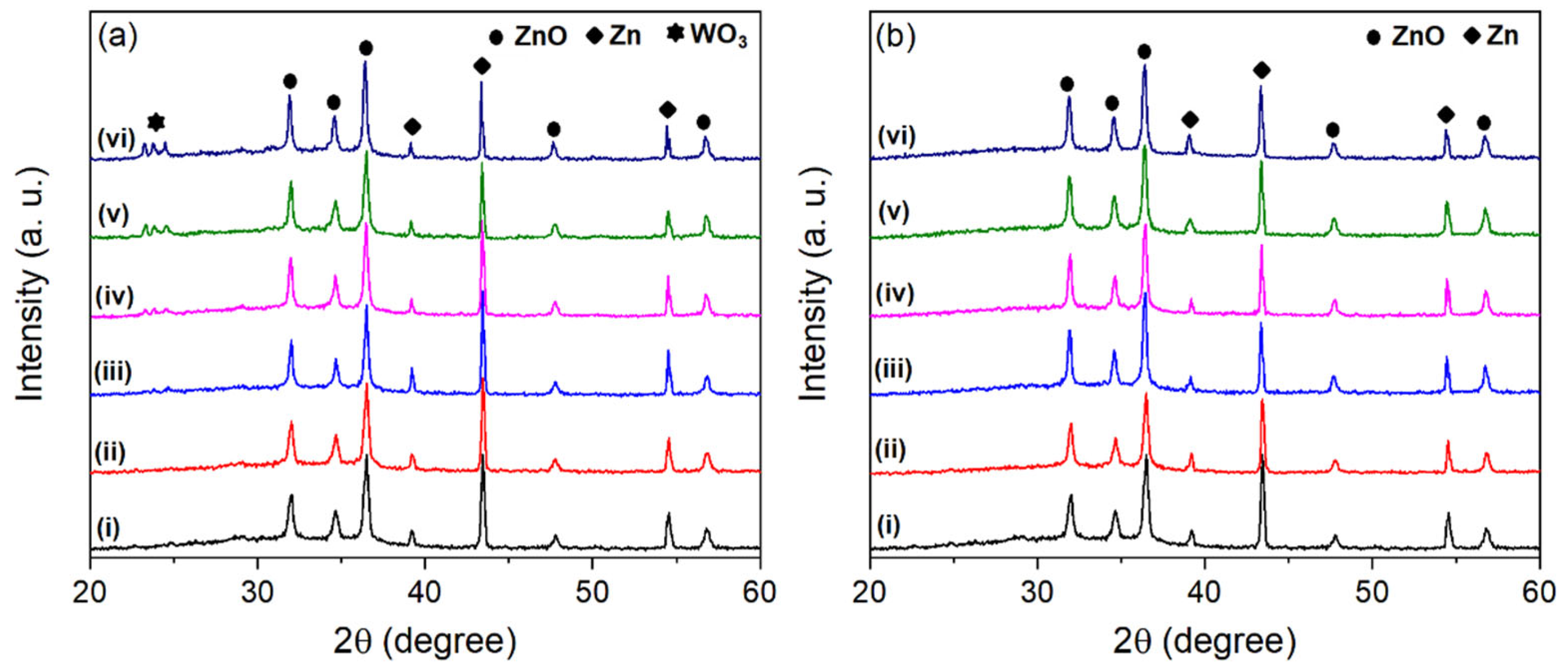
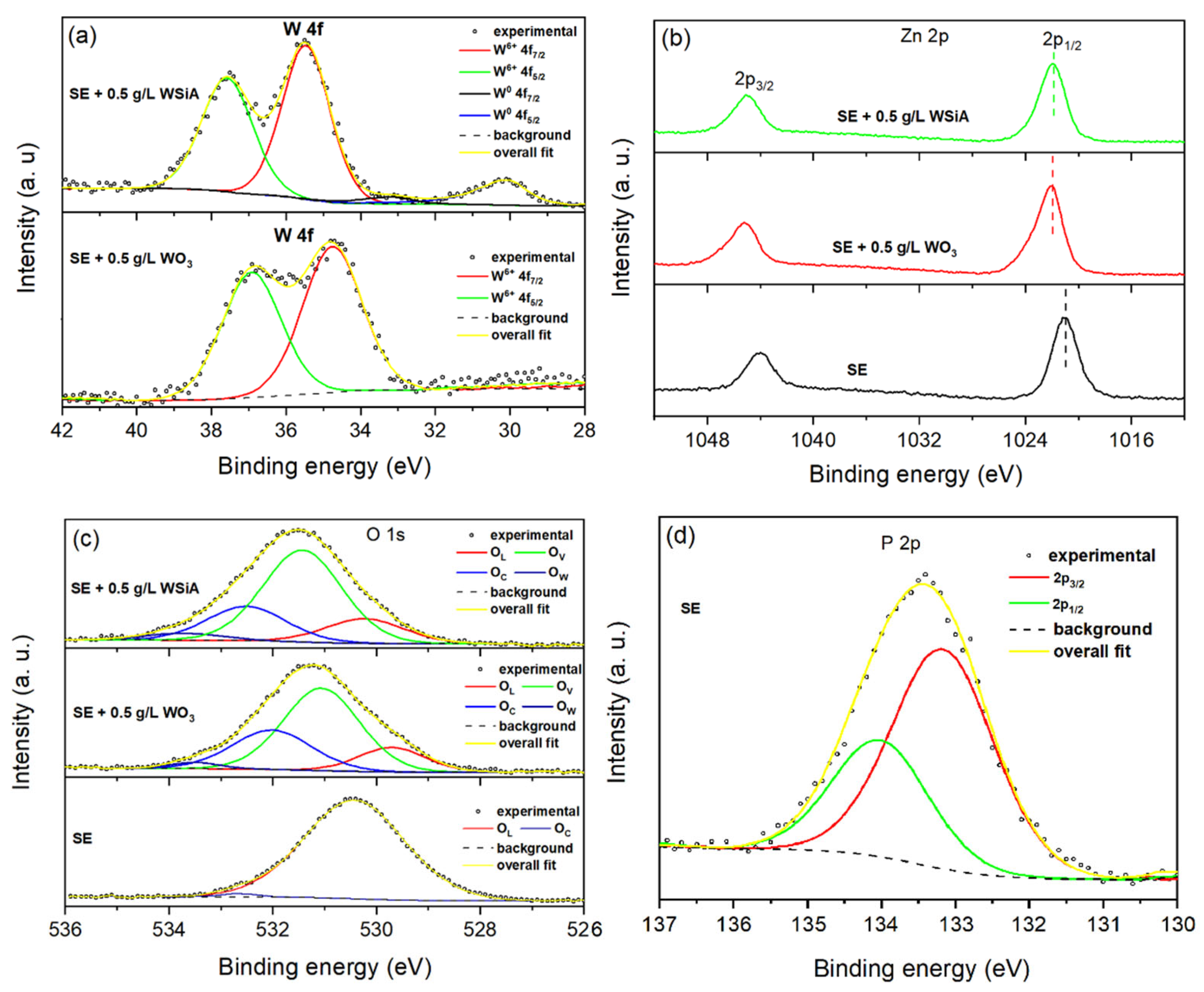
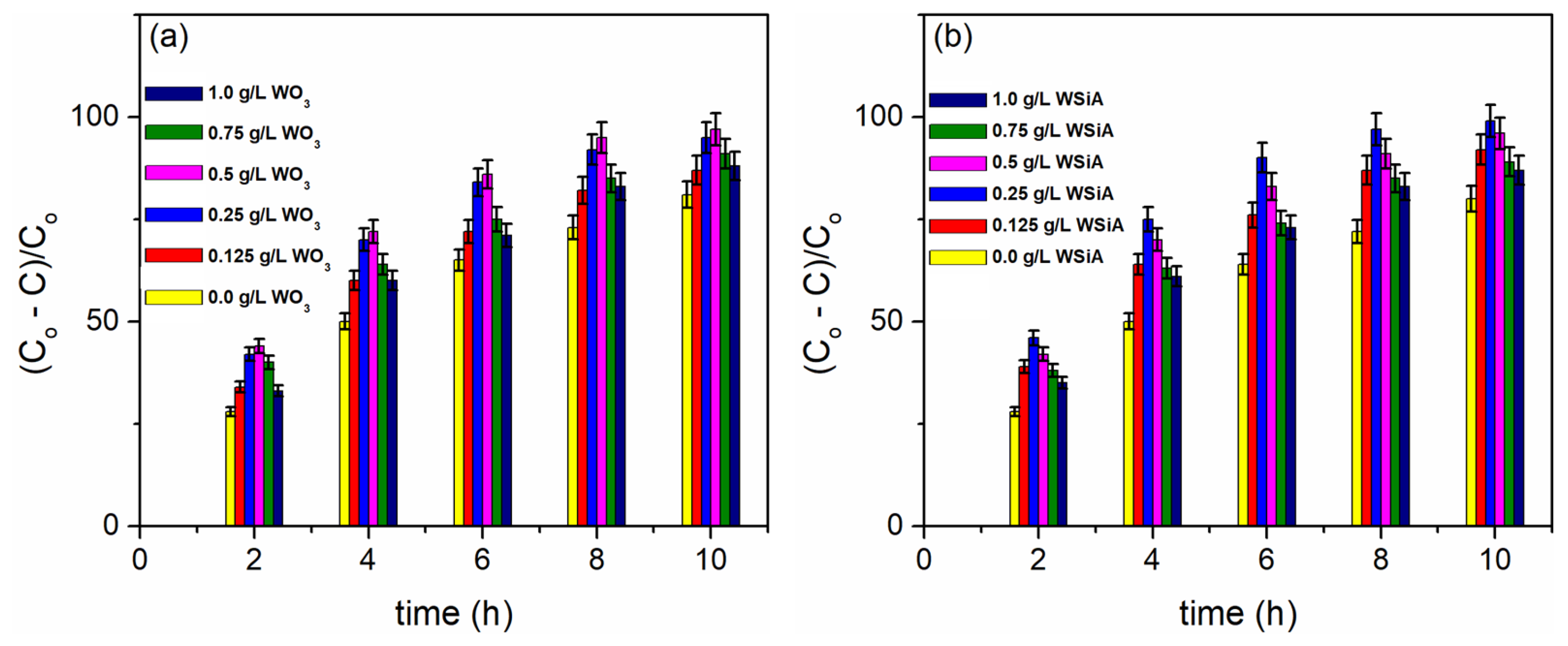
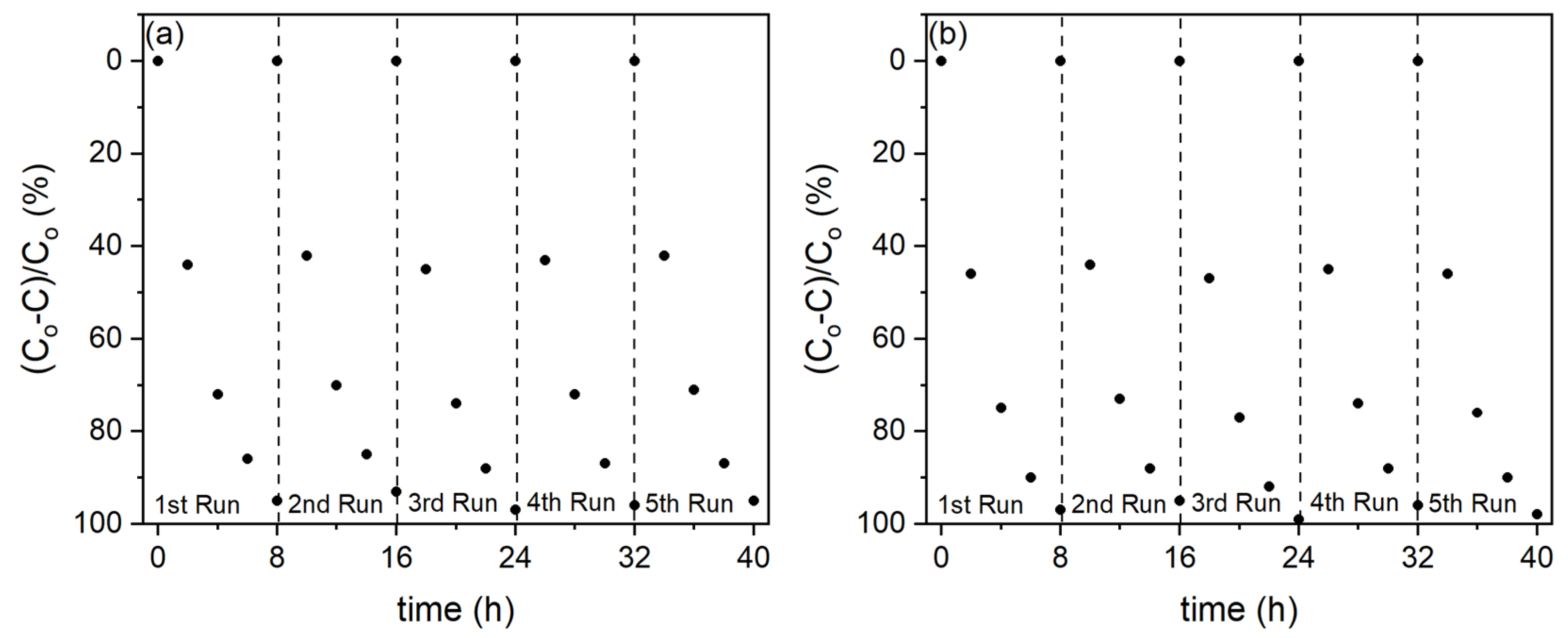
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ansari, A.A.; Lv, R.; Gai, S.; Parchur, A.K.; Solanki, P.R.; Archana; Ansari, Z.A.; Dhayal, M.; Yang, P.; Nazeeruddin, M.K.; et al. ZnO nanostructures—Future frontiers in photocatalysis, solar cells, sensing, supercapacitor, fingerprint technologies, toxicity, and clinical diagnostics. Coord. Chem. Rev. 2024, 515, 215942. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, W.; Li, Q.; Liu, H.; Wang, X. Preparations and applications of zinc oxide based photocatalytic materials. Adv. Sens. Energy Mater. 2023, 2, 100069. [Google Scholar] [CrossRef]
- Monika, P.; Krishna, R.H.; Hussain, Z.; Nandhini, K.; Pandurangi, S.J.; Malek, T.; Kumar, S.G. Antimicrobial hybrid coatings: A review on applications of nano ZnO based materials for biomedical applications. Biomater. Adv. 2025, 172, 214246. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. ZnO nanostructured materials and their potential applications: Progress, challenges and perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef]
- Gartner, M.; Stroescu, H.; Mitrea, D.; Nicolescu, M. Various applications of ZnO thin films obtained by chemical routes in the last decade. Molecules 2023, 28, 4674. [Google Scholar] [CrossRef]
- Bhadwal, N.; Mrad, R.B.; Behdinan, K. Review of zinc oxide piezoelectric nanogenerators: Piezoelectric properties, composite structures and power output. Sensors 2023, 23, 3859. [Google Scholar] [CrossRef]
- Chu, L.; Xu, C.; Zeng, W.; Nie, C.; Hu, Y. Fabrication and application of different nanostructured ZnO in ultraviolet photodetectors: A review. IEEE Sens. J. 2022, 22, 7451–7462. [Google Scholar] [CrossRef]
