Boosting Electrochemical Performances of Li-Rich Mn-Based Cathode Materials by La Doping via Enhanced Structural Stability
Abstract
1. Introduction
2. Experimental
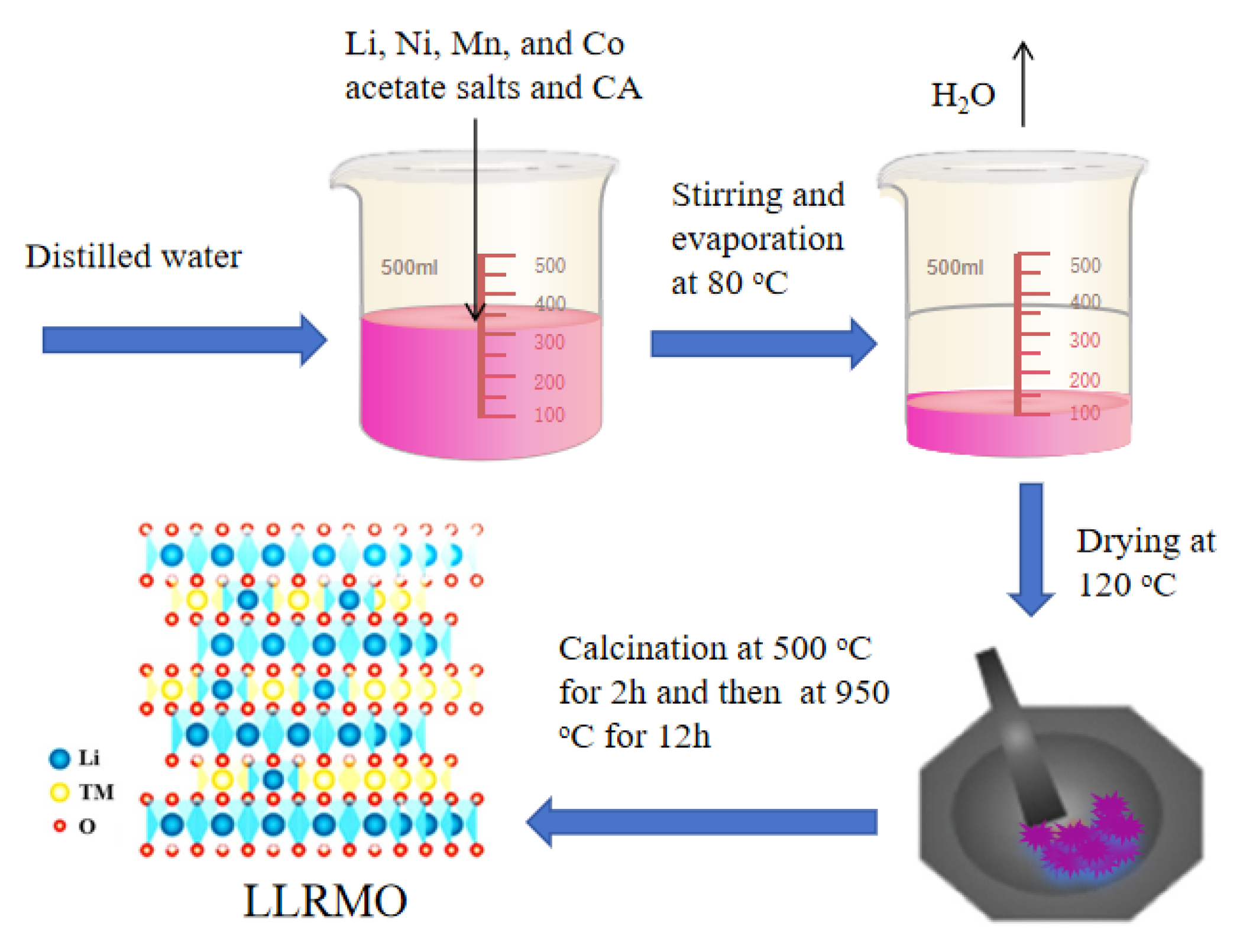
2.1. Material Preparation
2.2. Material Characterization
2.3. Electrochemical Measurements
3. Results
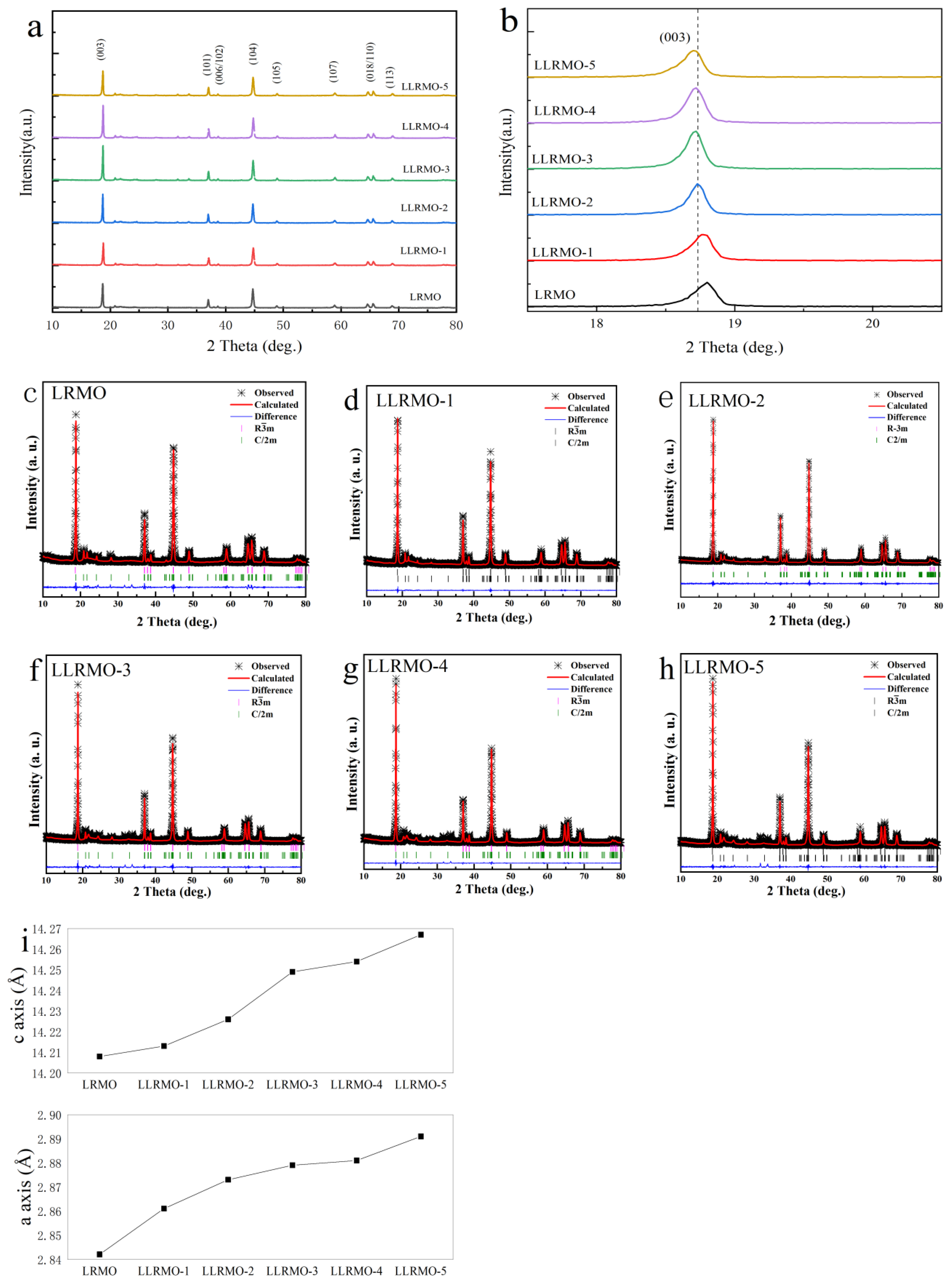
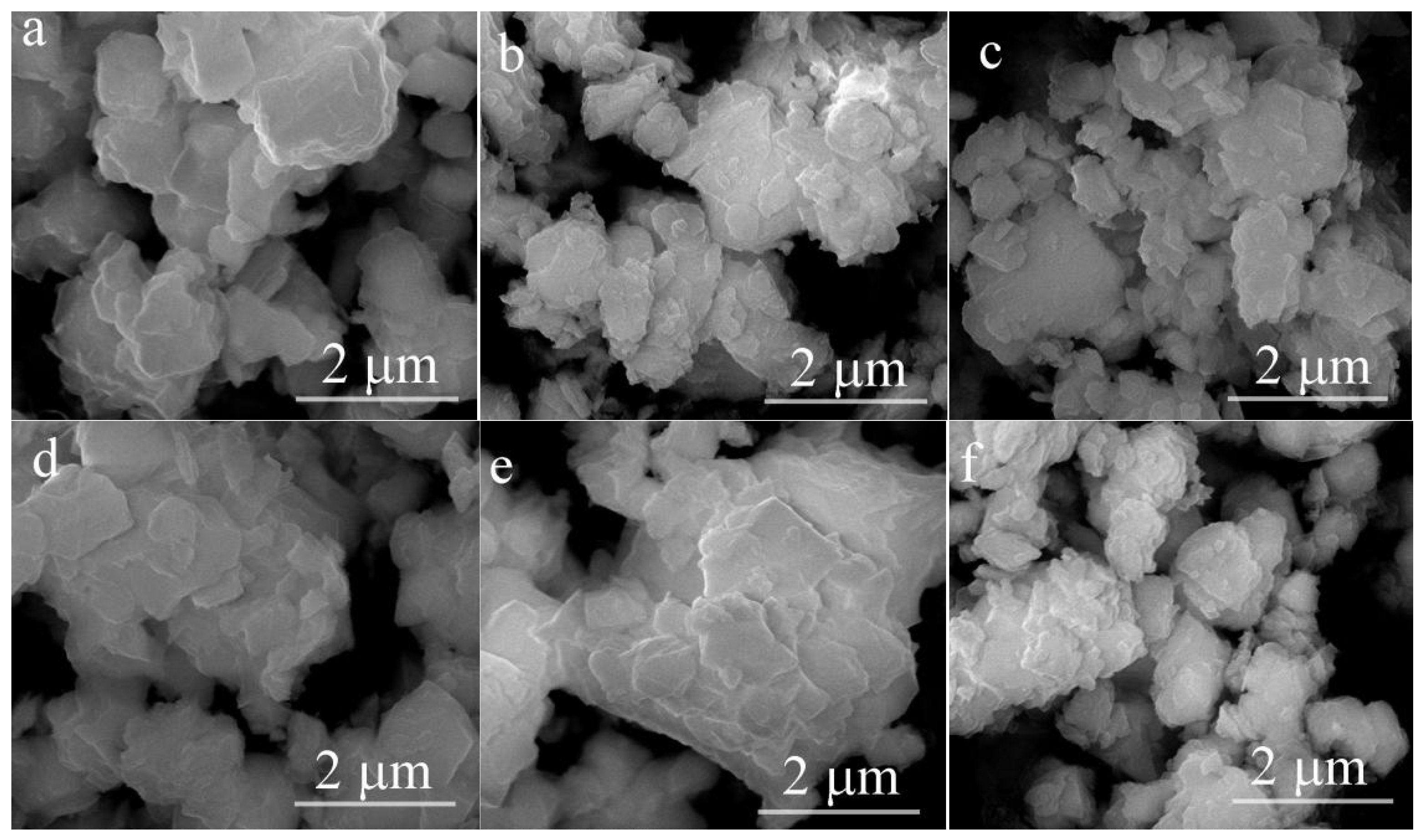
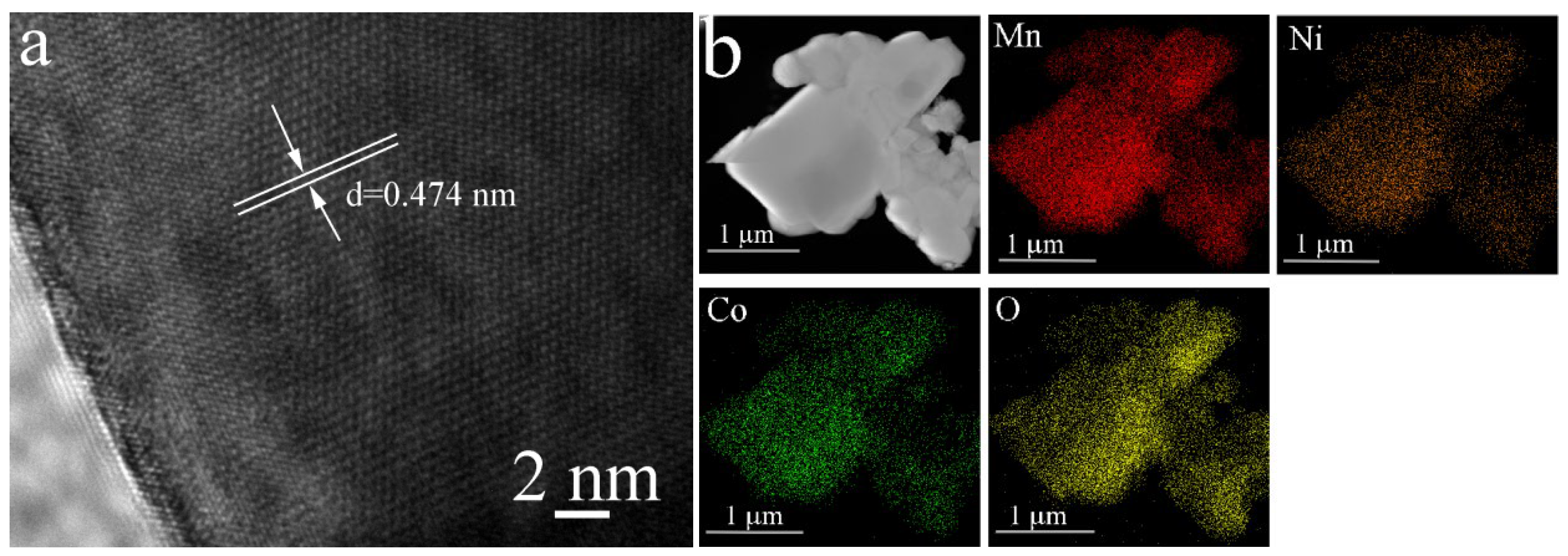
3.1. Structure and Morphology
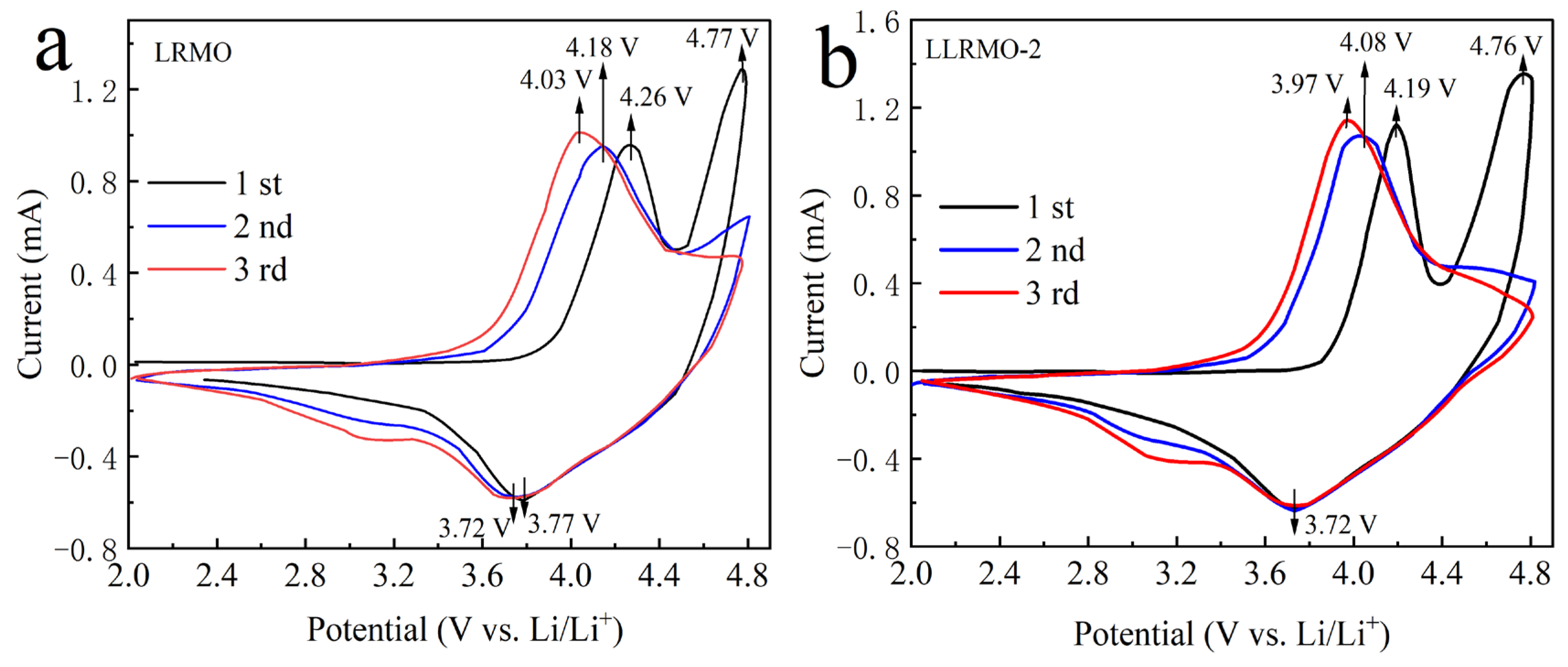
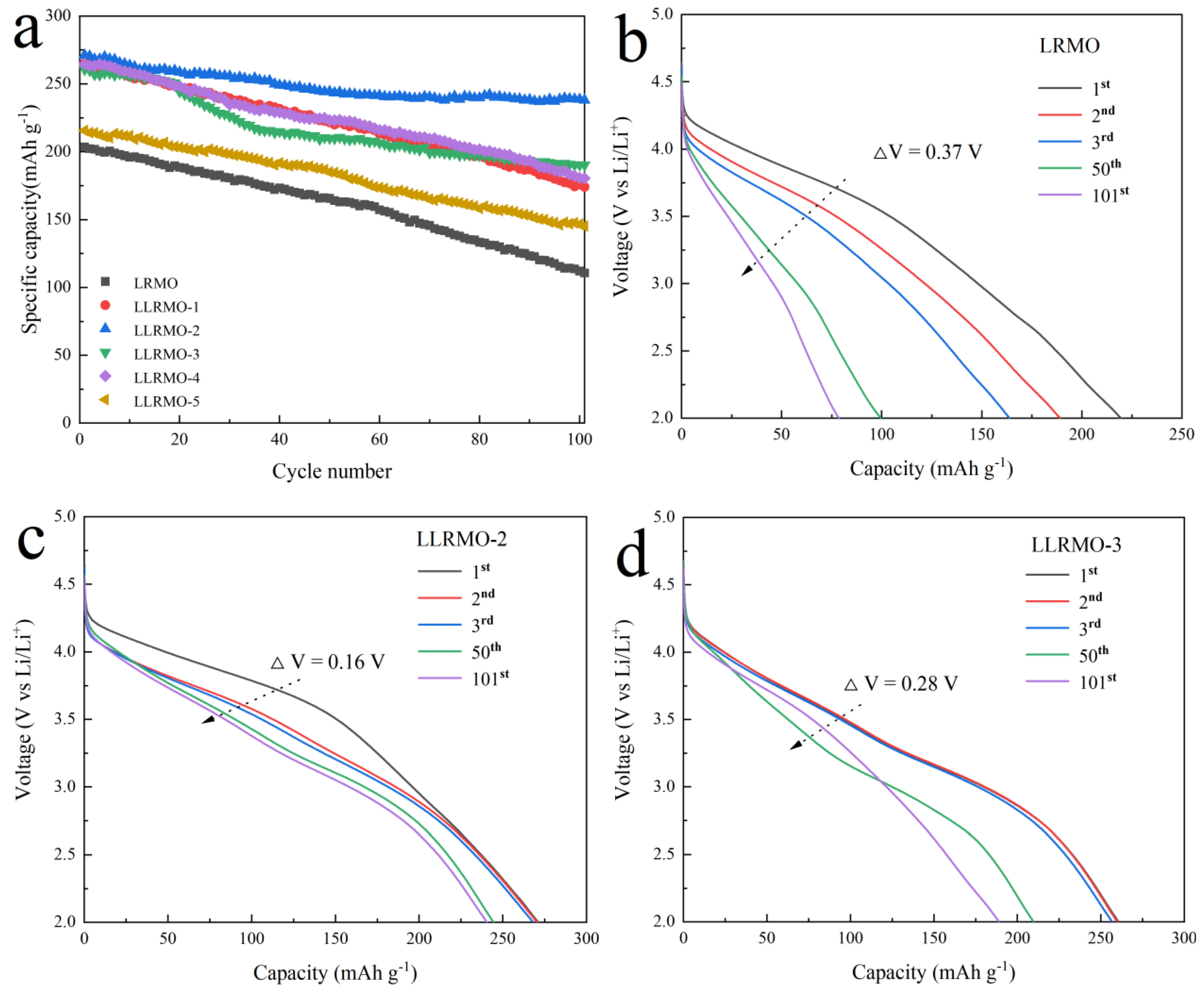
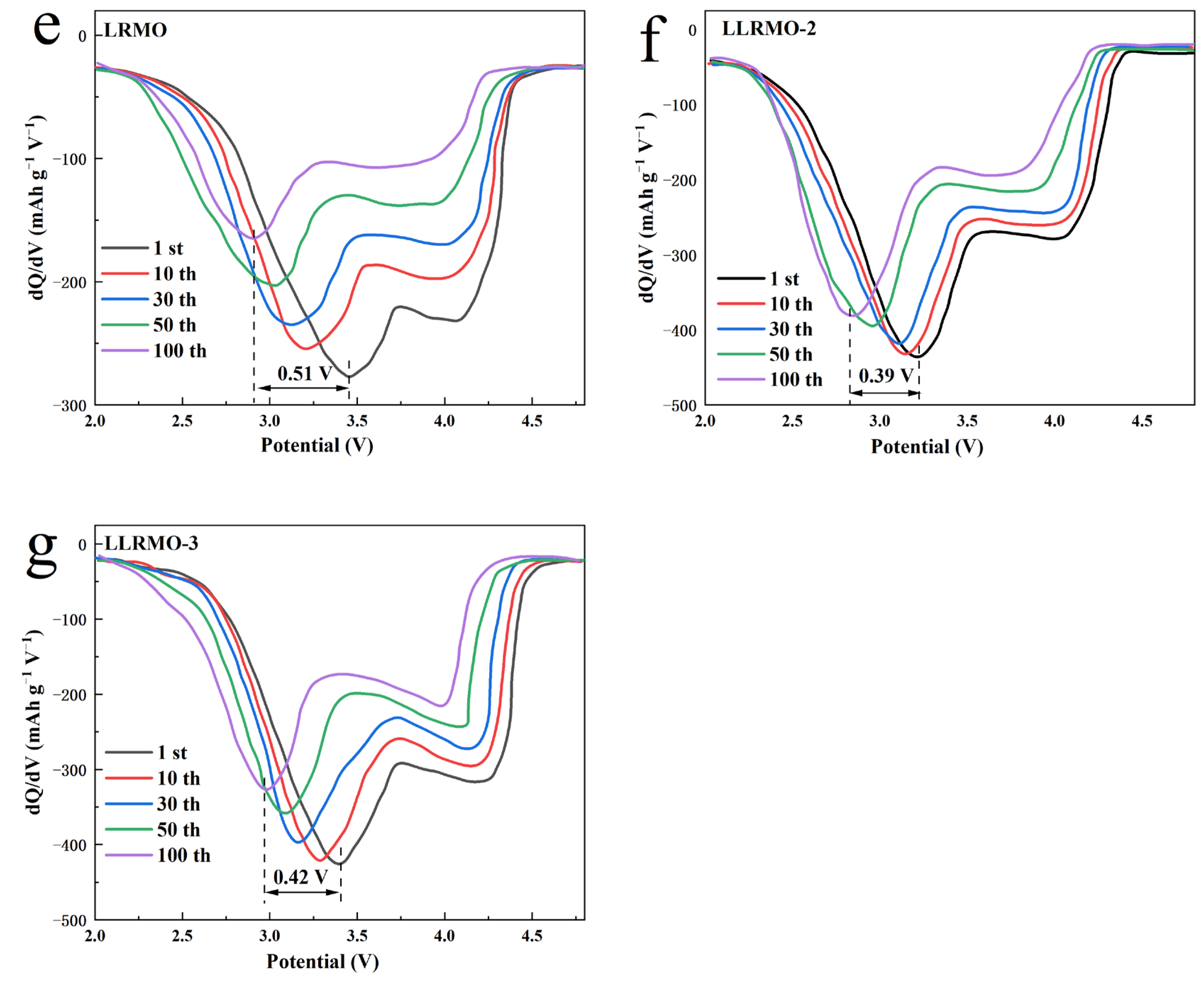
3.2. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodenough, J.B.; Gao, H. A perspective on the Li-ion battery. Sci. China Chem. 2019, 62, 1555–1556. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A. An Outlook on Lithium Ion Battery Technology. ACS Cent. Sci. 2017, 10, 1063–1069. [Google Scholar] [CrossRef]
- Xie, J.; Lu, Y. A retrospective on lithium-ion batteries. Nat. Commun. 2020, 11, 2499. [Google Scholar] [CrossRef]
- Cheng, Q.; He, W.; Zhang, X.; Li, M.; Wang, L. Modification of Li2MnSiO4 cathode materials for lithium-ion battery: A review. J. Mater. Chem. A 2017, 7, 10772. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.; Li, C.; Fu, L.; Liu, X. Nanostructured positive electrode materials for post-lithium ion batteries. Energy Environ. Sci. 2016, 6, 3570–3611. [Google Scholar] [CrossRef]
- Toprakci, O.; Toprakci, H.; Li, Y.; Jim, L.; Xue, L.; Lee, H.; Zhang, S.; Zhang, X. Synthesis and characterization of xLi2MnO3·(1−x)LiMn1/3Ni1/3Co1/3O2 composite cathode materials for rechargeable lithium-ion batteries. J. Power Sources 2013, 241, 522–528. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Hou, M.; Wang, F.; Che, R.; Xia, Y. General synthesis of xLi2MnO3·(1-x)LiMn1/3Ni1/3Co1/3O2 nanomaterials by a molten-salt method: Towards a high capacity and high power cathode for rechargeable lithium batteries. J. Mater. Chem. 2012, 22, 25380–25387. [Google Scholar] [CrossRef]
- Thackeray, M.; Johnson, C.; Vaughey, J.; Li, N.; Hackney, S. Advances in manganese-oxide composite electrodes for lithium-ion batteries. J. Mater. Chem. 2019, 15, 2257–2267. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef]
