Impact of Irradiation on Corrosion Performance of Hybrid Organic/Inorganic Coatings on Austenitic Stainless Steel
Abstract
1. Introduction
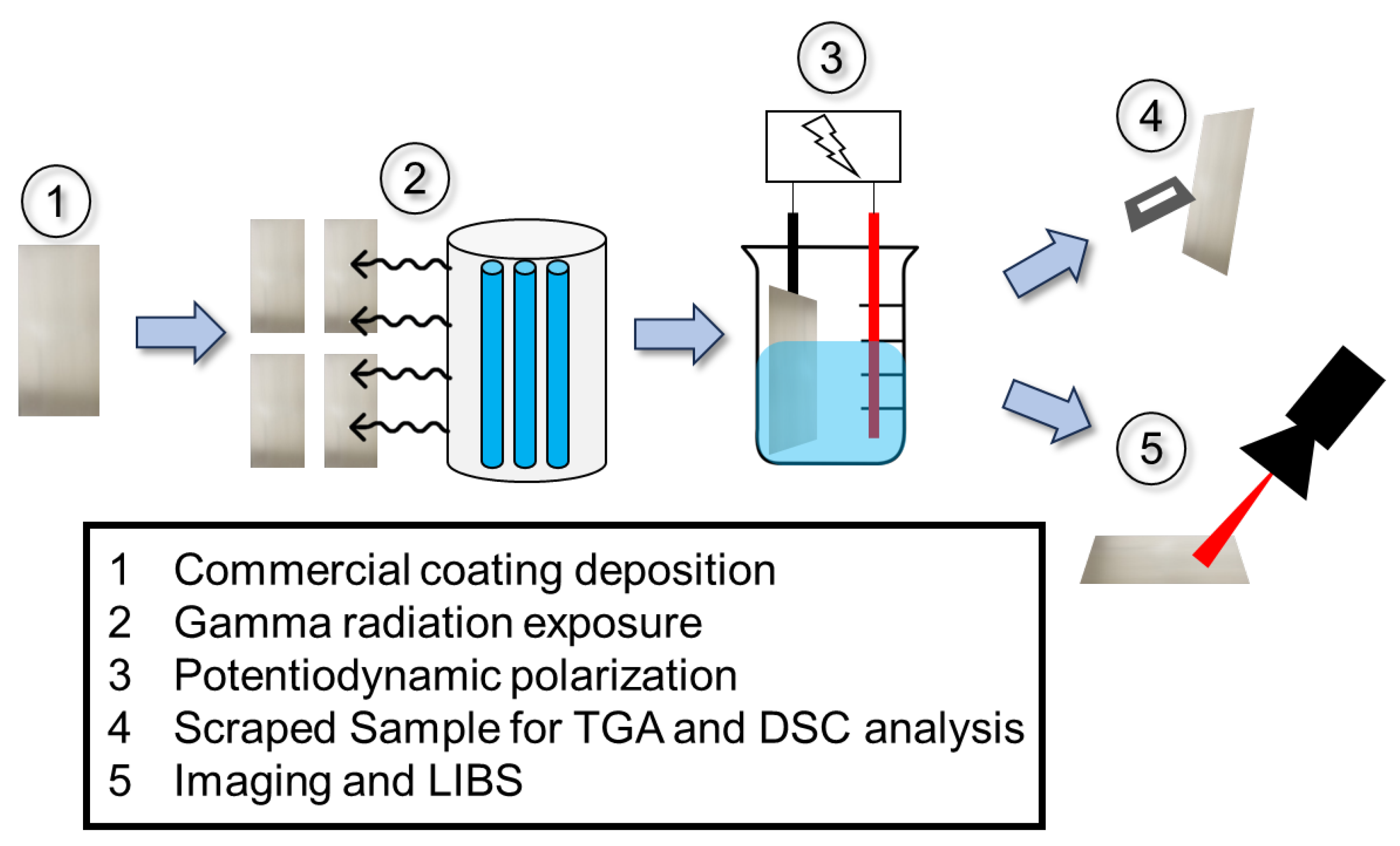
2. Materials and Methods
2.1. Materials
2.2. Thermal Gravimetric Analysis/Differential Scanning Calorimetry
2.3. Radiation Exposures
2.4. Electrochemical Testing
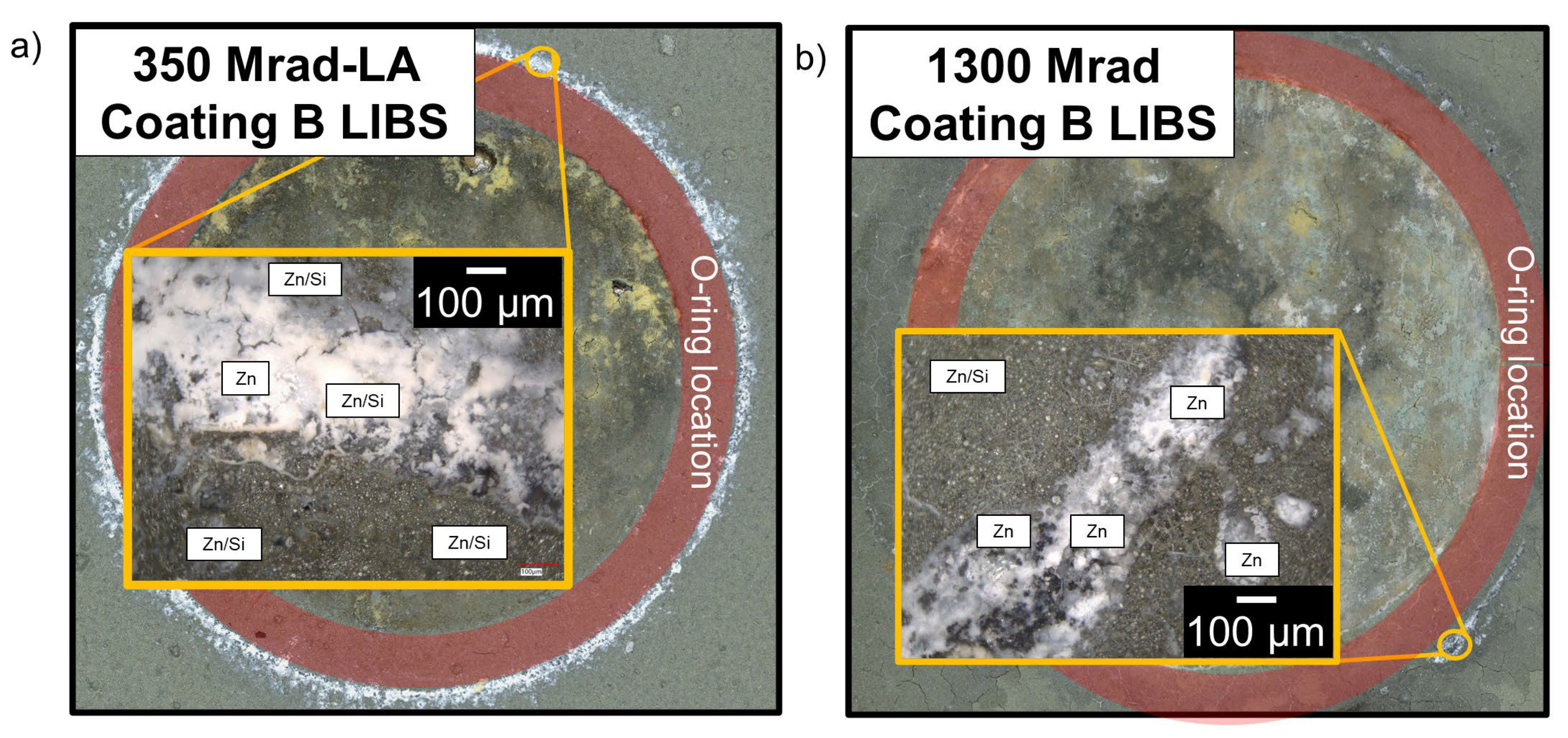
2.5. Post-Exposure Optical Imaging and Laser-Induced Breakdown Spectroscopy
3. Results
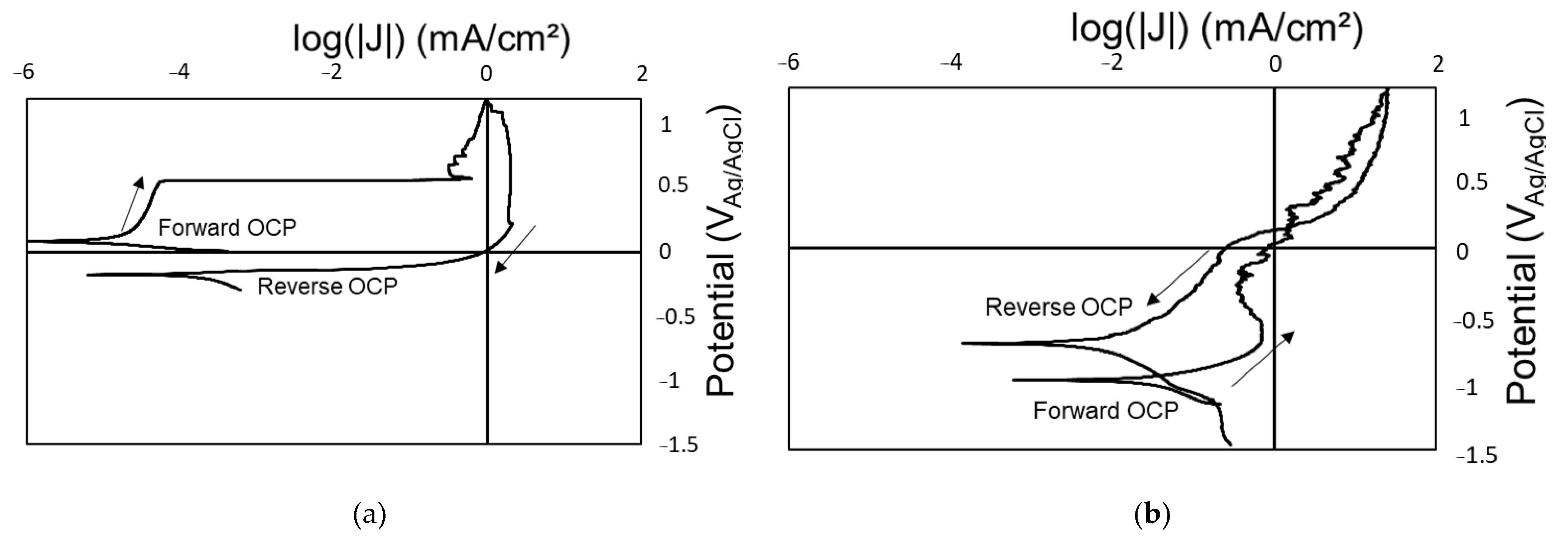
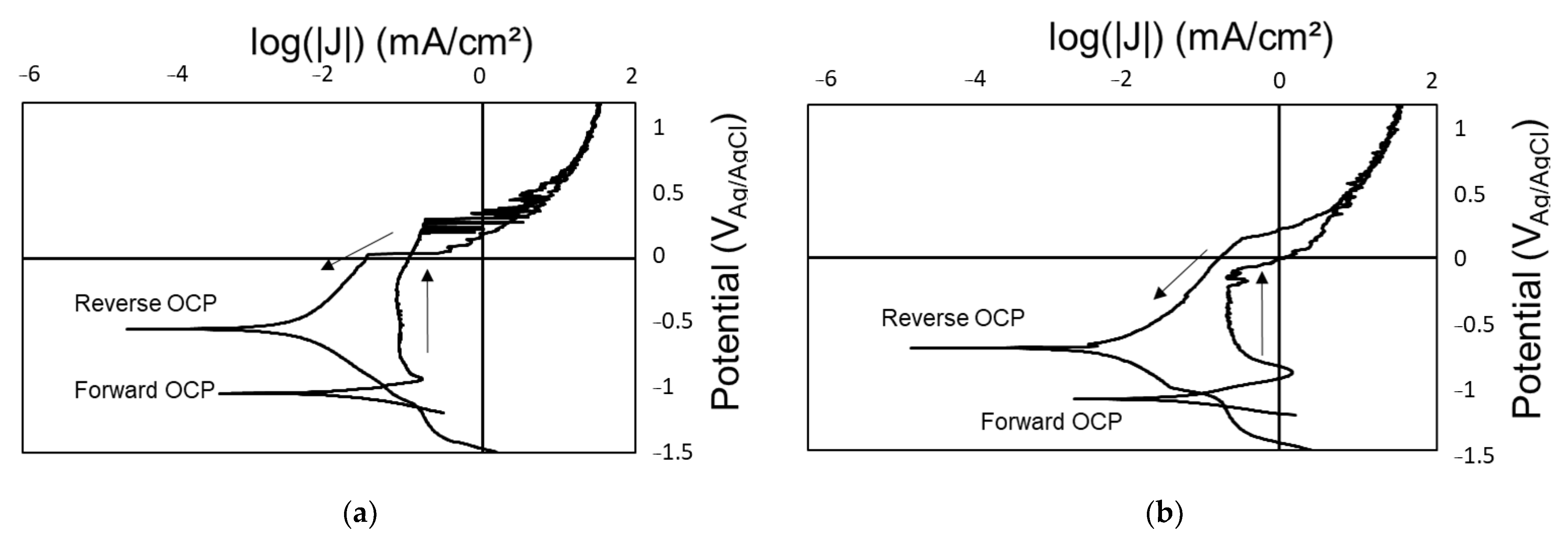
3.1. Baseline Performance
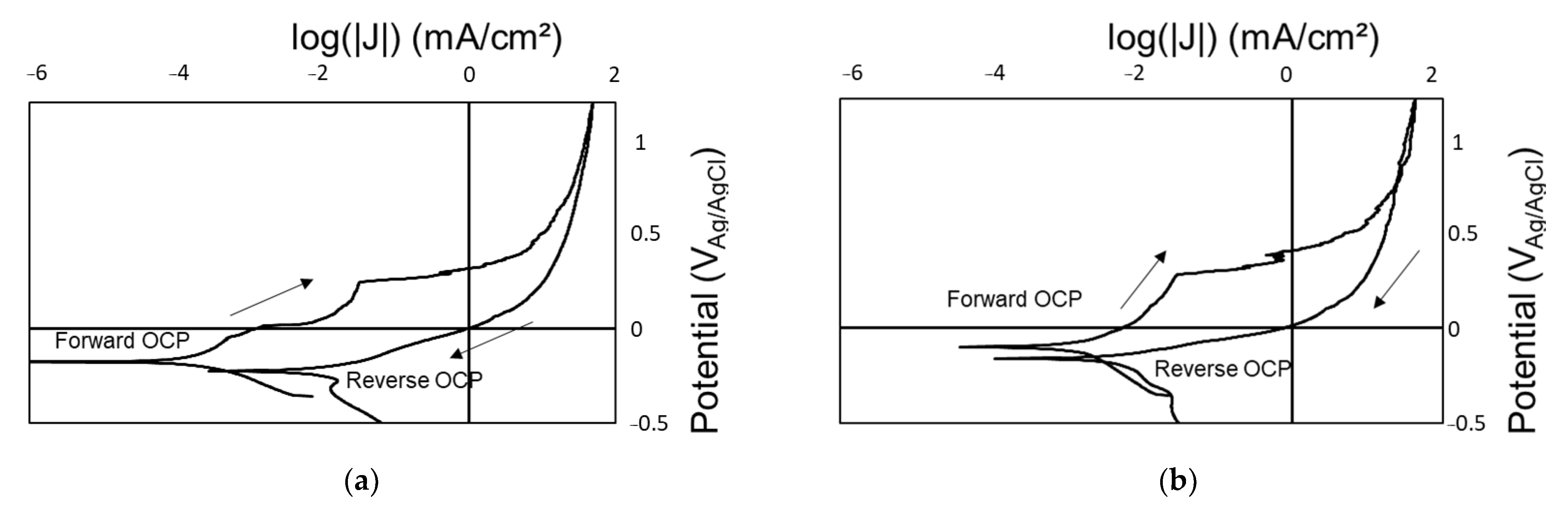
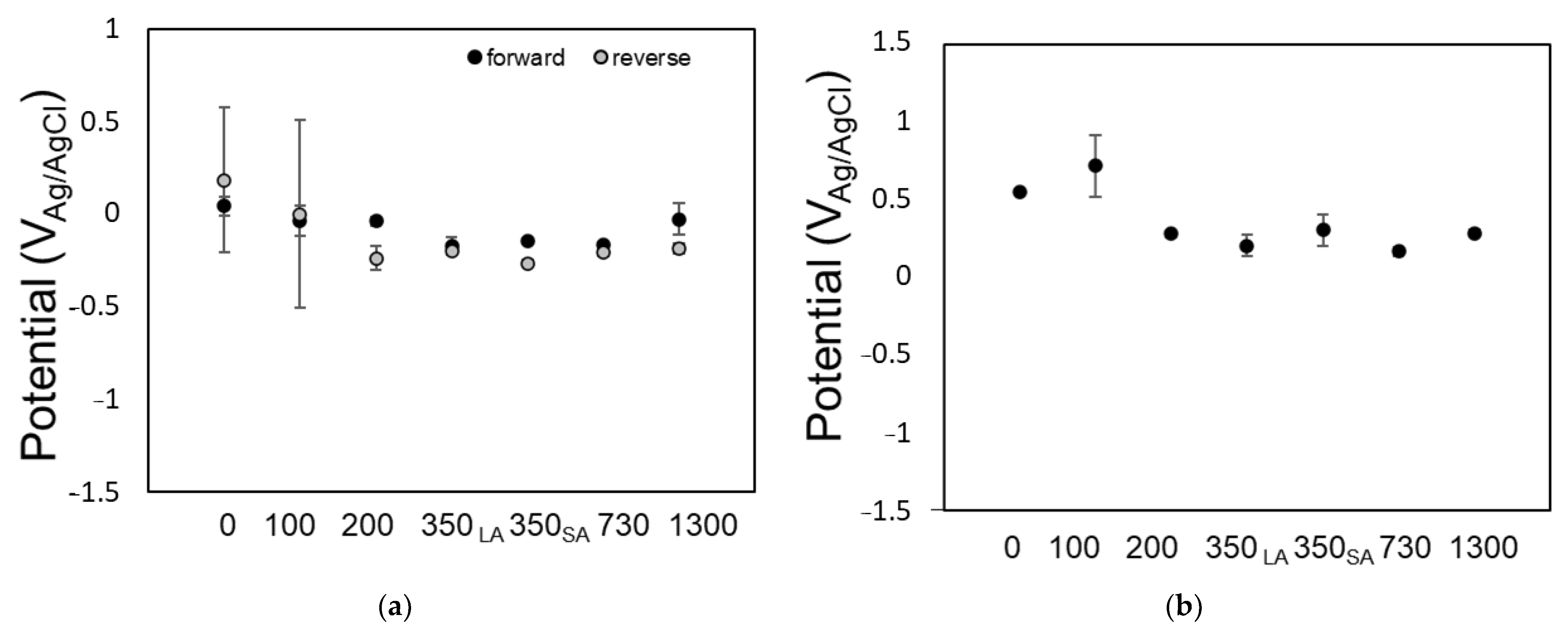
3.2. Impact of Radiation on Coating Performance
4. Discussion
4.1. Temperature Minimally Influences Polymer Degradation of Non-Irradiated Coatings
4.2. Total Radiation Dose Influences Coating Susceptibility and Electrochemical Activity
4.3. Radiation Dose Rates Examined Did Not Influence Coating Susceptibility
4.4. Limitations and Implications of the Presented Data
5. Conclusions
- The coating performance with respect to corrosion was influenced by the total radiation dose received during exposure:
- ○
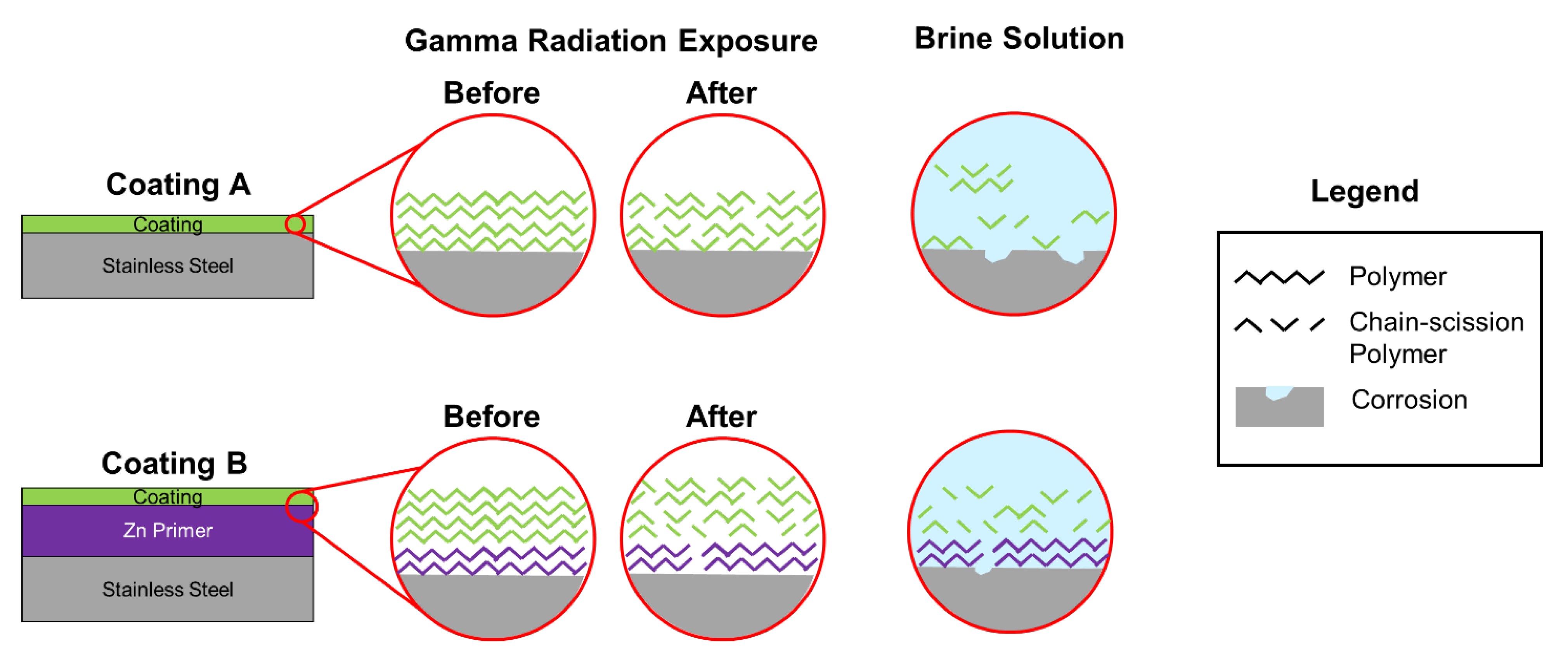
- The polymer–ceramic coating (Coating A) offered limited corrosion protection below 200 Mrad, but beyond this dose, all samples showed signs of corrosion. OCP values measured during the reverse scan were closer to that of a bare SS substrate. Additionally, LIBS identified SS with limited Si from the coating, suggesting degradation of the polymer coating. The organic portion of the polymer–silica coating is likely most susceptible to the total gamma radiation dose.
- ○
- The polymer–ceramic, Zn-rich primer coating (Coating B) showed improved corrosion resistance at elevated doses of gamma radiation compared to Coating A, ascribed to both the increased overall coating thickness and sacrificial nature of the secondary coating layer, the Zn-rich primer. OCP values obtained on the forward CPP are closer to that of Zn, whereas reverse scan OCP values suggest a mixed potential between Zn and SS, indicating some coating degradation and corrosion of the SS substrate.
