Dual Z-Scheme MoS2/g-C3N4/Bi2O3 Composite Coating on Carbon Fiber with Enhanced Photocatalytic Performance
Abstract
1. Introduction
2. Experimental Section
2.1. Preparation of Materials
2.1.1. Preparation of g-C3N4 Nanosheets
2.1.2. Preparation of MoS2
2.1.3. Preparation of g-C3N4/Bi2O3/CFb Composite Fiber Materials
2.1.4. Preparation of MoS2/g-C3N4/Bi2O3/CFb Composite Fiber Materials
2.2. Characterizations
2.3. Photocatalytic Tests
3. Results and Discussion
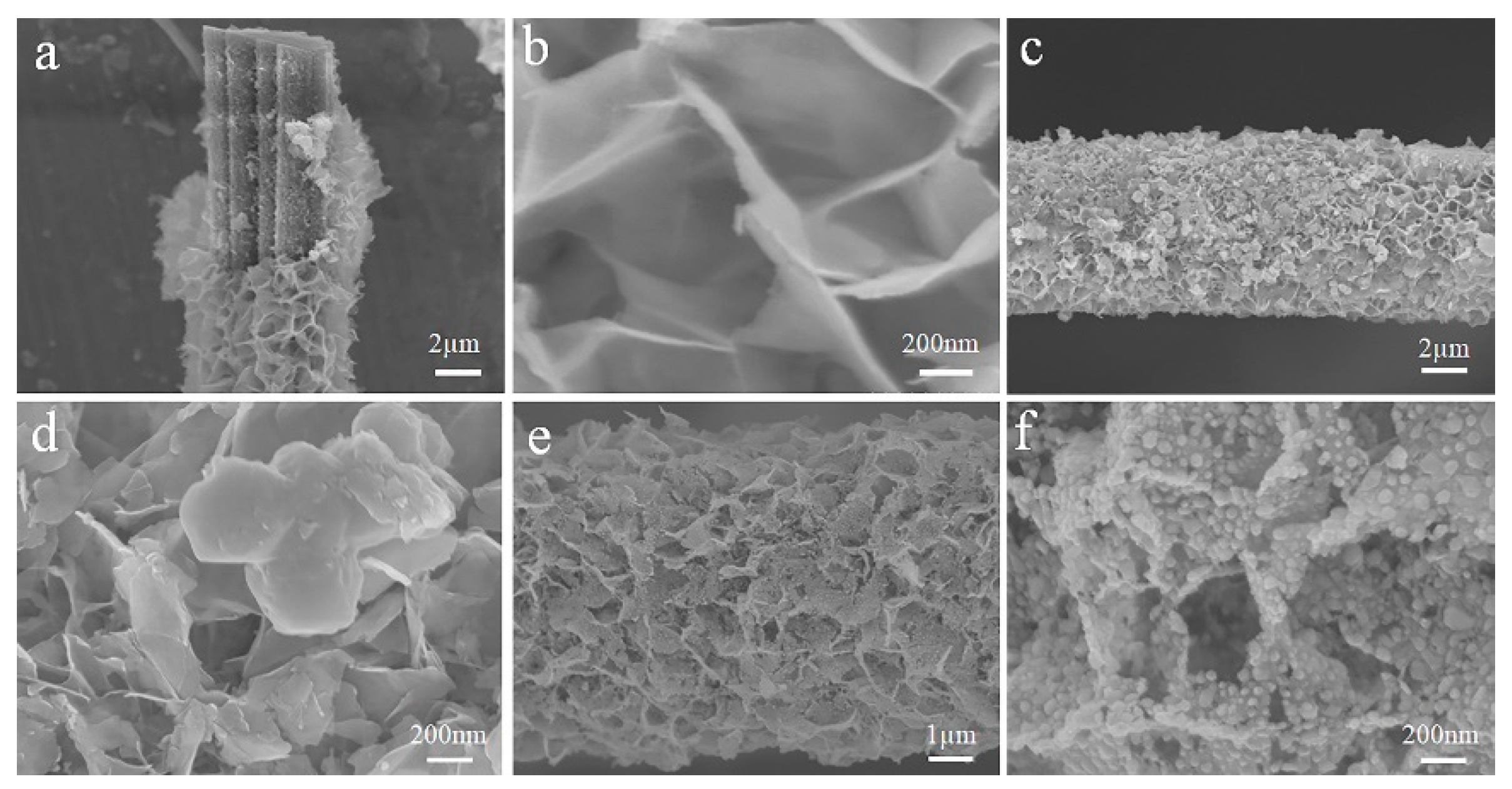
3.1. Analysis of Catalyst Morphology and Physical Structure
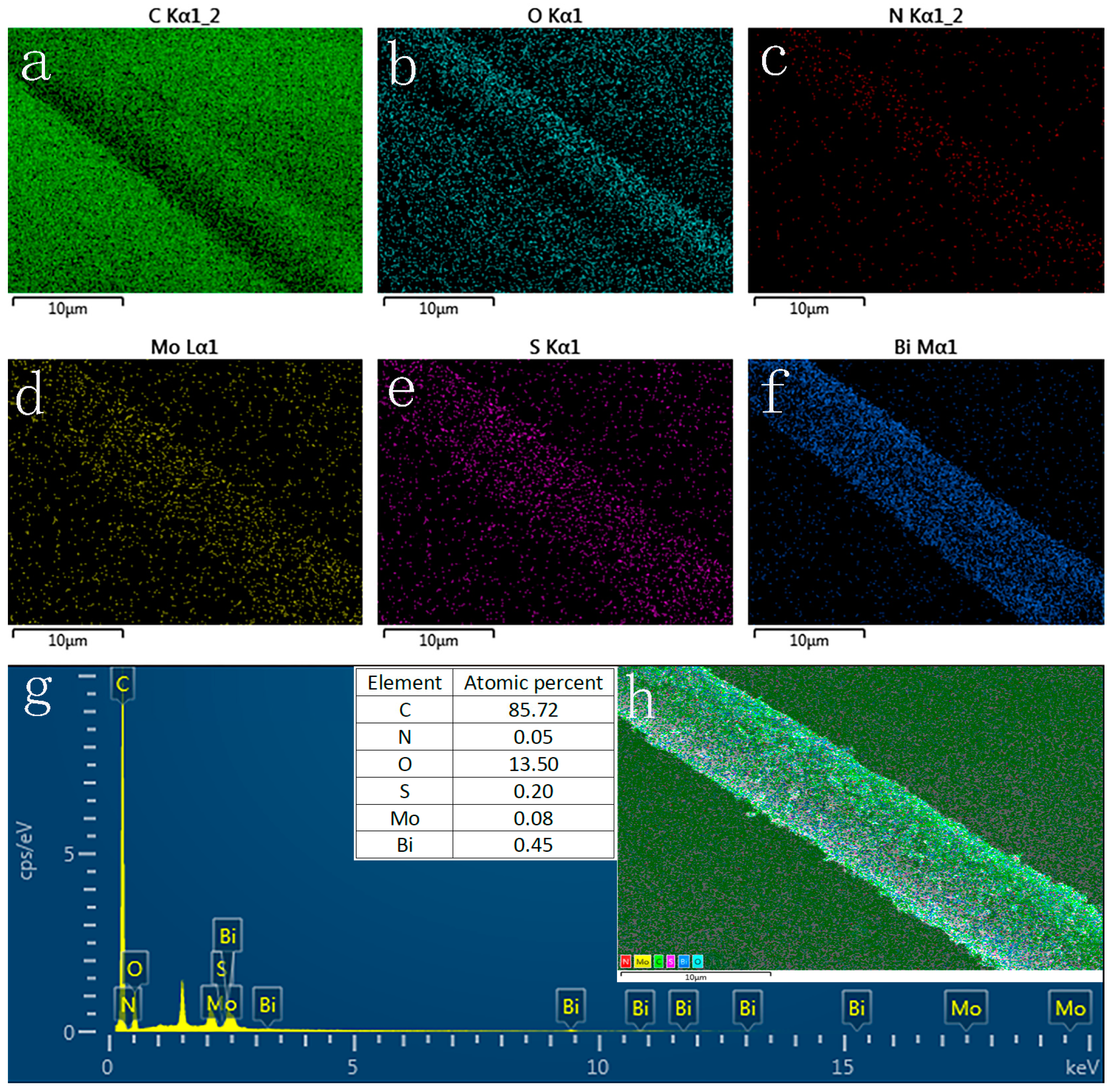
3.2. Surface and Valence Bond Analysis of Materials
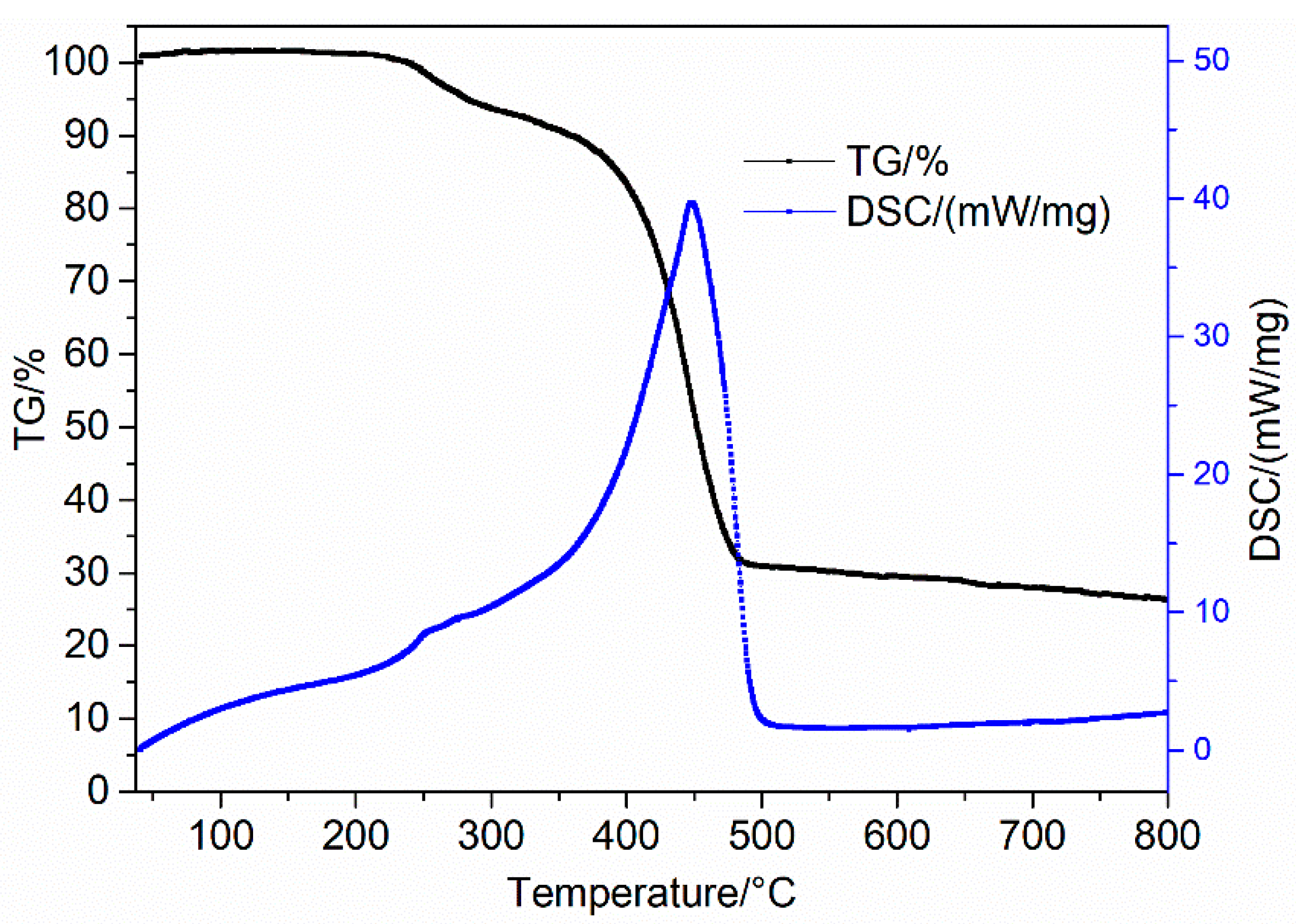
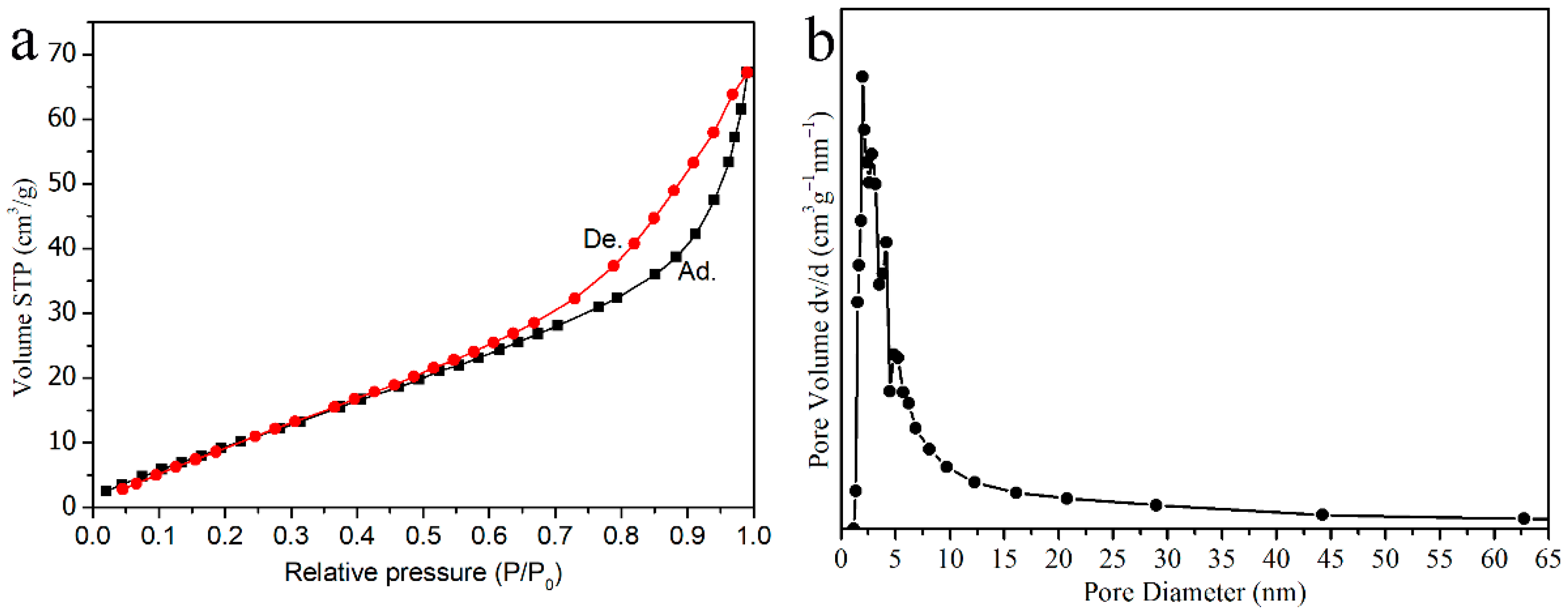
3.3. Thermogravimetric and Specific Surface Analysis of Materials
3.4. Results and Analysis of Degradation Experiments
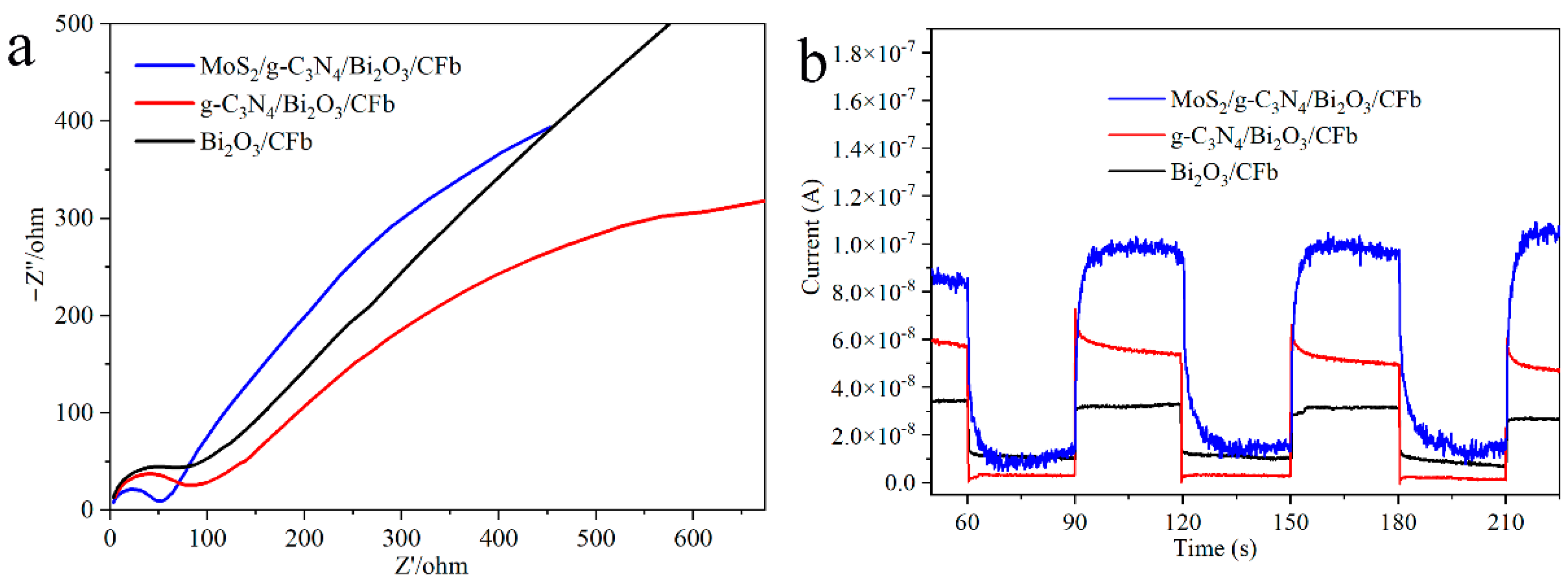
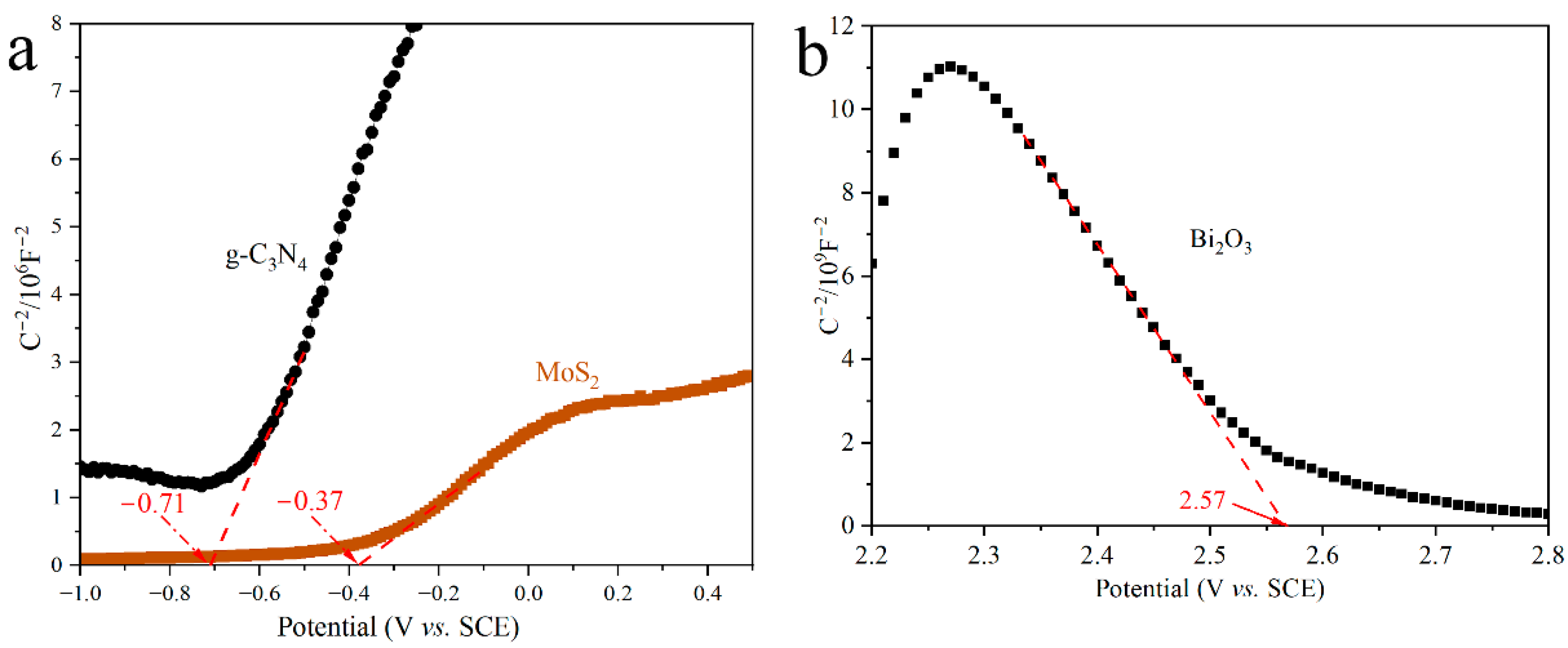
3.5. Optical and Photoelectrochemical Properties of Materials
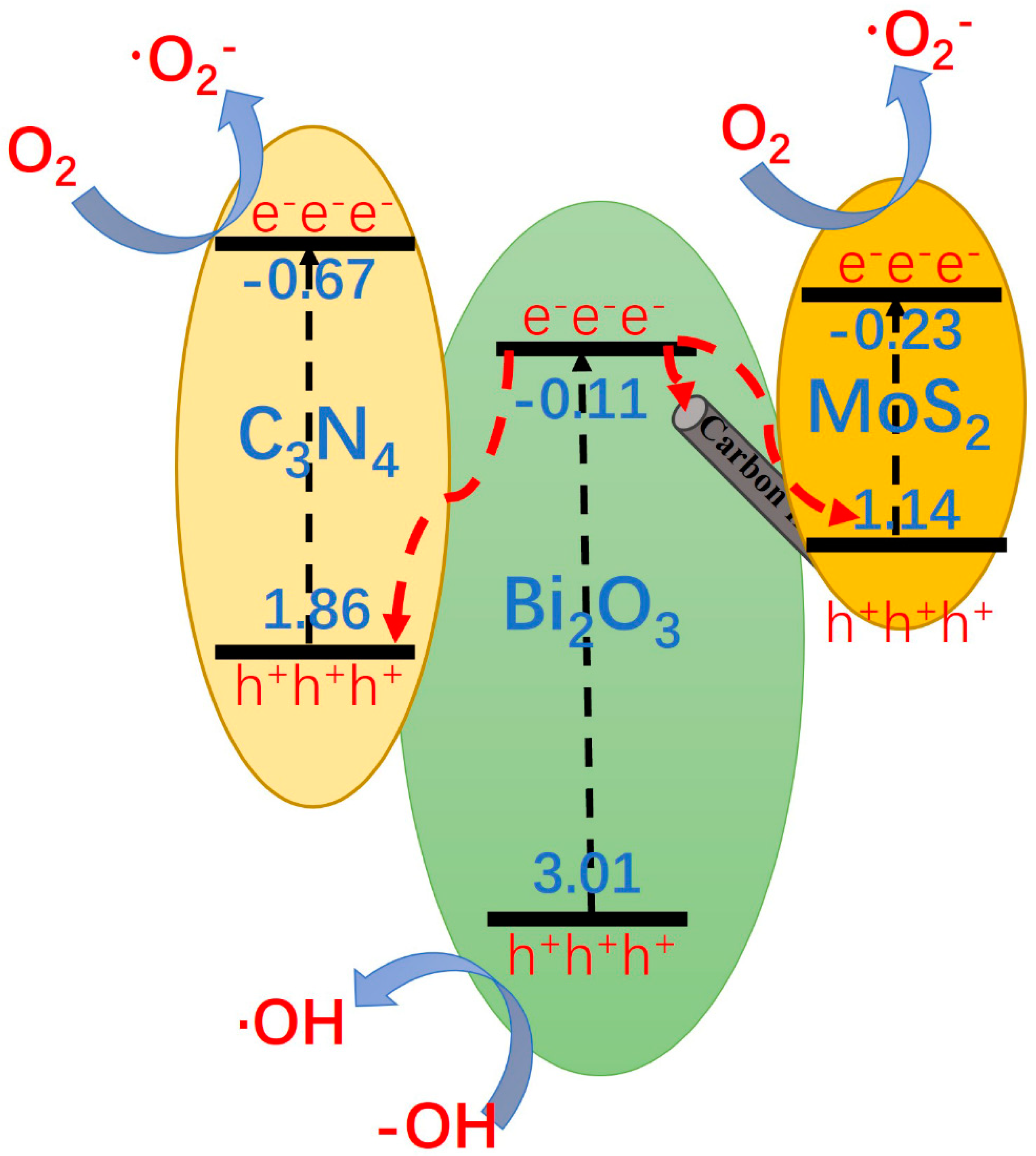
3.6. Analysis of Photocatalytic Degradation Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Messaoudi, N.; Miyah, Y.; Singh, N.; Gubernat, S.; Fatima, R.; Georgin, J.; El Mouden, A.; Saghir, S.; Knani, S.; Hwang, Y. A critical review of Allura Red removal from water: Advancements in adsorption and photocatalytic degradation technologies, and future perspectives. J. Environ. Chem. Eng. 2024, 12, 114843. [Google Scholar] [CrossRef]
- Wang, W.; Yang, R.; Li, T.; Komarneni, S.; Liu, B. Advances in recyclable and superior photocatalytic fibers: Material, construction, application and future perspective. Compos. Part B Eng. 2021, 205, 108512. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, M.-Q.; Liu, S.; Sun, Y.; Xu, Y.-J. Waltzing with the versatile platform of graphene to synthesize composite photocatalysts. Chem. Rev. 2015, 115, 10307–10377. [Google Scholar] [CrossRef]
