Effect of the Fluoride Species and Content of the PEO Electrolyte on the Corrosion Properties of the Layers Obtained on AZ31 for Biomedical Purposes
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Coating Characterisations
3.1.1. SEM Analysis
3.1.2. EDS Determination
3.1.3. XRD Analysis
3.2. Electrochemical Behaviour
- ZCPE is the impedance;
- Q0 is an admittance constant;
- j is the imaginary unit;
- ω is the angular frequency;
- n is the phase exponent.

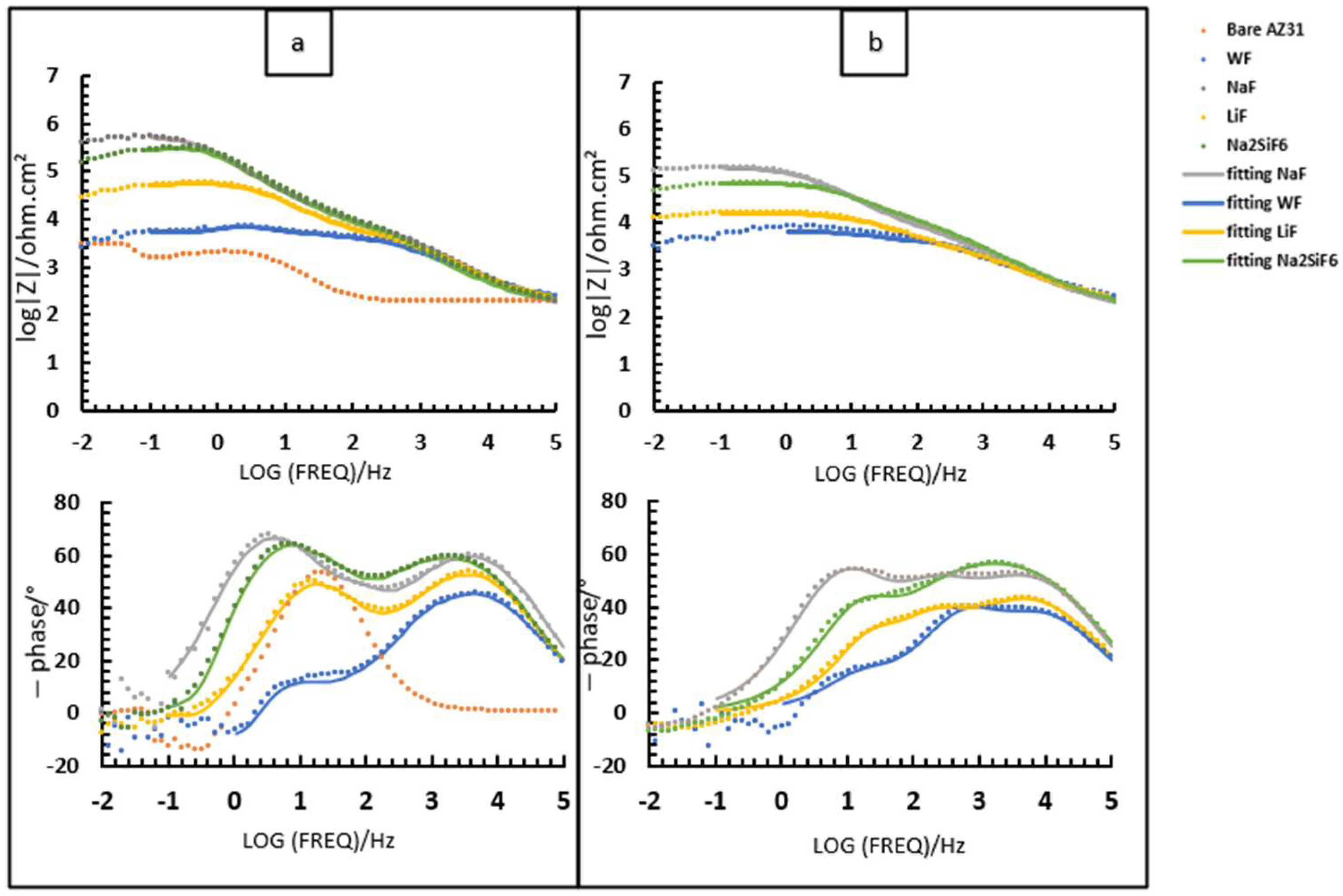
| Sample | Rs (Ohm·cm2) | Router (Ohm·cm2) | Q0oxide(CPE outer) (sn/(Ohm·cm2)) | nouter (CPEouter) (-) | Rinter(Ohm·cm2) | Q0d(CPEinter) (sn/(Ohm·cm2)) | Ninter (CPEcor) (-) | Q0d/inl(CPEdl/in) (sn/(Ohm·cm2)) | nin (CPEdl) (-) | Rct/in (Ohm·cm2) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 h 0.1 M NaCl saturated Mg(OH)2 | ||||||||||
| WF | 198.6 | 2839 | 4.34 × 10−7 | 0.76 | 1752 | 1.34 × 10−7 | 0.95 | 92.7 × 10−7 | 0.90 | 1951 |
| NaF | 147.7 | 10,193 | 2.68 × 10−7 | 0.79 | 34,328 | 2.17 × 10−7 | 0.95 | 2.48 × 10−7 | 0.95 | 513,000 |
| LiF | 184.1 | 6567 | 4.09 × 10−7 | 0.77 | 44,605 | 9.19 × 10−7 | 0.86 | 9.15 × 10−7 | 0.90 | 10,526 |
| Na2SiF6 | 170.5 | 19,472 | 4.68 × 10−7 | 0.76 | 275,000 | 2.63 × 10−7 | 0.91 | 1.02 × 10−7 | 0.90 | 142,000 |
| 24 h 0.1 M NaCl saturated Mg(OH)2 | ||||||||||
| WF | 206.6 | 2082 | 7.04 × 10−7 | 0.71 | 2082 | 1.98 × 10−7 | 0.92 | 73.9 × 10−7 | 0.9 | 2621 |
| NaF | 153.8 | 6310 | 4.93 × 10−7 | 0.74 | 21,584 | 1.54 × 10−7 | 0.88 | 3.39 × 10−7 | 0.92 | 128,000 |
| LiF | 181.6 | 3703 | 9.33 × 10−7 | 0.69 | 6164 | 3.69 × 10−7 | 0.90 | 0.014 × 10−7 | 0.97 | 6537 |
| Na2SiF6 | 161.5 | 20,578 | 5.84 × 10−7 | 0.72 | 19,444 | 1.61 × 10−7 | 0.95 | 4.21 × 10−7 | 0.95 | 35,353 |


3.3. Eudiometry
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PEO | Plasma Electrolytic Oxidation |
| WF | Without Fluoride |
References
- Reina, N.; Laffosse, J.M. Biomécanique de l’Os, Application Au Traitement et à La Consolidation Des Fractures. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 9, 1–17. [Google Scholar] [CrossRef]
- Uppal, G.; Thakur, A.; Chauhan, A.; Bala, S. Magnesium Based Implants for Functional Bone Tissue Regeneration—A Review. J. Magnes. Alloy. 2021, 10, 356–386. [Google Scholar] [CrossRef]
