Preparation of High-Entropy Silicide Coating on Tantalum Substrate by Silicon Infiltration Method and Its Antioxidant Performance
Abstract
1. Introduction
2. Experimental Details
2.1. Preparation of Precursor Powders
2.2. Preparation of Coating
2.3. Oxidation Test
3. Results and Discussion
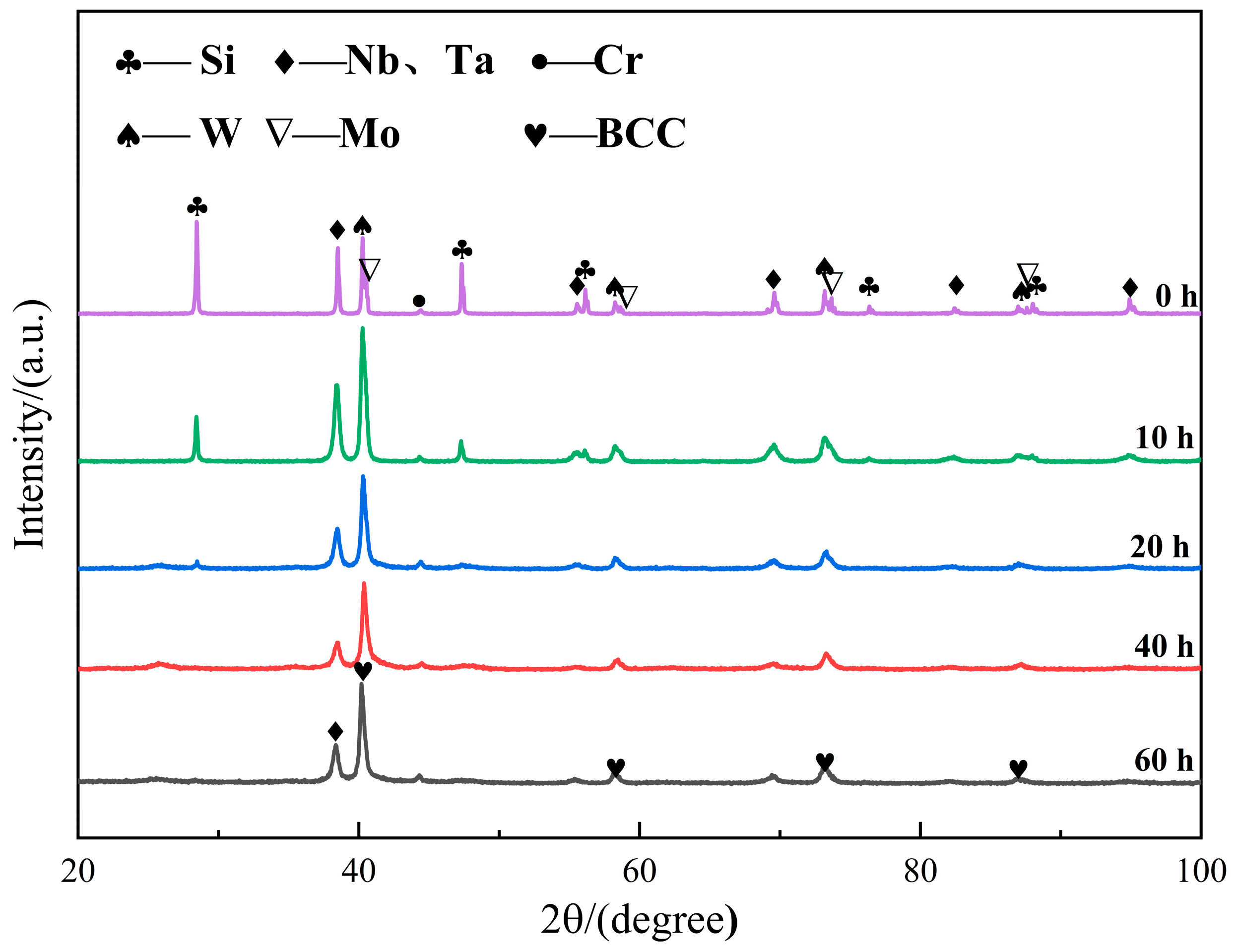
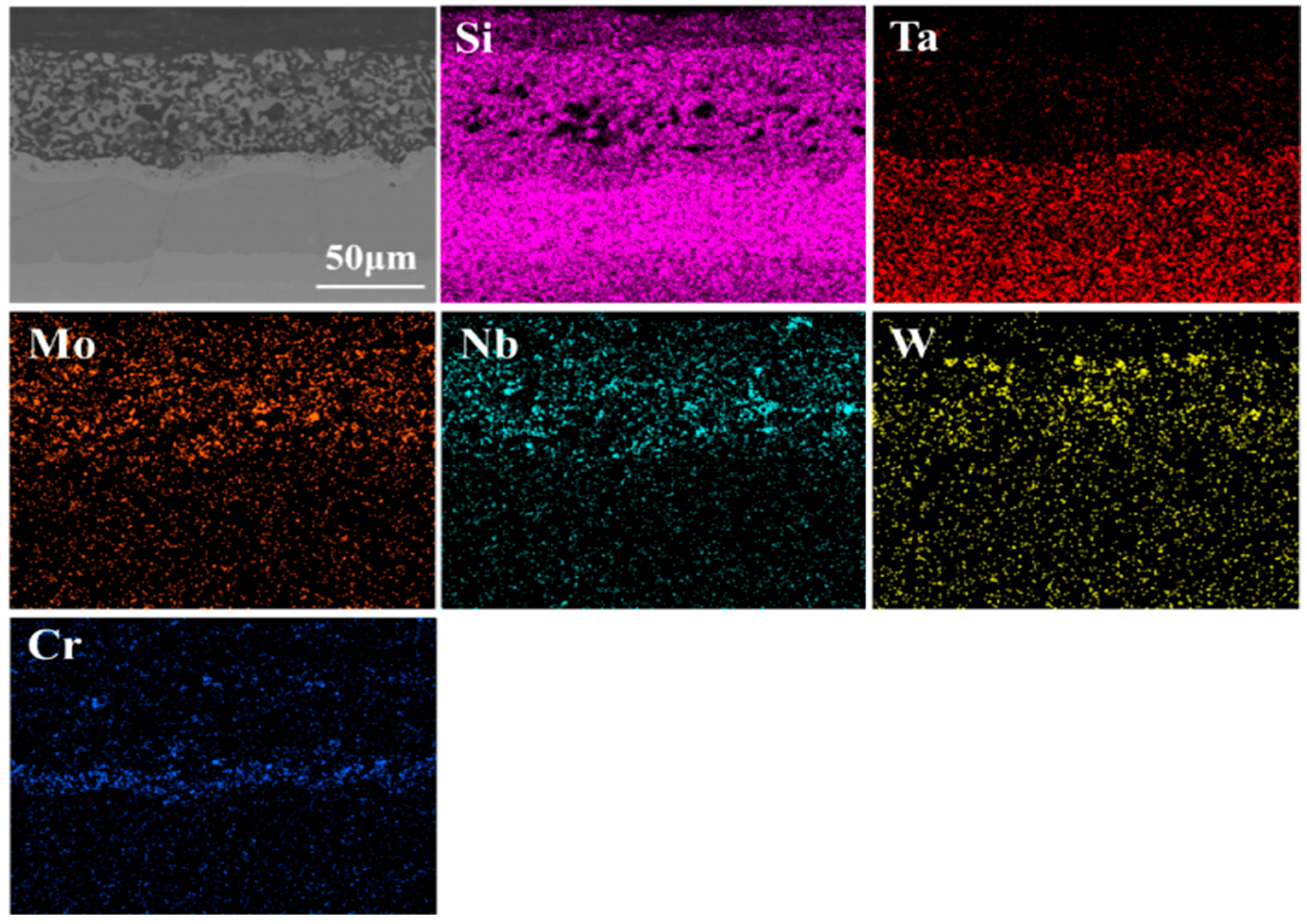
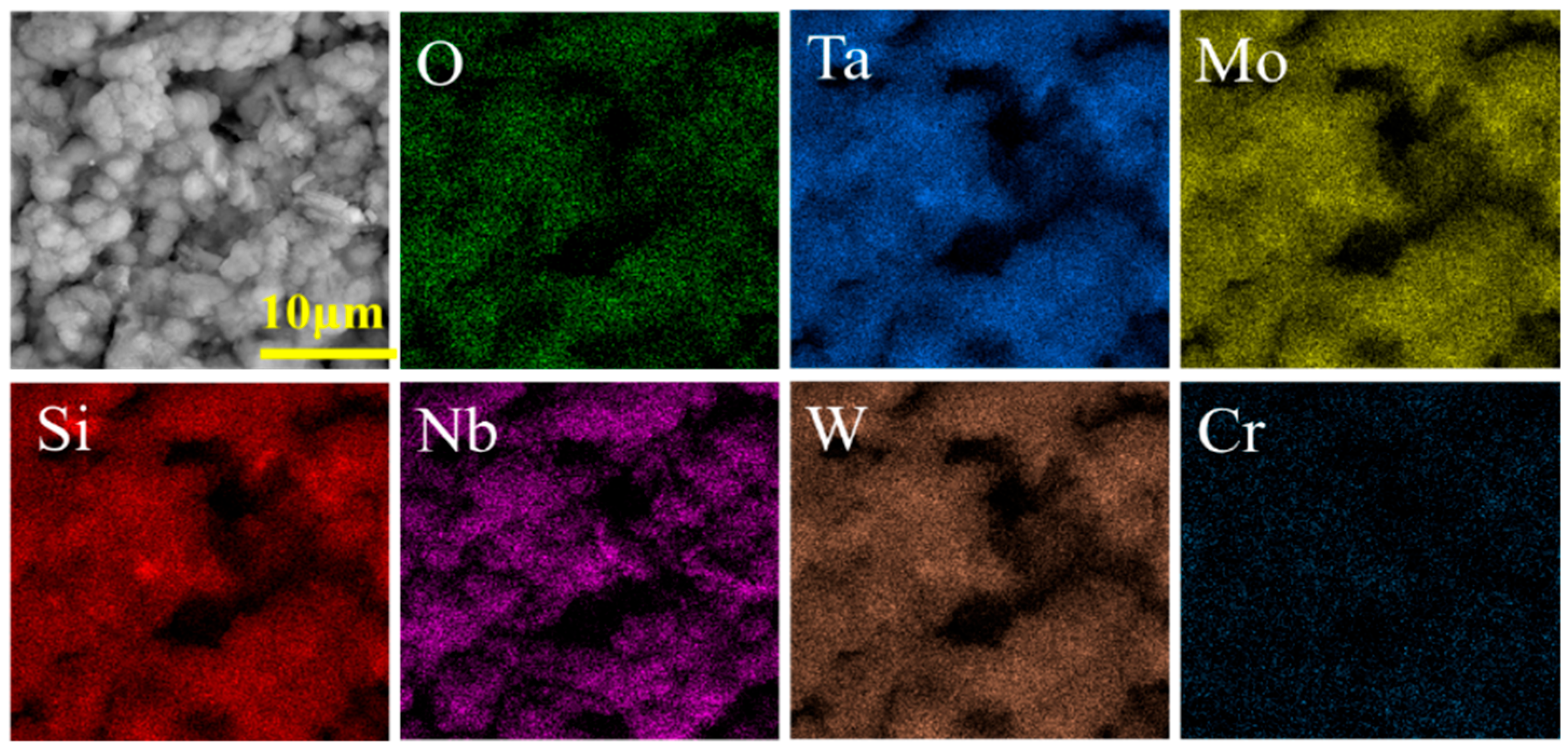
3.1. Microstructure and Composition of Coatings
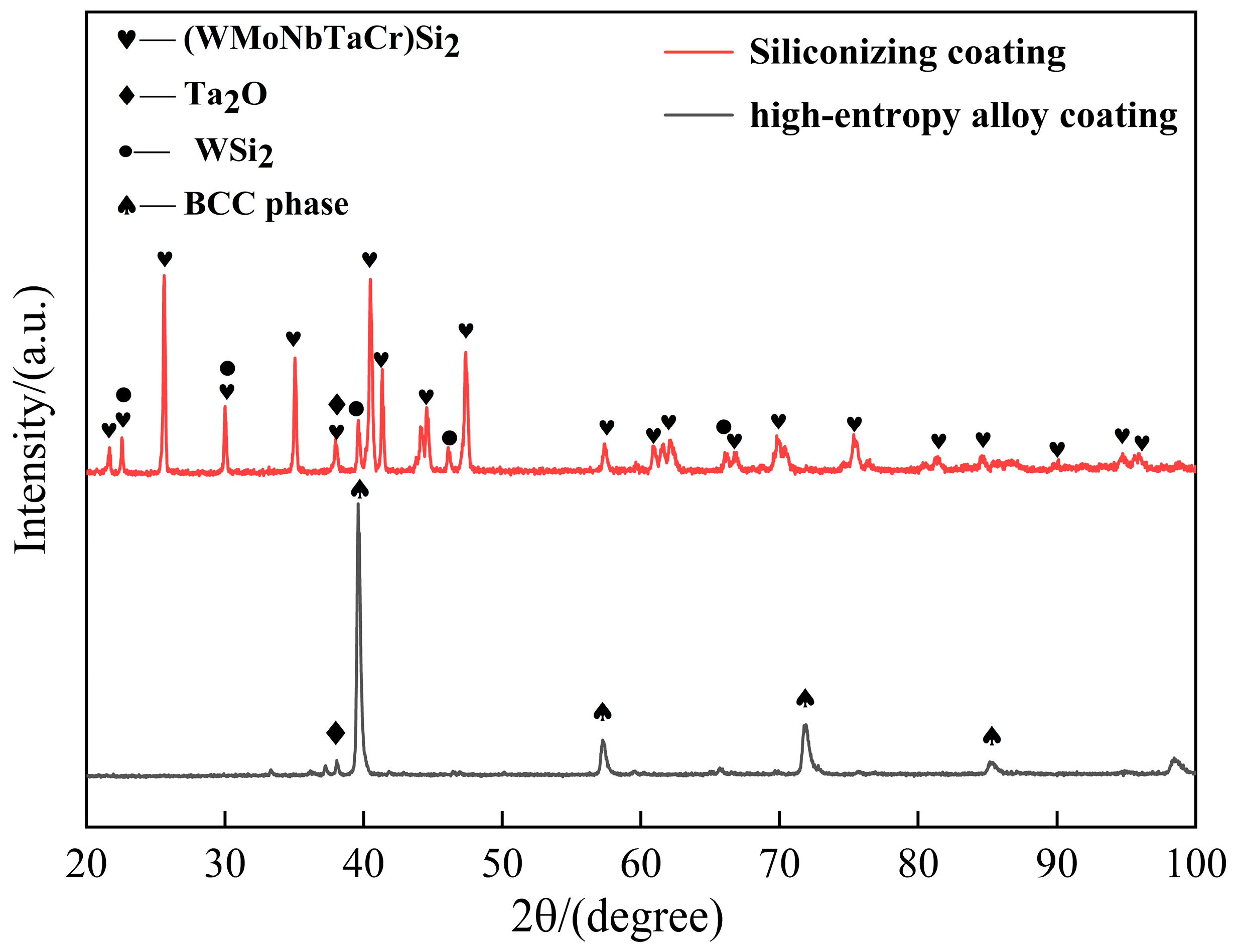
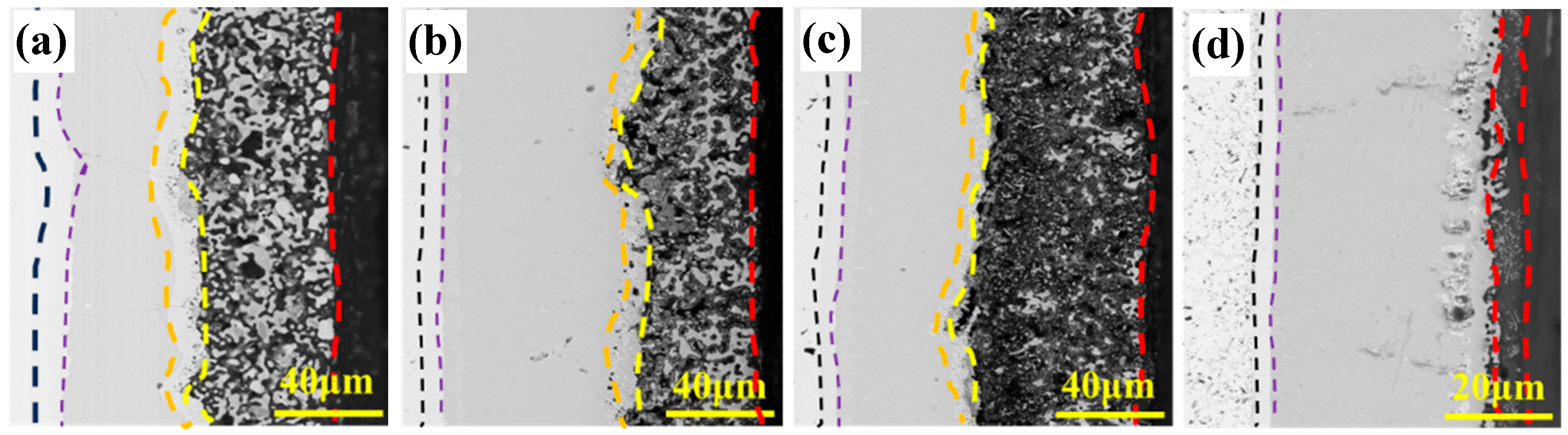
3.2. Oxidation Behavior of Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardonne, S.M.; Kumar, P.; Michaluk, C.A.; Schwartz, H.D. Tantalum and its alloys. Int. J. Refract. Met. Hard Mater. 1995, 13, 187–194. [Google Scholar] [CrossRef]
- Browning, P.N.; Alagic, S.; Carroll, B.; Kulkarni, A.; Matson, L.; Singh, J. Room and ultrahigh temperature mechanical properties of field assisted sintered tantalum alloys. Mater. Sci. Eng. A 2017, 680, 141–151. [Google Scholar] [CrossRef]