- Kaur, P.; Kriti; Rahul; Kaur, S.; Kumar, V.; Kandasami, A.; Singh, D.P. Temperature-dependent characteristics of ZnO phosphors from synchrotron-based vacuum ultraviolet photoluminescence spectroscopy. Eur. Phys. J. Plus 2022, 137, 142. [Google Scholar] [CrossRef]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A review on ZnO: Fundamental properties and applications. Mater. Today: Proc. 2022, 49, 3028–3035. [Google Scholar] [CrossRef]
- Zheng, A.L.T.; Abdullah, C.A.C.; Chung, E.L.T.; Andou, Y. Recent progress in visible light-doped ZnO photocatalyst for pollution control. Int. J. Environ. Sci. Technol. 2023, 20, 5753–5772. [Google Scholar] [CrossRef]
- Aftab, S.; Shabir, T.; Shah, A.; Nisar, J.; Shah, I.; Muhammad, H.; Shah, N.S. Highly efficient visible light active doped ZnO photocatalysts for the treatment of wastewater contaminated with dyes and pathogens of emerging concern. Nanomaterials 2022, 12, 486. [Google Scholar] [CrossRef]
- Mutalib, A.A.A.; Jaafar, N.F. ZnO photocatalysts applications in abating the organic pollutant contamination: A mini review. Total Environ. Res. Themes 2022, 3–4, 100013. [Google Scholar] [CrossRef]
- Tuama, A.N.; Alzubaidi, L.H.; Jameel, M.H.; Abass, K.H.; bin Mayzan, M.Z.H.; Salman, Z.N. Impact of electron–hole recombination mechanism on the photocatalytic performance of ZnO in water treatment: A review. J. Sol-Gel Sci. Technol. 2024, 110, 792–806. [Google Scholar] [CrossRef]
- Hezam, A.; Drmosh, Q.A.; Ponnamma, D.; Bajiri, M.A.; Qamar, M.; Namratha, K.; Zare, M.; Nayan, M.B.; Onaizi, S.A.; Byrappa, K. Strategies to enhance ZnO photocatalyst’s performance for water treatment: A comprehensive review. Chem. Rec. 2022, 22, e202100299. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, H.; Chen, T. Application of ZnO/WO3 composite nanofiber photocatalysts in textile wastewater treatment. Separations 2023, 10, 339. [Google Scholar] [CrossRef]
- Mubeen, K.; Irshad, A.; Safeen, A.; Aziz, U.; Safeen, K.; Ghani, T.; Khan, K.; Ali, Z.; ul Haq, I.; Shah, A. Band structure tuning of ZnO/CuO composites for enhanced photocatalytic activity. J. Saudi Chem. Soc. 2023, 27, 101639. [Google Scholar] [CrossRef]