- King, J.F. Materials Perspective Magnesium: Commodity or Exotic? Mater. Sci. Technol. 2007, 23, 1–14. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and Advances in Magnesium Alloy Corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Taheri, M.; Kish, J.R.; Birbilis, N.; Danaie, M.; McNally, E.A.; McDermid, J.R. Towards a Physical Description for the Origin of Enhanced Catalytic Activity of Corroding Magnesium Surfaces. Electrochim. Acta 2014, 116, 396–403. [Google Scholar] [CrossRef]
- Asmussen, R.M.; Binns, W.J.; Partovi-Nia, R.; Jakupi, P.; Shoesmith, D.W. The Stability of Aluminum-Manganese Intermetallic Phases under the Microgalvanic Coupling Conditions Anticipated in Magnesium Alloys. Mater. Corros. 2016, 67, 39–50. [Google Scholar] [CrossRef]
- Ballerini, G.; Bardi, U.; Bignucolo, R.; Ceraolo, G. About Some Corrosion Mechanisms of AZ91D Magnesium Alloy. Corros. Sci. 2005, 47, 2173–2184. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma Electrolytic Oxidation of Magnesium and Its Alloys: Mechanism, Properties and Applications. J. Magnes. Alloy 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Acta Biomaterialia Biomedical Coatings on Magnesium Alloys—A Review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef]
- Roknian, M.; Fattah-alhosseini, A.; Gashti, S.O. Plasma Electrolytic Oxidation Coatings on Pure Ti Substrate: Effects of Na3PO4 Concentration on Morphology and Corrosion Behavior of Coatings in Ringer’s Physiological Solution. J. Mater. Eng. Perform. 2018, 27, 1343–1351. [Google Scholar] [CrossRef]
- Hussein, R. Plasma Process Control for Improved PEO Coatings on Magnesium Alloys. Ph.D. Thesis, University of Windsor, Windsor, ON, Canada, 2015. [Google Scholar]
- Matykina, E.; Garcia, I.; Arrabal, R.; Mohedano, M.; Mingo, B.; Sancho, J.; Merino, M.C.; Pardo, A. Role of PEO Coatings in Long-Term Biodegradation of a Mg Alloy. Appl. Surf. Sci. 2016, 389, 810–823. [Google Scholar] [CrossRef]
- Monetta, T.; Parnian, P.; Acquesta, A. Recent Advances in the Control of the Degradation Rate of PEO Treated Magnesium and Its Alloys for Biomedical Applications. Metals 2020, 10, 907. [Google Scholar] [CrossRef]
- Cui, X.; Lin, X.; Liu, C.; Yang, R.; Li, M. Microstructure and Properties of MAO Coatings for AZ91D Magnesium Alloy in Varies Work Mode. Mater. Sci. Forum 2013, 747–748, 178–183. [Google Scholar] [CrossRef]
- Liang, J.; Guo, B.; Tian, J.; Liu, H.; Zhou, J.; Xu, T. Effect of Potassium Fluoride in Electrolytic Solution on the Structure and Properties of Microarc Oxidation Coatings on Magnesium Alloy. Appl. Surf. Sci. 2005, 252, 345–351. [Google Scholar] [CrossRef]
