Nanoengineering of Ultrathin Carbon-Coated T-Nb2O5 Nanosheets for High-Performance Lithium Storage
Abstract
1. Introduction
2. Results and Discussion
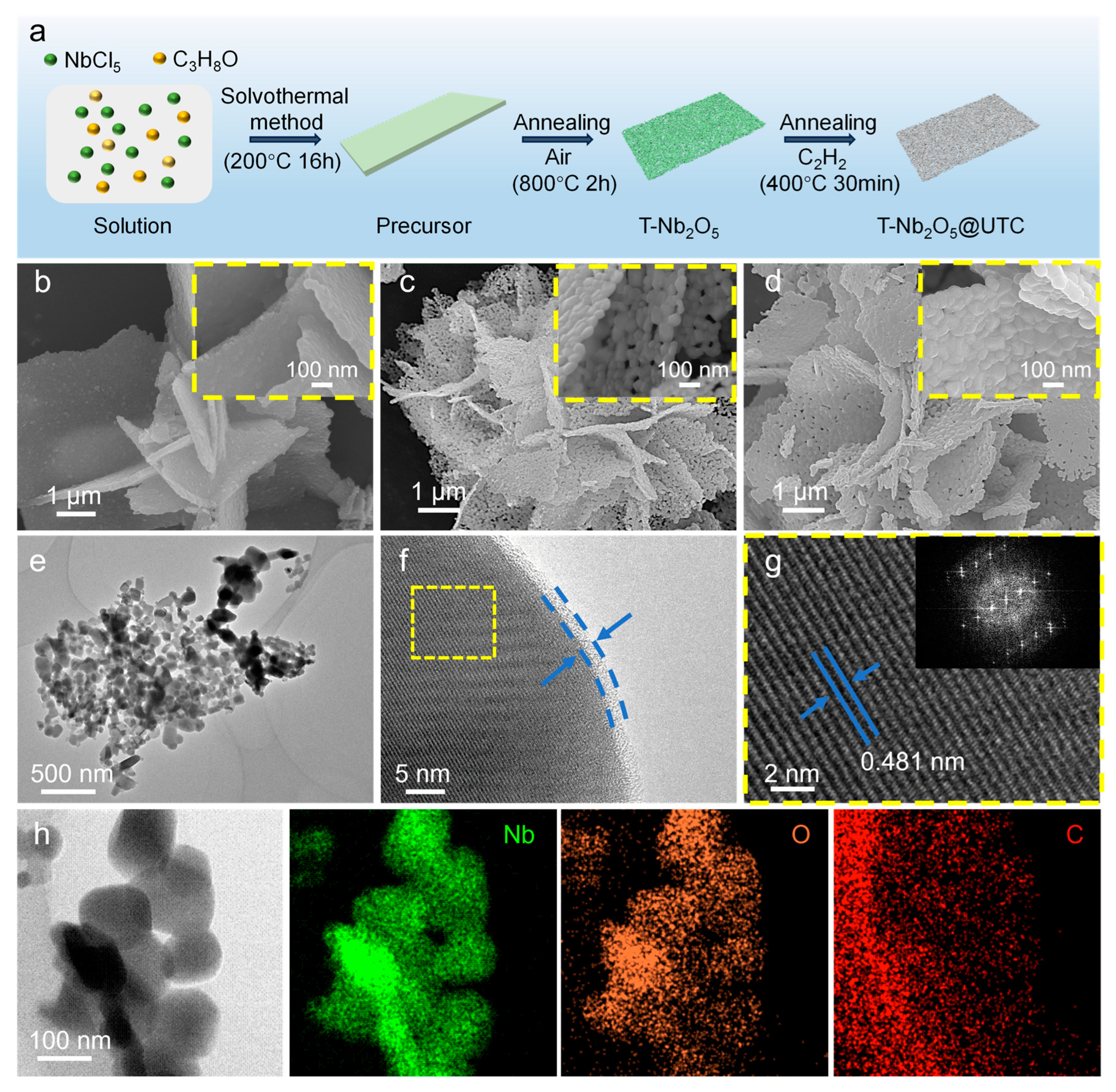
2.1. Preparation and Structural Characterization of T-Nb2O5@UTC
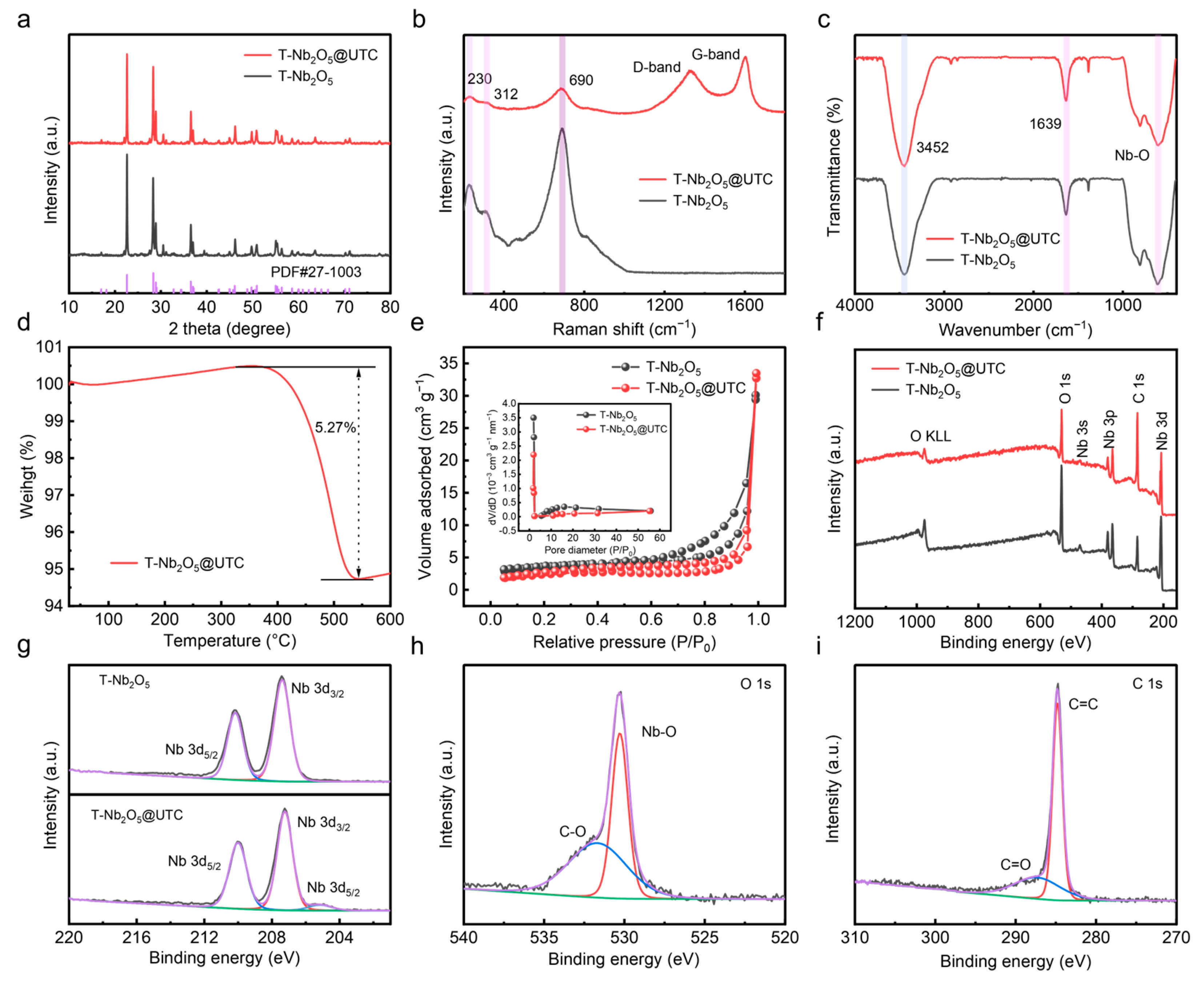
2.2. Crystal Structure and Vibrational Analysis of T-Nb2O5 and T-Nb2O5@UTC
2.3. Carbon Content and Surface Area Analysis of T-Nb2O5 and T-Nb2O5@UTC
2.4. Elemental Composition and Chemical State Analysis by XPS
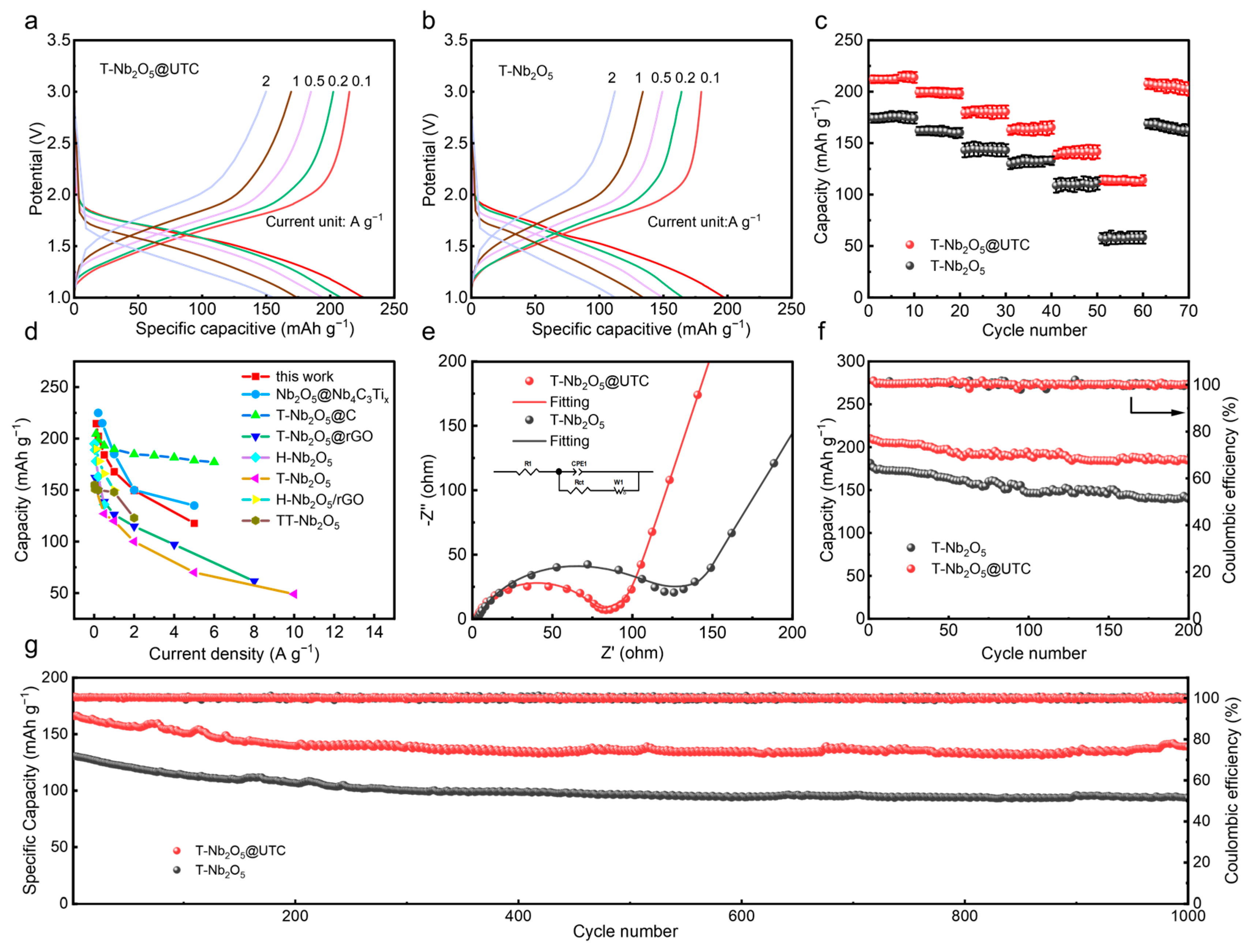
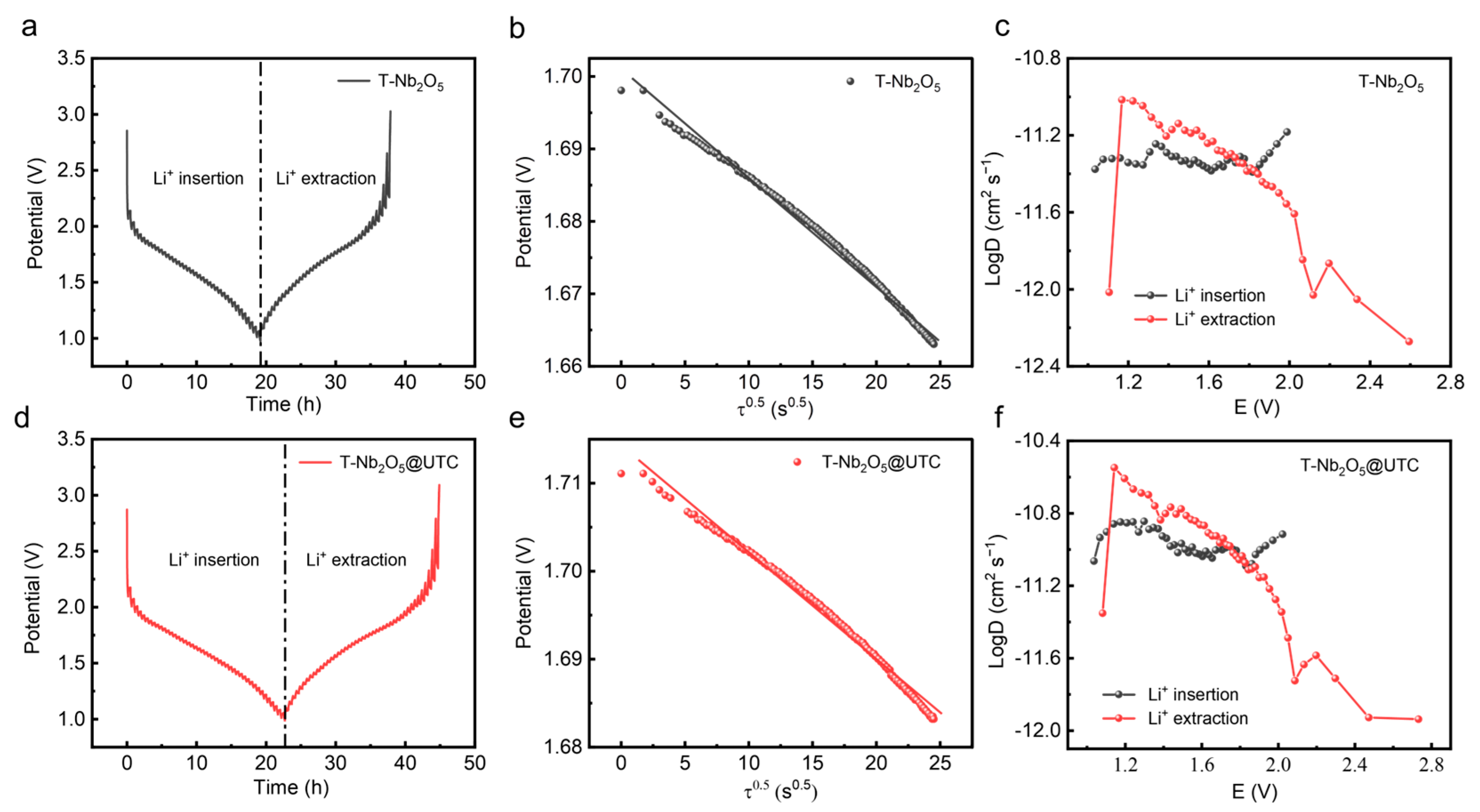
2.5. Electrochemical Performance Test
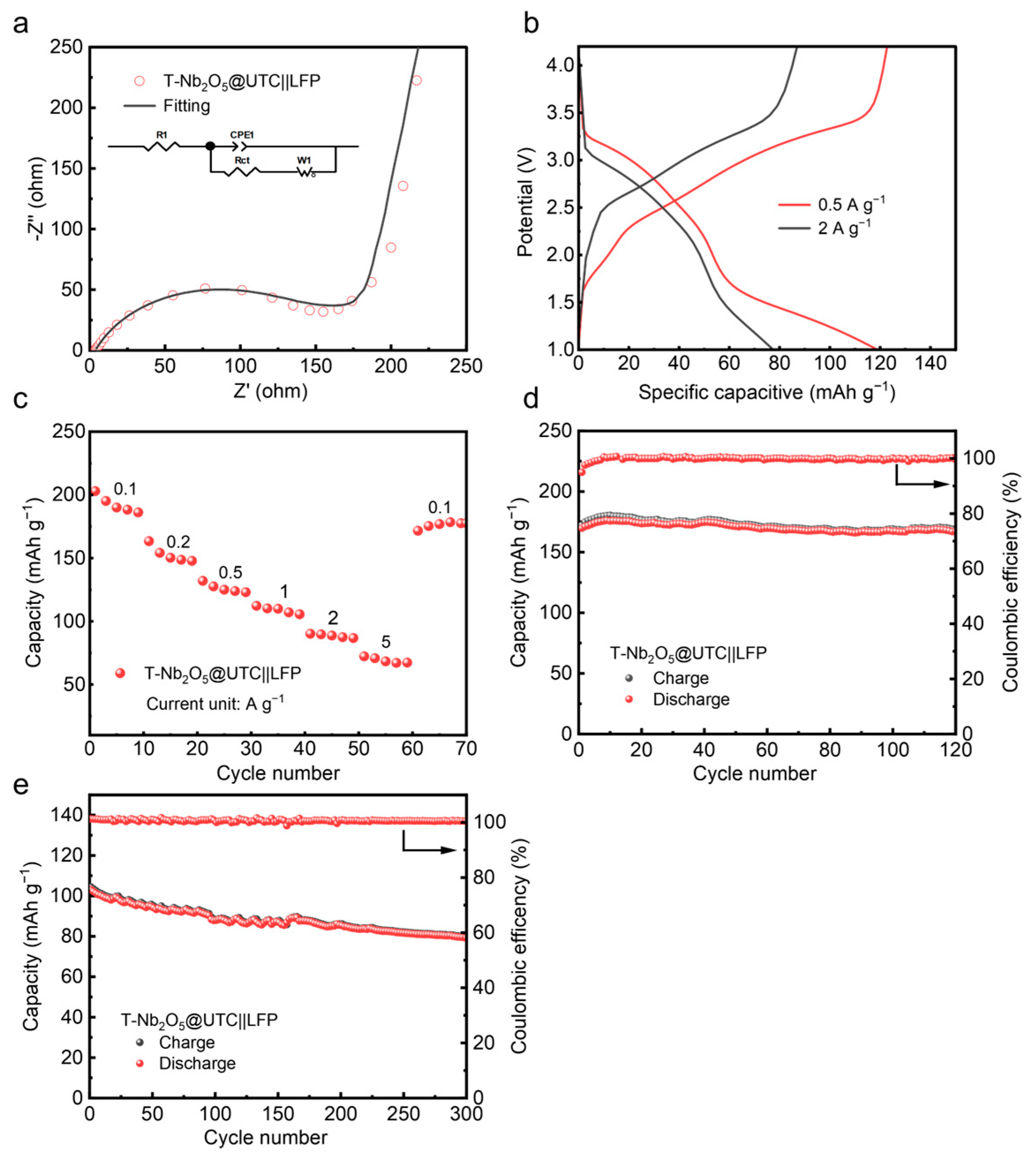
3. Electrochemical Performance of Full Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 2019, 4, 540–550. [Google Scholar] [CrossRef]
- Li, S.; Wang, K.; Zhang, G.; Li, S.; Xu, Y.; Zhang, X.; Zhang, X.; Zheng, S.; Sun, X.; Ma, Y. Fast Charging Anode Materials for Lithium-Ion Batteries: Current Status and Perspectives. Adv. Funct. Mater. 2022, 32, 2200796. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Xia, Y. Recent Progress in Polyanionic Anode Materials for Li (Na)-Ion Batteries. Electrochem. Energy Rev. 2021, 4, 447–472. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Wang, Q.; Wang, Q.; Zhu, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J.; Zhang, Q. High-Capacity and Kinetically Accelerated Lithium Storage in MoO3 Enabled by Oxygen Vacancies and Heterostructure. Adv. Energy Mater. 2021, 11, 2101712. [Google Scholar] [CrossRef]
- Tang, Z.; Zhou, S.; Huang, Y.; Wang, H.; Zhang, R.; Wang, Q.; Sun, D.; Tang, Y.; Wang, H. Improving the Initial Coulombic Efficiency of Carbonaceous Materials for Li/Na-Ion Batteries: Origins, Solutions, and Perspectives. Electrochem. Energy Rev. 2023, 6, 8. [Google Scholar] [CrossRef]