- Song, P.P.; Le, J.; Ye, F.; Sheng, X.C.; Zhang, X.W. Research on the Si-Ti High Temperature Oxidation-Resistant Coatings for Tantalum Alloys. Adv. Mater. Res. 2013, 785–786, 45–51. [Google Scholar] [CrossRef]
- Dong, Z.H.; Peng, X.; Wang, F.H. Oxidation of a ZrB2 coating fabricated on Ta–W alloy by electrophoretic deposition and laser melting. Mater. Lett. 2015, 148, 76–78. [Google Scholar] [CrossRef]
- Majumdar, S.; Sengupta, P.; Kale, G.B.; Sharma, I.G. Development of multilayer oxidation resistant coatings on niobium and tantalum. Surf. Coat. Technol. 2006, 200, 3713–3718. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Zhang, D.; Chen, X.; Huang, Y.; Peng, Y.; Xu, C.; Huang, S.; Pu, R.; Liu, S.; Zhao, X.; et al. A novel ultra-high-temperature oxidation protective MoSi2-TaSi2 ceramic coating for tantalum substrate. J. Eur. Ceram. Soc. 2019, 39, 2277–2286. [Google Scholar] [CrossRef]
- Yoon, J.K.; Kim, G.H.; Kim, H.S.; Shon, I.J.; Kim, J.S.; Doh, J.M. Microstructure and oxidation behavior of in situ formed TaSi2–Si3N4 nanocomposite coating grown on Ta substrate. Intermetallics 2008, 16, 1263–1272. [Google Scholar] [CrossRef]
- Li, S.; Xiao, L.; Liu, S.; Zhang, Y.; Xu, J.; Zhou, X.; Zhao, G.; Cai, Z.; Zhao, X. Ultra–high temperature oxidation resistance of a novel (Mo, Hf, W, Ti) Si2 ceramic coating with Nb interlayer on Ta substrate. J. Eur. Ceram. Soc. 2022, 42, 4866–4880. [Google Scholar] [CrossRef]
- Xiao, L.R.; Xiao, Y.X.; Zhao, X.J.; Zhou, X.J.; Zhao, G.; Zhong, Q.; Yu, H.; Wang, S.; Peng, Z.W.; Cai, Z.Y. The high temperature oxidation and thermal shock behavior of a dense WSi2-TaSi2 coating on Ta substrate prepared by a novel two-step process. Ceram. Int. 2023, 49, 26767–26777. [Google Scholar] [CrossRef]
- Meschter, P.J. Low-temperature oxidation of molybdenum disilicide. Metall. Trans. A 1992, 23, 1763–1772. [Google Scholar] [CrossRef]
