Preparation and Characterization of Composite Hydrogen Barrier Coatings with (Graphene–Epoxy Resin)/(Silicon Carbide–Epoxy Resin)/(Graphene–Epoxy Resin) Sandwich Structures
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Preparation
- (1)
- Substrate treatment
- (2)
- Preparation of the precursors
- (3)
- Preparation of the coatings
2.3. Characterization
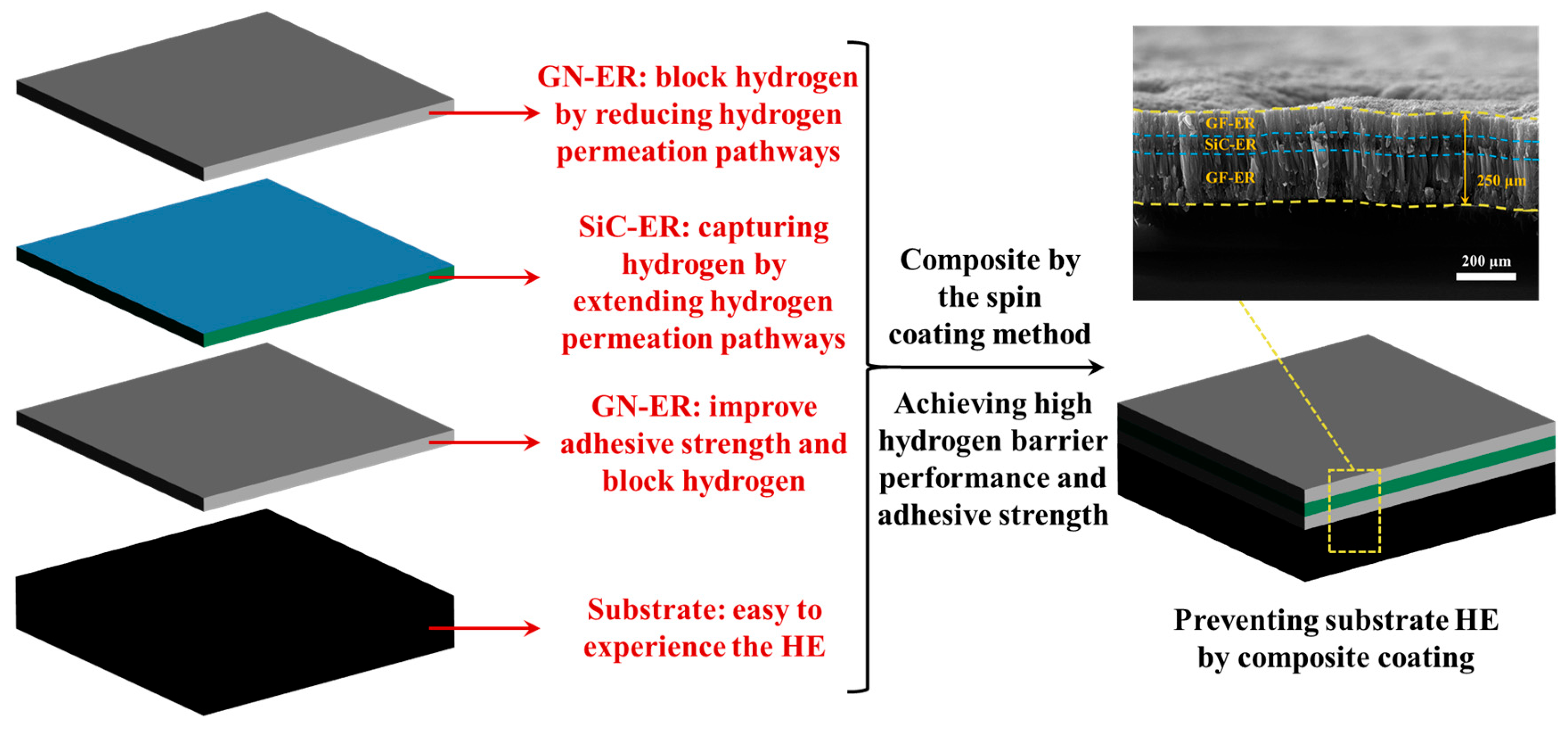
3. Results and Discussion
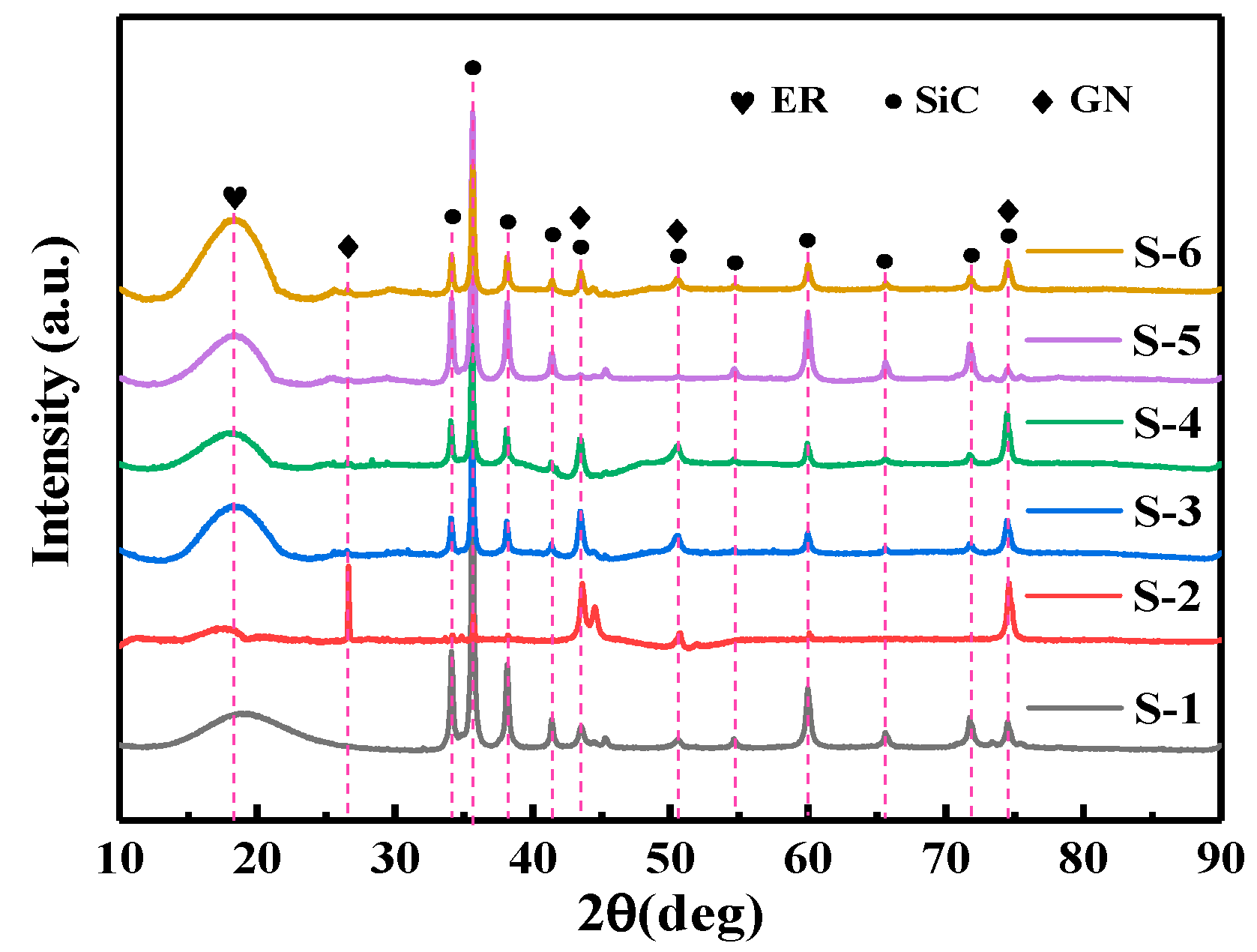
3.1. XRD Diffraction Analysis of the Coatings
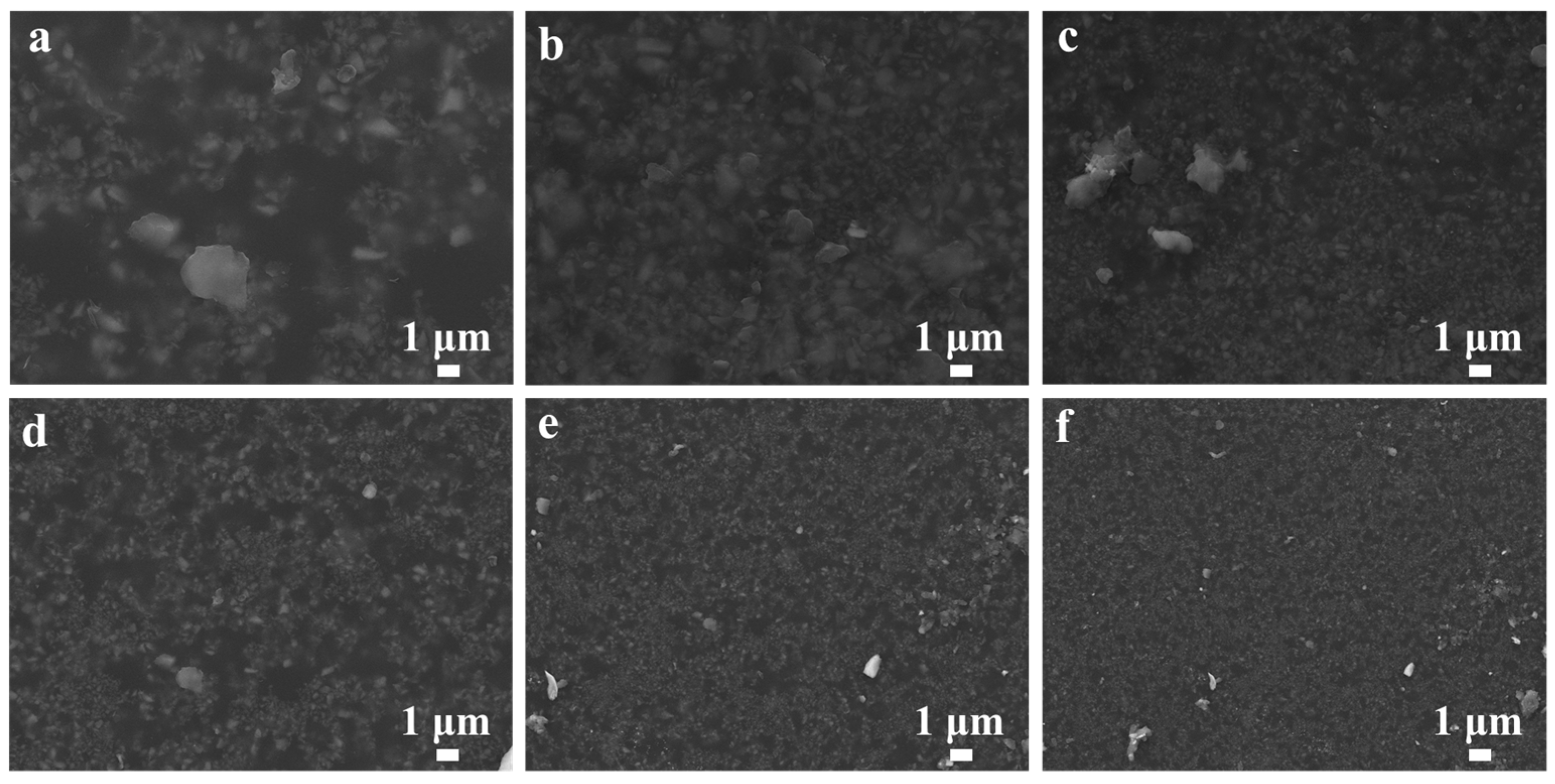
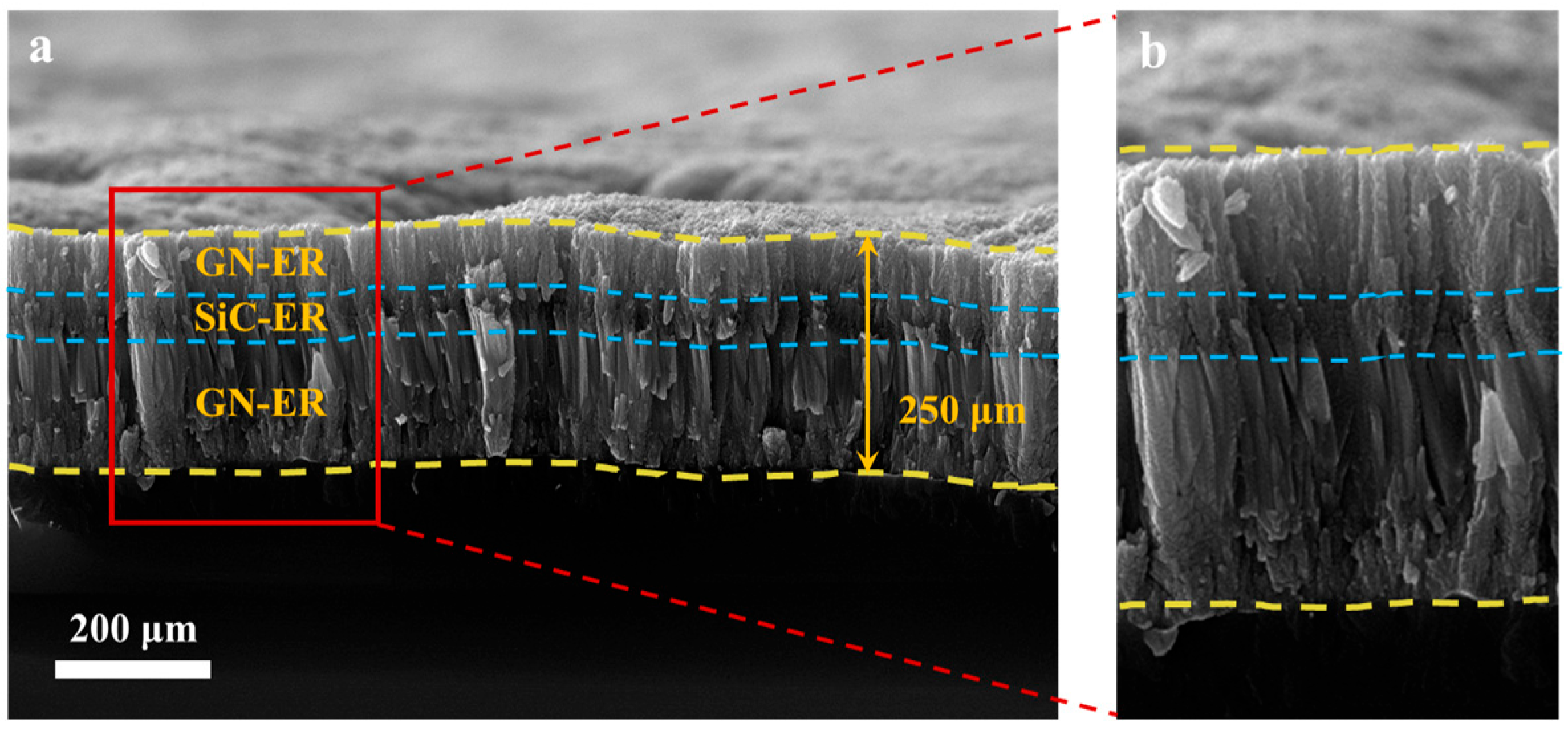
3.2. SEM Research on the Coatings
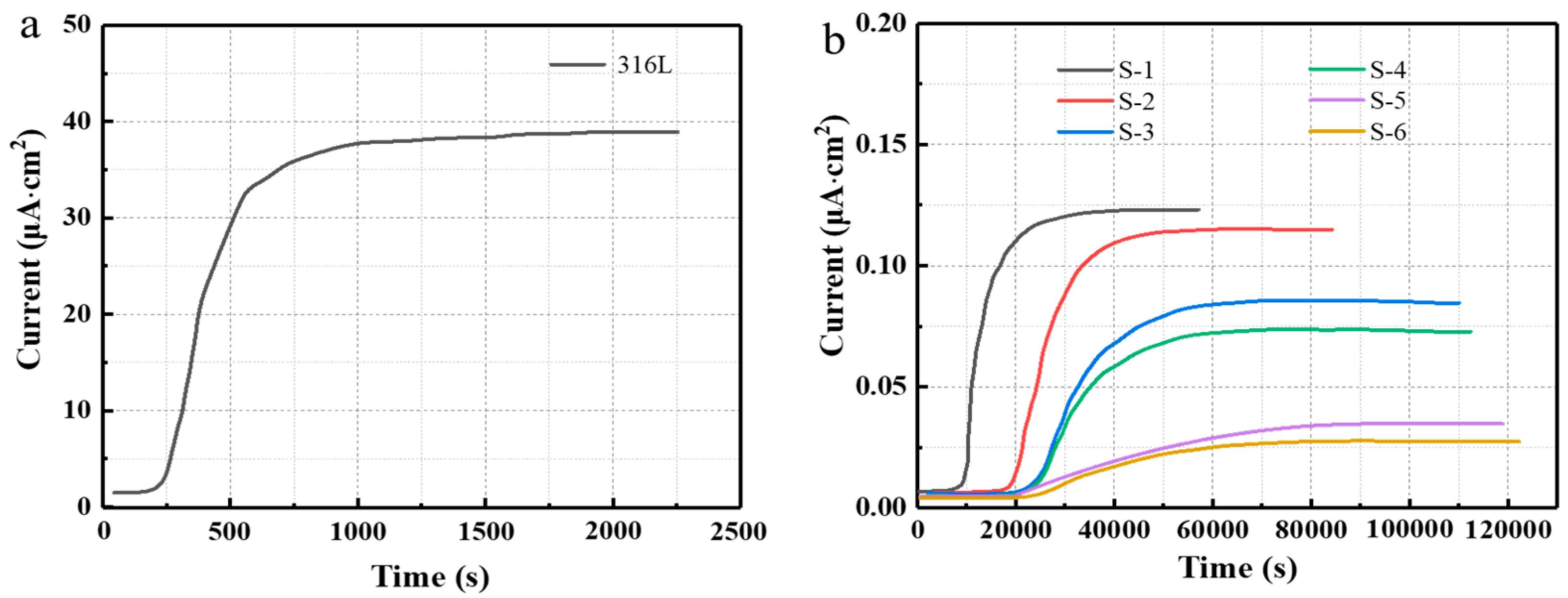
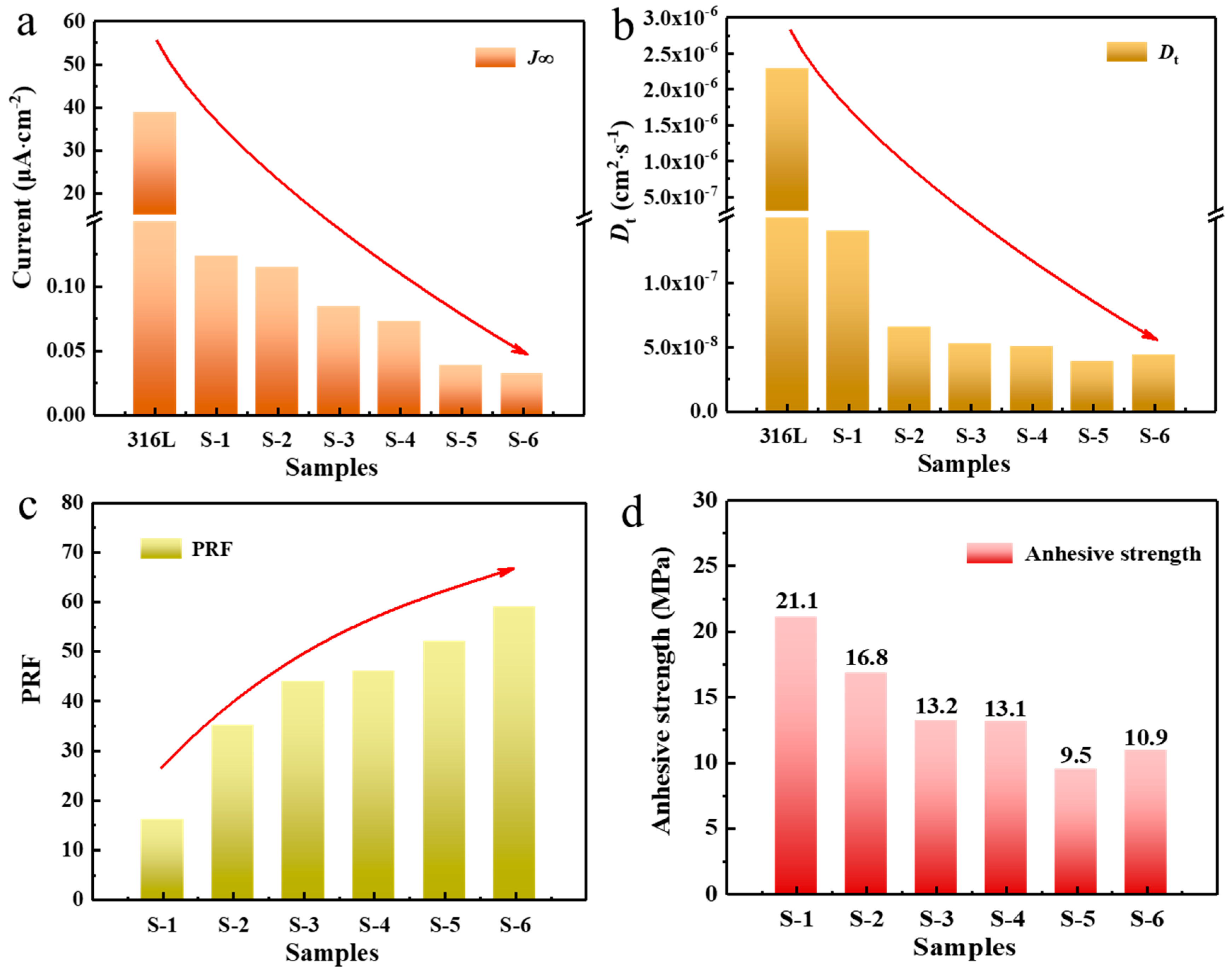
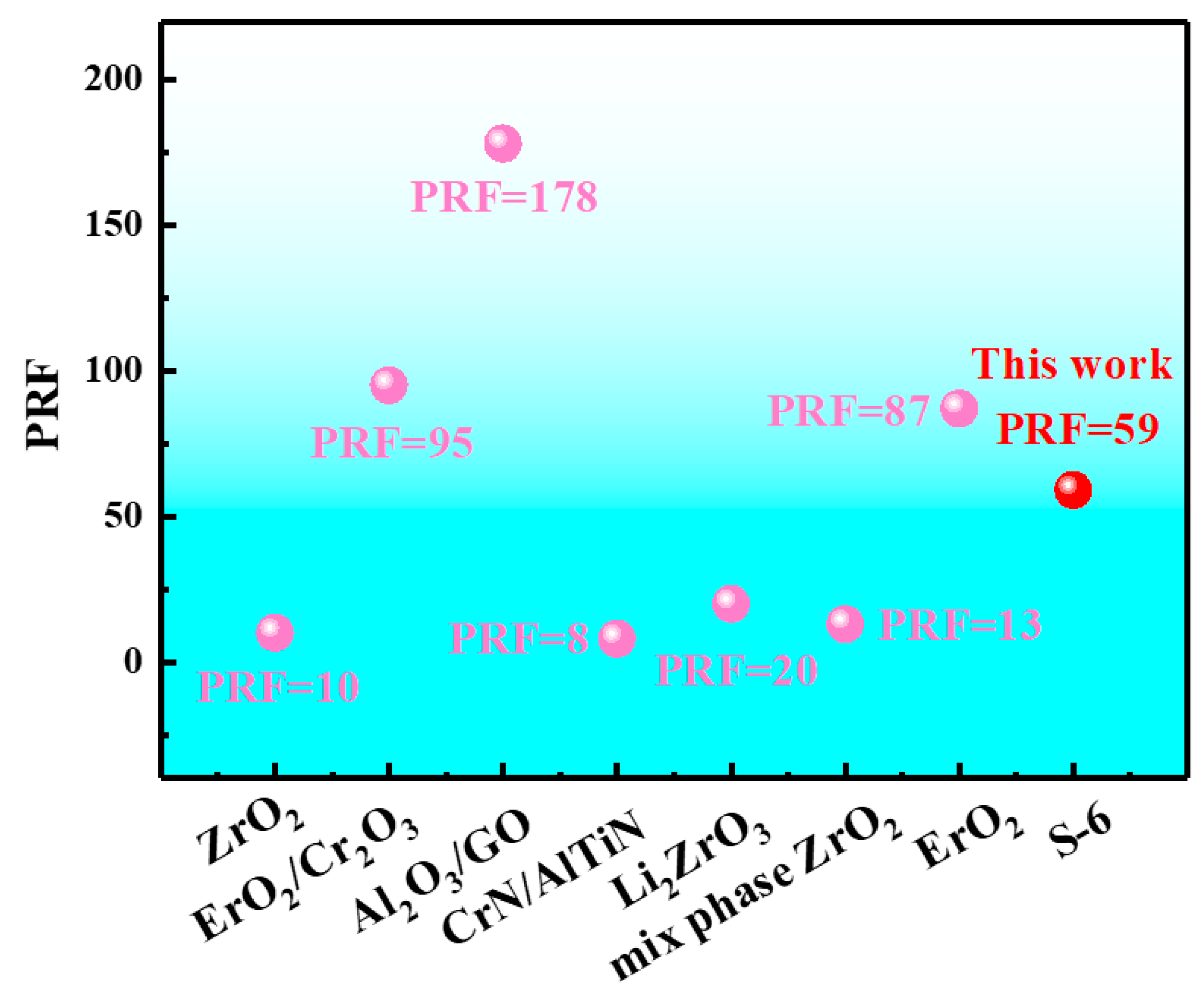
3.3. Hydrogen Barrier Performances of the Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.F.; Yin, J.; Zhang, J.; Wang, Y.F.; Song, X.L.; Zhang, Y.; Ren, X.C. Hydrogen embrittlement and failure mechanisms of multi-principal element alloys: A review. J. Mater. Sci. Technol. 2022, 122, 20–32. [Google Scholar] [CrossRef]
- Meda, U.S.; Bhat, N.; Pandey, A.; Subramanya, K.N.; Lourdu Antony Raj, M.A. Challenges associated with hydrogen storage systems due to the hydrogen embrittlement of high strength steels. Int. J. Hydrogen Energy 2023, 48, 17894–17913. [Google Scholar] [CrossRef]
- Kim, T.; Lee, J.; Kim, S.; Hong, E.; Lee, H. Hydrogen permeation barrier of carbon-doped TiZrN coatings by laser carburization. Corros. Sci. 2021, 19, 109700. [Google Scholar] [CrossRef]
- Bull, S.K.; Champ, T.A.; Raj, S.V.; O’Brien, R.C.; Musgrave, C.B.; Weimer, A.W. Atomic layer deposited boron nitride nanoscale films act as high temperature hydrogen barriers. Appl. Surf. Sci. 2021, 565, 150428. [Google Scholar] [CrossRef]
- Dehghanian, H.A.; Hosseinabadi, N. The hydrogen storage capacity of Al-Cu alloy with permeable alumina hydrogen permeation barrier (HPB) applied by plasma electrolytic oxidation (PEO). Int. J. Hydrogen Energy 2022, 47, 7339–7350. [Google Scholar] [CrossRef]
