A Review of the Performance, Sustainable Applications, and Research Challenges of Limestone-Calcined Clay-Cement (LC3) Systems
Abstract
1. Introduction


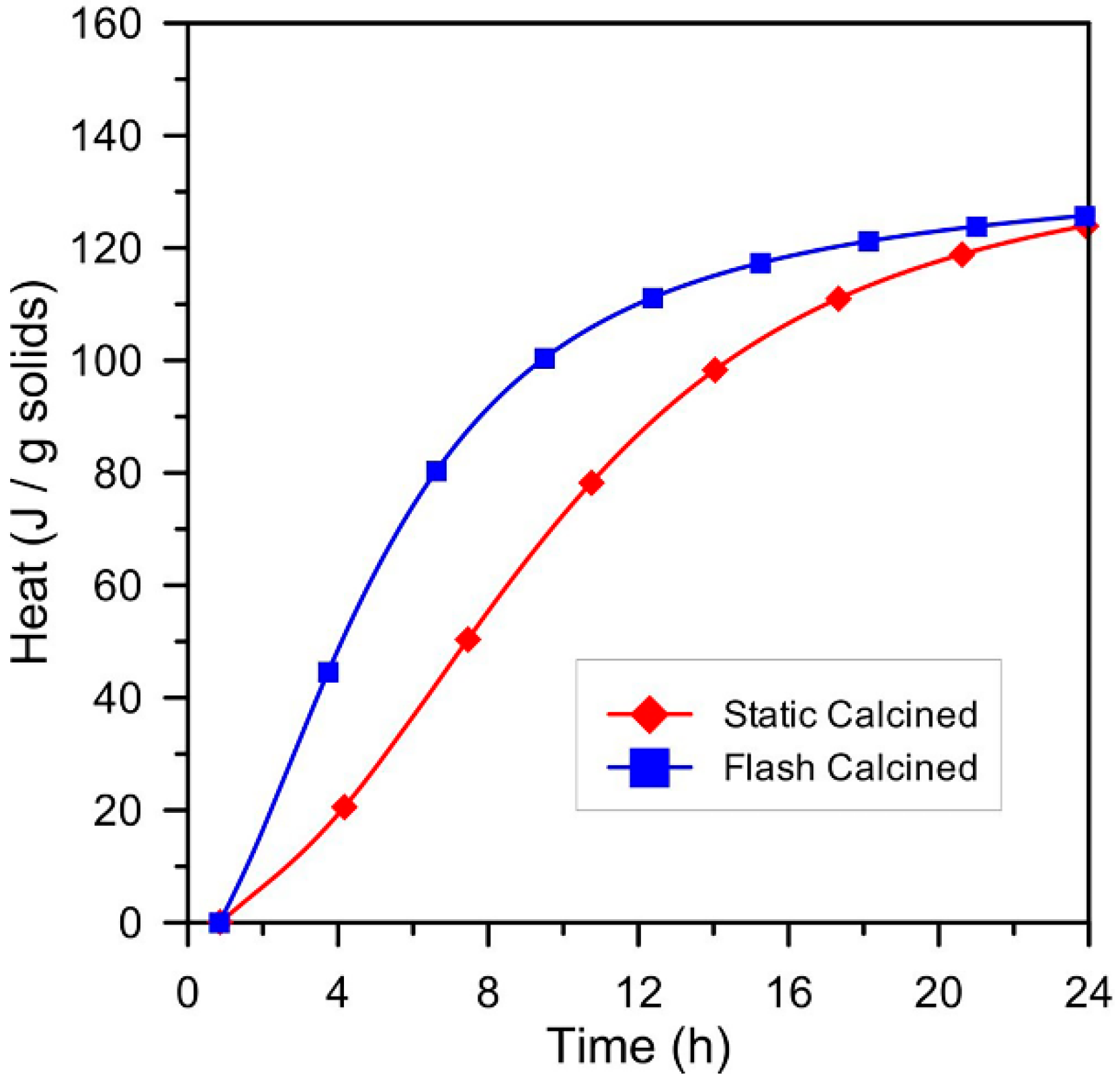
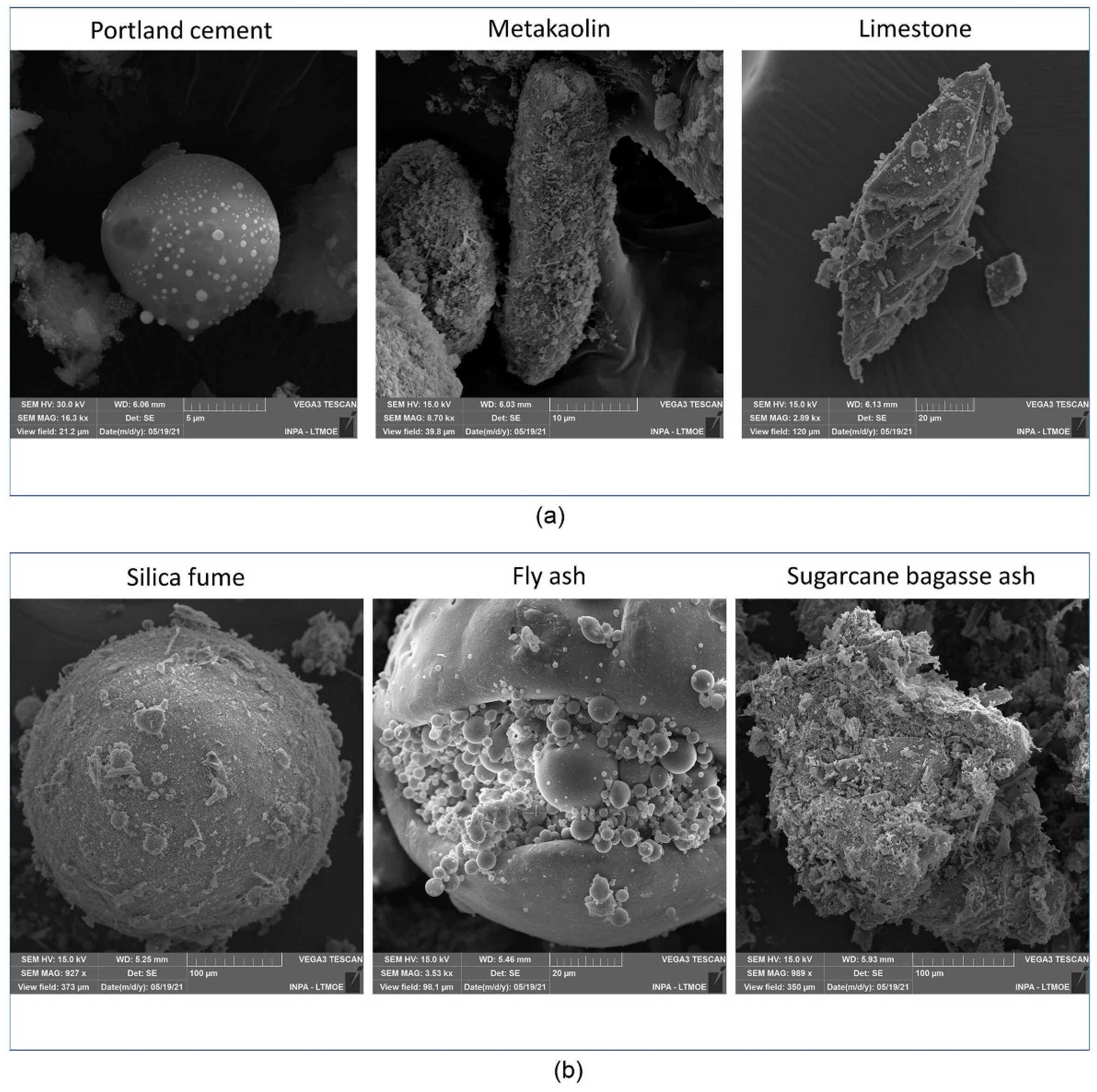
2. Chemical Composition and Hydration Reactions of LC3
