The Impact of the Antimicrobial Packaging Covered with Coatings Containing Carvacrol or Geraniol with the Addition of Zinc Oxide on the Quality of Sliced Plant-Based Sausages
Abstract
1. Introduction
2. Materials and Methods
2.1. Coating Preparation
2.2. Coatings’ Optical Property Examination
2.3. Coatings’ Surface Examination
2.4. Packaging and Storage
- a.
- Control samples (C)—neat BOPP bags: PBMA slices separated with BOPP spacers;
- b.
- AG bags—BOPP bags with the AG coating applied on the internal side of the bag: PBMA slices separated with spacers with the AG coating applied on both sides of the bag;
- c.
- AC bags—BOPP bags with the AC coating applied on the internal side of the bag: PBMA slices separated with spacers with the AC coating applied on both sides of the bag.

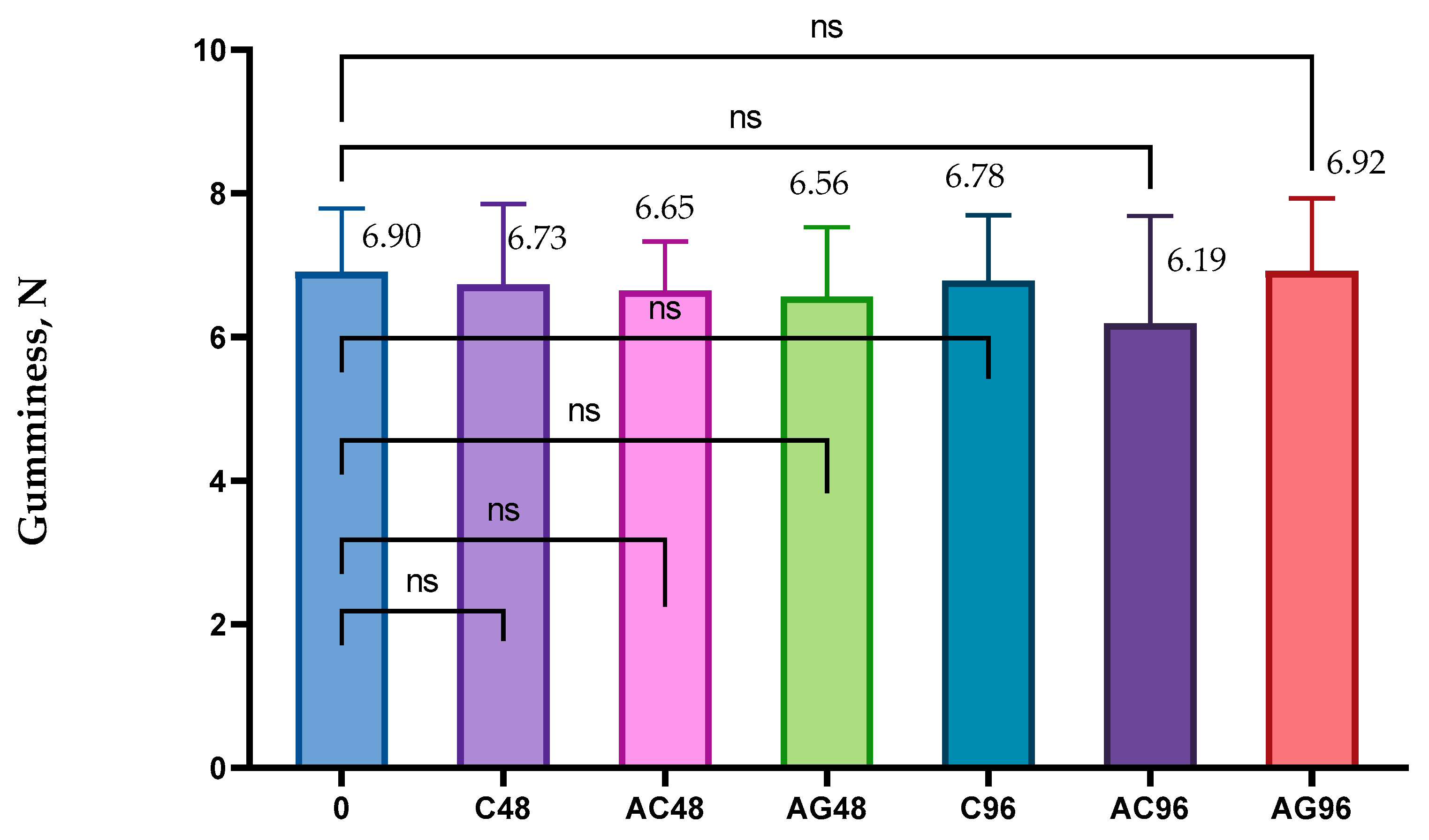
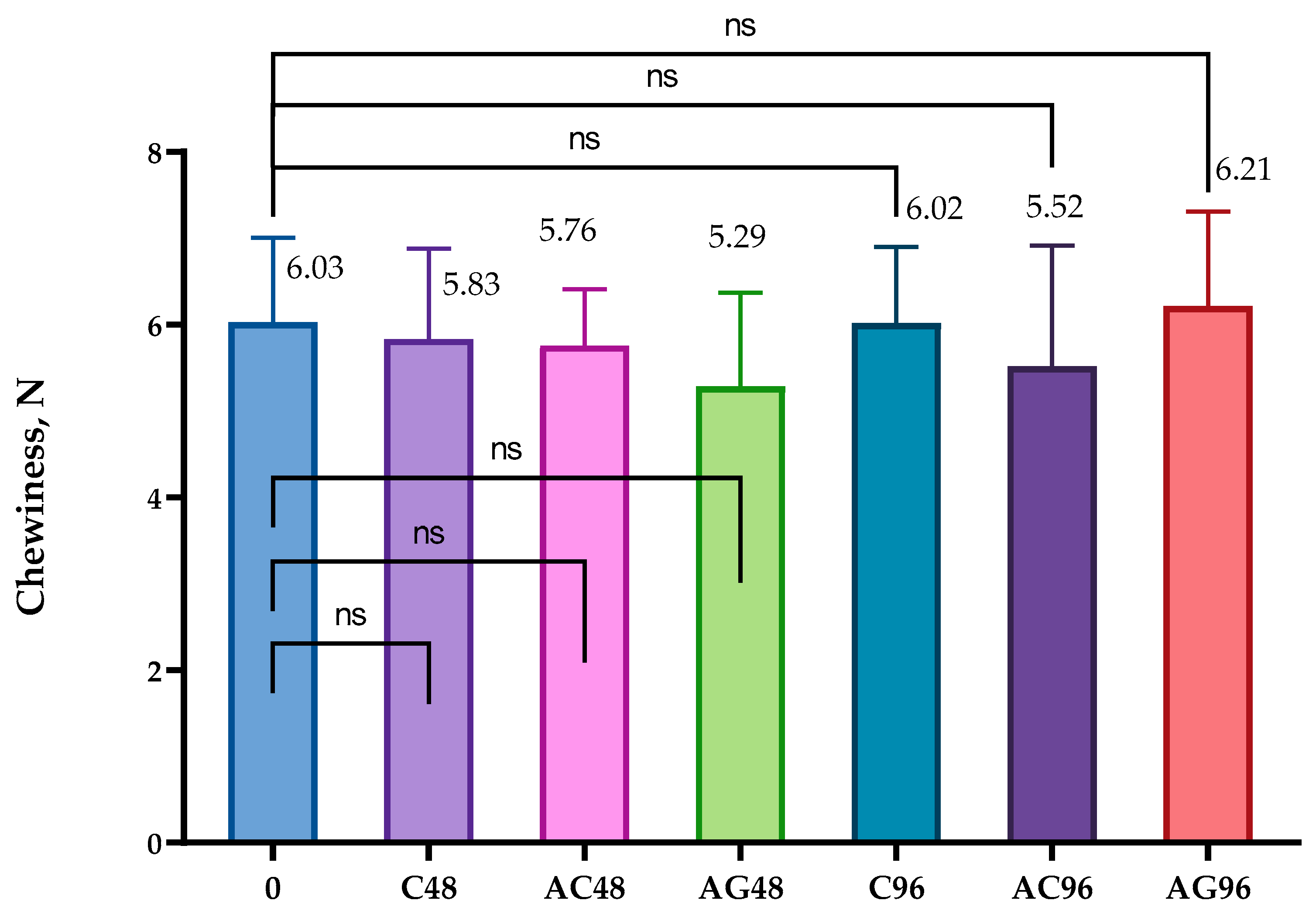
2.5. The Textural Analysis
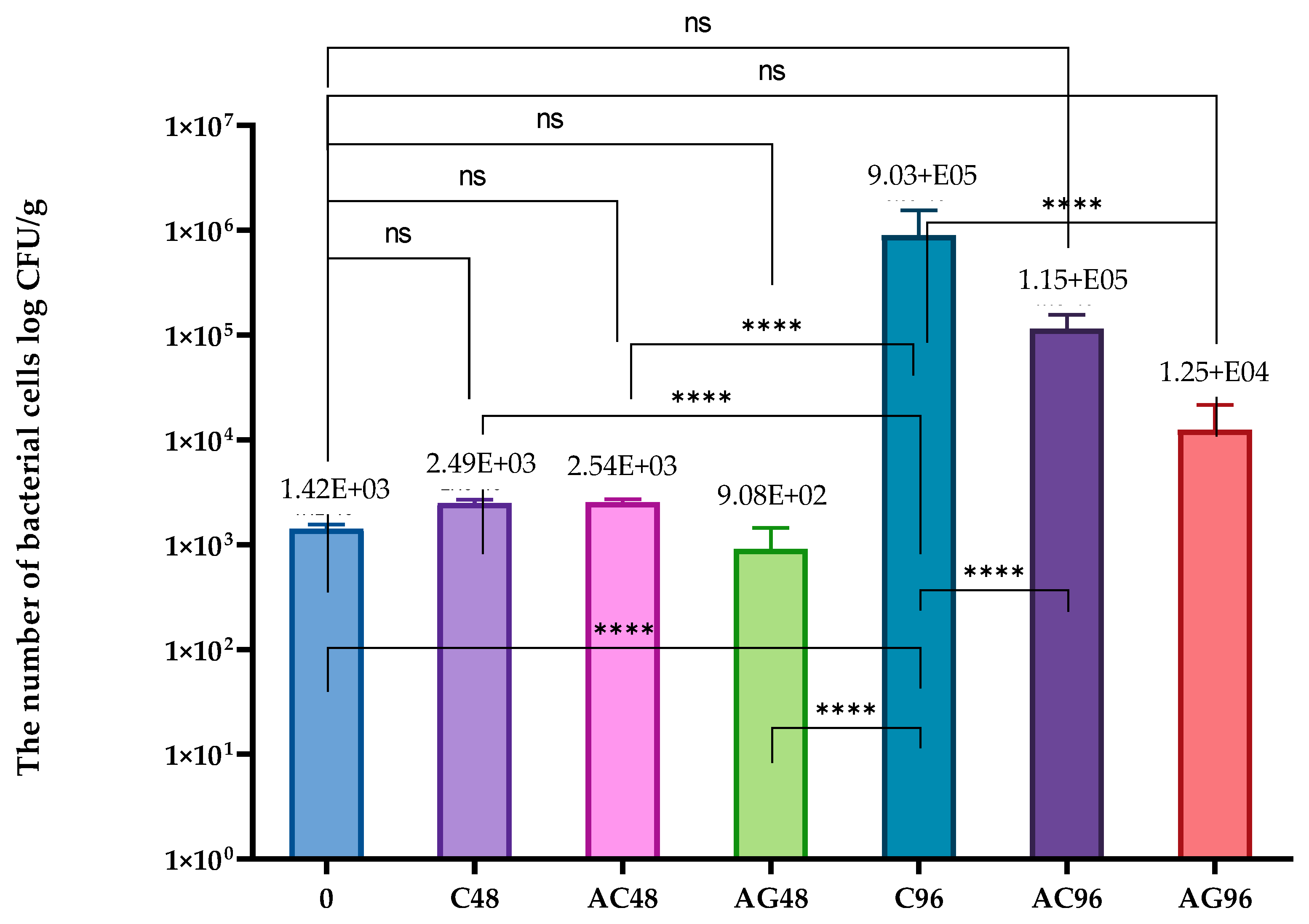
2.6. The Microbiological Purity Examination
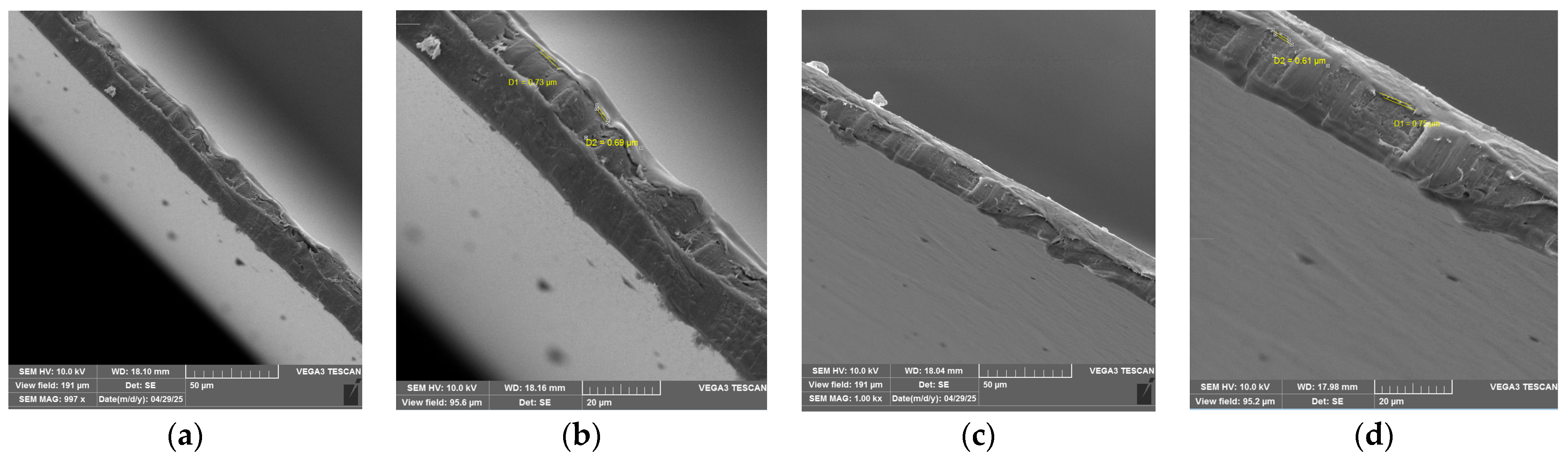
2.7. The Microstructure Analysis
2.8. Dry Mass Tests
2.9. L* a* b* Tests
2.10. Statistical Analysis
3. Results and Discussion
3.1. Optical Properties of Coatings
3.2. Coatings’ Surface Analysis
3.3. Microbial Analysis
3.4. The Textural Analysis’ Results
3.5. Microstructure Analysis
3.6. Dry Mass Examination