- Zheng, F.; Yang, C.; Xiong, X.; Xiong, J.; Hu, R.; Chen, Y.; Liu, M. Nanoscale surface modification of lithium-rich layered-oxide composite cathodes for suppressing voltage fade. Angew. Chem. Int. Ed. Engl. 2019, 54, 13058–13062. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q.; Deng, Y.; Shen, C.; Peng, X.; Huang, L.; Sun, S. Effect of synthetic routes on the rate performance of Li-rich layered Li1.2Mn0.56Ni0.12Co0.12O2. J. Mater. Chem. A 2015, 3, 5197–5203. [Google Scholar] [CrossRef]
- Guo, J.; Lai, Y.; Gao, X.; Li, S.; Zhang, H.; Guan, C.; Chen, L.; Yang, Z.; Li, S.; Zhang, Z. Triggering cationic/anionic hybrid redox stabilizes high-temperature Li-rich cathodes materials via three-in-one strategy. Energy Storage Mater. 2024, 69, 103383. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, T.; Xie, Q.; He, W.; Zhang, Y.; Zheng, H.; Lu, X.; Wei, W.; Sa, B.; Wang, L.; et al. Multiscale deficiency integration by Na-rich engineering for high stability Li-rich layered oxide cathodes. ACS Appl. Mater. Interfaces 2021, 13, 8239–8248. [Google Scholar] [CrossRef]
- Wang, E.; Xiao, D.; Wu, T.; Liu, X.; Zhou, Y.; Wang, B.; Lin, T.; Zhang, X.; Yu, H. Al/Ti synergistic doping enhanced cycle stability of Li-rich layered oxides. Adv. Funct. Mater. 2022, 32, 2201744. [Google Scholar] [CrossRef]
- Guo, L.; Tan, X.; Mao, D.; Zhao, T.; Song, L.; Liu, Y.; Kang, X.; Wang, H.; Sun, L.; Chu, W. Improved electrochemical activity of the Li2MnO3-like superstructure in high-nickel Li-rich layered oxide Li1.2Ni0.4Mn0.4O2 and its enhanced performances via tungsten doping. Electrochim. Acta 2021, 370, 137808. [Google Scholar] [CrossRef]
- Zhang, K.; Sheng, H.; Wu, X.; Fu, L.; Liu, Z.; Zhou, C.; Holze, R.; Wu, Y. Improving electrochemical properties by sodium doping for lithium-rich layered oxides. ACS Appl. Energy Mater. 2020, 3, 8953–8959. [Google Scholar] [CrossRef]
- Wang, E.; Xiao, D.; Wu, T.; Wang, B.; Wang, Y.; Wu, L.; Zhang, X.; Yu, H. Stabilizing Oxygen by High Valance Element Doping for High-Performance Li-Rich Layered Oxides. Battery Energy 2023, 2, 20220030. [Google Scholar] [CrossRef]
- He, W.; Liu, P.; Qu, B.; Zheng, Z.; Zheng, H.; Pan, D.; Li, P.; Li, S.; Huang, H.; Wang, L.; et al. Uniform Na+ Doping-Induced Defects in Li- and Mn-Rich Cathodes for High-Performance Lithium-Ion Batteries. Adv. Sci. 2019, 6, 1802114. [Google Scholar] [CrossRef]
- Wang, G.; Xie, C.; Wang, H.; Li, Q.; Xia, F.; Zeng, W.; Peng, H.; Tendeloo, G.; Tan, G.J.; Tian, J.; et al. Mitigated Oxygen Loss in Lithium-Rich Manganese-Based Cathode Enabled by Strong Zr-O Affinity. Adv. Funct. Mater. 2024, 34, 2313672. [Google Scholar] [CrossRef]