- Li, Z.L.; Yan, S.F.; Chen, W.D.; Zhang, Z.H.; Kang, Y.X.; Ma, W. The effect of current density on the anodic oxidation hydrogen barrier film on ZrH1.8 surface. Corros. Sci. 2024, 227, 111740. [Google Scholar] [CrossRef]
- Zhou, C.L.; Huang, Y.L.; Liu, X.H.; Huang, Y.; Wu, H.; Hua, Z.L.; Chu, P.K.; Yin, Y.S. Functionalized graphite nanosheet/organosilicon composite coatings with soft-hard structures: Excellent wear resistance and hydrogen barrier properties. Surf. Coat. Technol. 2025, 496, 131684. [Google Scholar] [CrossRef]
- Cho, J.; Kim, B.; Venkateshalu, S.; Chung, D.Y.; Lee, K.; Choi, S. Electrochemically activatable liquid organic hydrogen carriers and their applications. J. Am. Chem. Soc. 2023, 145, 16951–16965. [Google Scholar] [CrossRef]
- Lebedeva, O.; Kultin, D.; Каlenchuk, A.; Кustov, L. Advances and prospects in electrocatalytic hydrogenation of aromatic hydrocarbons for synthesis of “loaded” liquid organic hydrogen carriers. Curr. Opin. Electrochem. 2023, 38, 101207. [Google Scholar] [CrossRef]
- Rullo, F.; Beier, C.K.; Henseler, J.; Bösmann, A.; Preuster, P.; Wasserscheid, P.; Geißelbrecht, M. Pushing activity and stability of LOHC dehydrogenation catalysts by strict LOHC quality protocols. Int. J. Hydrogen Energy 2025, 98, 606–613. [Google Scholar] [CrossRef]
- Wu, M.; Chen, Y.; Peng, J.Q.; Yan, G.Q.; Sun, Y.P.; Zhang, J.D.; Zhang, S.L.; Wang, L.J. Hydrogen permeation resistance and characterization of Si-Al and Si-Zr composite sol oxide coating on surface of zirconium hydride. Rare Met. 2017, 36, 55–60. [Google Scholar] [CrossRef]
- Li, P.; Chen, K.; Zhao, L.L.; Zhang, H.Y.; Sun, H.X.; Yang, X.J.; Kim, N.H.; Lee, J.H.; Niu, Q.J. Preparation of modified graphene oxide/polyethyleneimine film with enhanced hydrogen barrier properties by reactive layer-by-layer self-assembly. Compos. Part B-Eng. 2019, 166, 663–672. [Google Scholar] [CrossRef]