- Zhang, P.; Yin, L.; Yang, X.; Wang, J.; Chi, M.; Qiu, J. Cotton-derived 3D carbon fiber aerogel to in situ support Bi2O3 nanoparticles as a separation-free photocatalyst for antibiotic removal. Carbon 2023, 201, 110–119. [Google Scholar] [CrossRef]
- Cao, N.; Gu, M.; Gao, M.; Li, C.; Liu, K.; Zhao, X.; Feng, J.; Ren, Y.; Wei, T. A three-layer photocatalyst carbon fibers/TiO2 seed/TiO2 nanorods with high photocatalytic degradation under visible light. Appl. Surf. Sci. 2020, 530, 147289. [Google Scholar] [CrossRef]
- Luo, Y.; Kim, K.-D.; Seo, H.O.; Kim, M.J.; Tai, W.S.; Lee, K.H.; Lim, D.C.; Kim, Y.D. Photocatalytic Decomposition of Toluene Vapor by Bare and TiO2-coated Carbon Fibers. Bull. Korean Chem. Soc. 2010, 31, 1661–1664. [Google Scholar] [CrossRef][Green Version]
- Yuan, R.S.; Guan, R.B.; Zheng, J.T. Effect of the pore size of TiO2-loaded activated carbon fiber on its photocatalytic activity. Scr. Mater. 2005, 52, 1329–1334. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, J. Carbon-doped Oxygen-deficient TiO2 Fibers Synthesized without Adding External Carbon Sources and Their Photocatalytic Activity. Chem. J. Chin. Univ. Chin. 2021, 42, 1284–1291. [Google Scholar] [CrossRef]
- Fu, Y.; Jin, X.; Ni, Q. Preparation and Characterization of Pd-TiO2 Photocatalytic Materials Supported by Carbon Fibers. Rare Met. Mater. Eng. 2010, 39, 1075–1078. [Google Scholar]
- Yang, Y.; Chen, H.; Zou, X.; Shi, X.-L.; Liu, W.-D.; Feng, L.; Suo, G.; Hou, X.; Ye, X.; Zhang, L.; et al. Flexible Carbon-Fiber/Semimetal Bi Nanosheet Arrays as Separable and Recyclable Plasmonic Photocatalysts and Photoelectrocatalysts. ACS Appl. Mater. Interfaces 2020, 12, 24845–24854. [Google Scholar] [CrossRef]