- Peng, P.; Chen, Y.; Zhou, Q.; Shen, L.; Wen, Y.; Du, F.; Chen, Y.; Zheng, J. Structure engineering with sodium doping for cobalt-free Li-rich layered oxide toward improving electrochemical stability. J. Colloid. Interf. Sci. 2024, 676, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wei, B.; Wang, M.; Zhang, D.; Uchiyama, T.; Liang, C.; Chen, L.; Uchimoto, Y.; Zhang, R.; Wang, P.; et al. Regulating oxygen covalent electron localization to enhance anionic redox reversibility of lithium-rich layered oxide cathodes. Energy Storage Mater. 2022, 46, 512–522. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y.; Chen, Y.; Peng, J.; Zhou, T.; Pang, W.; Didier, C.; Peterson, V.; Wang, H.; Li, Q.; et al. Insight of a Phase Compatible Surface Coating for Long-Durable Li-Rich Layered Oxide Cathode. Adv. Energy Mater. 2019, 9, 1901795. [Google Scholar] [CrossRef]
- Hall, D.; Gauthier, R.; Eldesoky, A.; Murray, V.; Dahn, J. New Chemical Insights Into the Beneficial Role of Al2O3 Cathode Coatings in Lithium-Ion Cells. ACS Appl. Mater. Interfaces 2019, 11, 14095–14100. [Google Scholar] [CrossRef]
- Ran, X.; Tao, J.; Chen, Z.; Yan, Z.; Yang, Y.; Li, J.X.; Lin, Y.; Huang, Z. Surface heterostructure induced by TiO2 modification in Li-rich cathode materials for enhanced electrochemical performance. Electrochim. Acta 2020, 353, 135959. [Google Scholar] [CrossRef]
- Jiao, C.; Wang, M.; Huang, B.; Zhang, M.; Xu, G.; Liu, Y.; Zhao, Y.; Hu, X. Surface modification single crystal Li-rich Li1.2Mn0.54Ni0.13Co0.13O2 as high performance cathode materials for Li-ion batteries. J. Alloy Compd. 2023, 937, 168389. [Google Scholar] [CrossRef]
- Zhuang, Y.; Du, F.; Zhu, L.; Cao, H.; Dai, H.; Adkins, J.; Zhou, Q.; Zheng, J. Trimethylsilyl (trimethylsiloxy) acetate as a novel electrolyte additive for improvement of electrochemical performance of lithium-rich Li1.2Ni0.2Mn0.6O2 cathode in lithium-ion batteries. Electrochim. Acta 2018, 290, 220–227. [Google Scholar] [CrossRef]
- Song, J.; Li, B.; Chen, Y.; Zuo, Y.; Ning, F.; Shang, H.; Feng, G.; Liu, N.; Shen, C.; Ai, X.; et al. A High-Performance Li-Mn-O Li-Rich Cathode Material with Rhombohedral Symmetry via Intralayer Li/Mn Disordering. Adv. Mater. 2020, 32, 2000190. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, F.; Que, L.; Deng, L.; Xia, Y.; Ke, W.; Han, Y.; Wang, Z. Revealing the Thermodynamics and Kinetics of in-Plane Disordered Li2MnO3 Structure in Li-Rich Cathodes. ACS Energy Lett. 2021, 6, 3836–3843. [Google Scholar] [CrossRef]