- Soliman, H.; Hamdy, A.S. Effect of Fluoride Ions Modifier and Ceramic Al2O3 Particles Additives on Plasma Electrolytic Oxidation of AZ31. Surf. Eng. 2017, 33, 767–772. [Google Scholar] [CrossRef]
- Rogov, A.B.; Shayapov, V.R. The Role of Cathodic Current in PEO of Aluminum: Influence of Cationic Electrolyte Composition on the Transient Current-Voltage Curves and the Discharges Optical Emission Spectra. Appl. Surf. Sci. 2017, 394, 323–332. [Google Scholar] [CrossRef]
- Prince, L.; Noirfalise, X.; Paint, Y.; Olivier, M. Corrosion Mechanisms of AZ31 Magnesium Alloy: Importance of Starting PH and Its Evolution. Mater. Corros. 2022, 3, 1615–1630. [Google Scholar] [CrossRef]
- Sharma, S.; Sangal, S.; Mondal, K. On the Optical Microscopic Method for the Determination of Ball-on-Flat Surface Linearly Reciprocating Sliding Wear Volume. Wear 2013, 300, 82–89. [Google Scholar] [CrossRef]
- Rendenbach, C.; Fischer, H.; Kopp, A.; Schmidt-Bleek, K.; Kreiker, H.; Stumpp, S.; Thiele, M.; Duda, G.; Hanken, H.; Beck-Broichsitter, B.; et al. Improved in Vivo Osseointegration and Degradation Behavior of PEO Surface-Modified WE43 Magnesium Plates and Screws after 6 and 12 Months. Mater. Sci. Eng. C 2021, 129, 112380. [Google Scholar] [CrossRef]
- Ralls, A.M.; Daroonparvar, M.; Menezes, P.L. Spark Plasma Sintering of Mg-Based Alloys: Microstructure, Mechanical Properties, Corrosion Behavior, and Tribological Performance. J. Magnes. Alloy 2024, 12, 405–442. [Google Scholar] [CrossRef]
- Zhang, W.; Xin, S.; Huang, Q.; Jiao, H. Study on the Thermal Control Performance of Mg-Li Alloy Micro-Arc Oxidation Coating in High-Temperature Environments. Surfaces 2024, 7, 969–978. [Google Scholar] [CrossRef]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to Plasma Electrolytic Oxidation-an Overview of the Process and Applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- Pezzato, L.; Brunelli, K.; Gross, S.; Magrini, M.; Dabalà, M. Effect of Process Parameters of Plasma Electrolytic Oxidation on Microstructure and Corrosion Properties of Magnesium Alloys. J. Appl. Electrochem. 2014, 44, 867–879. [Google Scholar] [CrossRef]
- Lujun, Z.; Hongzhan, L.; Qingmei, M.; Jiangbo, L.; Zhengxian, L. The Mechanism for Tuning the Corrosion Resistance and Pore Density of Plasma Electrolytic Oxidation (PEO) Coatings on Mg Alloy with Fluoride Addition. J. Magnes. Alloy 2023, 11, 2823–2832. [Google Scholar] [CrossRef]