- Huang, J.; Xie, H.; Luo, L.M.; Zan, X.; Liu, D.G.; Wu, Y.C. Preparation and properties of FeAl/Al2O3 composite tritium permeation barrier coating on surface of 316L stainless steel. Surf. Coat. Technol. 2020, 383, 125282. [Google Scholar] [CrossRef]
- Wang, F.Y.; Wang, J.; Lu, W.; Li, W.G.; Chu, D.L.; Wang, W.H. Multi-layer structured AlN/Er2O3 coating: Enhanced thermal stability and hydrogen isotope permeation resistance. Ceram. Int. 2024, 50, 40902–40910. [Google Scholar] [CrossRef]
- Liu, J.G.; Bi, H.S.; Zhang, Q.S.; Liu, S.Q.; Li, H.W.; Cui, G. Design, fabrication, and hydrogen blocking performance of alumina/zirconia functional gradient coatings. Ceram. Int. 2024, 50, 40902–40910. [Google Scholar] [CrossRef]
- Wan, H.X.; Cheng, Z.L.; Song, D.D.; Chen, C.F. Preparation and performance study of waterborne epoxy resin/non-covalent modified graphene oxide hydrogen barrier coatings. Int. J. Hydrogen Energy 2024, 53, 218–228. [Google Scholar] [CrossRef]
- Yuan, S.C.; Zhang, S.; Wei, J.T.; Gao, Y.; Zhu, Y.J.; Wang, H.Y. Materials selection, design, and regulation of polymer-based hydrogen barrier composite coatings, membranes and films for effective hydrogen storage and transportation: A comprehensive review. Int. J. Hydrogen Energy 2024, 91, 555–573. [Google Scholar] [CrossRef]
- Seo, O.B.; Saha, S.; Kim, N.H.; Lee, J.H. Preparation of functionalized mxene-stitched-graphene oxide/poly (ethylene-co-acrylic acid) nanocomposite with enhanced hydrogen gas barrier properties. J. Membrane. Sci. 2021, 640, 119839. [Google Scholar] [CrossRef]
- Saha, S.; Son, W.S.; Kim, N.H.; Lee., J.H. Fabrication of impermeable dense architecture containing covalently stitched graphene oxide/boron nitride hybrid nanofiller reinforced semi-interpenetrating network for hydrogen gas barrier applications. J. Mater. Chem. A 2022, 10, 4376–4391. [Google Scholar] [CrossRef]
- Nam, T.H.; Lee, J.H.; Choi, S.R.; Yoo, J.B.; Kim, J.G. Graphene coating as a protective barrier against hydrogen embrittlement. Int. J. Hydrogen Energy 2014, 39, 11810–11817. [Google Scholar] [CrossRef]