3. Fresh Mix Performance
4. Mechanical Properties
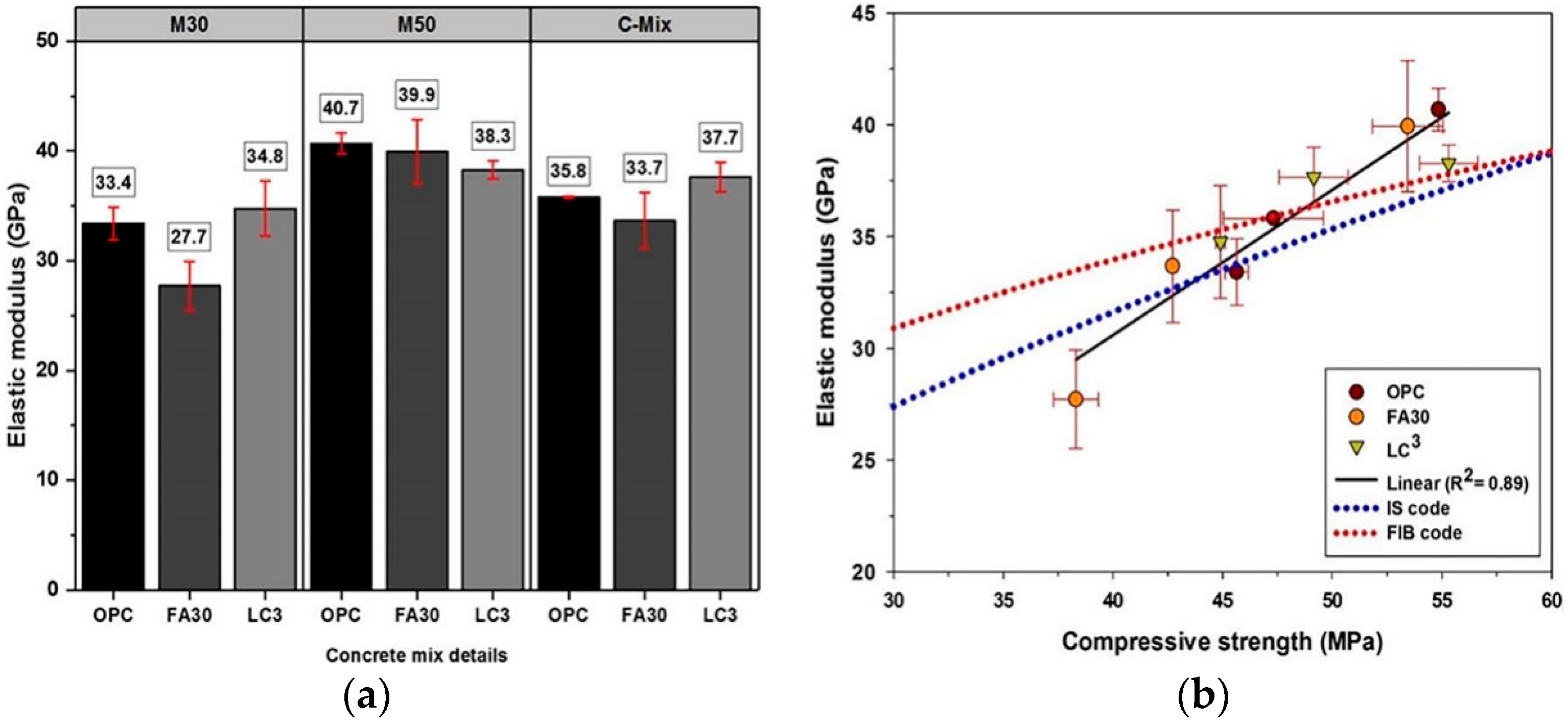
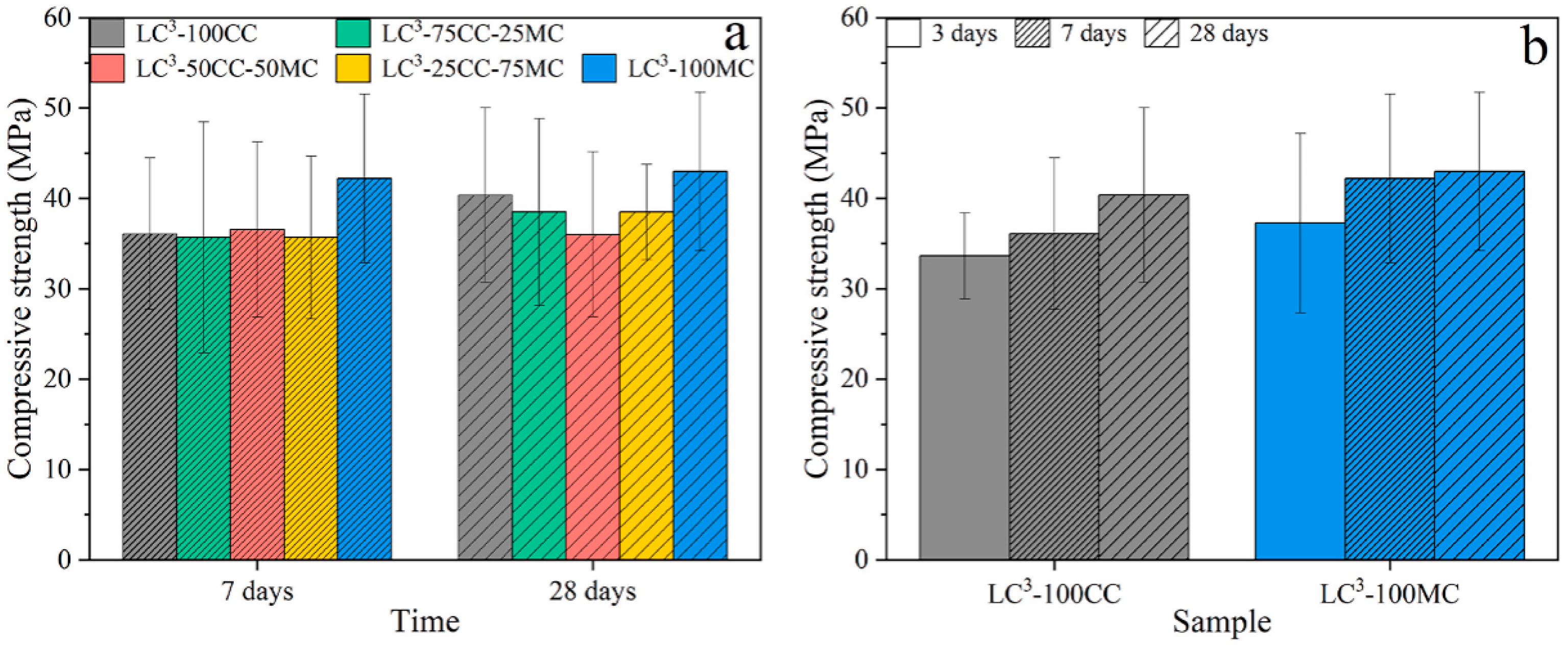
4.1. Compressive Properties
4.2. Other Mechanical Properties
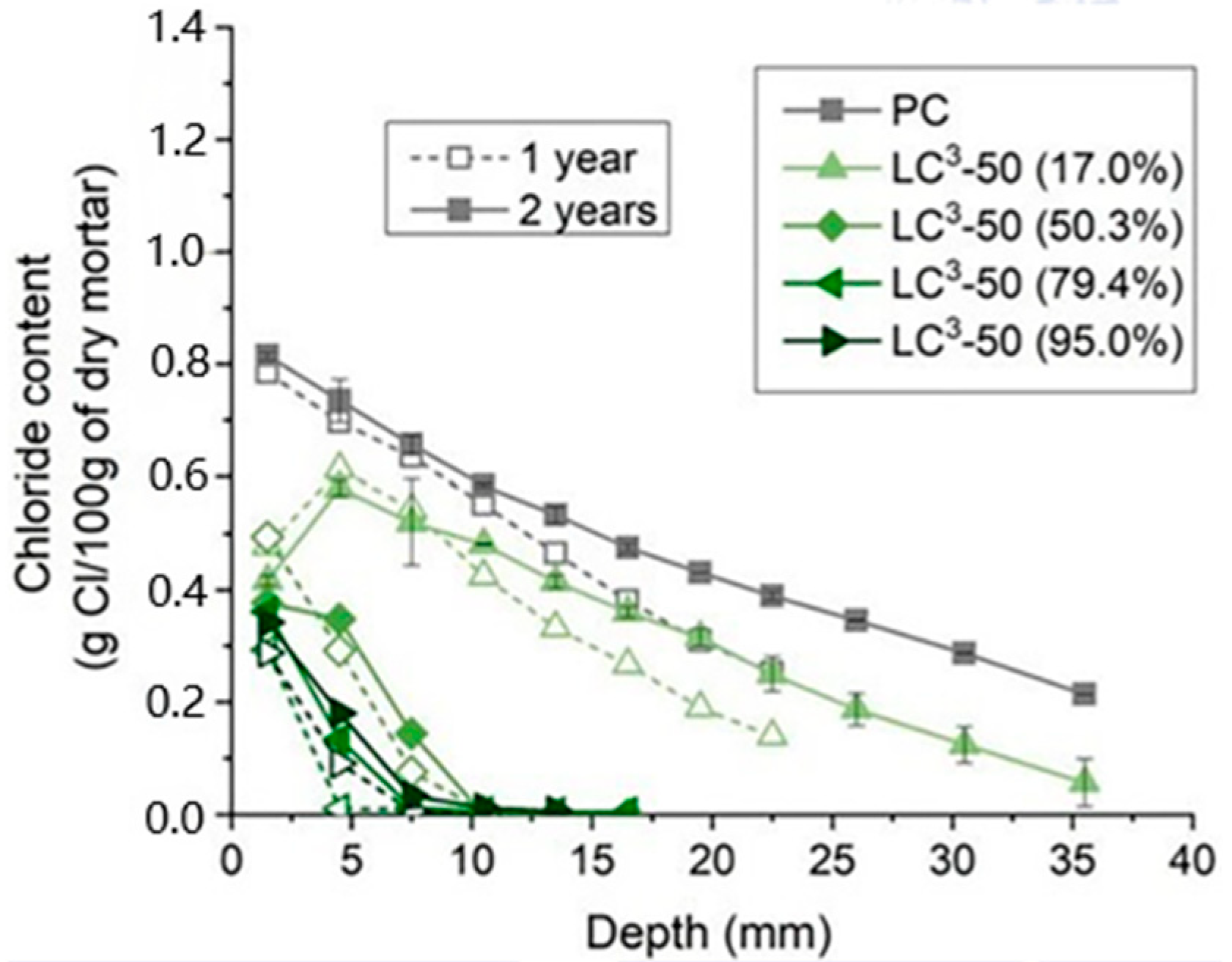