- Purniawan, A.; Faqih, M.A.A.; Wuryantoro, A.; Wicaksono, S.T.; Susanti, D.; Rasyida, A.; Ardhyananta, H. The Influence of Voltage and Time Variation on Plasma Electrolytic Oxidation (PEO) on the Morphology and Degradation Rate of AZ61 Magnesium Alloy. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5196304 (accessed on 24 March 2025).
- Taher, M.K.; Momoli, F.; Go, J.; Hagiwara, S.; Ramoju, S.; Hu, X.; Jensen, N.; Terrell, R.; Hemmerich, A.; Krewski, D. Systematic Review of Epidemiological and Toxicological Evidence on Health Effects of Fluoride in Drinking Water. Crit. Rev. Toxicol. 2024, 54, 2–34. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Yan, Z.; Wang, H.; Peng, J. Effect of Potassium Fluoride on Structure and Corrosion Resistance of Plasma Electrolytic Oxidation Films Formed on AZ31 Magnesium Alloy. J. Alloys Compd. 2009, 480, 469–474. [Google Scholar] [CrossRef]
- Hou, R.; Victoria-Hernandez, J.; Jiang, P.; Willumeit-Römer, R.; Luthringer-Feyerabend, B.; Yi, S.; Letzig, D.; Feyerabend, F. In Vitro Evaluation of the ZX11 Magnesium Alloy as Potential Bone Plate: Degradability and Mechanical Integrity. Acta Biomater. 2019, 97, 608–622. [Google Scholar] [CrossRef]
- Thanaa, T.T.; Fattah-alhosseini, A.; Alkaseem, M.; Kaseem, M. Improving the Surface Properties of Mg Based-Plasma Electrolytic Oxidation (PEO) Coatings under the Fluoride Electrolytes: A Review. Inorg. Chem. Commun. 2024, 170, 113163. [Google Scholar] [CrossRef]
- Castellanos, A.; Altube, A.; Vega, J.M.; García-Lecina, E.; Díez, J.A.; Grande, H.J. Effect of Different Post-Treatments on the Corrosion Resistance and Tribological Properties of AZ91D Magnesium Alloy Coated PEO. Surf. Coatings Technol. 2015, 278, 99–107. [Google Scholar] [CrossRef]
- Bordbar Khiabani, A.; Rahimi, S.; Yarmand, B.; Mozafari, M. Electrophoretic Deposition of Graphene Oxide on Plasma Electrolytic Oxidized-Magnesium Implants for Bone Tissue Engineering Applications. Mater. Today Proc. 2018, 5, 15603–15612. [Google Scholar] [CrossRef]
- Fu, L.; Yang, Y.; Zhang, L.; Wu, Y.; Liang, J.; Cao, B. Preparation and Characterization of Fluoride-Incorporated Plasma Electrolytic Oxidation Coatings on the AZ31 Magnesium Alloy. Coatings 2019, 9, 826. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information Lithium Fluoride. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lithium-fluoride (accessed on 12 April 2025).