- Ning, F.; Li, B.; Song, J.; Zuo, Y.; Shang, H.; Zhao, Z.; Yu, Z.; Chu, W.; Zhang, K.; Feng, G.; et al. Inhibition of Oxygen Dimerization by Local Symmetry Tuning in Li-Rich Layered Oxides for Improved Stability. Nat. Commun. 2020, 11, 4973. [Google Scholar] [CrossRef]
- Jiang, Y.; Liao, Z.; Yu, F.; Ke, W.; Li, X.; Xia, Y.; Xu, G.; Sun, G.; Xia, Y.; Yin, W.; et al. A Cable-Stayed Honeycomb Superstructure to Improve the Stability of Li-Rich Materials via Inhibiting Interlaminar Lattice Strain. Adv. Mater. 2024, 36, 2404982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Wang, X.; Liu, H.; Yan, Y.; Zhou, S.; Tang, Y.; Zeng, G.; Wu, X.; Liao, H.; et al. Role of Substitution Elements in Enhancing the Structural Stability of Li-Rich Layered Cathodes. J. Am. Chem. Soc. 2023, 145, 8700–8713. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Tan, D.; Li, P.; Li, H.; Wei, F.; Zhang, H. Research progress and prospect in element doping of lithium-rich layered oxides as cathode materials for lithium-ion batteries. J. Solid State Electr. 2023, 27, 1–23. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, Z.; Jamil, S.; Chen, J.; Zhang, X.; Wang, X.; Yang, Z.; Shu, H.; Yang, X. Effects of nanofiber architecture and antimony doping on the performance of lithium-rich layered oxides: Enhancing lithium diffusivity and lattice oxygen stability. ACS Appl. Mater. Interfaces 2018, 10, 16561–16571. [Google Scholar] [CrossRef]
- Wang, C.; Lin, Y.; Chiu, K. Alleviation of voltage fade of lithium-rich layered oxide cathodes of Li-ion battery by incorporation of Cr. J. Alloys Compd. 2017, 721, 600–608. [Google Scholar] [CrossRef]
- Kang, S.; Qin, H.; Fang, Y.; Li, X.; Wang, Y. Preparation and electrochemical performance of yttrium-doped Li[Li0.20Mn0.534Ni0.133Co0.133]O2 as cathode material for lithium-ion batteries. Electrochim. Acta 2014, 144, 22–30. [Google Scholar] [CrossRef]
- Xie, Z.; Wu, X.; Zhang, Y.; Li, G.; Ma, F.; Yan, W.; Chen, Y.; Li, F.; Zhou, M. Insight into the effect of Nb5+ on the crystal structure and electrochemical performance of the Li-rich cathode materials. J. Electroanal. Chem. 2022, 922, 116762. [Google Scholar] [CrossRef]