- Xiang, H.; Xing, Y.; Dai, F.Z.; Wang, H.; Su, L.; Miao, L.; Zhang, G.; Wang, Y.; Qi, X.; Yao, L.; et al. High-entropy ceramics: Present status, challenges, and a look forward. J. Adv. Ceram. 2021, 10, 385–441. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Liu, D. Complete elimination of pest oxidation by high entropy refractory metallic silicide (Mo0.2W0.2Cr0.2Ta0.2Nb0.2)Si2. Scr. Mater. 2020, 189, 25–29. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Xiao, Z. Criteria of predicting phase formation for MSi2-Type High- Entropy refractory metal silicides. Mater. Des. 2023, 231, 112060. [Google Scholar] [CrossRef]
- Akrami, S.; Edalati, P.; Fuji, M.; Edalati, K. High-entropy ceramics: Review of principles, production and applications. Mater. Sci. Eng. R Rep. 2021, 146, 100644. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2025, 46, 2817–2829. [Google Scholar] [CrossRef]
- Wan, Y.X.; Liang, X.B.; Cheng, Y.H.; Liu, Y.N.; He, P.F.; Zhang, Z.B.; Mo, J.Y. Superior high-temperature strength in a dual-BCC-phase NbMoTaWHf refractory high-entropy alloy. Intermetallics 2024, 175, 108515. [Google Scholar] [CrossRef]
- Kuang, J.; Zhang, P.; Wang, Q.; Wang, Z.; Liang, X.; Shen, B. Formation and oxidation behavior of refractory high-entropy silicide (NbMoTaW)Si2 coating. Corros. Sci. J. Environ. Degrad. Mater. Its Control 2022, 198, 110134. [Google Scholar] [CrossRef]
- Bhowmik, A.; Stone, H.J. A study on the influence of Mo, Al and Si additions on the microstructure of annealed dual phase Cr-Ta alloys. J. Mater. Sci. 2013, 48, 3283–3293. [Google Scholar] [CrossRef]
- Tian, D.X.; Zhao, G.; Wang, D.Z.; Liu, X.L. Structure and properties of multicomp- onent silicide ceramic coatings on Ta substrate prepared by slurry sintering method. Ceram. Int. 2024, 50, 24725–24733. [Google Scholar] [CrossRef]
- Yi, G.; Ding, Y.; Cheng, Y.H.; Zhang, P.; Wang, X.; Liang, X.B. Development and oxidation behavior of high entropy silicide (NbMoTaWV)Si2 coatings on NbMoTaWV alloy. J. Alloys Compd. 2022, 916, 165384. [Google Scholar] [CrossRef]