- Information National Center for Biotechnology Sodium Fluoride. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-Fluoride (accessed on 11 April 2025).
- Yang, H.; Dong, Y.; Li, X.; Gao, Y.; He, W.; Liu, Y.; Mu, X.; Zhao, Y. Anti-Corrosion Superhydrophobic Micro-GF/Micro-TiB2/Nano-SiO2 Based Coating with Braid Strengthening Structure Fabricated by a Single-Step Spray Deposition. J. Alloys Compd. 2024, 1008, 176725. [Google Scholar] [CrossRef]
- Moreno, L.; Mohedano, M.; Arrabal, R.; Matykina, E. Screening of Fluoride-Free PEO Coatings on Cast Mg3Zn0.4Ca Alloy for Orthopaedic Implants. Surf. Coatings Technol. 2024, 476, 130184. [Google Scholar] [CrossRef]
- Peng, H.; Wang, W.; Jiang, H.; Zan, R.; Sun, Y.; Yu, S.; Ni, J.; Wang, W.; Wang, T.; Wang, J.; et al. Effect of Galvanic Corrosion on the Degradability of Biomedical Magnesium. Front. Mater. 2021, 8, 767179. [Google Scholar] [CrossRef]












| Bath | KOH (M) | Na2SiO3 (M) | NaF (M) | LiF (M) | Na2SiF6 (M) | pH | Conductivity (mS·cm−1) |
|---|---|---|---|---|---|---|---|
| Reference bath | |||||||
| Without fluoride (WF) | 0.15 | 0.086 | - | - | - | 13.77 | 43.33 |
| Study of fluorides | |||||||
| LiF | 0.15 | 0.086 | - | 0.046 | - | 13.79 | 45.40 |
| NaF | 0.15 | 0.086 | 0.046 | - | - | 13.78 | 43.78 |
| Na2SiF6 | 0.15 | 0.086 | - | - | 0.0077 | 13.78 | 43.40 |
| Average Pore Size | Pore Density | |||||||
|---|---|---|---|---|---|---|---|---|
| WF (µm) | NaF (µm) | LiF (µm) | Na2SiF6 (µm) | WF (%) | NaF (%) | LiF (%) | Na2SiF6 (%) | |
| 4 min PEO deposit | 1.97 ± 0.40 | 2.35 ± 0.38 | 3.05 ± 0.76 | 1.92 ± 1.27 | 87.8 | 75.0 | 93.9 | 88.0 |
| 30 min PEO deposit | 2.92 ± 0.92 | 2.50 ± 1.48 | 4.53 ± 2.95 | 2.80 ± 0.93 | 39.4 | 34.6 | 3.1 | 42.8 |
| WF (µm) | NaF (µm) | LiF (µm) | Na2SiF6 (µm) | |
|---|---|---|---|---|
| 4 min PEO deposit | 8.5 ± 0.8 | 7.1 ± 1.8 | 7.2 ± 0.8 | 5.0 ± 1.9 |
| 30 min PEO deposit | 11.8 ± 2.3 | 13.4 ± 2.7 | 25.5 ± 5.4 | 15.9 ± 2.8 |
| Atomic % Error +/− 0.6 | F | Mg | Si | O |
|---|---|---|---|---|
| After 4 min deposit | ||||
| NaF | 2.8 | 25.4 | 8.4 | 48.8 |
| LiF | 1.6 | 23.8 | 9.7 | 44.3 |
| Na2SiF6 | 2.5 | 23.7 | 9.7 | 47.2 |
| After 30 min deposit | ||||
| NaF | 6.1 | 36.5 | 4.8 | 43.8 |
| LiF | 5.0 | 34.6 | 6.0 | 42.7 |
| Na2SiF6 | 5.6 | 36.5 | 3.8 | 40.4 |
| Sample | Area | Mg (%) | O (%) | Si (%) | F (%) | C (%) | Al (%) | K (%) |