- As TGA/DSC data showed that polymer degradation began at 250 °C, and the Zn-rich primer coating displayed Zn oxidation beyond 420 °C, temperature did not play a significant role in coating degradation, with radiation exposure performed at 25 and 75 °C.
- For the shutter array (SA) and linear array (LA) samples exposed to 350 Mrad, the change in dose rate from 176 to 1054 rad/s did not have a significant effect on coating performance.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katona, R.M.; Carpenter, J.C.; Knight, A.W.; Bryan, C.R.; Schaller, R.F.; Kelly, R.G.; Schindelholz, E.J. Importance of the hydrogen evolution reaction in magnesium chloride solutions on stainless steel. Corros. Sci. 2020, 177, 108935. [Google Scholar] [CrossRef]
- Katona, R.M.; Kelly, R.G.; Bryan, C.R.; Schaller, R.F.; Knight, A.W. Use of in situ Raman spectroelectrochemical technique to explore atmospheric corrosion in marine-relevant environments. Electrochem. Commun. 2020, 118, 106768. [Google Scholar] [CrossRef]
- Gao, H.; Lan, X.; Liu, L.; Xiao, X.; Liu, Y.; Leng, J. Study on performances of colorless and transparent shape memory polyimide film in space thermal cycling, atomic oxygen and ultraviolet irradiation environments. Smart Mater. Struct. 2017, 26, 095001. [Google Scholar] [CrossRef]
- Shulman, H.; Ginell, W.S. Nuclear and Space Radiation Effects on Materials; SP-8053; National Aeronautics and Space Administration: Washington, DC, USA, 1970. [Google Scholar]
- Schaller, R.; Knight, A.W.; Bryan, C.R.; Nation, B.L.; Montoya, T.; Katona, R.M. SNF Interim Storage Canister Corrosion and Surface Environment Investigations (FY2020 Status Report) M2SF-21SN010207055 (No. SAND-2020-12663R); Sandia National Lab. (SNL-NM): Albuquerque, NM, USA, 2020. [Google Scholar]