- Guerram, A.; Laouini, S.E.; Mohammed, H.A.; Hasan, G.G.; Tedjani, M.L.; Alharthi, F.; Menaa, F. Synergistic Performance of ZnO/SnO2 nanocomposites: Synthesis, characterization, and applications in photocatalysis and superoxide radical scavenger. J. Clust. Sci. 2024, 35, 2231–2242. [Google Scholar] [CrossRef]
- Shahzad, R.; Muneer, M.; Khalid, R.; Amin, H.M.A. ZnO-Bi2O3 Heterostructured composite for the photocatalytic degradation of orange 16 reactive dye: Synergistic effect of UV irradiation and hydrogen peroxide. Catalysts 2023, 13, 1328. [Google Scholar] [CrossRef]
- Nadikatla, S.K.; Chintada, V.B.; Gurugubelli, T.R.; Koutavarapu, R. Review of recent developments in the fabrication of ZnO/CdS heterostructure photocatalysts for degradation of organic pollutants and hydrogen production. Molecules 2023, 28, 4277. [Google Scholar] [CrossRef]
- Stojadinović, S.; Radisavljevic, Z.; Petrović, Z.; Radić, N. ZnO/Zn3(PO4)2/CeO2 photocatalysts formed on zinc by plasma electrolytic oxidation. Solid State Sci. 2024, 158, 107748. [Google Scholar] [CrossRef]
- Desseigne, M.; Dirany, N.; Chevallier, V.; Arab, M. Shape dependence of photosensitive properties of WO3 oxide for photocatalysis under solar light irradiation. Appl. Surf. Sci. 2019, 483, 313–323. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, T. Development of nanostructured based ZnO@WO3 photocatalyst and its photocatalytic and electrochemical properties: Degradation of Rhodamine B. Int. J. Electrochem. Sci. 2023, 18, 100055. [Google Scholar] [CrossRef]
- Sangkhanak, S.; Kunthakudee, N.; Hunsom, M.; Ramakul, P.; Serivalsatit, K.; Pruksathorn, K. Highly efficient ZnO/WO3 nanocomposites towards photocatalytic gold recovery from industrial cyanide-based gold plating wastewater. Sci. Rep. 2023, 13, 22752. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Z.; Mou, R. Preparation and characterization of WO3/ZnO composite photocatalyst and its application for degradation of oxytetracycline in aqueous solution. Inorg. Chem. Commun. 2022, 142, 109667. [Google Scholar] [CrossRef]
