Abstract
This study explores the corrosion behavior of pure copper in simulated oilfield-produced water and evaluates the inhibitory effect of cetylpyridinium chloride (CPC) on microbiologically influenced corrosion (MIC). Weight loss tests, potentiodynamic polarization, and pitting analyses revealed that sulfate-reducing bacteria (SRB) activity significantly accelerated corrosion, with the maximum pit depth reaching 7.54 µm in the absence of CPC—approximately 1.83 times greater than under abiotic conditions. The introduction of CPC substantially reduced corrosion rates and pit depths, with maximum pit depths decreasing to 2.97 µm, 1.11 µm, and 1.02 µm at 10, 50, and 80 mg/L CPC, respectively. CPC inhibited SRB biofilm formation, metabolic activity, and corrosion product accumulation, achieving an inhibition efficiency of up to 89% at 80 mg/L. These findings highlight CPC’s dual role as a biocide and a corrosion inhibitor, offering a promising approach to controlling MIC in oilfields and similar industrial environments.
1. Introduction
Corrosion imposes an extraordinary economic burden globally, with annual costs exceeding USD 4 trillion [1]. Among various forms of corrosion, microbiologically influenced corrosion (MIC) accounts for approximately 20% of these losses, making it one of the most significant contributors to the problem [2,3,4]. MIC poses severe challenges to industries, including oil and gas, water coolant systems, and fire sprinkler systems [5,6]. Sulfate-reducing bacteria (SRB) are particularly notorious in this regard, causing nearly half of all MIC failures, especially in environments that are rich in sulfate [7,8,9,10,11].
Copper (Cu) and its alloys are widely employed in diverse industrial applications, including heat exchangers and fire sprinkler systems, due to their excellent thermal conductivity and machinability [12,13,14]. However, copper is highly susceptible to MIC caused by SRB, leading to equipment failures and significant operational disruptions. Recent studies have reported alarming failure cases, such as SRB-induced corrosion of copper pipes and coils in air conditioners in Florida homes, which also released toxic hydrogen sulfide gas [15]. Mechanistically, SRB-generated H2S reduces the anodic reaction potential, accelerates copper corrosion, and forms poorly protective Cu2S films that exacerbate localized corrosion [16]. These effects, compounded by heterogeneities in the copper surface, result in accelerated damage and have drawn considerable attention from researchers [17].
Biocides are among the most effective measures to mitigate MIC by eliminating sessile and planktonic bacterial cells, thereby preventing biofilm formation on material surfaces [18,19,20]. However, traditional biocides often exhibit low antibacterial efficiency, produce harmful by-products, and require supplementary inhibitors or descaling agents, which increase environmental and economic costs [19,21,22]. This has created a pressing demand for bifunctional biocides that combine bactericidal and corrosion inhibition properties. While compounds like benzothiazoles and ionic liquids have shown promise, their high toxicity and large-dosage requirements hinder widespread adoption [23,24]. Similarly, plant extracts, though effective, are limited by complex extraction processes and high costs [25,26]. Consequently, an environmentally friendly, efficient, and practical bifunctional biocide is urgently needed.
Cetylpyridinium chloride (CPC), a quaternary ammonium salt, is a highly safe and eco-friendly biocide widely used in food, oral care, and personal care products [27,28]. CPC exhibits strong antibacterial activity by binding to negatively charged bacterial cell membranes and disrupting their integrity, leading to cell death [29]. Additionally, CPC has been reported to dissolve individual bacterial cells by causing cytoplasmic leakage [28]. While CPC’s corrosion inhibition effects on low-carbon steel have been studied [30], its effectiveness against SRB-induced MIC in copper remains unexplored.
In this study, the antibacterial and corrosion inhibition properties of CPC against SRB-induced MIC in pure copper are investigated. Corrosion electrochemical testing combined with material characterization testing technologies are employed to comprehensively assess CPC’s effects. The mechanisms underlying CPC’s dual action are elucidated, and its quantitative efficacy is evaluated. This work aims to address the limitations of traditional biocides and provide insights into the application of CPC as an effective solution for mitigating MIC in critical industries.