3.7. L* a* b* Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giezenaar, C.; Orr, R.E.; Godfrey, A.J.R.; Maggs, R.; Foster, M.; Hort, J. Profiling the novel plant-based meat alternative category: Consumer affective and sensory response in the context of perceived similarity to meat. Food Res. Int. 2024, 188, 114465. [Google Scholar] [CrossRef] [PubMed]
- Sultan, L.; Maganinho, M.; Padrão, P. Comparative assessment of the nutritional composition and degree of processing of meat products and their plant-based analogues. J. Food Compos. Anal. 2024, 133, 106390. [Google Scholar] [CrossRef]
- Shahid, M.; Shah, P.; Mach, K.; Rodgers-Hunt, B.; Finnigan, T.; Frost, G.; Neal, B.; Hadjikakou, M. The environmental impact of mycoprotein-based meat alternatives compared to plant-based meat alternatives: A systematic review. Future Foods 2024, 10, 100410. [Google Scholar] [CrossRef]
- Gréa, C.; Dittmann, A.; Wolff, D.; Werner, R.; Turban, C.; Roser, S.; Hoffmann, I.; Storcksdieck genannt Bonsmann, S. Comparison of the Declared Nutrient Content of Plant-Based Meat Substitutes and Corresponding Meat Products and Sausages in Germany. Nutrients 2023, 15, 3864. [Google Scholar] [CrossRef]
- Moonaisur, N.; Marx-Pienaar, N.; de Kock, H.L. Plant-based meat alternatives in South Africa: An analysis of products on supermarket shelves. Food Sci. Nutr. 2023, 12, 627. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macdiarmid, J.I. The food system and climate change: Are plant-based diets becoming unhealthy and less environmentally sustainable? Proc. Nutr. Soc. 2022, 81, 162. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Qureshi, S.; Akbar, M.H.; Siddiqui, S.A.; Gani, A.; Mushtaq, M.; Hassan, I.; Dhull, S.B. Plant-based meat alternatives: Compositional analysis, current development and challenges. Appl. Food Res. 2022, 2, 100154. [Google Scholar] [CrossRef]
- Asgar, M.A.; Fazilah, A.; Huda, N.; Bhat, R.; Karim, A.A. Nonmeat Protein Alternatives as Meat Extenders and Meat Analogs. Compr. Rev. Food Sci. Food Saf. 2010, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jackson, S.L.; Martinez, E.; Gillespie, C.; Yang, Q. Association between ultraprocessed food intake and cardiovascular health in US adults: A cross-sectional analysis of the NHANES 2011–2016. Am. J. Clin. Nutr. 2021, 113, 428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferreira Corrêa, P.; da Silva, C.F.; Ferreira, J.P.; Medeiros Campos Guerra, J. Vegetable-based frankfurter sausage production by different emulsion gels and assessment of physical-chemical, microbiological and nutritional properties. Food Chem. Adv. 2023, 3, 100354. [Google Scholar] [CrossRef]
- De las Heras-Delgado, S.; Shyam, S.; Cunillera, E.; Dragusan, N.; Salas-Salvadó, J.; Babio, N. Are plant-based alternatives healthier? A two-dimensional evaluation from nutritional and processing standpoints. Food Res. Int. 2023, 169, 112857. [Google Scholar] [CrossRef] [PubMed]