- Aziz, F.; Warsi, A.; Somaily, H.H.; Din, M.I.; Sabeeh, H.; Warsi, M.F.; Shakir, I. Zinc oxide-tungsten oxide (ZnO-WO3) composite for solar light-assisted degradation of organic dyes. Korean J. Chem. Eng. 2023, 40, 1497–1509. [Google Scholar] [CrossRef]
- Rania, M.; Keshu; Pandey, S.; Rishabh; Sharma, S.; Shanker, U. Sunlight assisted highly efficient photocatalytic remediation of organic pollutants by green biosynthesized ZnO@ WO3 nanocomposite. J. Photochem. Photobiol. A Chem. 2024, 446, 115160. [Google Scholar] [CrossRef]
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2019, 64, 127–162. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Karbasi, M.; Chaharmahali, E.N.R.; Hosseini, R.; Giannakis, S.; Bahramian, H.; Kaseem, M.; Fattah-alhosseini, A. A review on plasma electrolytic oxidation coatings for organic pollutant degradation: How to prepare them and what to expect of them? J. Environ. Chem. Eng. 2023, 11, 110027. [Google Scholar] [CrossRef]
- Saji, V.S. Plasma electrolytic oxidation (PEO) layers grown on metals and alloys as supported photocatalysts. Next Energy 2025, 8, 100259. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R.; Kaseem, M. Exploring nanoparticle contributions to enhanced photocatalytic activity of PEO coatings on titanium: A review of the recent advancements. Nano-Struct. Nano-Objects 2024, 39, 101273. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R.; Alizad, S.; Babaei, K.; Stojadinović, S. A review on the revealed improved photocatalytic activity of PEO coatings applied on Al alloys. Nano-Struct. Nano-Objects 2024, 39, 101233. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Stojadinović, S.; Chaharmahali, R.; Gnedenkov, A. An examination of the enhanced photocatalytic performance of PEO coatings applied on Mg alloys: A review. J. Magnes. Alloys 2024, 12, 4422–4435. [Google Scholar] [CrossRef]