- Fan, Y.J.; Huang, Y.F.; Cui, B.; Zhou, Q. Graphene coating on nickel as effective barriers against hydrogen embrittlement. Surf. Coat. Technol. 2019, 374, 610–616. [Google Scholar] [CrossRef]
- Zhao, L.L.; Zhang, H.Y.; Kim, N.H.; Hui, D.; Lee, J.H.; Li, Q.; Sun, H.X.; Li, P. Preparation of graphene oxide/polyethyleneimine layer-by-layer assembled film for enhanced hydrogen barrier property. Compos. Part B-Eng. 2016, 92, 252–258. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Park, W.B.; Layek, R.K.; Uddin, M.E.; Kim, N.H.; Kim, H.G.; Lee, J.H. Hexylamine functionalized reduced graphene oxide/polyurethane nanocomposite-coated nylon for enhanced hydrogen gas barrier film. J. Membr. Sci. 2016, 500, 106–114. [Google Scholar] [CrossRef]
- Liu, H.Y.; Bandyopadhyay, P.; Kshetri, T.; Kim, N.H.; Ku, B.C.; Moon, B.; Lee, J.H. Layer-by-layer assembled polyelectrolyte-decorated graphene multilayer film for hydrogen gas barrier application. Compos. Part B-Eng. 2017, 114, 339–347. [Google Scholar] [CrossRef]
- Alkrunz, M.; Shajahan, S.; Elkaffas, R.; Schiffer, A.; Zheng, L.X.; Liao, K.; Khan, M.Y.; Anjum, D.; Zweiri, Y.; Samad, Y.A. Fabrication and characterization of rippled graphene/LDPE composites with enhanced hydrogen barrier properties. Int. J. Hydrogen Energy 2024, 85, 794–803. [Google Scholar] [CrossRef]
- Liu, M.F.; Lin, K.L.; Zhou, M.Y.; Wallwork, A.; Bissett, M.A.; Young, R.J.; Kinloch, I.A. Mechanism of gas barrier improvement of graphene/polypropylene nanocomposites for new-generation light-weight hydrogen storage. Compos. Sci. Technol. 2024, 249, 110483. [Google Scholar] [CrossRef]
- Wan, H.X.; Song, X.X.; Cheng, Z.L.; Min, W.L.; Song, D.D.; Chen, C.F. Construction and properties of graphene oxide hydrogen-blocking coatings. Int. J. Hydrogen Energy 2024, 84, 410–419. [Google Scholar] [CrossRef]