- Wang, L.; Huang, F.; Zhu, G.; Dai, Z. Nb2O5 nanocrystals decorated graphene composites as anode materials for high-performance dual-ion batteries. Nano Res. 2024, 17, 1535–1541. [Google Scholar] [CrossRef]
- Wang, X.; Bai, Y.; He, R.; Yao, F.; Chang, L.; Nie, P. In situ construction T-Nb2O5 nanolayer on porous carbon cloth as Binder-Free anode for Lithium-Ion battery with long cycle life. Appl. Surf. Sci. 2024, 670, 160635. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Liu, R.; Li, X.; Wu, Y.; Ran, F. “Fast-Charging” Anode Materials for Lithium-Ion Batteries from Perspective of Ion Diffusion in Crystal Structure. ACS Nano 2024, 18, 2611–2648. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, S.; Cao, X.; Deng, S.; Pan, G.; Liu, Q.; Wang, X.; Xia, X.; Tu, J. Popcorn-like niobium oxide with cloned hierarchical architecture as advanced anode for solid-state lithium ion batteries. Energy Storage Mater. 2020, 25, 695–701. [Google Scholar] [CrossRef]
- Griffith, K.J.; Forse, A.C.; Griffin, J.M.; Grey, C.P. High-Rate Intercalation without Nanostructuring in Metastable Nb2O5 Bronze Phases. J. Am. Chem. Soc. 2016, 138, 8888–8899. [Google Scholar] [CrossRef]
- Han, H.; Jacquet, Q.; Jiang, Z.; Sayed, F.N.; Jeon, J.-C.; Sharma, A.; Schankler, A.M.; Kakekhani, A.; Meyerheim, H.L.; Park, J.; et al. Li iontronics in single-crystalline T-Nb2O5 thin films with vertical ionic transport channels. Nat. Mater. 2023, 22, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- She, L.; Liu, D.; Zhao, Y.; Dong, L.; Wu, Z.; Xue, X.; Tian, Y.; Du, W.; Zheng, C.; He, S.; et al. Advances on Defect Engineering of Niobium Pentoxide for Electrochemical Energy Storage. Small 2025, 21, 2410211. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Zhang, B.; Wang, Y.; Liu, X.; Yu, C.; Xu, T.; Mofarah, S.S.; Yu, Y.; Liu, Y.; Sun, H.; et al. Nanoscale niobium oxides anode for electrochemical lithium and sodium storage: A review of recent improvements. J. Nanostruct. Chem. 2021, 11, 33–68. [Google Scholar] [CrossRef]
- Ding, X.; Meng, F.; Zhou, Q.; Li, X.; Kuai, H.; Xiong, X. Complementary niobium-based heterostructure for ultrafast and durable lithium storage. Nano Energy 2024, 121, 109188. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, K.; Wang, L.; Chen, S.; Li, C. Pillaring Electronic Nano-Wires to Slice T-Nb2O5 Laminated Particles for Durable Lithium-Ion Batteries. Small 2024, 20, 2308727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Liu, P.; Chou, S.L.; Wang, J.Z.; Liu, H.; Wang, G.; Zhao, H. Highly Ordered Single Crystalline Nanowire Array Assembled Three-Dimensional Nb3O7(OH) and Nb2O5 Superstructures for Energy Storage and Conversion Applications. ACS Nano 2016, 10, 507–514. [Google Scholar] [CrossRef]
- Lian, Y.; Wang, D.; Hou, S.; Ban, C.; Zhao, J.; Zhang, H. Construction of T-Nb2O5 nanoparticles on/in N-doped carbon hollow tubes for Li-ion hybrid supercapacitors. Electrochim. Acta 2020, 330, 135204. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, K.; Xie, C.; Yang, P. Vertically implanting MoSe2 nanosheets on superior thin C-doped g-C3N4 nanosheets towards interface-enhanced electrochemical activities. Carbon 2024, 220, 118884. [Google Scholar] [CrossRef]