- Nation, B.L.; Knight, A.W.; Maguire, M.; Verma, S.; Click, N.; Debrun, G.; McCready, T.; Katona, R.; Schaller, R.F.; Bryan, C.R. FY23 Status: Corrosion-Resistant Coatings on Spent Nuclear Fuel Canisters to Mitigate and Repair Potential Stress Corrosion Cracking M3SF-23SN010207052 (No. SAND-2023-09205R); Sandia National Lab. (SNL-NM): Albuquerque, NM, USA, 2023. [Google Scholar]
- Karasz, E.K.; Montoya, T.D.; Taylor, J.M.; Ross, K.A.; Schaller, R.F. Accelerated corrosion testing of cold spray coatings on 304L in chloride environments. Front. Met. Alloys 2022, 1, 1021000. [Google Scholar] [CrossRef]
- Qu, H.J.; Srinivasan, J.; Zhao, Y.; Mao, K.S.; Taylor, J.M.; Marino, G.; Montoya, T.; Johnson, K.; Locke, J.S.; Schaller, R.; et al. Stress corrosion cracking mechanism of cold spray coating on a galvanically similar substrate. Mater. Sci. Eng. A 2022, 849, 143404. [Google Scholar] [CrossRef]
- Yeom, H.; Sridharan, K. Cold spray technology in nuclear energy applications: A review of recent advances. Ann. Nucl. Energy 2021, 150, 107835. [Google Scholar] [CrossRef]
- Yeom, H.; Dabney, T.; Pocquette, N.; Ross, K.; Pfefferkorn, F.E.; Sridharan, K. Cold spray deposition of 304L stainless steel to mitigate chloride-induced stress corrosion cracking in canisters for used nuclear fuel storage. J. Nucl. Mater. 2020, 538, 152254. [Google Scholar] [CrossRef]
- Wang, W.; Tamakloe, S.; Deng, Z.; Li, L.; Cai, W.; Lu, K. Effects of processing temperature on the corrosion and tribocorrosion resistance of perhydropolysilazane-derived coatings on AISI 304 steel. Surf. Coat. Technol. 2022, 439, 128463. [Google Scholar] [CrossRef]
- Du, Y.J.; Damron, M.; Tang, G.; Zheng, H.; Chu, C.J.; Osborne, J.H. Inorganic/organic hybrid coatings for aircraft aluminum alloy substrates. Prog. Org. Coat. 2001, 41, 226–232. [Google Scholar] [CrossRef]
- Zheng, S.; Li, J. Inorganic-organic sol gel hybrid coatings for corrosion protection of metals. J. Sol-Gel Sci. Technol. 2010, 54, 174–187. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol-gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Arman, S.Y.; Mehdipour, M. Anticorrosion properties of an epoxy zinc-rich composite coating reinforced with zinc, aluminum, and iron oxide pigments. J. Coat. Technol. Res. 2014, 11, 727–737. [Google Scholar] [CrossRef]