- Ma, J.; Chen, J.; Wang, B.; Cai, S. The in-situ growth of BiVO4 coatings on carbon fibers and their photocatalytic performance. Mater. Res. Bull. 2016, 77, 253–257. [Google Scholar] [CrossRef]
- Han, C.; Jing, M.-X.; Yang, H.; Shen, X.-Q.; Qiao, G.-J. An Overlapped Nano-Fe2O3/TiO2 Composite Coating on Activated Carbon Fiber Membrane with Enhanced Photocatalytic Performance. J. Nanosci. Nanotechnol. 2019, 19, 7123–7130. [Google Scholar] [CrossRef] [PubMed]
- Zahid, A.H.; Han, Q. A review on the preparation, microstructure, and photocatalytic performance of Bi2O3 in polymorphs. Nanoscale 2021, 13, 17687–17724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, Y.; Jiang, X.; Chen, S.; Meng, S.; Fu, X. Design of a direct Z-scheme photocatalyst: Preparation and characterization of Bi2O3/g-C3N4 with high visible light activity. J. Hazard. Mater. 2014, 280, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Peng, Y.; Yang, Y.; Chen, S.; Shang, J.; Yang, C.; Xu, M.; Bai, J.; Zhao, Z.; Hu, X. Visible-light-driven photocatalytic for degrading tetracycline wastewater by BiOI/Bi2O3 Z-scheme heterojunction. J. Environ. Chem. Eng. 2024, 12, 114395. [Google Scholar] [CrossRef]
- Wang, X.; Du, C.; Yu, Y.; Li, W.; Li, T.; Wang, S.; Mao, S.; Wang, Y.; Zhao, J.; Xiong, C. Efficient photocatalytic degradation with a lattice-matched α-Bi2O3/Co3O4 Z-scheme heterojunction: An integrated experimental and DFT study. J. Water Process Eng. 2024, 65, 105829. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, S.; Zhao, Y.; Deng, Y.; Yang, W.; Ye, Y.; Wang, K. Construction of Z-scheme Bi2O3/CeO2 heterojunction for enhanced photocatalytic capacity of TiO2 NTs. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 304, 123405. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; Huang, B.; Chen, Y.; Li, X.; Chen, L.; Dan, Y. Bi2O3@MoS2 heterojunction for enhanced photoelectrocatalytic hydrogen evolution. J. Electroanal. Chem. 2024, 957, 118127. [Google Scholar] [CrossRef]
- Fu, J.; Cao, S.; Yu, J. Dual Z-scheme charge transfer in TiO2–Ag–Cu2O composite for enhanced photocatalytic hydrogen generation. J. Mater. 2015, 1, 124–133. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Kumari, A.; Guo, C.; Naushad, M.; Vo, D.-V.N.; Iqbal, J.; Stadler, F.J. Construction of dual Z-scheme g-C3N4/Bi4Ti3O12/Bi4O5I2 heterojunction for visible and solar powered coupled photocatalytic antibiotic degradation and hydrogen production: Boosting via I−/I3− and Bi3+/Bi5+ redox mediators. Appl. Catal. B Environ. 2021, 284, 119808. [Google Scholar] [CrossRef]
- Li, X.; Sun, H.; Xie, Y.; Liang, Y.; Gong, X.; Qin, P.; Jiang, L.; Guo, J.; Liu, C.; Wu, Z. Principles, synthesis and applications of dual Z-scheme photocatalysts. Coord. Chem. Rev. 2022, 467, 214596. [Google Scholar] [CrossRef]
- Malefane, M.E.; Mafa, P.J.; Managa, M.; Nkambule, T.T.; Kuvarega, A.T. Understanding the principles and applications of dual Z-scheme heterojunctions: How far can we go? J. Phys. Chem. Lett. 2023, 14, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Jiang, T.; Han, Y.; Okoth, O.K.; Cheng, L.; Zhang, J. Construction of dual Z-scheme Bi2S3/Bi2O3/WO3 ternary film with enhanced visible light photoelectrocatalytic performance. Appl. Surf. Sci. 2020, 505, 144632. [Google Scholar] [CrossRef]