- Mou, S.; Liu, S.; Dai, W.; Sun, Y.; Dong, F. Fluorine-induced D-band center shift in Nb2O5 nanosheets for efficient electrochemical formamide synthesis. Appl. Catal. B Environ. 2025, 365, 124963. [Google Scholar] [CrossRef]
- Cheon, S.; Lee, J.; Heo, J.; Im, J.; Cho, S.O. Nanoporous Nb2O5 with an Amorphous Structure for the Application as a Binder-Free Anode of a Hybrid Li-Ion Capacitor. ACS Appl. Nano Mater. 2024, 7, 23188–23195. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Z.; Zhang, D.; Zheng, Z.; Chen, G.; Zhang, N.; Liu, X.; Ma, R. Carbon coated Nb2O5 nanosheets via dopamine-induced phase transition for high-rate lithium-ion battery. J. Power Sources 2022, 530, 231274. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Cui, G.; Zhu, T.; Zhang, J.; Yu, C.; Cui, J.; Wu, J.; Tan, H.H.; Zhang, Y.; et al. Carbon-Coated Self-Assembled Ultrathin T-Nb2O5 Nanosheets for High-Rate Lithium-Ion Storage with Superior Cycling Stability. ACS Appl. Energy Mater. 2020, 3, 12037–12045. [Google Scholar] [CrossRef]
- He, Z.; Zhang, C.; Zhu, Z.; Yu, Y.; Zheng, C.; Wei, F. Advances in Carbon Nanotubes and Carbon Coatings as Conductive Networks in Silicon-based Anodes. Adv. Funct. Mater. 2024, 34, 2408285. [Google Scholar] [CrossRef]
- Yu, J.; Yang, J.; Feng, X.; Jia, H.; Wang, J.; Lu, W. Uniform Carbon Coating on Silicon Nanoparticles by Dynamic CVD Process for Electrochemical Lithium Storage. Ind. Eng. Chem. Res. 2014, 53, 12697–12704. [Google Scholar] [CrossRef]
- Arya, A.K.; Raman, R.K.S.; Saxena, S. Multilayer CVD Graphene Coatings Developed with Suitable Geometrical Parameters for Improved Corrosion Resistance of Ni and a Ni–Cu Alloy in Chloride Environment. Small 2025, 21, 2405813. [Google Scholar] [CrossRef]