- Yu, R.; Wang, G.; Liu, M.; Zhang, X.; Wang, X.; Shu, H.; Yang, X.; Huang, W. Mitigating voltage and capacity fading of lithium-rich layered cathodes by lanthanum doping. J. Power Sources 2016, 335, 65–75. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Zhao, L.; Zhou, F.; Zhang, F.; He, D. Improving the electrochemical performance of lithium-rich oxide layer material with Mg and La cooping. J. Alloy Compd. 2019, 782, 451–460. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Liu, M.; Chen, Y.; Gu, Y. Enhanced electrochemical performance of La-doped Li-rich layered cathode material. J. Alloy Compd. 2020, 848, 156620. [Google Scholar] [CrossRef]
- Lu, C.-H.; Li, W.; Subburaj, T.; Ou, C.; Kumar, P.S. Influence of bio-derived agar addition on the electrochemical performance of LiFePO4 cathode powders for Li-ion batteries. Ceram. Int. 2019, 45, 12218–12224. [Google Scholar] [CrossRef]
- Dong, P.; Xia, S.; Zhang, Y.-J.; Zhang, Y.; Qiu, Z.; Yao, Y. Influence of Complexing Agents on the Structure and Electrochemical Properties of LiNi0.80Co0.15Al0.05O2 Cathode Synthesized by Sol-Gel Method: A Comparative Study. Int. J. Electrochem. Sci. 2017, 12, 561–575. [Google Scholar]
- Chen, C.; Wu, H.; Zhou, D.; Xu, D.; Zhou, Y.; Guo, J. Sol-gel synthesis of nano Li1.2Mn0.54Ni0.13Co0.13O2 cathode materials using DL-lactic acid as chelating agent. Ceram. Int. 2021, 47, 6270–6278. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, C.; Feng, Y.; Wei, B.; Chen, L.; Zhang, R.; Ivey, D.; Wang, P.; Wei, W. Achieving high structure and voltage stability in cobalt-free Li-rich layered oxide cathodes via selective dual-cation doping. Energy Storage Mater. 2020, 32, 37–45. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, B.; Cao, H.; Xia, Y.; Liu, Z. Electrochemical properties of 0.6Li[Li1/3Mn2/3]O2-0.4LiNixMnyCo1-x-yO2 cathode materials for lithium-ion batteries. J. Power Sources 2012, 218, 128–133. [Google Scholar] [CrossRef]
- Zheng, J.; Ye, Y.; Liu, T.; Xiao, Y.; Wang, C.; Wang, F.; Pan, F. Ni/Li Disordering in Layered Transition Metal Oxide: Electrochemical Impact, Origin, and Control. Acc. Chem. Res. 2019, 52, 2201–2209. [Google Scholar] [CrossRef]
- Mao, G.; Yang, Y.; Yao, Y.; Jiao, W.; Yao, Y.; Yu, W.; Tong, H.; He, H. Boron and Magnesium Ions Co-doping Synergistically Enhances the Electrochemical Performance of Nickel-rich Cathode Materials. J. Electroanal. Chem. 2023, 946, 17730. [Google Scholar] [CrossRef]