- Stojadinović, S.; Radić, N. Photocatalytic performance of ZnO and ZnO/Zn3(PO4)2 coatings formed by plasma electrolytic oxidation of zinc. Solid State Sci. 2024, 153, 107578. [Google Scholar] [CrossRef]
- Stojadinović, S.; Radić, N.; Perković, M. Highly efficient ZrO2 photocatalysts in the presence of UV radiation synthesized in a very short time by plasma electrolytic oxidation of zirconium. Opt. Mater. 2023, 146, 114608. [Google Scholar] [CrossRef]
- Stojadinović, S.; Radić, N.; Vasilić, R. Application of micro-arc discharges during anodization of tantalum for synthesis of photocatalytic active Ta2O5 coatings. Micromachines 2023, 14, 701. [Google Scholar] [CrossRef]
- Stojadinović, S.; Radić, N.; Perković, M. Nb2O5 and AlNbO4 coatings formed by plasma electrolytic oxidation of niobium: Synthesis, characterization, and photocatalytic activity. J. Mater. Sci. Mater. Electron. 2024, 35, 410. [Google Scholar] [CrossRef]
- Fattah-alhosseinia, A.; Karbasi, M.; Fardosi, A.; Kaseem, M. Optimization of electrolyte composition for enhanced photocatalytic performance of the ceramic coating produced on brass by plasma electrolytic oxidation. Ceram. Int. 2024, 50, 25822–25831. [Google Scholar] [CrossRef]
- Hanafi, M.F.; Sapawe, N. Areview on the water problem associate with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mater. Today Proc. 2020, 31, A141–A150. [Google Scholar] [CrossRef]
- Lu, X.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma electrolytic oxidation coatings with particle additions—A review. Surf. Coat. Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Salimi, H.; Fattah-alhosseini, A.; Karbasi, M.; Nikoomanzari, E. Development of WO3-incorporated porous ceramic coating: A key role of WO3 nanoparticle concentration on methylene blue photodegradation upon visible light illumination. Ceram. Int. 2023, 49, 32181–32192. [Google Scholar] [CrossRef]