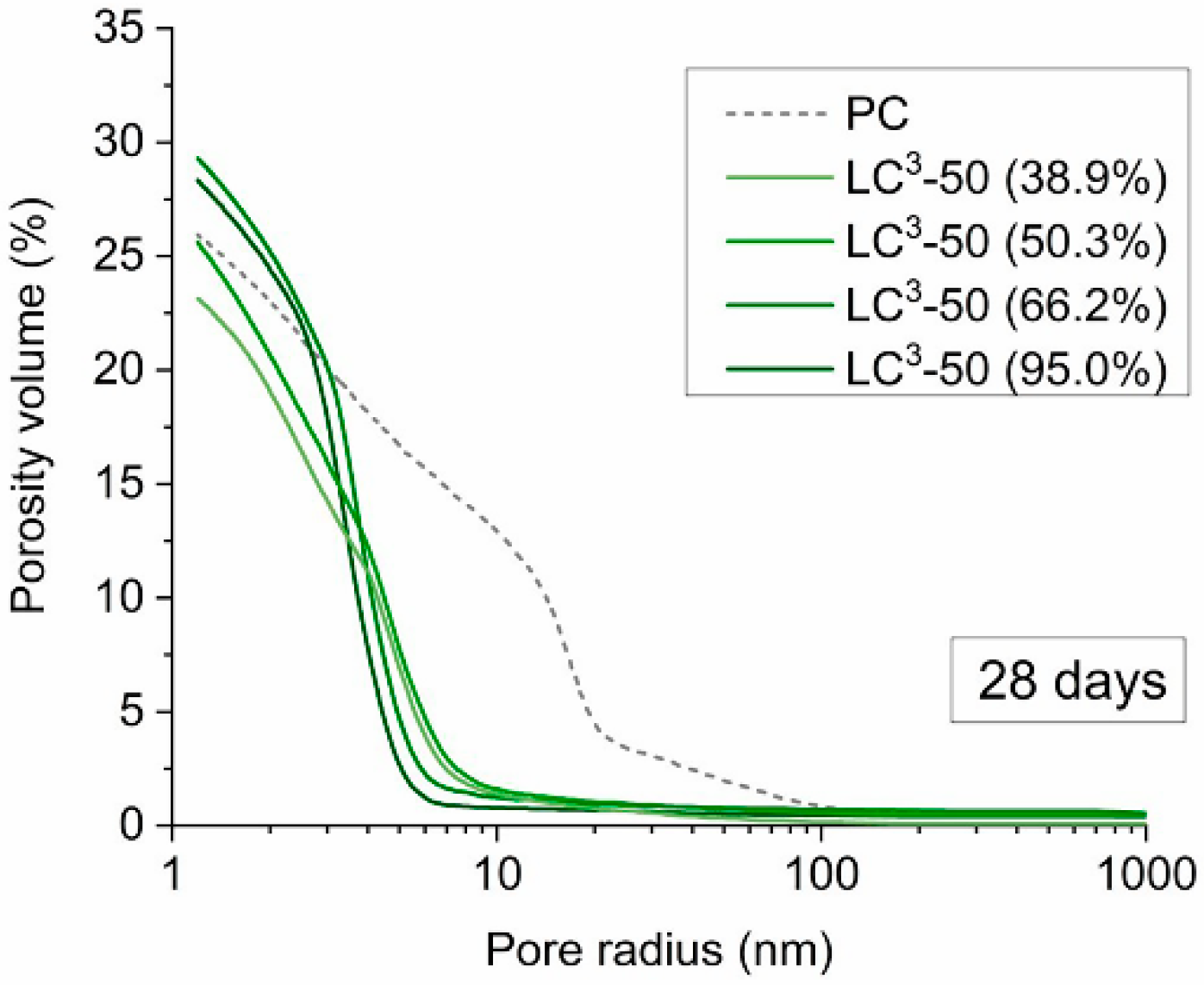
5. Durability
6. Applications of LC3
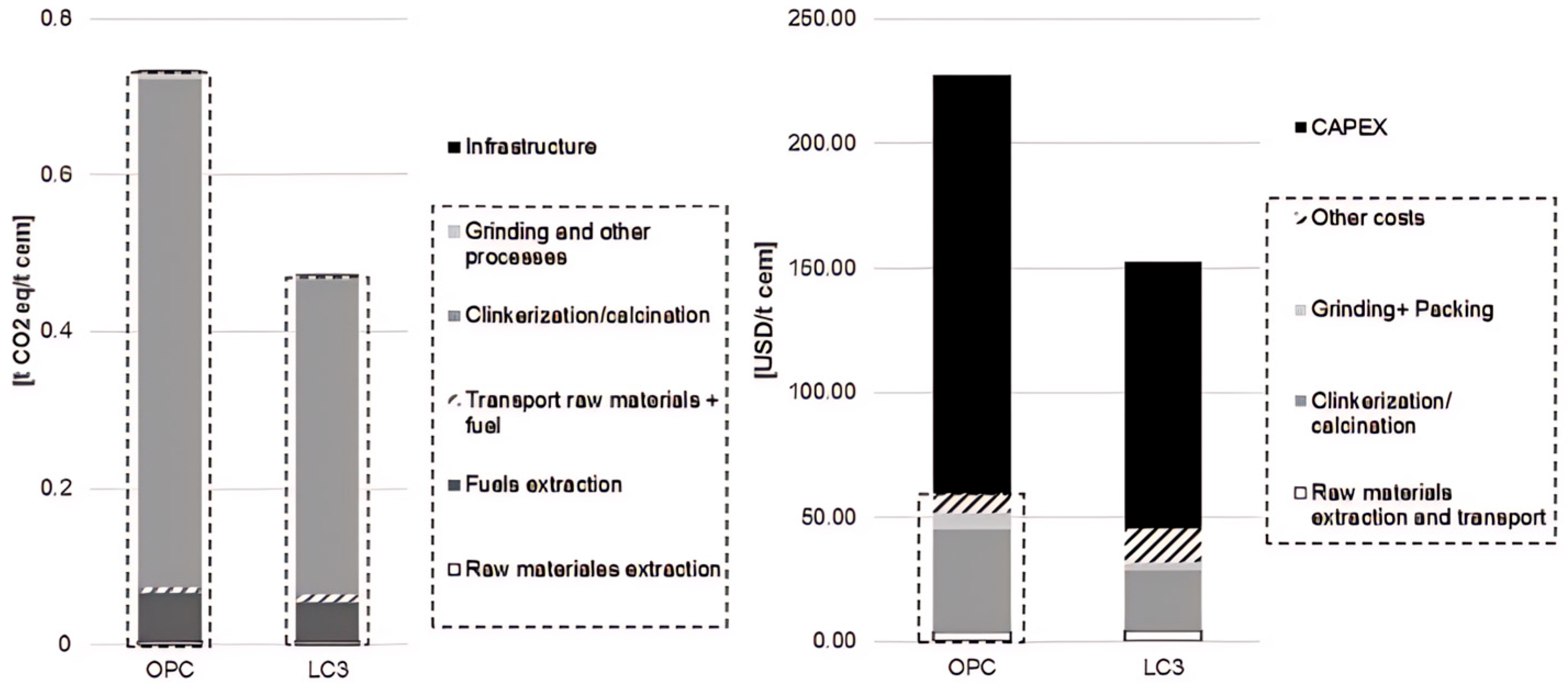
7. Discussion of Economic Feasibility and Environmental Aspects
8. Potential Research Areas for LC3
- Optimization of Clay Purification and Calcination Processes
- 2.