- Shiji, M.; Snigdha, S.; Jyothis, M.; Radhakrishnan, E.K. Biodegradable and active nanocomposite pouches rein-forced with silver nanoparticles for improved packaging of chicken sausages. Food Packag. Shelf Life 2019, 19, 155–166. [Google Scholar] [CrossRef]
- Ordon, M.; Burdajewicz, W.; Pitucha, J.; Tarnowiecka-Kuca, A.; Mizielińska, M. Influence of Active Packaging Covered with Coatings Containing Mixtures of Glycyrrhiza L. and Scutellaria baicalensis Extracts on the Microbial Purity and Texture of Sliced Chicken Sausages. Coatings 2023, 13, 795. [Google Scholar] [CrossRef]
- Sampson, G.L.; Ruelle, S.B.; Phan, L.; Williams-Hill, D.; Hellberg, R.S. Effectiveness of selected pre-enrichment broths for the detection of Salmonella spp. in meat analogs. Food Control 2023, 143, 109282. [Google Scholar] [CrossRef]
- Liu, Z.; Shaposhnikov, M.; Zhuang, S.; Tu, T.; Wang, H.; Wang, L. Growth and survival of common spoilage and pathogenic bacteria in ground beef and plant-based meat analogues. Food Res. Int. 2023, 164, 112408. [Google Scholar] [CrossRef]
- Carhuancho-Colca, K.P.; Silva-Paz, R.J.; Elías-Peñafiel, C.; Salvá-Ruiz, B.K.; Encina-Zelada, C.R. Comparison of Vegetarian Sausages: Proximal Composition, Instrumental Texture, Rapid Descriptive Sensory Method and Overall Consumer Liking. Foods 2024, 13, 1733. [Google Scholar] [CrossRef]