|---|---|---|---|---|---|---|---|---|
| NaF | 1 (Barrier) | 47.6 ± 0.5 | 31.0 ± 0.7 | 6.8 ± 0.3 | 5.5 ± 0.7 | 6.8 ± 0.4 | 1.8 ± 0.3 | 0.5 ± 0.2 |
| 2 (Outer) | 32.7 ± 0.4 | 44.3 ± 0.7 | 13.8 ± 0.2 | 1.9 ± 0.7 | 6.2 ± 0.2 | 0.9 ± 0.1 | 0.2 ± 0.1 | |
| LiF | 1 (Barrier) | 43.2 ± 0.5 | 35.1 ± 0.7 | 8.7 ± 0.3 | 5.4 ± 0.9 | 6.5 ± 0.4 | 1.4 ± 0.3 | 0.3 ± 0.1 |
| 2 (Outer) | 33.9 ± 0.4 | 43.9 ± 0.6 | 17.4 ± 0.3 | / | 3.6 ± 0.3 | 1.1 ± 0.2 | / | |
| Na2SiF6 | 1 (Barrier) | 45.5 ± 0.5 | 33.1 ± 0.8 | 5.4 ± 0.3 | 5.4 ± 0.9 | 7.0 ± 0.4 | 1.5 ± 0.2 | 0.8 ± 0.2 |
| 2 (Outer) | 36.0 ± 0.4 | 42.9 ± 0.7 | 15.2 ± 0.3 | 1.3 ± 0.4 | 3.5 ± 0.2 | 1.0 ± 0.1 | / |
| Sample | Rs (Ohm·cm2) | Router (Ohm·cm2) | Q0oxide(CPE outer) (sn/(Ohm·cm2)) | nouter (CPEoutide) (-) | Rinter (Ohm·cm2) | Q0inter (CPEinter) (sn/(Ohm·cm2)) | nd/in (CPEcor) (-) | Q0dl/in(CPEdl) (sn/(Ohm·cm2)) | ndl/in (CPEdl) (-) | Rct/in (Ohm·cm2) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 h PBS | ||||||||||
| NaF | 115 | 10,000 | 4.03 × 10−7 | 0.77 | 146,000 | 7.13 × 10−7 | 0.84 | 2.29 × 10−7 | 0.90 | 50,016 |
| LiF | 125 | 6275 | 3.58 × 10−7 | 0.79 | 8099 | 1.31 × 10−7 | 0.99 | 5.28 × 10−7 | 0.90 | 58,930 |
| Na2SiF6 | 123.3 | 7242 | 3.55 × 10−7 | 0.79 | 11,372 | 0.73 × 10−7 | 0.99 | 3.22 × 10−7 | 0.90 | 156,000 |
| 24 h PBS | ||||||||||
| NaF | 130.1 | 141.6 | 6.11 × 10−7 | 0.84 | 2489 | 11.0 × 10−7 | 0.85 | 7.49 × 10−7 | 0.89 | 45,672 |
| LiF | 131.4 | 374.3 | 32.5 × 10−7 | 0.87 | 23,763 | 21.4 × 10−7 | 0.88 | 164 × 10−7 | 0.90 | 7368 |
| Na2SiF6 | 119.7 | 237.3 | 49.1 × 10−7 | 0.89 | 9455 | 33.3 × 10−7 | 0.90 | 393 × 10−7 | 0.47 | 11,500 |
| Sample | Rs (Ohm·cm2) | Router (Ohm·cm2) | Q0outer(CPE outer) (sn/(Ohm·cm2)) | nouter (CPEouter) (-) | Rinter(Ohm·cm2) | Q0inter(CPEinter) (sn/(Ohm·cm2)) | ninter (CPEcor) (-) | Q0d/inl(CPEdl/in) (sn/(Ohm·cm2)) | nin (CPEdl) (-) | Rct/in (Ohm·cm2) |
|---|---|---|---|---|---|---|---|---|---|---|
| NaCl 0.1 M saturated in Mg(OH)2 | ||||||||||
| WF | 192.7 | 17,692 | 3.15 × 10−7 | 0.76 | 47,574 | 9.61 × 10−7 | 0.75 | 23.8 × 10−7 | 0.87 | 117,000 |
| NaF | 139.8 | 10,050 | 3.87 × 10−7 | 0.79 | 29,683 | 1.45 × 10−7 | 0.98 | 3.72 × 10−7 | 0.95 | 3,090,000 |
| LiF | 214.4 | 7535 | 2.19 × 10−7 | 0.93 | 45,569 | 2.17 × 10−7 | 0.93 | 2.39 × 10−7 | 0.98 | 407,000 |
| Na2SiF6 | 158.5 | 3972 | 5.59 × 10−7 | 0.71 | 4938 | 16.3 × 10−7 | 0.96 | 26.8 × 10−7 | 0.90 | 448,000 |