- McMahon, M.E.; Burns, J.T.; Scully, J.R. Development of new criteria for evaluating the effectiveness of Zn-rich primers in protecting Al-Mg alloys. Prog. Org. Coat. 2019, 135, 392–409. [Google Scholar] [CrossRef]
- McMahon, M.E.; Santucci Jr, R.J.; Glover, C.F.; Kannan, B.; Walsh, Z.R.; Scully, J.R. A Review of Modern Assessment Methods for Metal and Metal-Oxide Based Primers for Substrate Corrosion Protection. Front. Mater. 2019, 6, 190. [Google Scholar] [CrossRef]
- McMahon, M.E.; Scully, J.R.; Burns, J.T. Mitigation of Intergranular Cracking in Al-Mg Alloys via Zn-Based Electrode Potential Control in Sodium Chloride Solution. Corrosion 2019, 75, 911–928. [Google Scholar] [CrossRef]
- Panossian, Z.; Mariaca, L.; Morcillo, M.; Flores, S.; Rocha, J.; Peña, J.J.; Herrera, F.; Corvo, F.; Sanchez, M.; Rincon, O.T.; et al. Steel cathodic protection afforded by zinc, aluminium and zinc/aluminium alloy coatings in the atmosphere. Surf. Coat. Technol. 2005, 190, 244–248. [Google Scholar] [CrossRef]
- Abdeldaym, A.; Magida, M.M.; Elnahas, H.H. Preparation and evaluation of structural, optical, dielectric and thermal characteristics of unirradiated and irradiated polyurethane/magnesium silicate composites. J. Mater. Sci. Mater. Electron. 2021, 32, 5755–5769. [Google Scholar] [CrossRef]
- Gorna, K.; Gogolewski, S. The effect of gamma radiation on molecular stability and mechanical properties of biodegradable polyurethanes for medical applications. Polym. Degrad. Stab. 2003, 79, 465–474. [Google Scholar] [CrossRef]
- Kim, J.H.; Hwang, J.H.; Suh, J.; Tongay, S.; Kwon, S.; Hwang, C.C.; Wu, J.; Park, J.Y. Work function engineering of single layer graphene by irradiation-induced defects. Appl. Phys. Lett. 2013, 103, 171604. [Google Scholar]