- Wang, Q.; Li, N.; Tan, M.; Deng, M.; Yang, G.; Li, Q.; Du, H. Novel dual Z-scheme Bi/BiOI-Bi2O3-C3N4 heterojunctions with synergistic boosted photocatalytic degradation of phenol. Sep. Purif. Technol. 2023, 307, 122733. [Google Scholar] [CrossRef]
- Pan, J.; Wang, P.; Chen, Z.; Yu, Q.; Wang, P.; Zhu, M.; Zhao, W.; Wang, J.; Zheng, Y.; Li, C. The Pt-free 1T/2H-MoS2/CdS/MnOx hollow core-shell nanocomposites toward overall water splitting via HER/OER synergy of 1T-MoS2/MnOx. Mater. Today Chem. 2021, 21, 100528. [Google Scholar] [CrossRef]
- Ou, W.; Pan, J.; Liu, Y.; Li, S.; Li, H.; Zhao, W.; Wang, J.; Song, C.; Zheng, Y.; Li, C. Two-dimensional ultrathin MoS2-modified black Ti3+–TiO2 nanotubes for enhanced photocatalytic water splitting hydrogen production. J. Energy Chem. 2020, 43, 188–194. [Google Scholar] [CrossRef]
- Zhao, T.; Yang, Z.; Liu, Y.; Li, Y.; Zeng, K.; Zhao, C.; Yu, J.; Cai, L.; Zhang, R. Novel Bi2S3/Bi2WO6 nanomaterials with 2D/3D spatial structure stably degrade veterinary antibiotics under visible light. J. Phys. Chem. Solids 2021, 155, 110106. [Google Scholar] [CrossRef]
- Mishra, B.P.; Babu, P.; Parida, K. Phosphorous, boron and sulfur doped g-C3N4 nanosheet: Synthesis, characterization, and comparative study towards photocatalytic hydrogen generation. Mater. Today Proc. 2021, 35, 258–262. [Google Scholar] [CrossRef]
- Song, B.; Wang, T.; Wang, L.; Liu, H.; Mai, X.; Wang, X.; Wang, N.; Huang, Y.; Ma, Y.; Lu, Y. Interfacially reinforced carbon fiber/epoxy composite laminates via in-situ synthesized graphitic carbon nitride (g-C3N4). Compos. Part B Eng. 2019, 158, 259–268. [Google Scholar] [CrossRef]
- Thanh Ngoc, N.; Vinh Van, T.; Vu Khac Hoang, B.; Kim, M.; Park, D.; Hur, J.; Kim, I.T.; Lee, H.U.; Ko, S.; Lee, Y.-C. A Novel Photocatalyst Composite of Magnesium Aminoclay and TiO2 Immobilized into Activated Carbon Fiber (ACF) Matrix for Pollutant Removal. J. Nanosci. Nanotechnol. 2020, 20, 6844–6849. [Google Scholar] [CrossRef]