- Kim, J.; Cho, Y.-W.; Woo, S.-G.; Lee, J.-N.; Lee, G.-H. Advancements in Chemical Vapor Deposited Carbon Films for Secondary Battery Applications. Small 2025, 2410570. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, B.; Song, Z.; Xiao, X.; Cao, Z.; Xiong, D.; Deng, W.; Hou, H.; Yang, Y.; Zou, G.; et al. Enabling Electron Delocalization by Conductor Heterostructure for Highly Reversible Sodium Storage. Adv. Funct. Mater. 2024, 34, 2312905. [Google Scholar] [CrossRef]
- Bhakar, A.; Taxak, M.; Rai, S.K.J.A.C. Significance of diffraction peak shapes in determining crystallite size distribution:A peak shape analysis procedure for pseudo-Voigt profiles and its application. J. Appl. Crystallogr. 2023, 56, 1466–1479. [Google Scholar] [CrossRef]
- Hu, J.; Li, J.; Wang, K.; Xia, H. Self-assembly Nb2O5 microsphere with hollow and carbon coated structure as high rate capability lithium-ion electrode materials. Electrochim. Acta 2020, 331, 135364. [Google Scholar] [CrossRef]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-ray Diffraction Techniques for Mineral Characterization: A Review for Engineers of the Fundamentals, Applications, and Research Directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Z.; Shadike, Z.; Lei, M.; Liu, J.; Li, C. Defect-Concentration-Mediated T-Nb2O5 Anodes for Durable and Fast-Charging Li-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2107060. [Google Scholar] [CrossRef]
- Li, T.; Huang, S.; Kane, N.; Wang, J.-H.; Luo, Z.; Zhang, W.; Nam, G.; Zhao, B.; Qi, Y.; Liu, M. Operando Raman and DFT Analysis of (De)lithiation in Fast-Charging, Shear-Phase H-Nb2O5. ACS Energy Lett. 2023, 8, 3131–3140. [Google Scholar] [CrossRef]