- Wang, L.; Wang, K.; Erkan, N.; Yuan, Y.; Chen, J.; Nie, B.; Li, F.; Okamoto, K. Metal material surface wettability increase induced by electron beam irradiation. Appl. Surf. Sci. 2020, 511, 145555. [Google Scholar] [CrossRef]
- Velo-Gala, I.; Lopez-Penalver, J.J.; Sánchez-Polo, M.; Rivera-Utrilla, J. Surface modifications of activated carbon by gamma irradiation. Carbon 2014, 67, 236–249. [Google Scholar] [CrossRef]
- Clough, R. High-energy radiation and polymers: A review of commercial processes and emerging applications. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2001, 185, 8–33. [Google Scholar] [CrossRef]
- Ito, S.; Miyazaki, Y.; Hirai, N.; Ohki, Y. Effects of gamma irradiation on the degradation of silicone rubber by steam exposure. J. Nucl. Sci. Technol. 2021, 58, 166–172. [Google Scholar] [CrossRef]
- Zimmermann, J.; Schalm, T.; Sadeghi, M.Z.; Schröder, K.U. Empirical investigations on the effects of ionizing radiation on epoxy structural adhesives and resins: An overview. Int. J. Adhes. Adhes. 2022, 117, 103014. [Google Scholar] [CrossRef]
- Knight, A.; Nation, B.; Maguire, M.; Schaller, R.; Bryan, C. Corrosion-Resistant Coatings on Spent Nuclear Fuel Canisters to Mitigate and Repair Potential Stress Corrosion Cracking (FY22 Status) M3SF-22SN010207062 (No. SAND2022-14233R); Sandia National Lab. (SNL-NM): Albuquerque, NM, USA, 2022. [Google Scholar]
- Dodge, H. Gamma Irradiation Facility (No. SAND2021-12046R); Sandia National Lab. (SNL-NM): Albuquerque, NM, USA, 2021. [Google Scholar]
- ASTM-D4082; Standard Test Method for Effects of Gamma Radiation on Coatings for Use in Nuclear Power Plants. ASTM International: West Conshohocken, PA, USA, 2023; pp. 1–2.
- ASTM D659-86e1; Method of Evaluating Degree of Chalking of Exterior Paints. ASTM International: West Conshohocken, PA, USA, 2021; pp. 70–71.
- ASTM D661-93(2019); Standard Test Method for Evaluating Degree of Cracking of Exterior Paints. ASTM International: West Conshohocken, PA, USA, 2019; pp. 1–3.