- Refinement of LC3 Hydration Thermodynamic and Kinetic Models
- 3.
- Workability Regulation and Compatibility of Admixtures
- 4.
- Development of Alternative Al/Si Sources and Impurity Control
- 5.
- Long-Term Performance Evaluation and Standardization Framework
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, J.; Cen, K.; Geng, Y. China’s Cement Demand and CO2 Emissions toward 2030: From the Perspective of Socioeconomic, Technology and Population. Environ. Sci. Pollut. Res. 2019, 26, 6409–6423. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Wang, J.; Fang, Y.; Wang, L. Carbon Dioxide as an Admixture for Better Performance of OPC-Based Concrete. J. CO2 Util. 2018, 25, 31–38. [Google Scholar] [CrossRef]
- Shanks, W.; Dunant, C.F.; Drewniok, M.P.; Lupton, R.C.; Serrenho, A.; Allwood, J.M. How Much Cement Can We Do without? Lessons from Cement Material Flows in the UK. Resour. Conserv. Recycl. 2019, 141, 441–454. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Snellings, R.; Bernal, S.A. Supplementary Cementitious Materials: New Sources, Characterization, and Performance Insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Aprianti, S.E. A Huge Number of Artificial Waste Material Can Be Supplementary Cementitious Material (SCM) for Concrete Production—A Review Part II. J. Clean. Prod. 2017, 142, 4178–4194. [Google Scholar] [CrossRef]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement Substitution by a Combination of Metakaolin and Limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Ramezanianpour, A.M.; Hooton, R.D. A Study on Hydration, Compressive Strength, and Porosity of Portland-Limestone Cement Mixes Containing SCMs. Cem. Concr. Compos. 2014, 51, 1–13. [Google Scholar] [CrossRef]
- Wang, D.; Shi, C.; Farzadnia, N.; Shi, Z.; Jia, H.; Ou, Z. A Review on Use of Limestone Powder in Cement-Based Materials: Mechanism, Hydration and Microstructures. Constr. Build. Mater. 2018, 181, 659–672. [Google Scholar] [CrossRef]
- Zajac, M.; Rossberg, A.; Le Saout, G.; Lothenbach, B. Influence of Limestone and Anhydrite on the Hydration of Portland Cements. Cem. Concr. Compos. 2014, 46, 99–108. [Google Scholar] [CrossRef]
- Alujas, A.; Fernández, R.; Quintana, R.; Scrivener, K.L.; Martirena, F. Pozzolanic Reactivity of Low Grade Kaolinitic Clays: Influence of Calcination Temperature and Impact of Calcination Products on OPC Hydration. Appl. Clay Sci. 2015, 108, 94–101. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Investigation of the Calcined Kaolinite Content on the Hydration of Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 124–135. [Google Scholar] [CrossRef]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The Origin of the Pozzolanic Activity of Calcined Clay Minerals: A Comparison between Kaolinite, Illite and Montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Sabir, B.B.; Wild, S.; Bai, J. Metakaolin and Calcined Clays as Pozzolans for Concrete: A Review. Cem. Concr. Compos. 2001, 23, 441–454. [Google Scholar] [CrossRef]
- Scrivener, K.L. Options for the Future of Cement. Indian Concr. J. 2014, 88, 11–21. [Google Scholar]
- Mallik, A.; Joseph, S.; Krishnan, S. Pilot Scale Manufacture of Limestone Calcined Clay Cement: The Indian Experience. Indian Concr. J. 2014, 88, 22–28. [Google Scholar]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined Clay Limestone Cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Yu, J.; Mishra, D.K.; Hu, C.; Leung, C.K.; Shah, S.P. Mechanical, Environmental and Economic Performance of Sustainable Grade 45 Concrete with Ultrahigh-Volume Limestone-Calcined Clay (LCC). Resour. Conserv. Recycl. 2021, 175, 105846. [Google Scholar] [CrossRef]
- Du, W.; Jiang, L.; Liu, Q.; Chen, W.; Ding, Q. Study on the Influence of Thermoplastic Microcapsules on the Sulfate Resistance and Self-Healing Performance of Limestone Calcined Clay Cement Concrete. Molecules 2024, 29, 4797. [Google Scholar] [CrossRef]
- Wang, H.; Hou, P.; Li, Q.; Adu-Amankwah, S.; Chen, H.; Xie, N.; Zhao, P.; Huang, Y.; Wang, S.; Cheng, X. Synergistic Effects of Supplementary Cementitious Materials in Limestone and Calcined Clay-Replaced Slag Cement. Constr. Build. Mater. 2021, 282, 122648. [Google Scholar] [CrossRef]
- Cardinaud, G.; Rozière, E.; Martinage, O.; Loukili, A.; Barnes-Davin, L.; Paris, M.; Deneele, D. Calcined Clay—Limestone Cements: Hydration Processes with High and Low-Grade Kaolinite Clays. Constr. Build. Mater. 2021, 277, 122271. [Google Scholar] [CrossRef]
- Huang, W.; Kazemi-Kamyab, H.; Sun, W.; Scrivener, K. Effect of Replacement of Silica Fume with Calcined Clay on the Hydration and Microstructural Development of Eco-UHPFRC. Mater. Des. 2017, 121, 36–46. [Google Scholar] [CrossRef]
- Avet, F.; Boehm-Courjault, E.; Scrivener, K. Investigation of C-a-S-H Composition, Morphology and Density in Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2019, 115, 70–79. [Google Scholar] [CrossRef]
- Mañosa, J.; Huete-Hernández, S.; Alvarez-Coscojuela, A.; Maldonado-Alameda, A.; Chimenos, J.M. Comparative Study of Limestone Calcined Clay Cement Produced with Mechanically Activated Kaolin and Calcined Kaolin. J. Build. Eng. 2024, 97, 110748. [Google Scholar] [CrossRef]
- Zunino, F.; Scrivener, K. The Reaction between Metakaolin and Limestone and Its Effect in Porosity Refinement and Mechanical Properties. Cem. Concr. Res. 2021, 140, 106307. [Google Scholar] [CrossRef]
- Li, X.; Wanner, A.; Hesse, C.; Friesen, S.; Dengler, J. Clinker-Free Cement Based on Calcined Clay, Slag, Portlandite, Anhydrite, and CSH Seeding: An SCM-Based Low-Carbon Cementitious Binder Approach. Constr. Build. Mater. 2024, 442, 137546. [Google Scholar] [CrossRef]
- Ghrici, M.; Kenai, S.; Said-Mansour, M. Mechanical Properties and Durability of Mortar and Concrete Containing Natural Pozzolana and Limestone Blended Cements. Cem. Concr. Compos. 2007, 29, 542–549. [Google Scholar] [CrossRef]
- Scrivener, K.; Avet, F.; Maraghechi, H.; Zunino, F.; Ston, J.; Hanpongpun, W.; Favier, A. Impacting Factors and Properties of Limestone Calcined Clay Cements (LC3). Green Mater. 2019, 7, 3–14. [Google Scholar] [CrossRef]
- De Weerdt, K.; Haha, M.B.; Le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration Mechanisms of Ternary Portland Cements Containing Limestone Powder and Fly Ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Celik, K.; Meral, C.; Gursel, A.P.; Mehta, P.K.; Horvath, A.; Monteiro, P.J.M. Mechanical Properties, Durability, and Life-Cycle Analysis of Self-Consolidating Concrete Mixtures Made with Blended Portland Cements Containing Fly Ash and Limestone Powder. Cem. Concr. Compos. 2015, 56, 59–72. [Google Scholar] [CrossRef]