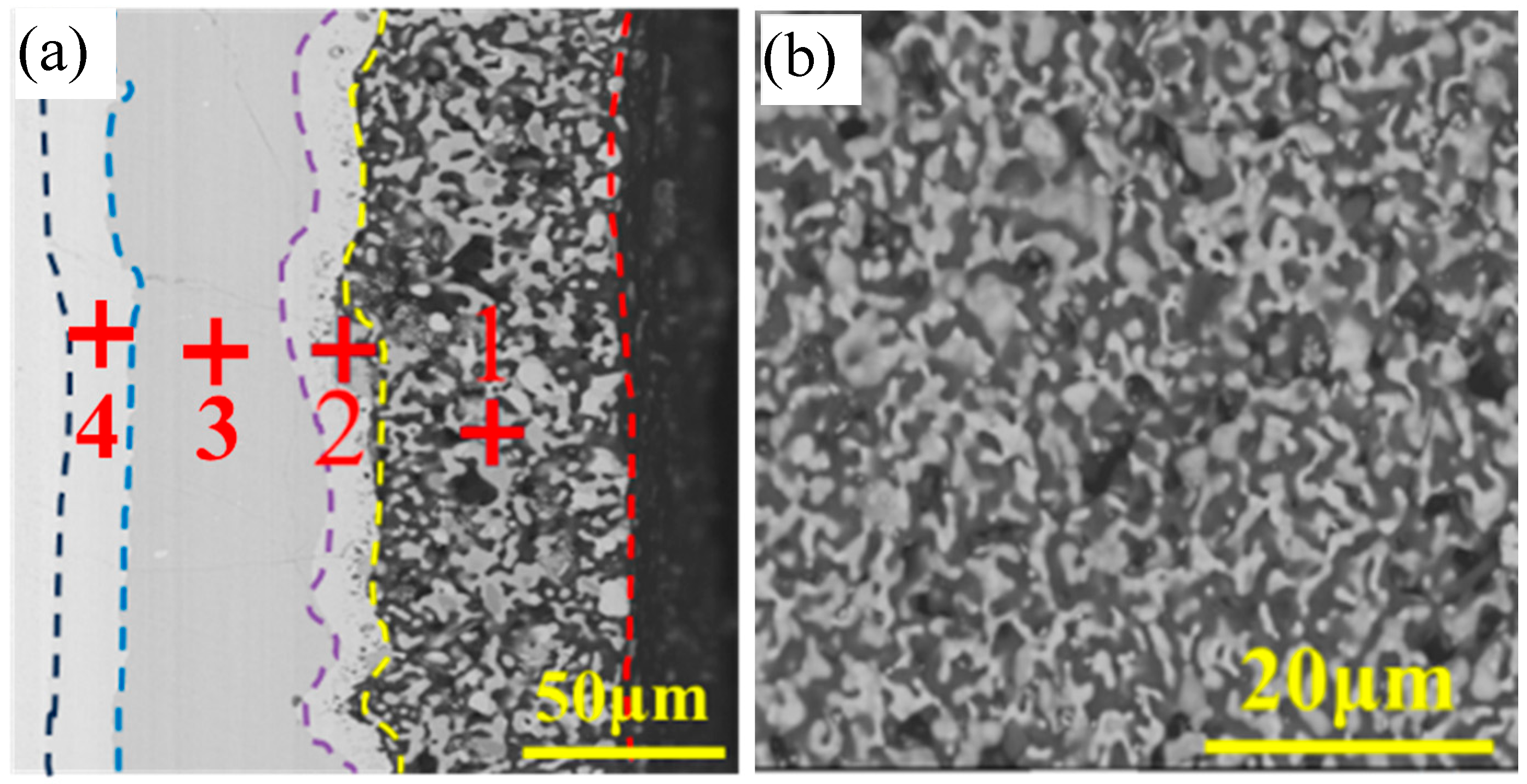





| Points | Composition (At%) | Possible Phase | ||||||
|---|---|---|---|---|---|---|---|---|
| Si | Cr | Nb | Mo | Ta | W | O | ||
| 1 | 69.4 | 3.5 | 6.5 | 4.4 | 6.5 | 5.7 | 4.0 | MeSi2 |
| 2 | 40.8 | 14.8 | 0.4 | 0.4 | 37.8 | 0.1 | 5.7 | low silicides of Cr and Ta |
| 3 | 65.4 | 0.3 | 0.0 | 0.3 | 30.6 | 0.0 | 3.4 | TaSi2 |
| 4 | 41.8 | 0.5 | 0.0 | 0.4 | 51.8 | 0.2 | 5.3 | Ta5Si3 |
| Composition (At%) | Possible Phase | |||||||
|---|---|---|---|---|---|---|---|---|
| Si | Cr | Nb | Mo | Ta | W | O | ||
| 1 | 56 | 0.1 | 5.2 | 4.4 | 16.6 | 5.7 | 12 | MeSi2 + Me5Si3 |
| 2 | 31.5 | 0 | 0.7 | 0.3 | 1.5 | 0.1 | 65.9 | SiO2 + Ta2O5 |
| 3 | 27.7 | 0 | 1.3 | 0.3 | 3.8 | 0.3 | 66.6 | SiO2 + Nb2O5 |
| 4 | 34.7 | 0.1 | 0.2 | 0.6 | 47.4 | 0.3 | 16.7 | TaSi2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Tian, D.; Mao, J.; Zhao, G.; Wang, D. Preparation of High-Entropy Silicide Coating on Tantalum Substrate by Silicon Infiltration Method and Its Antioxidant Performance. Coatings 2025, 15, 476. https://doi.org/10.3390/coatings15040476
Liu X, Tian D, Mao J, Zhao G, Wang D. Preparation of High-Entropy Silicide Coating on Tantalum Substrate by Silicon Infiltration Method and Its Antioxidant Performance. Coatings. 2025; 15(4):476. https://doi.org/10.3390/coatings15040476
Chicago/Turabian StyleLiu, Xinli, Dexiang Tian, Jiali Mao, Gang Zhao, and Dezhi Wang. 2025. "Preparation of High-Entropy Silicide Coating on Tantalum Substrate by Silicon Infiltration Method and Its Antioxidant Performance" Coatings 15, no. 4: 476. https://doi.org/10.3390/coatings15040476
APA StyleLiu, X., Tian, D., Mao, J., Zhao, G., & Wang, D. (2025). Preparation of High-Entropy Silicide Coating on Tantalum Substrate by Silicon Infiltration Method and Its Antioxidant Performance. Coatings, 15(4), 476. https://doi.org/10.3390/coatings15040476