- Zehra, T.; Kaseem, M.; Patil, S.A.; Shrestha, N.K.; Fattah-alhosseini, A.; Kaseem, M. Anionic assisted incorporation of WO3 nanoparticles for enhanced electrochemical properties of AZ31 Mg alloy coated via plasma electrolytic oxidation. J. Alloys Compd. 2022, 916, 165445. [Google Scholar] [CrossRef]
- Kaseem, M.; Hussain, T.; Rehman, Z.U.; Ko, Y.G. Stabilization of AZ31 Mg alloy in sea water via dual incorporation of MgO and WO3 during micro-arc oxidation. J. Alloys Compd. 2021, 853, 157036. [Google Scholar] [CrossRef]
- Stojadinović, S.; Tadić, N.; Vasilić, R. Formation and characterization of ZnO films on zinc substrate by plasma electrolytic oxidation. Surf. Coat. Technol. 2016, 307, 650–657. [Google Scholar] [CrossRef]
- Klisch, M. 12-Tungstosilicic Acid (12-TSA) as a tungsten precursor in alcoholic solution for deposition of xWO3(1−x)SiO2 thin films (x ≤ 0.7) exhibiting electrochromic coloration ability. J. Sol-Gel Sci. Technol. 1998, 12, 21–33. [Google Scholar] [CrossRef]
- Bouvard, O.; Krammer, A.; Schüler, A. In situ core-level and valence-band photoelectron spectroscopy of reactively sputtered tungsten oxide films. Surf. Interface Anal. 2016, 48, 660–663. [Google Scholar] [CrossRef]
- Wakizaka, M.; Chun, W.-J.; Imaoka, T.; Yamamoto, K. Metallic tungsten nanoparticles that exhibitan electronic state like carbides during the carbothermal reduction of WCl6 by hydrogen. Inorg. Chem. 2020, 59, 15690–15695. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887. [Google Scholar] [CrossRef]
- Vines, F.; Iglesias-Juez, A.; Fernandez-García, M.; Illas, F. Understanding W doping in Wurtzite ZnO. J. Phys. Chem. C 2018, 122, 19082–19089. [Google Scholar] [CrossRef]
- Kwoka, M.; Kulis-Kapuscinska, A.; Zappa, D.; Comini, E.; Szuber, J. Novel insight on the local surface properties of ZnO nanowires. Nanotechnology 2020, 31, 465705. [Google Scholar] [CrossRef]
- Adhikari, S.; Sarkar, D.; Madras, G. Highly efficient WO3–ZnO mixed oxides for photocatalysis. RSC Adv. 2015, 5, 11895–11904. [Google Scholar] [CrossRef]