- Zeraatpisheh, F.; Tabatabaei, Y.F.; Shahidi, F. Investigation of effect of cold plasma on microbial load and physicochemical properties of ready-to-eat sliced chicken sausage. J. Food Sci. Technol. 2022, 59, 3928–3937. [Google Scholar] [CrossRef]
- Sharma, H.; Mendiratta, S.K.; Agrawal, R.K.; Talukder, S.; Kumar, S. Studies on the potential application of various blends of essential oils as antioxidant and antimicrobial preservatives in emulsion based chicken sausages. Br. Food J. 2018, 120, 1398. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165. [Google Scholar] [CrossRef]
- Ordon, M.; Burdajewicz, W.; Sternal, J.; Okręglicki, M.; Mizielińska, M. The Antibacterial Effect of the Films Coated with the Layers Based on Uncaria tomentosa and Formitopsis betulina Extracts and ZnO Nanoparticles and Their Influence on the Secondary Shelf-Life of Sliced Cooked Ham. Appl. Sci. 2023, 13, 8853. [Google Scholar] [CrossRef]
- Duthoo, E.; Rasschaert, G.; Leroy, F.; Weckx, S.; Heyndrickx, M.; De Reu, K. The Microbiota of Modified-Atmosphere-Packaged Cooked Charcuterie Products throughout Their Shelf-Life Period, as Revealed by a Complementary Combination of Culture-Dependent and Culture-Independent Analysis. Microorganisms 2021, 9, 1223. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, G.; Candeliere, F.; Amaretti, A.; Licciardello, F.; Rossi, M.; Raimondi, S. Microbiota Survey of Sliced Cooked Ham During the Secondary Shelf Life. Front. Microbiol. 