- Song, B.; Wang, T.; Sun, H.; Liu, H.; Mai, X.; Wang, X.; Wang, L.; Wang, N.; Huang, Y.; Guo, Z. Graphitic carbon nitride (g-C3N4) interfacially strengthened carbon fiber epoxy composites. Compos. Sci. Technol. 2018, 167, 515–521. [Google Scholar] [CrossRef]
- Shen, X.; Song, B.; Shen, X.; Shen, C.; Shan, S.; Xue, Q.; Chen, X.; Li, S. Rationally designed S-scheme heterojunction of C3N4/Bi2MoO6/carbon fiber cloth as a recyclable, macroscopic and efficient photocatalyst for wastewater treatment. Chem. Eng. J. 2022, 445, 136703. [Google Scholar] [CrossRef]
- Kumari, P.; Ghosh, S.K.; Perla, V.K.; Saha, C.; Singh, H.; Mallick, K. Nanostructured bismuth phosphate-based asymmetric supercapacitor: Electrochemical evaluation and oscillator application. J. Mater. Sci. Mater. Electron. 2024, 35, 1882. [Google Scholar] [CrossRef]
- Jia, Y.; Li, S.; Gao, J.; Zhu, G.; Zhang, F.; Shi, X.; Huang, Y.; Liu, C. Highly efficient (BiO)2CO3-BiO2-x-graphene photocatalysts: Z-Scheme photocatalytic mechanism for their enhanced photocatalytic removal of NO. Appl. Catal. B Environ. 2019, 240, 241–252. [Google Scholar] [CrossRef]
- Li, X.; He, C.; Zuo, S.; Yan, X.; Dai, D.; Zhang, Y.; Yao, C. Photocatalytic nitrogen fixation over fluoride/attapulgite nanocomposite: Effect of upconversion and fluorine vacancy. Sol. Energy 2019, 191, 251–262. [Google Scholar] [CrossRef]
- Gong, H.; Zhang, X.; Wang, G.; Liu, Y.; Li, Y.; Jin, Z. Dodecahedron ZIF-67 anchoring ZnCdS particles for photocatalytic hydrogen evolution. Mol. Catal. 2020, 485, 110832. [Google Scholar] [CrossRef]