- Longhi, M.; Zini, L.P.; Kunst, S.R.; Zattera, A.J. Influence of the type of epoxy resin and concentration of glycidylisobutyl-poss in the properties of nanocomposites. Polym. Polym. Compos. 2017, 25, 593–602. [Google Scholar] [CrossRef]
- Joseph, E.J.; Panneerselvam, K. Manufacturing and characterization of tungsten particulate-reinforced aw106 epoxy resin composites. Trans. Indian Inst. Met. 2021, 74, 817–825. [Google Scholar] [CrossRef]
- Sujith, R.; Chauhan, P.K.; Gangadhar, J.; Maheshwari, A. Graphene nanoplatelets as nanofillers in mesoporous silicon oxycarbide polymer derived ceramics. Sci. Rep. 2018, 8, 17633. [Google Scholar] [CrossRef]
- Wei, S.N.; Guan, L.; Song, B.Z.; Fan, B.B.; Zhao, B.; Zhang, R. Seeds-induced synthesis of SiC by microwave heating. Ceram. Int. 2019, 45, 9771–9775. [Google Scholar] [CrossRef]
- Yang, H.Y.; Li, M.Y.; Zhou, X.G.; Wang, H.L.; Yu, J.S. Flexural modulus of SiC/SiC composites sintered by microwave and conventional heating. Ceram. Int. 2019, 45, 10142–10148. [Google Scholar] [CrossRef]
- Li, B.B.; Mao, B.X.; Wang, X.B.; He, T. Fabrication and frictional wear property of bamboo-like SiC nanowires reinforced SiC coating. Surf. Coat. Technol. 2019, 45, 10142–10148. [Google Scholar] [CrossRef]
- Lü, X.X.; Li, L.B.; Sun, J.J.; Yang, J.H.; Jiao, J. Microstructure and tensile behavior of (BN/SiC)n coated SiC fibers and SiC/SiC minicomposites. J. Eur. Ceram. Soc. 2023, 43, 1828–1842. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Hu, Z.R.; Tong, G.Q.; Lin, D.; Nian, Q.; Shao, J.Y.; Hu, Y.W.; Saeib, M.; Jin, S.Y.; Cheng, G.J. Laser sintered graphene nickel nanocomposites. J. Mater. Process. Technol. 2016, 231, 143–150. [Google Scholar] [CrossRef]
- Houshi, M.N.; Aakyiir, M.; Ma, J. Low-temperature, rapid preparation of functionalized graphene platelets. Compos. Commun. 2020, 22, 100500. [Google Scholar] [CrossRef]
- Chen, G.J.; Ng, K.Y.S.; Lin, C.C. Effects of nitrogen-doping or alumina films on graphene as anode materials of lithium-ion batteries verified by in situ XRD. J. Nanomater. 2022, 2022, 1758789. [Google Scholar] [CrossRef]