2022, 8, 842390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mizielińska, M.; Nawrotek, P.; Stachurska, X.; Ordon, M.; Bartkowiak, A. Packaging Covered with Antiviral and Antibacterial Coatings Based on ZnO Nanoparticles Supplemented with Geraniol and Carvacrol. Int. J. Mol. Sci. 2021, 22, 1717. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Papalia, T. Antimicrobial Activity of Carvacrol: Current Progress and Future Prospectives. Recent. Pat. Anti-Infect. Drug Discov. 2012, 7, 28. [Google Scholar] [CrossRef]
- Zanetti, M.; Ternus, Z.R.; Dalcanton, F.; de Mello, M.M.J.; de Oliveira, D.; Araujo, P.H.H.; Riella, H.G.; Fiori, M.A. Microbiological Characterization of Pure Geraniol and Comparison with Bactericidal Activity of the Cinnamic Acid in Gram-Positive and Gram-Negative Bacteria. J. Microb. Biochem. Technol. 2015, 7, 186. [Google Scholar] [CrossRef]
- Lorenzi, V.; Muselli, A.; Bernardini, A.F.; Berti, L.; Pagès, J.M.; Amaral, L.; Bolla, J.M. Geraniol Restores Antibiotic Activities against Multidrug-Resistant Isolates from Gram-Negative Species. Antimicrob. Agents. Chemother. 2009, 53, 2209. [Google Scholar] [CrossRef]
- PN-ISO 11036:1999; Sensory Analysis. Methodology. Texture Profiling. Available online: https://www.pkn.pl/polskie-normy/wykazy-pn/wykaz-opublikowanych-pn (accessed on 20 September 2022).