- Zunino, F.; Scrivener, K. Microstructural Developments of Limestone Calcined Clay Cement (LC3) Pastes after Long-Term (3 Years) Hydration. Cem. Concr. Res. 2022, 153, 106693. [Google Scholar] [CrossRef]
- Chand, G. Microstructural Study of Sustainable Cements Produced from Industrial By-Products, Natural Minerals and Agricultural Wastes: A Critical Review on Engineering Properties. Clean. Eng. Technol. 2021, 4, 100224. [Google Scholar] [CrossRef]
- Krishnan, S.; Kanaujia, S.K.; Mithia, S.; Bishnoi, S. Hydration Kinetics and Mechanisms of Carbonates from Stone Wastes in Ternary Blends with Calcined Clay. Constr. Build. Mater. 2018, 164, 265–274. [Google Scholar] [CrossRef]
- Tang, J.; Wei, S.; Li, W.; Ma, S.; Ji, P.; Shen, X. Synergistic Effect of Metakaolin and Limestone on the Hydration Properties of Portland Cement. Constr. Build. Mater. 2019, 223, 177–184. [Google Scholar] [CrossRef]
- Krishnan, S.; Bishnoi, S. A Numerical Approach for Designing Composite Cements with Calcined Clay and Limestone. Cem. Concr. Res. 2020, 138, 106232. [Google Scholar] [CrossRef]
- Go, S.-S.; Chung, C.-W.; Struble, L.J.; Lee, H.-C. Pozzolanic Activity of Hwangtoh Clay. Constr. Build. Mater. 2010, 24, 2638–2645. [Google Scholar] [CrossRef]
- Danner, T.; Norden, G.; Justnes, H. Characterisation of Calcined Raw Clays Suitable as Supplementary Cementitious Materials. Appl. Clay Sci. 2018, 162, 391–402. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Santhanam, M. Assessment of Pore Structure Evolution in the Limestone Calcined Clay Cementitious System and Its Implications for Performance. Cem. Concr. Compos. 2017, 84, 36–47. [Google Scholar] [CrossRef]
- Avet, F.; Li, X.; Scrivener, K. Determination of the Amount of Reacted Metakaolin in Calcined Clay Blends. Cem. Concr. Res. 2018, 106, 40–48. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Nepal, J.; Das, B.B. Properties, Compatibility, Environmental Benefits and Future Directions of Limestone Calcined Clay Cement (LC3) Concrete: A Review. J. Build. Eng. 2023, 79, 107794. [Google Scholar] [CrossRef]
- Nie, S.; Skibsted, J. Aluminum Distribution in C-(a)-S-H and Calcium Aluminate Hydrate Phases of Portland Cement—Metakaolin—Limestone Blends Studied by 27Al and 29Si NMR Spectroscopy. Cem. Concr. Res. 2024, 186, 107664. [Google Scholar] [CrossRef]
- Shao, Z.; Cao, M.; Zheng, X. Limestone Particle Sizes and Sulfate Impact on the Early Hydration of Limestone Calcined Clay Cement. J. Build. Eng. 2024, 97, 110848. [Google Scholar] [CrossRef]
- Overmann, S.; Eggimann, M.; Vollpracht, A.; Matschei, T. Impact of Clay Reactivity on the Hydration of Calcined Clay Limestone Cements. Constr. Build. Mater. 2024, 449, 138455. [Google Scholar] [CrossRef]
- Atasever, M.; Erdoğan, S.T. Effects of Clay Type and Component Fineness on the Hydration and Properties of Limestone Calcined Clay Cement. Mater. Struct. 2024, 57, 183. [Google Scholar] [CrossRef]
- Balestra, C.E.T.; Dragunski, D.C.; Neckel, R.M.; Silvestro, L.; Savaris, G.; Schneider, R. Fresh Properties of Low-Carbon Cement Pastes Incorporating Industrial by Products. J. Mater. Civ. Eng. 2025, 37, 4024443. [Google Scholar] [CrossRef]
- Atasever, M.; Erdoğan, S.T. Characterization of Limestone Calcined Clay Cement Made with Calcium Sulfoaluminate Clinker. Int. J. Civ. Eng. 2024, 22, 2407–2419. [Google Scholar] [CrossRef]
- Yin, B.B.; Li, G.; Zhang, Y.; Liew, K.M. Sustainability-Driven Atomistic Model for Exploring the Mechanical Properties of Low Carbon Limestone Calcined Clay Cement (LC3). J. Clean. Prod. 2023, 412, 137394. [Google Scholar] [CrossRef]
- Yang, P.; Dhandapani, Y.; Santhanam, M.; Neithalath, N. Simulation of Chloride Diffusion in Fly Ash and Limestone-Calcined Clay Cement (LC3) Concretes and the Influence of Damage on Service-Life. Cem. Concr. Res. 2020, 130, 106010. [Google Scholar] [CrossRef]
- Park, K.B.; Jee, N.Y.; Yoon, I.S.; Lee, H.S. Prediction of Temperature Distribution in High-Strength Concrete Using Hydration Model. ACI Mater. J. 2008, 105, 180. [Google Scholar]
- Wang, X.-Y.; Lee, H.-S.; Park, K.-B.; Kim, J.-J.; Golden, J.S. A Multi-Phase Kinetic Model to Simulate Hydration of Slag–Cement Blends. Cem. Concr. Compos. 2010, 32, 468–477. [Google Scholar] [CrossRef]
- Tironi, A.; Scian, A.N.; Irassar, E.F. Blended Cements with Limestone Filler and Kaolinitic Calcined Clay: Filler and Pozzolanic Effects. J. Mater. Civ. Eng. 2017, 29, 4017116. [Google Scholar] [CrossRef]
- Avet, F.; Snellings, R.; Alujas Diaz, A.; Ben Haha, M.; Scrivener, K. Development of a New Rapid, Relevant and Reliable (R3) Test Method to Evaluate the Pozzolanic Reactivity of Calcined Kaolinitic Clays. Cem. Concr. Res. 2016, 85, 1–11. [Google Scholar] [CrossRef]
- Shah, V.; Parashar, A.; Mishra, G.; Medepalli, S.; Krishnan, S.; Bishnoi, S. Influence of Cement Replacement by Limestone Calcined Clay Pozzolan on the Engineering Properties of Mortar and Concrete. Adv. Cem. Res. 2020, 32, 101–111. [Google Scholar] [CrossRef]
- Akhlaghi, O.; Aytas, T.; Tatli, B.; Sezer, D.; Hodaei, A.; Favier, A.; Scrivener, K.; Menceloglu, Y.Z.; Akbulut, O. Modified Poly (Carboxylate Ether)-Based Superplasticizer for Enhanced Flowability of Calcined Clay-Limestone-Gypsum Blended Portland Cement. Cem. Concr. Res. 2017, 101, 114–122. [Google Scholar] [CrossRef]
- Bhandari, I.; Kumar, R.; Sofi, A.; Nighot, N.S. A Systematic Study on Sustainable Low Carbon Cement—Superplasticizer Interaction: Fresh, Mechanical, Microstructural and Durability Characteristics. Heliyon 2023, 9, e19176. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.D.; Khan, M.S.H.; Castel, A. Engineering Properties of Limestone Calcined Clay Concrete. J. Adv. Concr. Technol. 2018, 16, 343–357. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Afroz, S.; Zhang, Y.; Kim, T.; Li, W.; Castel, A. Autogenous and Total Shrinkage of Limestone Calcined Clay Cement (LC3) Concretes. Constr. Build. Mater. 2022, 314, 125720. [Google Scholar] [CrossRef]
- Nair, N.; Mohammed Haneefa, K.; Santhanam, M.; Gettu, R. A Study on Fresh Properties of Limestone Calcined Clay Blended Cementitious Systems. Constr. Build. Mater. 2020, 254, 119326. [Google Scholar] [CrossRef]
- Muzenda, T.R.; Hou, P.; Kawashima, S.; Sui, T.; Cheng, X. The Role of Limestone and Calcined Clay on the Rheological Properties of LC3. Cem. Concr. Compos. 2020, 107, 103516. [Google Scholar] [CrossRef]
- Ferreiro, S.; Herfort, D.; Damtoft, J.S. Effect of Raw Clay Type, Fineness, Water-to-Cement Ratio and Fly Ash Addition on Workability and Strength Performance of Calcined Clay—Limestone Portland Cements. Cem. Concr. Res. 2017, 101, 1–12. [Google Scholar] [CrossRef]
- Chen, Y.; Rodriguez, C.R.; Li, Z.; Chen, B.; Çopuroğlu, O.; Schlangen, E. Effect of Different Grade Levels of Calcined Clays on Fresh and Hardened Properties of Ternary-Blended Cementitious Materials for 3D Printing. Cem. Concr. Compos. 2020, 114, 103708. [Google Scholar] [CrossRef]