- ASTM D714-02(2017); Standard Test Method for Evaluating Degree of Blistering Paints. ASTM International: West Conshohocken, PA, USA, 2017; pp. 1–6.
- ASTM D772-24; Standard Test Method for Evaluating Degree of Flaking (Scaling) of Exterior Paints. ASTM International: West Conshohocken, PA, USA, 2024; pp. 1–5.
- Mouanga, M.; Berçot, P. Comparison of corrosion behaviour of zinc in NaCl and in NaOH solutions; Part II: Electrochemical analyses. Corros. Sci. 2010, 52, 3993–4000. [Google Scholar] [CrossRef]
- El-Sayed, A.; Shilkamy, H.; Elrouby, M. The passivity breakdown of zinc antimony alloy as an anode in the alkaline batteries. Sci. Rep. 2022, 12, 18925. [Google Scholar] [CrossRef]
- Van Loo, M.; Laiderman, D.D.; Bruhn, R.R. Filiform Corrosion. Corrosion 1953, 9, 277–283. [Google Scholar] [CrossRef]
- Frankel, G.S. Pitting Corrosion in ASM International; ASM Handbook Online: Materials Park, OH, USA, 2003; pp. 236–241. [Google Scholar] [CrossRef]
- Hassanajili, S.; Khademi, M.; Keshavarz, P. Influence of various types of silica nanoparticles on permeation properties of polyurethane/silica mixed matrix membranes. J. Membr. Sci. 2014, 453, 369–383. [Google Scholar] [CrossRef]
- Xu, L.; Fu, Y.; Du, M. Investigation on Structures and Properties of Shape Memory Polyurethane/Silica Nanocomposites. Chin. J. Chem. 2011, 29, 703–710. [Google Scholar] [CrossRef]
- Kalogeras, I.M. Encyclopedia of Polymer Blends, 1st ed.; Wiley: Hoboken, NJ, USA, 2016; Volume 3. [Google Scholar]
- Jang, E.S.; Khan, S.B.; Seo, J.; Akhtar, K.; Choi, J.; Kim, K.I.; Han, H. Synthesis and characterization of novel UV-Curable PU-Si hybrids: Influence of silica on thermal, mechanical, and water sorption properties of polyurethane acrylates. Macromol. Res. 2011, 19, 1006–1013. [Google Scholar] [CrossRef]
- Zaborowska, A.; Wyszkowska, E.; Clozel, M.; Vanazzi, M.; Di Fonzo, F.; Turek, M.; Jóźwik, I.; Kosińska, A.; Jagielski, J. Influence of ion irradiation on the nanomechanical properties of thin alumina coatings deposited on 316L SS by PLD. Surf. Coat. Technol. 2020, 386, 125491. [Google Scholar] [CrossRef]