- PN-EN ISO 4833-2:2013-12; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms. Available online: https://www.pkn.pl/polskie-normy/wykazy-pn/wykaz-opublikowanych-pn (accessed on 20 September 2022).
- PN-ISO 17410:2004; Horizontal Method for Enumeration of Psychrotrophic Microorganisms. Available online: https://www.pkn.pl/polskie-normy/wykazy-pn/wykaz-opublikowanych-pn (accessed on 20 September 2022).
- PN-EN ISO 6888-1; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species). Available online: https://www.pkn.pl/polskie-normy/wykazy-pn/wykaz-opublikowanych-pn (accessed on 20 September 2022).
- PN-ISO 4832:2007; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coliforms—Colony-Count Technique. Available online: https://www.pkn.pl/polskie-normy/wykazy-pn/wykaz-opublikowanych-pn (accessed on 20 September 2022).
- PN-EN ISO 6579-1:2017-04; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella. Available online: https://www.pkn.pl/polskie-normy/wykazy-pn/wykaz-opublikowanych-pn (accessed on 20 September 2022).
- PN EN ISO 11290-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp. Available online: https://www.pkn.pl/polskie-normy/wykazy-pn/wykaz-opublikowanych-pn (accessed on 20 September 2022).
- EC (2007). Commission Regulation No 1441/2007 of 5 December 2007 Amending Regulation (EC) No 2073/2005 on Microbiological Criteria for Foodstuffs. Available online: https://faolex.fao.org/docs/pdf/eur75857.pdf (accessed on 20 September 2022).
- Grzybowski, J.; Reiss, J. Praktyczna Bakteriologia Lekarska i Sanitarna; Dom Wydawniczy Bellona: Warszawa, Poland, 2001; p. 346. [Google Scholar]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- El Atki, Y.; Aouam, I.; Taroq, A.; Kamari FEl Timinouni, M.; Lyoussi, B.; Abdellaoui, A. Antibacterial Effect of Combination of Cinnamon Essential Oil and Thymol, Carvacrol, Eugenol, or Geraniol. J. Rep. Pharm. Sci. 2020, 9, 104. [Google Scholar] [CrossRef]
- Andrade-Ochoa, S.; Chacón-Vargas, K.F.; Sánchez-Torres, L.E.; Rivera-Chavira, B.E.; Nogueda-Torres, B.; Nevárez-Moorillón, G.V. Differential Antimicrobial Effect of Essential Oils and Their Main Components: Insights Based on the Cell Membrane and External Structure. Membranes 2021, 11, 405. [Google Scholar] [CrossRef]
- Jiang, S.; Lin, K.; Cai, M. ZnO Nanomaterials: Current Advancements in Antibacterial Mechanisms and Applications. Front. Chem. 2020, 21, 580. [Google Scholar] [CrossRef]
- Shahrampour, D.; Razavi, S.; Sadeghi, A. Evaluation of green tea extract incorporated antimicrobial/antioxidant/biodegradable films based on polycaprolactone/polylactic acid and its application in cocktail sausage preservation. Food Meas. 2023, 17, 1058. [Google Scholar] [CrossRef]
- Van Paepeghem, C.; Taghlaoui, F.; De Loy-Hendrickx, A.; Vermeulen, A.; Devlieghere, F.; Jacxsens, L.; Uyttendaele, M. Prevalence and growth potential of Listeria monocytogenes in innovative, pre-packed, plant-based ready-to-eat food products on the Belgian market. Int. J. Food Microbiol. 2024, 410, 110506. [Google Scholar] [CrossRef]