- Yu, J.; Wu, H.-L.; Mishra, D.K.; Li, G.; Leung, C.K. Compressive Strength and Environmental Impact of Sustainable Blended Cement with High-Dosage Limestone and Calcined Clay (LC2). J. Clean. Prod. 2021, 278, 123616. [Google Scholar] [CrossRef]
- Hou, P.; Muzenda, T.R.; Li, Q.; Chen, H.; Kawashima, S.; Sui, T.; Yong, H.; Xie, N.; Cheng, X. Mechanisms Dominating Thixotropy in Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2021, 140, 106316. [Google Scholar] [CrossRef]
- Beigh, M.A.B.; Nerella, V.N.; Schröfl, C.; Mechtcherine, V. Studying the Rheological Behavior of Limestone Calcined Clay Cement (LC3) Mixtures in the Context of Extrusion-Based 3D-Printing. In Calcined Clays for Sustainable Concrete; Bishnoi, S., Ed.; RILEM Bookseries; Springer: Singapore, 2020; Volume 25, pp. 229–236. ISBN 978-981-15-2805-7. [Google Scholar]
- Du, H.; Pang, S.D. High-Performance Concrete Incorporating Calcined Kaolin Clay and Limestone as Cement Substitute. Constr. Build. Mater. 2020, 264, 120152. [Google Scholar] [CrossRef]
- Marangu, J.M. Physico-Chemical Properties of Kenyan Made Calcined Clay -Limestone Cement (LC3). Case Stud. Constr. Mater. 2020, 12, e00333. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Sakthivel, T.; Santhanam, M.; Gettu, R.; Pillai, R.G. Mechanical Properties and Durability Performance of Concretes with Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 136–151. [Google Scholar] [CrossRef]
- Krishnan, S.; Emmanuel, A.C.; Bishnoi, S. Hydration and Phase Assemblage of Ternary Cements with Calcined Clay and Limestone. Constr. Build. Mater. 2019, 222, 64–72. [Google Scholar] [CrossRef]
- Mishra, G.; Emmanuel, A.C.; Bishnoi, S. Influence of Temperature on Hydration and Microstructure Properties of Limestone-Calcined Clay Blended Cement. Mater. Struct. 2019, 52, 91. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Alujas Diaz, A.; Vizcaíno Andrés, L.M.; Martirena Hernández, J.F.; Antoni, M.G. Effect of Fineness in Clinker-Calcined Clays-Limestone Cements. Adv. Cem. Res. 2015, 27, 546–556. [Google Scholar]
- Dhandapani, Y.; Vignesh, K.; Raja, T.; Santhanam, M. Development of the Microstructure in LC3 Systems and Its Effect on Concrete Properties. In Calcined Clays for Sustainable Concrete; Martirena, F., Favier, A., Scrivener, K., Eds.; RILEM Bookseries; Springer: Dordrecht, The Netherlands, 2018; Volume 16, pp. 131–140. ISBN 978-94-024-1206-2. [Google Scholar]
- Mo, Z.; Wang, R.; Gao, X. Hydration and Mechanical Properties of UHPC Matrix Containing Limestone and Different Levels of Metakaolin. Constr. Build. Mater. 2020, 256, 119454. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Jing, Y.; Yang, G.; Song, J.; You, W.; Liu, X. Mechanical Properties and Chloride Resistance of Ultra-High-Performance Seawater Sea-Sand Concrete with Limestone and Calcined Clay Cement (UHPSSC-LC3) under Various Curing Conditions. J. Build. Eng. 2024, 97, 110767. [Google Scholar] [CrossRef]
- Li, Q. The Microstructure and Mechanical Properties of Cementitious Materials Comprised of Limestone, Calcined Clay and Clinker. Ceram. Silik. 2019, 63, 356–364. [Google Scholar] [CrossRef]
- Wang, L.; Ur Rehman, N.; Curosu, I.; Zhu, Z.; Beigh, M.A.B.; Liebscher, M.; Chen, L.; Tsang, D.C.W.; Hempel, S.; Mechtcherine, V. On the Use of Limestone Calcined Clay Cement (LC3) in High-Strength Strain-Hardening Cement-Based Composites (HS-SHCC). Cem. Concr. Res. 2021, 144, 106421. [Google Scholar] [CrossRef]
- Long, W.-J.; Wu, Z.; Khayat, K.H.; Wei, J.; Dong, B.; Xing, F.; Zhang, J. Design, Dynamic Performance and Ecological Efficiency of Fiber-Reinforced Mortars with Different Binder Systems: Ordinary Portland Cement, Limestone Calcined Clay Cement and Alkali-Activated Slag. J. Clean. Prod. 2022, 337, 130478. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, D.; Wang, T.; Wu, H.; Li, V.C. Mechanical and Self-Healing Behavior of Low Carbon Engineered Cementitious Composites Reinforced with PP-Fibers. Constr. Build. Mater. 2020, 259, 119805. [Google Scholar] [CrossRef]
- Yu, J.; Wu, H.-L.; Leung, C.K. Feasibility of Using Ultrahigh-Volume Limestone-Calcined Clay Blend to Develop Sustainable Medium-Strength Engineered Cementitious Composites (ECC). J. Clean. Prod. 2020, 262, 121343. [Google Scholar] [CrossRef]
- Zhang, D.; Jaworska, B.; Zhu, H.; Dahlquist, K.; Li, V.C. Engineered Cementitious Composites (ECC) with Limestone Calcined Clay Cement (LC3). Cem. Concr. Compos. 2020, 114, 103766. [Google Scholar] [CrossRef]
- Ferreiro, S.; Canut, M.M.C.; Lund, J.; Herfort, D. Influence of Fineness of Raw Clay and Calcination Temperature on the Performance of Calcined Clay-Limestone Blended Cements. Appl. Clay Sci. 2019, 169, 81–90. [Google Scholar] [CrossRef]
- Maraghechi, H.; Avet, F.; Wong, H.; Kamyab, H.; Scrivener, K. Performance of Limestone Calcined Clay Cement (LC3) with Various Kaolinite Contents with Respect to Chloride Transport. Mater. Struct. 2018, 51, 125. [Google Scholar] [CrossRef]
- Zolfagharnasab, A.; Ramezanianpour, A.A.; Bahman-Zadeh, F. Investigating the Potential of Low-Grade Calcined Clays to Produce Durable LC3 Binders against Chloride Ions Attack. Constr. Build. Mater. 2021, 303, 124541. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Influence of pH on the Chloride Binding Capacity of Limestone Calcined Clay Cements (LC3). Cem. Concr. Res. 2020, 131, 106031. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Castel, A. Reinforcement Corrosion in Limestone Flash Calcined Clay Cement-Based Concrete. Cem. Concr. Res. 2020, 132, 106051. [Google Scholar] [CrossRef]
- Pillai, R.G.; Gettu, R.; Santhanam, M.; Rengaraju, S.; Dhandapani, Y.; Rathnarajan, S.; Basavaraj, A.S. Service Life and Life Cycle Assessment of Reinforced Concrete Systems with Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2019, 118, 111–119. [Google Scholar] [CrossRef]
- Sui, S.; Georget, F.; Maraghechi, H.; Sun, W.; Scrivener, K. Towards a Generic Approach to Durability: Factors Affecting Chloride Transport in Binary and Ternary Cementitious Materials. Cem. Concr. Res. 2019, 124, 105783. [Google Scholar] [CrossRef]
- Limbachiya, M.; Meddah, M.S.; Ouchagour, Y. Use of Recycled Concrete Aggregate in Fly-Ash Concrete. Constr. Build. Mater. 2011, 27, 439–449. [Google Scholar] [CrossRef]
- Guo, M.; Gong, G.; Yue, Y.; Xing, F.; Zhou, Y.; Hu, B. Performance Evaluation of Recycled Aggregate Concrete Incorporating Limestone Calcined Clay Cement (LC3). J. Clean. Prod. 2022, 366, 132820. [Google Scholar] [CrossRef]
- EN 196-1:2016; Methods of Testing Cement—Part 1: Determination of Strength. iTeh: Newark, DE, USA, 2016.