- García Ferré, F.; Mairov, A.; Ceseracciu, L.; Serruys, Y.; Trocellier, P.; Baumier, C.; Kaïtasov, O.; Brescia, R.; Gastaldi, D.; Vena, P.; et al. Radiation endurance in Al2O3 nanoceramics. Sci. Rep. 2016, 6, 33478. [Google Scholar] [CrossRef]
- Shintani, H.; Nakamura, A. Degradation and cross-linking of polyurethane irradiated by gamma-rays. Polym. Degrad. Stab. 1991, 32, 191–208. [Google Scholar] [CrossRef]
- Pierpoint, S.; Silverman, J.; Al-Sheikhly, M. Effects of ionizing radiation on the aging of polyester based polyurethane binder. Radiat. Phys. Chem. 2001, 62, 163–169. [Google Scholar] [CrossRef]
- Ferreño, D.; Mañanes, A.; Rábago, D.; Casado, J.A.; González, J.A.; Gómez, S.; Carrascal, I.A.; Ruiz, E.; Diego, S.; Gómez, F.; et al. Mechanical behavior and microstructural changes in polyurethane exposed to high doses of X rays, gamma rays or neutron irradiation. Polym. Test. 2018, 67, 359–369. [Google Scholar] [CrossRef]
- Sui, H.; Liu, X.; Zhong, F.; Li, X.; Wang, B.; Ju, X. Relationship between free volume and mechanical properties of polyurethane irradiated by gamma rays. J. Radioanal. Nucl. Chem. 2014, 300, 701–706. [Google Scholar] [CrossRef]
- Shintani, H.; Kikuchi, H.; Nakamura, A. Effects of gamma-ray irradiation on the change of characteristics of polyurethane. J. Appl. Polym. Sci. 1990, 41, 661–675. [Google Scholar] [CrossRef]
- Thierry, D.; Larché, N.; Leballeur, C.; Wijesinghe, S.L.; Zixi, T. Corrosion potential and cathodic reduction efficiency of stainless steel in natural seawater. Mater. Corros. 2015, 66, 453–458. [Google Scholar]
- Saeedikhani, M.; Wijesinghe, S.L.; Blackwood, D.J. Barrier and sacrificial protection mechanisms of zinc rich primers. Eng. J. 2019, 23, 223–233. [Google Scholar] [CrossRef]
- Takahashi, H.; Hashimoto, N. Radiation-induced segratation and grain boundary migration in Fe-Cr-Ni model alloy under irradiation. Mater. Trans. JIM 1993, 34, 1027–1030. [Google Scholar]