- Oishi, M.; Fujimoto, T.; Takanashi, Y.; Orikasa, Y.; Kawamura, A.; Ina, T.; Yamashige, H.; Takamatsu, D.; Sato, K.; Murayama, H.; et al. Charge compensation mechanisms in Li1.16Ni0.15Co0.19Mn0.50O2 positive electrode material for Li-ion batteries analyzed by a combination of hard and soft X-ray absorption near edge structure. J. Power Sources 2023, 222, 45–51. [Google Scholar] [CrossRef]
- Wang, Y.; Bie, X.; Nikolowski, K.; Ehrenberg, H.; Du, F.; Hinterstein, M.; Wang, C.; Chen, G.; Wei, Y. Relationships between Structural Changes and Electrochemical Kinetics of Li-Excess Li1.13Ni0.3Mn0.57O2 during the First Charge. J. Phys. Chem. C 2013, 117, 3279–3286. [Google Scholar] [CrossRef]
- Sun, Y.; Shiosaki, Y.; Xia, Y.; Noguchi, H. The preparation and electrochemical performance of solid solutions LiCoO2-Li2MnO3 as cathode materials for lithium ion batteries. J. Power Sources 2006, 159, 1353–1359. [Google Scholar] [CrossRef]
- Tang, Q.; Liu, C.; Lv, D.; Zhao, L.; Jiang, L.; Wang, J. Biotemplated Fe/La-co-doped TiO2 for photocatalytic depth treatment of compressed leachate from refuse transfer station. Environ. Sci. Pollut. R. 2024, 31, 40941–40957. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Q.; Zhu, H.; Lin, F.; Ji, Y.; Li, B.; Duan, J.; Li, L.; Chen, Z. Effect of different composition on voltage attenuation of Li-rich cathode material for Lithium-ion batteries. Materials 2021, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Z.; Liu, Y.; Li, L.; Fu, S. Facile and scalable fabrication of K+-doped Li1.2Ni0.2Co0.08Mn0.52O2 cathode with ultra high capacity and enhanced cycling stability for lithium ion batteries. Solid State Ion. 2019, 332, 47–54. [Google Scholar] [CrossRef]
- Etefagh, R.; Rozati, S.; Mohammad, D.; Ahmad, A.; Poursalehi, F.; Keshmarzi, M.K. Enhanced Li-storage performance of In-doped Li1.21[Mn0.54Ni0.125Co0.125]O2 as Li-and Mn-rich cathode materials for lithium-ion batteries. J. Appl. Electrochem. 2022, 52, 461–475. [Google Scholar] [CrossRef]
- Wang, H.; Liu, F.; Yu, R.; Xiao, Z.; Zhu, Z.; Zhou, L.; Wu, J. Co-gradient Li-rich cathode relieving the capacity decay in Lithium-ion batteries. Nano Energy 2022, 100, 107439. [Google Scholar] [CrossRef]