- Liu, J.C.; Su, Z.J.; Xu, J.M.; Zhang, Y.B. Thermal storage performance of NaNO3/cordierite-mullite ceramic (CMC) composites deriving from metallurgical slag. Ceram. Int. 2023, 49, 38094–38102. [Google Scholar] [CrossRef]
- Yang, W.T.; Zheng, G.P. The enhanced energy storage properties of (1-x)(0.94BNT-0.06BT)-x(Bi0.2Na0.2K0.2La0.2Sr0.2)TiO3 solid solutions. Ceram. Int. 2024, 50, 7834–7842. [Google Scholar] [CrossRef]
- Chen, Y.H.; Sun, J.X.; Chai, Z.H.; Zhang, B.Q.; Cao, Y.; Zhang, Y.H. High thermal conductivity and high energy density compatible latent heat thermal energy storage enabled by porous Al2O3@Graphite ceramics composites. Ceram. Int. 2024, 50, 19864–19872. [Google Scholar] [CrossRef]
- Dou, Z.M.; Yang, Y.; Zhou, L.; Luo, G.G.; Li, Y.H.; Hu, J.; Wang, S.X.; Wang, W.; Ruan, S.J.; Zhang, G.Z.; et al. Enhancing energy storage performance in multilayer ceramic capacitors with (Pb,La)(Zr,Sn,Ti)O3-based antiferroelectric solid solutions. Appl. Mater. Today 2024, 41, 102488. [Google Scholar] [CrossRef]
- Shen, Y.Q.; Xu, X.H.; Wu, J.F.; Yu, J.Q.; Qiu, S.X.; Zhang, D. Effect of ZrO2-MoO3 on the properties of in situ synthesized corundum-mullite composite thermal storage ceramics. Ceram. Int. 2025, 51, 6110–6124. [Google Scholar] [CrossRef]
- Chen, W.L.; Meng, X.N.; Wu, D.Q.; Yao, D.C.; Zhang, D.D. The effect of vacuum annealing on microstructure, adhesion strength and electrochemical behaviors of multilayered AlCrTiSiN coatings. Appl. Surf. Sci. 2019, 467–468, 391–401. [Google Scholar] [CrossRef]
- Warcaba, M.; Kowalski, K.; Kopia, A.; Moskalewicz, T. Impact of surface topography, chemistry and properties on the adhesion of sodium alginate coatings electrophoretically deposited on titanium biomaterials. Metall. Mater. Trans. A 2021, 52, 4454–4467. [Google Scholar] [CrossRef]
- Wan, H.X.; Min, W.L.; Song, D.D.; Chen, C.F. Research progress of hydrogen blocking coatings. Mater. Chem. Phys. 2024, 328, 130028. [Google Scholar] [CrossRef]