- Nakata, K.; Katano, Y.; Masaoka, I.; Shiraishi, K. Grain boundary migration during electron irradiation in austenitic stainless steels. J. Nucl. Mater. 1985, 133–134, 575–579. [Google Scholar] [CrossRef]
- Zheng, Z.B.; Zheng, Y.G. Effects of surface treatments on the corrosion and erosion-corrosion of 304 stainless steel in 3.5% NaCl solution. Corros. Sci. 2016, 112, 657–668. [Google Scholar] [CrossRef]






| Exposure Cell | Average Dose Rate | Total Dose (Mrad) | Temperature During Exposure (°C) | Duration of Exposure (Hours) |
|---|---|---|---|---|
| Linear Array | 176 rad/s | 105 | 25 | 165 |
| 211 | 25 | 333 | ||
| 350 | 25 | 575 | ||
| Shutter Array | 1054 rad/s | 351 | 77 | 92 |
| 724 | 77 | 196 | ||
| 1305 | 77 | 379 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Click, N.; Knight, A.; Nation, B.; Maguire, M.; Verma, S.; DeBrun, G.; McCready, T.; Goff, A.; Rotert, A.; Hanson, D.; et al. Impact of Irradiation on Corrosion Performance of Hybrid Organic/Inorganic Coatings on Austenitic Stainless Steel. Coatings 2025, 15, 312. https://doi.org/10.3390/coatings15030312
Click N, Knight A, Nation B, Maguire M, Verma S, DeBrun G, McCready T, Goff A, Rotert A, Hanson D, et al. Impact of Irradiation on Corrosion Performance of Hybrid Organic/Inorganic Coatings on Austenitic Stainless Steel. Coatings. 2025; 15(3):312. https://doi.org/10.3390/coatings15030312
Chicago/Turabian StyleClick, Natalie, Andrew Knight, Brendan Nation, Makeila Maguire, Samay Verma, Gavin DeBrun, Tyler McCready, Adam Goff, Audrey Rotert, Don Hanson, and et al. 2025. "Impact of Irradiation on Corrosion Performance of Hybrid Organic/Inorganic Coatings on Austenitic Stainless Steel" Coatings 15, no. 3: 312. https://doi.org/10.3390/coatings15030312
APA StyleClick, N., Knight, A., Nation, B., Maguire, M., Verma, S., DeBrun, G., McCready, T., Goff, A., Rotert, A., Hanson, D., & Schaller, R. F. (2025). Impact of Irradiation on Corrosion Performance of Hybrid Organic/Inorganic Coatings on Austenitic Stainless Steel. Coatings, 15(3), 312. https://doi.org/10.3390/coatings15030312