| PBS | ||||||||||
| WF | 116.9 | 210.1 | 15.9 × 10−7 | 0.84 | 79,275 | 18.4 × 10−7 | 0.84 | 376 × 10−7 | 0.64 | 37,610 |
| NaF | 121.2 | 499.6 | 7.93 × 10−7 | 0.76 | 5500 | 5.98 × 10−7 | 0.84 | 4.09 × 10−7 | 0.92 | 589,000 |
| LiF | 151.5 | 663.7 | 5.31 × 10−7 | 0.76 | 4940 | 6.12 × 10−7 | 0.84 | 6.59 × 10−7 | 0.90 | 727,000 |
| Na2SiF6 | 127.2 | 1047 | 38.8 × 10−7 | 0.76 | 3642 | 12.7 × 10−7 | 0.95 | 13.8 × 10−7 | 0.98 | 186,000 |
| P.E.% | NaF (%) | LiF (%) | Na2SiF6 (%) | WF (%) | |
|---|---|---|---|---|---|
| NaCl 0.1 M saturated in Mg(OH)2 | |||||
| 4 min deposit | 2 h | 99.26 | 91.96 | 98.59 | 24.51 |
| 4 min deposit | 24 h | 97.15 | 72.26 | 93.69 | 27.15 |
| 30 min deposit | 99.89 | 99.62 | 99.39 | 99.00 | |
| PBS | |||||
| 4 min deposit | 2 h | 99.75 | 99.42 | 99.77 | / |
| 4 min deposit | 24 h | 77.99 | 62.62 | 17.28 | / |
| 30 min deposit | 97.74 | 98.05 | 92.72 | 88.87 | |
| Sample | Corrosion Rate After a Period of 150 h (mm/year) | |
|---|---|---|
| 0.1 M NaCl saturated in Mg(OH)2 | PBS | |
| Bare AZ31 | 5.86 | 1.17 |
| WF | 1.41 | 0.94 |
| NaF | 0.23 | 0.47 |
| Na2SiF6 | 0.49 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tournay-Dufrenne, I.; Pasté, C.; Mégret, A.; Dangreau, L.; Olivier, M.-G. Effect of the Fluoride Species and Content of the PEO Electrolyte on the Corrosion Properties of the Layers Obtained on AZ31 for Biomedical Purposes. Coatings 2025, 15, 498. https://doi.org/10.3390/coatings15050498
Tournay-Dufrenne I, Pasté C, Mégret A, Dangreau L, Olivier M-G. Effect of the Fluoride Species and Content of the PEO Electrolyte on the Corrosion Properties of the Layers Obtained on AZ31 for Biomedical Purposes. Coatings. 2025; 15(5):498. https://doi.org/10.3390/coatings15050498
Chicago/Turabian StyleTournay-Dufrenne, Isis, Célia Pasté, Alexandre Mégret, Lisa Dangreau, and Marie-Georges Olivier. 2025. "Effect of the Fluoride Species and Content of the PEO Electrolyte on the Corrosion Properties of the Layers Obtained on AZ31 for Biomedical Purposes" Coatings 15, no. 5: 498. https://doi.org/10.3390/coatings15050498
APA StyleTournay-Dufrenne, I., Pasté, C., Mégret, A., Dangreau, L., & Olivier, M.-G. (2025). Effect of the Fluoride Species and Content of the PEO Electrolyte on the Corrosion Properties of the Layers Obtained on AZ31 for Biomedical Purposes. Coatings, 15(5), 498. https://doi.org/10.3390/coatings15050498