- Mizielińska, M.; Kowalska, U.; Jarosz, M.; Sumińska, P. A Comparison of the Effects of Packaging Containing Nano ZnO or Polylysine on the Microbial Purity and Texture of Cod (Gadus morhua) Fillets. Nanomaterials 2018, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Patiño, J.H.; Henríquez, L.E.; Restrepo, D.A.; Lantero, M.I.; García, M.A. Influence of polyamide composite casings with silver–zinc crystals on the quality of beef and chicken sausages during their storage. J. Food Sci. Technol. 2022, 59, 75. [Google Scholar] [CrossRef] [PubMed]
- Azlin-Hasim, S.; Cruz-Romero, M.C.; Morris, M.A.; Cummins, E.; Kerry, J.P. Effects of a combination of antimicrobial silver low density polyethylene nanocomposite films and modified atmosphere packaging on the shelf life of chicken breast fillets. Food Packag. Shelf Life 2015, 4, 26. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, J.; Bian, L.; Chang, T.; Zhang, C. Preparation of a novel curdlan/bacterial cellulose/cinnamon essential oil blending film for food packaging application. Int. J. Biol. Macromol. 2022, 212, 211. [Google Scholar] [CrossRef]







| Time [h] | Dry Mass [%] | ||
|---|---|---|---|
| C | AC | AG | |
| 0 | 41.24 ± 3.30 * | 41.24 ± 3.30 * | 41.24 ± 3.30 * |
| 48 | 30.75 ± 3.82 * | 30.58 ± 2.91 * | 31.26 ± 3.96 * |
| 96 | 42.76 ± 1.40 * | 44.72 ± 0.60 * | 44.84 ± 0.01 * |
| Time [h] | C | AC | AG | |
|---|---|---|---|---|
| ΔElab | 0 | 0.44 ± 0.15 * | 0.44 ± 0.15 * | 0.44 ± 0.15 * |
| ΔL | −0.04 ± 0.17 * | −0.04 ± 0.17 * | −0.04 ± 0.17 * | |
| ΔElab | 48 | 0.56 ± 0.16 * | 0.93 ± 0.56 * | 0.65 ± 0.27 * |
| ΔL | −0.46 ± 0.04 * | 0.05 ± 0.38 * | 0.07 ± 0.34 * | |
| ΔElab | 96 | 0.51 ± 0.06 * | 1.14 ± 0.95 * | 2.24 ± 0.47 * |
| ΔL | −0.18 ± 0.41 * | 0.43 ± 0.73 * | 1.34 ± 0.44 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizielińska, M.; Tarnowska, M.; Jankowski, W. The Impact of the Antimicrobial Packaging Covered with Coatings Containing Carvacrol or Geraniol with the Addition of Zinc Oxide on the Quality of Sliced Plant-Based Sausages. Coatings 2025, 15, 576. https://doi.org/10.3390/coatings15050576
Mizielińska M, Tarnowska M, Jankowski W. The Impact of the Antimicrobial Packaging Covered with Coatings Containing Carvacrol or Geraniol with the Addition of Zinc Oxide on the Quality of Sliced Plant-Based Sausages. Coatings. 2025; 15(5):576. https://doi.org/10.3390/coatings15050576
Chicago/Turabian StyleMizielińska, Małgorzata, Marcelina Tarnowska, and Wojciech Jankowski. 2025. "The Impact of the Antimicrobial Packaging Covered with Coatings Containing Carvacrol or Geraniol with the Addition of Zinc Oxide on the Quality of Sliced Plant-Based Sausages" Coatings 15, no. 5: 576. https://doi.org/10.3390/coatings15050576
APA StyleMizielińska, M., Tarnowska, M., & Jankowski, W. (2025). The Impact of the Antimicrobial Packaging Covered with Coatings Containing Carvacrol or Geraniol with the Addition of Zinc Oxide on the Quality of Sliced Plant-Based Sausages. Coatings, 15(5), 576. https://doi.org/10.3390/coatings15050576