- Cao, X.; Pan, A.; Liu, S.; Zhou, J.; Li, S.; Cao, G.; Liu, J.; Liang, S. Chemical Synthesis of 3D Graphene-Like Cages for Sodium-Ion Batteries Applications. Adv. Energy Mater. 2017, 7, 1700797. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, L.; Li, C.; Huang, H.; Sun, X.; Sun, K. A practical Li ion battery anode material with high gravimetric/volumetric capacities based on T-Nb2O5/graphite composite. Chem. Eng. J. 2017, 328, 844–852. [Google Scholar] [CrossRef]
- Xu, X.; Robertson, S.J.; Yang, T.; Chen, F.; Geng, X.; Wang, Y.; Ji, F.; Sun, C.; Chen, S.; Shao, M.; et al. Interfacial space charge design with desired electron density to enhance sodium storage of MoS2@Nb2O5 anode. Nano Energy 2024, 127, 109739. [Google Scholar] [CrossRef]
- Deng, S.; Zhu, H.; Wang, G.; Luo, M.; Shen, S.; Ai, C.; Yang, L.; Lin, S.; Zhang, Q.; Gu, L.; et al. Boosting fast energy storage by synergistic engineering of carbon and deficiency. Nat. Commun. 2020, 11, 132. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Zhang, L.; Hu, Z.; Yu, L.; Jiang, T.; Lu, C.; Yan, C.; Sun, J.; Liu, Z.J.A.M. Caging Nb2O5 nanowires in PECVD-derived graphene capsules toward bendable sodium-ion hybrid supercapacitors. Adv. Mater. 2018, 30, 1800963. [Google Scholar] [CrossRef]
- Zheng, Y.; Qiu, W.; Wang, L.; Liu, J.; Chen, S.; Li, C. Triple Conductive Wiring by Electron Doping, Chelation Coating and Electrochemical Conversion in Fluffy Nb2O5 Anodes for Fast-Charging Li-Ion Batteries. Adv. Sci. 2022, 9, 2202201. [Google Scholar] [CrossRef]
- Lotfabad, E.M.; Ding, J.; Cui, K.; Kohandehghan, A.; Kalisvaart, W.P.; Hazelton, M.; Mitlin, D.J.A.N. High-density sodium and lithium ion battery anodes from banana peels. ACS Nano 2014, 8, 7115–7129. [Google Scholar] [CrossRef]