- Naciri, Y.; Chennah, A.; Jaramillo-Páez, C.; Navío, J.A.; Bakiz, B.; Taoufyq, A.; Ezahri, M.; Villain, S.; Guinneton, F.; Benlhachemi, A. Preparation, characterization and photocatalytic degradation of Rhodamine B dye over a novel Zn3(PO4)2/BiPO4 catalyst. J. Environ. Chem. Eng. 2019, 7, 103075. [Google Scholar] [CrossRef]
- Malik, M.S.; Roy, D.; Chun, D.-M.; Abd-Elrahim, A.G. One-step dry coating of hybrid ZnO–WO3 nanosheet photoanodes for photoelectrochemical water splitting with composition-dependent performance. Micromachines 2023, 14, 2189. [Google Scholar] [CrossRef]
- Qin, H.; Chen, L.; Yu, X.; Wu, M.; Yan, Z. Preparation and photocatalytic performance of ZnO/WO3/TiO2 composite coatings formed by plasma electrolytic oxidation. J. Mater. Sci. Mater. Electron. 2018, 29, 2060–2071. [Google Scholar] [CrossRef]
- Sajjad, A.K.L.; Sajjad, S.; Iqbal, A.; Ryma, N.A. ZnO/WO3 nanostructure as an efficient visible light catalyst. Ceram. Int. 2018, 44, 9364–9371. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojadinović, S.; Pjević, D.; Radić, N. Photocatalytic Properties of ZnO/WO3 Coatings Formed by Plasma Electrolytic Oxidation of a Zinc Substrate in a Tungsten-Containing Electrolyte. Coatings 2025, 15, 657. https://doi.org/10.3390/coatings15060657
Stojadinović S, Pjević D, Radić N. Photocatalytic Properties of ZnO/WO3 Coatings Formed by Plasma Electrolytic Oxidation of a Zinc Substrate in a Tungsten-Containing Electrolyte. Coatings. 2025; 15(6):657. https://doi.org/10.3390/coatings15060657
Chicago/Turabian StyleStojadinović, Stevan, Dejan Pjević, and Nenad Radić. 2025. "Photocatalytic Properties of ZnO/WO3 Coatings Formed by Plasma Electrolytic Oxidation of a Zinc Substrate in a Tungsten-Containing Electrolyte" Coatings 15, no. 6: 657. https://doi.org/10.3390/coatings15060657
APA StyleStojadinović, S., Pjević, D., & Radić, N. (2025). Photocatalytic Properties of ZnO/WO3 Coatings Formed by Plasma Electrolytic Oxidation of a Zinc Substrate in a Tungsten-Containing Electrolyte. Coatings, 15(6), 657. https://doi.org/10.3390/coatings15060657