- He, R.; Zhou, J.; Fu, H.; Zhang, S.; Jiang, C. Room-temperature in situ fabrication of Bi2O3/g-C3N4 direct Z-scheme photocatalyst with enhanced photocatalytic activity. Appl. Surf. Sci. 2018, 430, 273–282. [Google Scholar] [CrossRef]
- Ranjith, R.; Vignesh, S.; Balachandar, R.; Suganthi, S.; Raj, V.; Ramasundaram, S.; Sundar, J.K.; Shkir, M.; Oh, T.H. Construction of novel g-C3N4 coupled efficient Bi2O3 nanoparticles for improved Z-scheme photocatalytic removal of environmental wastewater contaminant: Insight mechanism. J. Environ. Manag. 2023, 330, 117134. [Google Scholar] [CrossRef]
- Lu, S.; Wu, T.; Liu, Y.; Luo, H.; Jiang, F.; Nie, X.; Chen, H. All-solid Z-scheme Bi/γ-Bi2O3/O-doped g-C3N4 heterojunction with Bi as electron shuttle for visible-light photocatalysis. J. Alloys Compd. 2022, 911, 164980. [Google Scholar] [CrossRef]
- Kumar, R.; Sudhaik, A.; Kumar, D.; Devi, R.; Devi, E.; Chawla, A.; Raizada, P.; Hussain, C.M.; Ahamad, T.; Singh, P. Synergistic photocatalytic activity of Bi2O3/g-C3N4/ZnO ternary heterojunction with dual Z-scheme charge transfer towards textile dye degradation. J. Ind. Eng. Chem. 2025, 144, 575–584. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, X.; Shu, S.; Zhang, R.; Chen, J.; Wang, Y. Constructing a Z-scheme (3-Bi2O3/g-C3N4 heterojunction with enhancing photocatalytic activity for sulfamethoxypyridazine degradation. Mater. Sci. Semicond. Process. 2025, 189, 109273. [Google Scholar] [CrossRef]
- Goud, B.S.; Koyyada, G.; Jung, J.H.; Reddy, G.R.; Shim, J.; Nam, N.D.; Vattikuti, S.P. Surface oxygen vacancy facilitated Z-scheme MoS2/Bi2O3 heterojunction for enhanced visible-light driven photocatalysis-pollutant degradation and hydrogen production. Int. J. Hydrogen Energy 2020, 45, 18961–18975. [Google Scholar] [CrossRef]