- Zhou, Z.; Luo, Z.; He, Z.; Zheng, J.; Li, Y.; Yan, C.; Mao, J. Suppress voltage decay of lithium-rich materials by coating layers with different crystalline states. J. Energy Chem. 2021, 60, 591–598. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y.; Martha, S.K.; Wu, Z.; Andrews, J.C.; Ice, G.E.; Pianetta, P.; Nanda, J. Nanoscale morphological and chemical changes of high voltage lithium-manganese rich NMC composite cathodes with cycling. Nano Lett. 2014, 14, 4334–4341. [Google Scholar] [CrossRef]
- Chen, M.; Chen, D.; Liao, Y.; Zhong, X.; Li, W.; Zhang, Y. Layered lithium-rich oxide nanoparticles doped with spinel phase: Acidic sucrose-assistant synthesis and excellent performance as cathode of lithium ion battery. ACS Appl. Mater. Interfaces 2016, 8, 4575–4584. [Google Scholar] [CrossRef]
- Fu, C.; Meng, L.; Wang, J.; Wang, Q.; Yang, K.; Zhang, W.; Li, L. Bonding the Terminal Isocyanate-Related Functional Group to the Surface Manganese Ions to Enhance Li-Rich Cathode’s Cycling Stability. ACS Appl. Mater. Interfaces 2021, 13, 17565–17576. [Google Scholar] [CrossRef]
- Lu, G.; Peng, W.; Zhang, Y.; Wang, X.; Shi, X.; Song, D.; Zhang, H.; Zhang, L. Study on the formation, development and coating mechanism of new phases on interface in LiNbO3-coated LiCoO2. Electrochim. Acta 2021, 368, 137639. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, J.; Liang, J.; Yin, Y.; Zhang, J.; Yu, X.; Guo, Y. Suppressing Surface Lattice Oxygen Release of Li-Rich Cathode Materials via Heterostructured Spinel Li4Mn5O12 Coating. Adv. Mater. 2018, 30, 1801751. [Google Scholar] [CrossRef]
- Ni, L.; Chen, H.; Gao, J.; Mei, Y.; Wang, H.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Multiscale Crystal Field Effect for High-Performance Ultrahigh-Ni Layered Cathode. ACS Nano 2023, 17, 12759–12773. [Google Scholar] [CrossRef]
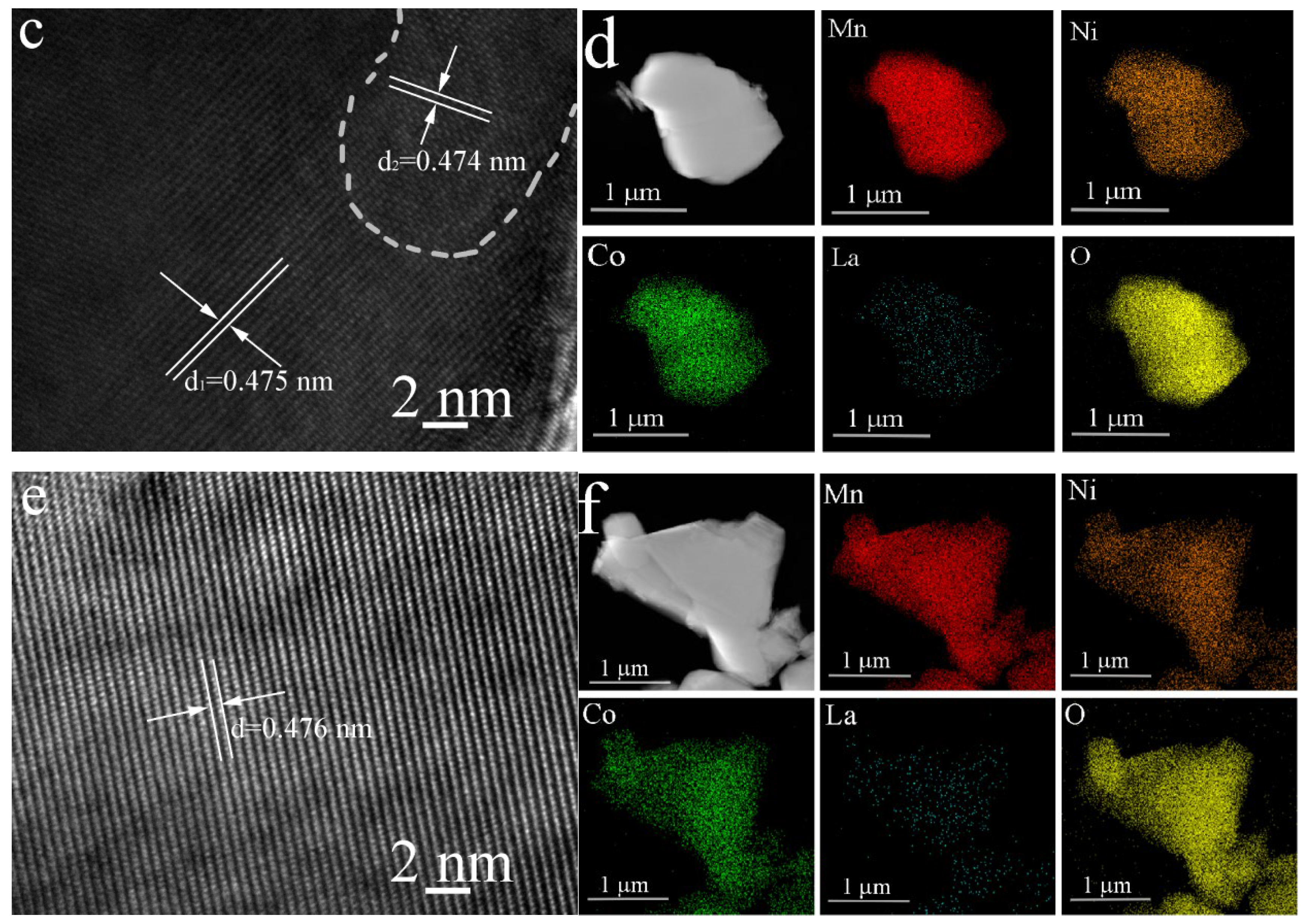




| Li | Mn | Ni | Co | La | Formula | |
|---|---|---|---|---|---|---|
| LRMO | 9.61 | 34.21 | 8.01 | 8.10 | 0 | Li1.2Mn0.54Ni0.12Co0.12O2 |
| LLRMO-1 | 9.57 | 33.82 | 7.94 | 7.98 | 0.75 | Li1.2Mn0.54Ni0.12Co0.12La0.004O2 |
| LLRMO-2 | 9.54 | 33.60 | 7.80 | 7.85 | 1.28 | Li1.2Mn0.53Ni0.12Co0.12La0.008O2 |
| LLRMO-3 | 9.39 | 32.63 | 7.83 | 7.92 | 1.32 | Li1.2Mn0.53Ni0.12Co0.12La0.008O2 |
| LLRMO-4 | 9.24 | 32.45 | 7.86 | 7.97 | 1.32 | Li1.2Mn0.54Ni0.12Co0.12La0.0081O2 |
| LLRMO-5 | 9.14 | 32.24 | 7.89 | 8.03 | 1.30 | Li1.2Mn0.53Ni0.12Co0.12La0.0083O2 |
| Sample | a (Å) | c (Å) | V (Å3) | c/a | I(003)/I(104) | Degree of Li/Ni Mixing |
|---|---|---|---|---|---|---|
| LRMO | 2.842 | 14.208 | 99.383 | 4.999 | 1.28 | 3.108 |