- Mukherjee, R.; Krishnan, R.; Lu, T.M. Nanostructured electrodes for high-power lithium ion batteries. Nano Energy 2012, 1, 518–533. [Google Scholar] [CrossRef]
- Shi, S.; Wang, G.; Wan, G.; Tang, Y.; Zhao, G.; Deng, Z.; Chai, J.; Wei, C.; Wang, G. Titanium niobate (Ti2Nb10O29) anchored on nitrogen-doped carbon foams as flexible and self-supported anode for high-performance lithium ion batteries. J. Colloid Interface Sci. 2021, 587, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Lei, T.; Zhu, W.; Xiao, L.; Liu, J. In-Plane Assembled Orthorhombic Nb2O5 Nanorod Films with High-Rate Li+ Intercalation for High-Performance Flexible Li-Ion Capacitors. Adv. Funct. Mater. 2018, 28, 1704330. [Google Scholar] [CrossRef]
- Meng, J.; He, Q.; Xu, L.; Zhang, X.; Liu, F.; Wang, X.; Li, Q.; Xu, X.; Zhang, G.; Niu, C.; et al. Identification of Phase Control of Carbon-Confined Nb2O5 Nanoparticles toward High-Performance Lithium Storage. Adv. Energy Mater. 2019, 9, 1802695. [Google Scholar] [CrossRef]
- Lübke, M.; Sumboja, A.; Johnson, I.D.; Brett, D.J.L.; Shearing, P.R.; Liu, Z.; Darr, J.A. High power nano-Nb2O5 negative electrodes for lithium-ion batteries. Electrochim. Acta 2016, 192, 363–369. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Zhu, W.; Lian, J.; Huang, Y.; Yu, Y.-Y.; Qiu, J.; Zhao, Y.; Yong, Y.-C.; Li, H. Controllable synthesis of uniform mesoporous H-Nb2O5/rGO nanocomposites for advanced lithium ion hybrid supercapacitors. J. Mater. Chem. A 2019, 7, 693–703. [Google Scholar] [CrossRef]