- Jan, A.; Ferrari, L.; Mikanovic, N.; Ben-Haha, M.; Franzoni, E. Chloride Ingress and Carbonation Assessment of Mortars Prepared with Recycled Sand and Calcined Clay-Based Cement. Constr. Build. Mater. 2024, 456, 139337. [Google Scholar] [CrossRef]
- Dixit, A.; Du, H.; Pang, S.D. Performance of Mortar Incorporating Calcined Marine Clays with Varying Kaolinite Content. J. Clean. Prod. 2021, 282, 124513. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Kim, T.; Castel, A. Mitigation of Alkali-Silica Reaction by Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2020, 137, 106176. [Google Scholar] [CrossRef]
- Shi, Z.; Ferreiro, S.; Lothenbach, B.; Geiker, M.R.; Kunther, W.; Kaufmann, J.; Herfort, D.; Skibsted, J. Sulfate Resistance of Calcined Clay—Limestone—Portland Cements. Cem. Concr. Res. 2019, 116, 238–251. [Google Scholar] [CrossRef]
- Sharma, M.; Bishnoi, S.; Martirena, F.; Scrivener, K. Limestone Calcined Clay Cement and Concrete: A State-of-the-Art Review. Cem. Concr. Res. 2021, 149, 106564. [Google Scholar] [CrossRef]
- Cheng, L.; Kurihara, R.; Ohkubo, T.; Kitagaki, R.; Teramoto, A.; Suda, Y.; Maruyama, I. Plugging Effect of Fine Pore Water in OPC and LC3 Paste during Accelerated Carbonation Monitored via Single-Sided Nuclear Magnetic Resonance Spectroscopy. Cem. Concr. Res. 2024, 186, 107688. [Google Scholar] [CrossRef]
- Khan, M.S.H.; Nguyen, Q.D.; Castel, A. Performance of Limestone Calcined Clay Blended Cement-Based Concrete against Carbonation. Adv. Cem. Res. 2020, 32, 481–491. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, S.; Zhang, J.; Liu, T.; Wang, L.; Jin, L. Enhancing Carbonation Resistance of Low-Activity Coal Gangue-Based LC3 Cementitious Materials with Ca(OH)2 and Gypsum Additives. J. Build. Eng. 2024, 98, 111275. [Google Scholar] [CrossRef]
- Lin, R.-S.; Han, Y.; Wang, X.-Y. Macro–Meso–Micro Experimental Studies of Calcined Clay Limestone Cement (LC3) Paste Subjected to Elevated Temperature. Cem. Concr. Compos. 2021, 116, 103871. [Google Scholar] [CrossRef]
- Meddah, M.S.; Lmbachiya, M.C.; Dhir, R.K. Potential Use of Binary and Composite Limestone Cements in Concrete Production. Constr. Build. Mater. 2014, 58, 193–205. [Google Scholar] [CrossRef]
- Liang, L.; Ding, Y.; Nishiwaki, T.; Nikolayevich, K.S.; Cai, Z.; Yu, K. Limestone Calcined Clay-Based Engineered Cementitious Composites (LC3-ECC) with Recycled Sand: Macro Performance and Micro Mechanism. Constr. Build. Mater. 2024, 453, 139036. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Zhang, T.; Zhang, M. Hardening Properties and Microstructure of 3D Printed Engineered Cementitious Composites Based on Limestone Calcined Clay Cement. Cem. Concr. Compos. 2024, 152, 105641. [Google Scholar] [CrossRef]
- Yan, K.-T.; Wang, X.-P.; Ding, Y.; Li, L.-Z.; Bazarov, D.; Deng, B.-Y.; Nikolayevich, K.-S.; Yu, K.-Q. 3D-Printed LC3-Based Lightweight Engineered Cementitious Composites: Fresh State, Harden Material Properties and Beam Performance. J. Build. Eng. 2024, 93, 109838. [Google Scholar] [CrossRef]
- Chen, Y.; Chaves Figueiredo, S.; Li, Z.; Chang, Z.; Jansen, K.; Çopuroğlu, O.; Schlangen, E. Improving Printability of Limestone-Calcined Clay-Based Cementitious Materials by Using Viscosity-Modifying Admixture. Cem. Concr. Res. 2020, 132, 106040. [Google Scholar] [CrossRef]
- Kang, S.-H.; Jeong, Y.; Tan, K.H.; Moon, J. High-Volume Use of Limestone in Ultra-High Performance Fiber-Reinforced Concrete for Reducing Cement Content and Autogenous Shrinkage. Constr. Build. Mater. 2019, 213, 292–305. [Google Scholar] [CrossRef]
- Long, W.-J.; Lin, C.; Tao, J.-L.; Ye, T.-H.; Fang, Y. Printability and Particle Packing of 3D-Printable Limestone Calcined Clay Cement Composites. Constr. Build. Mater. 2021, 282, 122647. [Google Scholar] [CrossRef]
- Bhattacherjee, S.; Basavaraj, A.S.; Rahul, A.V.; Santhanam, M.; Gettu, R.; Panda, B.; Schlangen, E.; Chen, Y.; Copuroglu, O.; Ma, G.; et al. Sustainable Materials for 3D Concrete Printing. Cem. Concr. Compos. 2021, 122, 104156. [Google Scholar] [CrossRef]
- Reddy, V.A.; Solanki, C.H.; Kumar, S.; Reddy, K.R.; Du, Y.-J. New Ternary Blend Limestone Calcined Clay Cement for Solidification/Stabilization of Zinc Contaminated Soil. Chemosphere 2019, 235, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, N.; Ye, W.; Rehman, Z.U.; Ijaz, Z. Novel Application of Low Carbon Limestone Calcined Clay Cement (LC3) in Expansive Soil Stabilization: An Eco-Efficient Approach. J. Clean. Prod. 2022, 371, 133492. [Google Scholar] [CrossRef]
- Hagemann, S.E.; Gastaldini, A.L.G.; Cocco, M.; Jahn, S.L.; Terra, L.M. Synergic Effects of the Substitution of Portland Cement for Water Treatment Plant Sludge Ash and Ground Limestone: Technical and Economic Evaluation. J. Clean. Prod. 2019, 214, 916–926. [Google Scholar] [CrossRef]
- Krishnan, S.; Emmanuel, A.C.; Shah, V.; Parashar, A.; Mishra, G.; Maity, S.; Bishnoi, S. Industrial Production of Limestone Calcined Clay Cement: Experience and Insights. Green Mater. 2019, 7, 15–27. [Google Scholar] [CrossRef]
- Vizcaíno-Andrés, L.M.; Sánchez-Berriel, S.; Damas-Carrera, S.; Pérez-Hernández, A.; Scrivener, K.L.; Martirena-Hernández, J.F. Industrial Trial to Produce a Low Clinker, Low Carbon Cement. Mater. Constr. 2015, 65, e045. [Google Scholar] [CrossRef]
- Marangu, J.M.; Mugambi, L.M.; Ratumo, J.T.; Valentini, L. Preliminary Assessment of Limestone Calcined Clay Cement (LC3) in Soil Stabilization for Geotechnical Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 179–188. [Google Scholar]
- Muchui Mugambi, L.; Mujombi, S.; Mutai, V.; Ratumo Toeri, J.; Mwiti Marangu, J.; Valentini, L. Potential of Limestone Calcined Clay Cement (LC3) in Soil Stabilization for Application in Roads and Pavements Construction. Case Stud. Constr. Mater. 2024, 21, e03706. [Google Scholar] [CrossRef]
- Joseph, S.; Bishnoi, S.; Maity, S. An Economic Analysis of the Production of Limestone Calcined Clay Cement in India. Indian Concr. J. 2016, 90, 22–27. [Google Scholar]