| LLRMO-1 | 2.861 | 14.213 | 100.752 | 4.968 | 1.29 | 2.280 |
| LLRMO-2 | 2.873 | 14.226 | 101.692 | 4.952 | 1.41 | 1.932 |
| LLRMO-3 | 2.879 | 14.249 | 102.283 | 4.949 | 1.33 | 1.992 |
| LLRMO-4 | 2.881 | 14.254 | 102.459 | 4.946 | 1.32 | 2.076 |
| LLRMO-5 | 2.891 | 14.267 | 103.267 | 4.934 | 1.30 | 2.472 |
| Cathode Material | Initial Discharge Capacity (mAh g−1) | Capacity Retention | Current Density (mA g−1) | Voltage Range (V) | Ref. |
|---|---|---|---|---|---|
| Li1.2Mn0.533Ni0.267O2 | 240.3 | 75.4% after 200 cycles | 25 | 2.0–4.7 | [22] |
| Li1.2Mn0.54Ni0.13Co0.13O2 | 286.4 | 93.2% after 100 cycles | 20 | 2.0–4.6 | [38] |
| Li(Li0.18Mn0.52Co0.13Ni0.13)O2 | 281.8 | 74.11% after 150 cycles | 100 | 2.0–4.8 | [39] |
| Li[Li0.2Mn0.54Ni0.13Co0.13]O2 | 196.9 (2nd) | 86.7% after 50 cycles | 25 | 2.0–4.8 | [40] |
| Li1.2Ni0.13Mn0.54Co0.13O2 | 272.1 | 87.8 % after 100 cycles | 100 | 2.0–4.8 | This work |
| Sample | Rs (Ω) | Rct (Ω) | DLi+ (cm2 s−1) |
|---|---|---|---|
| LRMO | 4.643 | 867.8 | 4.06 × 10−13 |
| LLRMO-1 | 4.55 | 674.3 | 3.98 × 10−12 |
| LLRMO-2 | 3.78 | 374.2 | 9.36 × 10−12 |
| LLRMO-3 | 4.45 | 734.1 | 3.12 × 10−12 |
| LLRMO-4 | 4.49 | 788.9 | 2.19 × 10−12 |
| LLRMO-5 | 3.89 | 844.2 | 5.31 × 10−13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, S.; Li, B.; Guo, Z.; Teng, R.; Ren, L.; Li, H.; Zhao, W.; Wei, F. Boosting Electrochemical Performances of Li-Rich Mn-Based Cathode Materials by La Doping via Enhanced Structural Stability. Coatings 2025, 15, 643. https://doi.org/10.3390/coatings15060643
Dou S, Li B, Guo Z, Teng R, Ren L, Li H, Zhao W, Wei F. Boosting Electrochemical Performances of Li-Rich Mn-Based Cathode Materials by La Doping via Enhanced Structural Stability. Coatings. 2025; 15(6):643. https://doi.org/10.3390/coatings15060643
Chicago/Turabian StyleDou, Shumei, Bo Li, Zhuolu Guo, Ruoxin Teng, Lijun Ren, Huiqin Li, Weiwei Zhao, and Fenyan Wei. 2025. "Boosting Electrochemical Performances of Li-Rich Mn-Based Cathode Materials by La Doping via Enhanced Structural Stability" Coatings 15, no. 6: 643. https://doi.org/10.3390/coatings15060643
APA StyleDou, S., Li, B., Guo, Z., Teng, R., Ren, L., Li, H., Zhao, W., & Wei, F. (2025). Boosting Electrochemical Performances of Li-Rich Mn-Based Cathode Materials by La Doping via Enhanced Structural Stability. Coatings, 15(6), 643. https://doi.org/10.3390/coatings15060643