- Pei, C.; Yin, Y.; Liao, X.; Xiong, F.; An, Q.; Jin, M.; Zhao, Y.; Mai, L. Structural properties and electrochemical performance of different polymorphs of Nb2O5 in magnesium-based batteries. J. Energy Chem. 2021, 58, 586–592. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Y.; Wang, L.; Cui, F.; Liu, B.; Hu, W.; Lu, Y.; Zhu, G. Porphyrin-framed PAF Based Single-Ion Lithium Salt Boosting Quasi Solid-State Lithium-Ion Battery Performance at Low Temperatures. Adv. Energy Mater. 2024, 2404008. [Google Scholar] [CrossRef]
- Ji, Q.; Xu, Z.; Gao, X.; Cheng, Y.-J.; Wan, X.; Zuo, X.; Chen, G.Z.; Hu, B.; Zhu, J.; Bruce, P.G.; et al. Carbon-emcoating architecture boosts lithium storage of Nb2O5. Sci. China Mater. 2021, 64, 1071–1086. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, H.; Du, C.; Zhao, H.; Yu, L.; Yan, Y.; Zhao, J.; Wan, G.; Wang, L.; Wang, G. Nanoengineering of Ultrathin Carbon-Coated T-Nb2O5 Nanosheets for High-Performance Lithium Storage. Coatings 2025, 15, 315. https://doi.org/10.3390/coatings15030315
Xiong H, Du C, Zhao H, Yu L, Yan Y, Zhao J, Wan G, Wang L, Wang G. Nanoengineering of Ultrathin Carbon-Coated T-Nb2O5 Nanosheets for High-Performance Lithium Storage. Coatings. 2025; 15(3):315. https://doi.org/10.3390/coatings15030315
Chicago/Turabian StyleXiong, Hualin, Changlong Du, Hongan Zhao, Lei Yu, Yongzhu Yan, Jinchuan Zhao, Gengping Wan, Liyong Wang, and Guizhen Wang. 2025. "Nanoengineering of Ultrathin Carbon-Coated T-Nb2O5 Nanosheets for High-Performance Lithium Storage" Coatings 15, no. 3: 315. https://doi.org/10.3390/coatings15030315
APA StyleXiong, H., Du, C., Zhao, H., Yu, L., Yan, Y., Zhao, J., Wan, G., Wang, L., & Wang, G. (2025). Nanoengineering of Ultrathin Carbon-Coated T-Nb2O5 Nanosheets for High-Performance Lithium Storage. Coatings, 15(3), 315. https://doi.org/10.3390/coatings15030315