- Sánchez Berriel, S.; Favier, A.; Rosa Domínguez, E.; Sánchez Machado, I.R.; Heierli, U.; Scrivener, K.; Martirena Hernández, F.; Habert, G. Assessing the Environmental and Economic Potential of Limestone Calcined Clay Cement in Cuba. J. Clean. Prod. 2016, 124, 361–369. [Google Scholar] [CrossRef]
- Cancio, Y.; Sanchez, S.; Martirena, F.; Sanchez, I.R.; Scrivener, K.; Habert, G. Economic and Ecological Assessment of Cuban Housing Solutions Using Alternative Cement. In Proceedings of the Sustainable Built Environment Regional Conference (SBE16)-ETH-Zürich, Zürich, Switzerland, 13–17 June 2016. [Google Scholar]
- Gettu, R.; Patel, A.; Rathi, V.; Prakasan, S.; Basavaraj, A.S.; Palaniappan, S.; Maity, S. Influence of Supplementary Cementitious Materials on the Sustainability Parameters of Cements and Concretes in the Indian Context. Mater. Struct. 2019, 52, 10. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Zhang, Z.; Ma, Y.; Wang, H. Recent Progress of Utilization of Activated Kaolinitic Clay in Cementitious Construction Materials. Compos. Part B 2021, 211, 108636. [Google Scholar] [CrossRef]
- Cancio Díaz, Y.; Sánchez Berriel, S.; Heierli, U.; Favier, A.R.; Sánchez Machado, I.R.; Scrivener, K.L.; Martirena Hernández, J.F.; Habert, G. Limestone Calcined Clay Cement as a Low-Carbon Solution to Meet Expanding Cement Demand in Emerging Economies. Dev. Eng. 2017, 2, 82–91. [Google Scholar] [CrossRef]
- Al-Fakih, A.; Al-Shugaa, M.A.; A. Al-Osta, M.; Thomas, B.S. Mechanical, Environmental, and Economic Performance of Engineered Cementitious Composite Incorporated Limestone Calcined Clay Cement: A Review. J. Build. Eng. 2023, 79, 107901. [Google Scholar] [CrossRef]
- Hanein, T.; Thienel, K.-C.; Zunino, F.; Marsh, A.T.M.; Maier, M.; Wang, B.; Canut, M.; Juenger, M.C.G.; Ben Haha, M.; Avet, F.; et al. Clay Calcination Technology: State-of-the-Art Review by the RILEM TC 282-CCL. Mater. Struct. 2022, 55, 3. [Google Scholar] [CrossRef]
- Gettu, R.; Pillai, R.G.; Santhanam, M.; Basavaraj, A.S.; Rathnarajan, S.; Dhanya, B.S. Sustainability-Based Decision Support Framework for Choosing Concrete Mixture Proportions. Mater. Struct. 2018, 51, 165. [Google Scholar] [CrossRef]
- Mañosa, J.; Calderón, A.; Salgado-Pizarro, R.; Maldonado-Alameda, A.; Chimenos, J.M. Research Evolution of Limestone Calcined Clay Cement (LC3), a Promising Low-Carbon Binder—A Comprehensive Overview. Heliyon 2024, 10, e25117. [Google Scholar] [CrossRef]
- Han, T.; Ponduru, S.A.; Cook, R.; Huang, J.; Sant, G.; Kumar, A. A Deep Learning Approach to Design and Discover Sustainable Cementitious Binders: Strategies to Learn from Small Databases and Develop Closed-Form Analytical Models. Front. Mater. 2022, 8, 796476. [Google Scholar] [CrossRef]
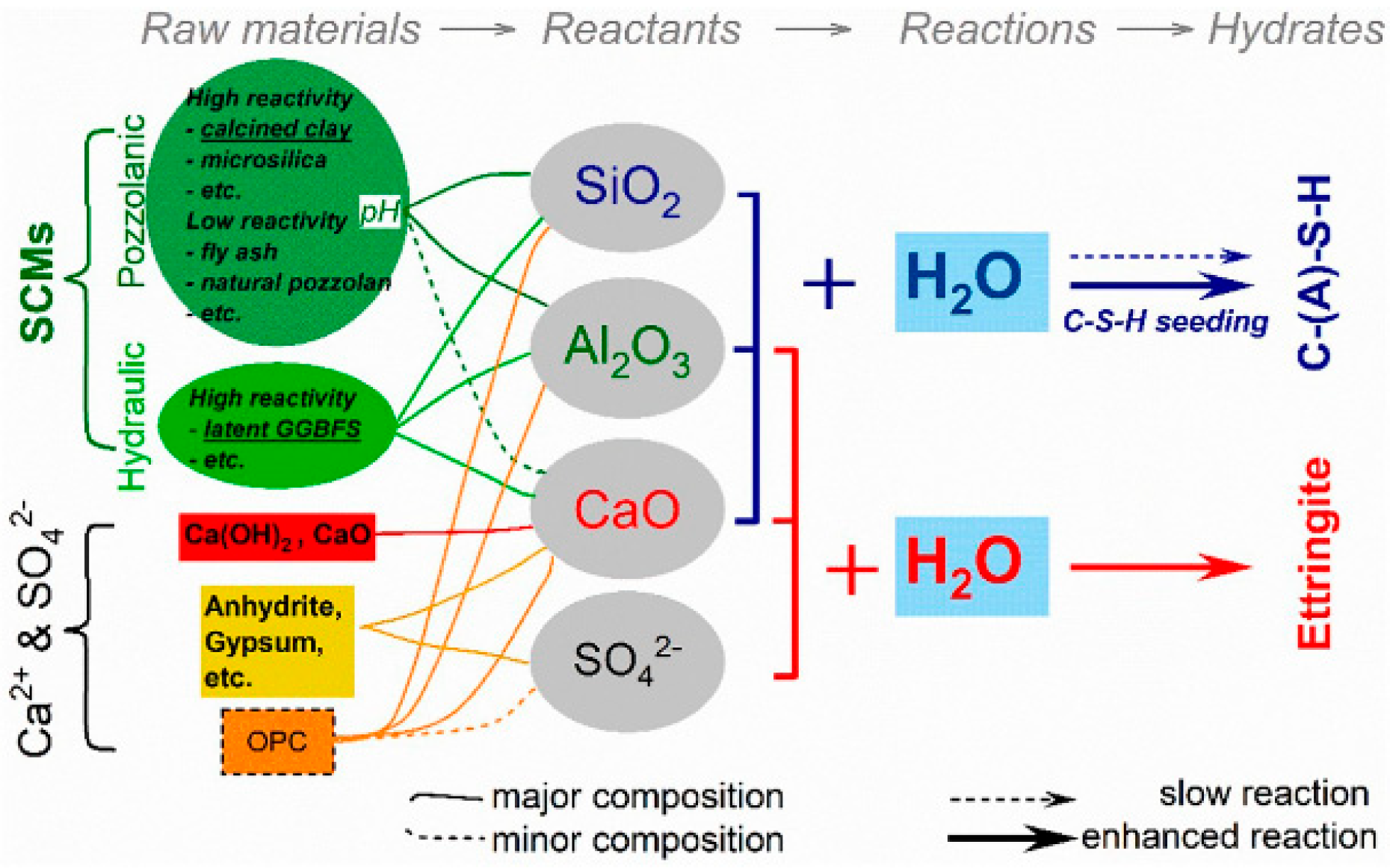



| Type | Composition | Chemical Reaction | Hydration Products |
|---|---|---|---|
| OPC | (1) Clinker (95%) | ||
| (a) Tricalcium Silicate (C3S) | C3S + 6H2O → C3S2H3 + 3Ca(OH)2 | CSH and CH | |
| (b) Dicalcium Silicate (C2S) | C2S + 4H2O → C3S2H3 + Ca(OH)2 | CSH and CH | |
| (c) Tricalcium Aluminate (C3A) | C3A + 6H2O → C3AH6 | CAH | |
| (d) Tetra-calcium Aluminoferrit (C4AF) | C4AF + 7H2O → C4(A, F) H13 | AFm and AFt | |
| (2) Gypsum (GY) (5%) | C3A + 3C$ + 26H2O → C6AS3H32 | Ettringite | |
| Calcium sulfate dihydrate (CaSO4⋅2H2O) | C6AS3H32 + 2C3A + 4H→ 3C4ASH12 | Monosulfate | |
| LC3 | (1) Clinker (50%) | ||
| (a) Tricalcium Silicate (C3S) | C3S + 6H2O →C3S2H3 + 3Ca(OH)2 | CSH and CH | |
| (b) Dicalcium Silicate (C2S) | C2S + 4H2O → C3S2H3 + Ca(OH)2 | CSH and CH | |
| (c) Tricalcium Aluminate (C3A) | C3A + 6H2O → C3AH6(further reaction) C3A + 3CaSO4·2H2O +26H → C6A$3H32 | ||
| (d) Tetra-calcium Aluminoferrit (C4AF) | C4AF + 4CH +14H →C4(A, F) H13 | ||
| (2) Gypsum (5%) | |||
| Calcium sulfate (C$) | C4AF + 4CH + C$ + 2Cc +12H → C4(A, F) cH13 | Iron-rich monocarbonate phase | |
| (3) Calcined clay (30%) | |||
| Metakaolin (AS2) | AS2 + 3.1CH + 4.6H →C1.5A0.1SH4 + C2ASH8 +0.9C4AH13 | C-(A)-S-H, calcium aluminate, AFm | |
| (4) Limestone powder (15%) | AS2 + 5.4CH + 0.4Cc +1.2C$ + 20H →2C1.5A0.1SH4 + 0.4C4AcH12 + 0.4C6A$3H32 | Monocarbonate or hemicarbonate as AFm phases | |
| Calcite (Cc) | C3A + 0.5 Cc + 0.5CH +0.5C$ + 11.5H →C4Ac0.5H12 |
| Property | Granulated Blast Furnace Slag (GGBFS) | Fly Ash (FA) | Calcined Clay | Steel Slag |
|---|---|---|---|---|
| Early-Age Strength | Moderate | Low | High (LC3 system) | Moderate |
| Long-Term Strength | Significant increase (+20%~30%) | Slow growth | Continuous growth | Stable improvement |
| Heat of Hydration | Reduction: 30%~50% | Reduction: 40%~60% | Moderate reductio | Significant reduction |
| Durability | Excellent impermeability, strong carbonation resistance | High sulfate resistance, weak freeze–thaw resistance | Strong chemical erosion resistance | Improved freeze–thaw resistance (when composited) |
| Sustainability | Waste utilization, 37% carbon reduction | Waste utilization, 30% carbon reduction | 40%~50% carbon reduction | 20%~30% carbon reduction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, J.; Guo, S.; Wang, H. A Review of the Performance, Sustainable Applications, and Research Challenges of Limestone-Calcined Clay-Cement (LC3) Systems. Coatings 2025, 15, 611. https://doi.org/10.3390/coatings15050611
Shao J, Guo S, Wang H. A Review of the Performance, Sustainable Applications, and Research Challenges of Limestone-Calcined Clay-Cement (LC3) Systems. Coatings. 2025; 15(5):611. https://doi.org/10.3390/coatings15050611
Chicago/Turabian StyleShao, Jingjing, Shun Guo, and Haibo Wang. 2025. "A Review of the Performance, Sustainable Applications, and Research Challenges of Limestone-Calcined Clay-Cement (LC3) Systems" Coatings 15, no. 5: 611. https://doi.org/10.3390/coatings15050611
APA StyleShao, J., Guo, S., & Wang, H. (2025). A Review of the Performance, Sustainable Applications, and Research Challenges of Limestone-Calcined Clay-Cement (LC3) Systems. Coatings, 15(5), 611. https://doi.org/10.3390/coatings15050611