- Lv, J.; Zhu, C.X.; Qiu, H.N.; Zhang, J.; Gu, C.D.; Feng, J. Robust icephobic epoxy coating using maleic anhydride as a crosslinking agent. Prog. Org. Coat. 2020, 142, 105561. [Google Scholar] [CrossRef]
- Li, B.; Qian, S.; Sun, C.; Wang, M.; Zhan, X. Mechanical properties and wear resistance of Cf/epoxy resin composites with in-situ grown SiC nanowires on carbon fibers. Compos. Interface 2023, 30, 923–939. [Google Scholar] [CrossRef]
- Nakamura, H.; Isobe, K.; Nakamichi, M.; Yamanishi, T. Evaluation of deuterium permeation reduction factor of various coatings deposited on ferritic/martensitic steels for development of tritium permeation barrier. Fusion Eng. Des. 2010, 85, 1531–1536. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Wang, S.M.; Li, S.; He, D.; Liu, X.P.; Jiang, L.J.; Huang, H.T. Deuterium permeation properties of Er2O3/Cr2O3 composite coating prepared by MOCVD on 316L stainless steel. Fusion Eng. Des. 2016, 113, 205–210. [Google Scholar] [CrossRef]
- Yang, H.; Shao, Z.M.; Wang, W.; Ji, X.; Li, C.J. A composite coating of GO-Al2O3 for tritium permeation barrier. Fusion Eng. Des. 2020, 156, 111689. [Google Scholar] [CrossRef]
- Hu, L.L.; Wei, G.; Yin, R.; Hong, M.Q.; Cheng, T.; Zhang, D.X.; Zhao, S.Q.; Yang, B.; Zhang, G.K.; Cai, G.X.; et al. Significant hydrogen isotopes permeation resistance via nitride nano-multilayer coating. Int. J. Hydrogen Energy 2020, 45, 19583–19589. [Google Scholar] [CrossRef]
- Mukai, K.; Kenjo, S.; Iwamatsu, N.; Mahmoud, B.; Chikada, T.; Yagi, J.; Konishi, S. Hydrogen permeation from F82H wall of ceramic breeder pebble bed: The effect of surface corrosion. Int. J. Hydrogen Energy 2022, 47, 6154–6163. [Google Scholar] [CrossRef]
- Wang, Z.G.; Chen, W.D.; Yan, S.F.; Zhong, X.K.; Ma, W.; Song, X.W.; Wang, Y.M.; Ouyang, J.H. Direct fabrication and characterization of zirconia thick coatings on zirconium hydride as a hydrogen permeation barrier. Coatings 2023, 13, 884. [Google Scholar] [CrossRef]