- Ji, R.; Ma, C.; Ma, W.; Liu, Y.; Zhu, Z.; Yan, Y. Z-scheme MoS2/Bi2O3 heterojunctions: Enhanced photocatalytic degradation performance and mechanistic insight. New J. Chem. 2019, 43, 11876–11886. [Google Scholar] [CrossRef]
- Qureshi, M.T.; Mushtaq, A.; Farooq, U.; Basit, A.; Iqbal, T.; Younas, A.; Al Elaimi, M.; Affan, H.; Othman, M.S.; Yunus, G. Facile Strategies to Fabricate MoS2/Bi2O3 Nanocomposites; A Nano Engineered Material for Photoassisted Degradation of Ciprofloxacin and Their Antibacterial Performance. J. Inorg. Organomet. Polym. Mater. 2024, 1–16. [Google Scholar] [CrossRef]
- Li, C.X.; Song, S.Y.; Shi, D.S.; Yhig, Y.; Zhang, X.X.; Wei, J.Q.; Hong, B.; Xu, J.C.; Jin, H.X.; Wang, P.F.; et al. Improved photocatalytic activity of Z-scheme β-Bi2O3/Bi2WO6 nanocomposites from band bending of p-n heterojunction. J. Nanopart. Res. 2020, 22, 123. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, J.; Pan, J.; Zhou, B.; Li, C. Dual Z-Scheme MoS2/g-C3N4/Bi2O3 Composite Coating on Carbon Fiber with Enhanced Photocatalytic Performance. Coatings 2025, 15, 447. https://doi.org/10.3390/coatings15040447
Niu J, Pan J, Zhou B, Li C. Dual Z-Scheme MoS2/g-C3N4/Bi2O3 Composite Coating on Carbon Fiber with Enhanced Photocatalytic Performance. Coatings. 2025; 15(4):447. https://doi.org/10.3390/coatings15040447
Chicago/Turabian StyleNiu, Jiantao, Jiaqi Pan, Bin Zhou, and Chaorong Li. 2025. "Dual Z-Scheme MoS2/g-C3N4/Bi2O3 Composite Coating on Carbon Fiber with Enhanced Photocatalytic Performance" Coatings 15, no. 4: 447. https://doi.org/10.3390/coatings15040447
APA StyleNiu, J., Pan, J., Zhou, B., & Li, C. (2025). Dual Z-Scheme MoS2/g-C3N4/Bi2O3 Composite Coating on Carbon Fiber with Enhanced Photocatalytic Performance. Coatings, 15(4), 447. https://doi.org/10.3390/coatings15040447