- Raia, S.; Rayjada, P.A.; Dhorajiya, P.B.; Patel, R.B.; Sharma, S.K.; Sircar, A.; Bhattacharyay, R. Deuterium permeation studies through bare and Er2O3 coated SS 316L. Fusion Eng. Des. 2024, 206, 114587. [Google Scholar] [CrossRef]
| Serial Numbers | Coating Types | Numbers of the Samples |
|---|---|---|
| 1 | SiC-ER | S-1 |
| 2 | GN-ER | S-2 |
| 3 | (SiC-ER)/(GN-ER) | S-3 |
| 4 | (GN-ER)/(SiC-ER) | S-4 |
| 5 | (SiC-ER)/(GN-ER)/(SiC-ER) | S-5 |
| 6 | (GN-ER)/(SiC-ER)/(GN-ER) | S-6 |
| Samples | t0.632 (s) | J∞ (μA·cm−2) | Dt (10−8 cm2·s−1) | PRF |
|---|---|---|---|---|
| 316L | 729 | 38.893 | 228.62 | / |
| S-1 | 11,932 | 0.12313 | 13.97 | 16 |
| S-2 | 25,578 | 0.11491 | 6.52 | 35 |
| S-3 | 31,718 | 0.084587 | 5.25 | 44 |
| S-4 | 33,264 | 0.072745 | 5.01 | 46 |
| S-5 | 38,015 | 0.034740 | 4.38 | 52 |
| S-6 | 42,987 | 0.029100 | 3.88 | 59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, K.; Jiang, B. Preparation and Characterization of Composite Hydrogen Barrier Coatings with (Graphene–Epoxy Resin)/(Silicon Carbide–Epoxy Resin)/(Graphene–Epoxy Resin) Sandwich Structures. Coatings 2025, 15, 518. https://doi.org/10.3390/coatings15050518
Cai K, Jiang B. Preparation and Characterization of Composite Hydrogen Barrier Coatings with (Graphene–Epoxy Resin)/(Silicon Carbide–Epoxy Resin)/(Graphene–Epoxy Resin) Sandwich Structures. Coatings. 2025; 15(5):518. https://doi.org/10.3390/coatings15050518
Chicago/Turabian StyleCai, Ke, and Bailing Jiang. 2025. "Preparation and Characterization of Composite Hydrogen Barrier Coatings with (Graphene–Epoxy Resin)/(Silicon Carbide–Epoxy Resin)/(Graphene–Epoxy Resin) Sandwich Structures" Coatings 15, no. 5: 518. https://doi.org/10.3390/coatings15050518
APA StyleCai, K., & Jiang, B. (2025). Preparation and Characterization of Composite Hydrogen Barrier Coatings with (Graphene–Epoxy Resin)/(Silicon Carbide–Epoxy Resin)/(Graphene–Epoxy Resin) Sandwich Structures. Coatings, 15(5), 518. https://doi.org/10.3390/coatings15050518