2. Experimental Methods
2.1. Preparation of Coupons
The test material used in this study was a commercially pure T2 copper plate (Cu > 99.9 wt.%). The microstructure of the copper used is shown in Figure 1 through the optic microscope (Axio lab. A1, Germany) and Electron Back-Scattered Diffraction (EBSD; FEI Company, Quattro s-FEI, OR, USA). Square-shaped coupons with dimensions of 10 mm × 10 mm × 3 mm were prepared by cutting the copper plate. A copper wire was soldered to the back of each coupon to facilitate electrical connections. The non-working surfaces were sealed with epoxy resin, leaving only the 10 mm × 10 mm working surface exposed. To ensure consistent surface roughness, the working surfaces were sequentially ground with silicon carbide sandpaper of 120, 240, 400, 600, and 800 grit sizes. After grinding, the coupons were rinsed with acetone, cleaned with anhydrous ethanol, and dried by using cold air. Finally, the prepared coupons were sterilized under a UV lamp for 20 min before use.
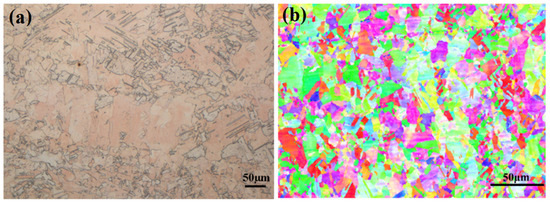
Figure 1.
The microstructures of pure copper: (a) metallographic structures; (b) inverse pole (IP) figures.
2.2. Bacteria and Culture Medium
The sulfate-reducing bacteria (Desulfovibrio desulfuricans) used in this study were obtained from the China General Microbiological Culture Collection Center (CGMCC; Beijing, China) [31]. The bacteria were cultured in a medium with the following composition (g/L): MgSO4 0.2, K2HPO4 0.01, NaCl 10, (NH4)2Fe(SO4)2 0.2, yeast extract 1, sodium lactate 4, and vitamin C 0.1 [19]. The pH of the medium was adjusted to 7.0–7.2 by using 1 mol/L NaOH solution. Simulated oilfield-produced water, whose chemical composition is detailed in Table 1, was used as the test solution. Both the culture medium and the simulated oilfield-produced water were sterilized by autoclaving them at 121 °C for 20 min. After sterilization, the solutions were purged with high-purity nitrogen gas for 2 h to remove dissolved oxygen.

Table 1.
The chemical composition of the simulated oilfield-produced water.
For inoculation, D. desulfuricans was pre-activated in the culture medium at a constant temperature for 12 h in an incubator. Subsequently, 5% (v/v) of the bacterial suspension was added to the culture medium. After 48 h of activation, 5% (v/v) of the activated culture medium was introduced into the simulated oilfield-produced water to prepare the test solution. All anaerobic operations, including inoculation, were conducted in a sterile anaerobic chamber filled with filter-sterilized high-purity N2 gas.
2.3. SRB Cell Count
Planktonic cells were counted by using a hemocytometer with an optical microscope (Axio Lab. A1, Zeiss, Oberkochen, Germany) at 400× magnification. Sessile cells that had adhered to the coupon surfaces were quantified by using the most probable number (MPN) method. After a 14-day immersion test, the SRB cells on the specimen surfaces were dislodged by vigorous mixing in 10 mL of physiological saline by using an XH-B vortex mixer. A 1 mL aliquot of the resulting solution was inoculated into an anaerobic tube containing 9 mL of sterile culture medium. Serial dilutions were then prepared for further analysis.
2.4. Weight Loss Tests
After a 14-day immersion, surface corrosion products and biofilms were removed by using a 10% H2SO4 solution, following ASTM standard G1-03 [32]. The weight loss of the coupons before and after immersion was measured with an analytical balance (Toledo AB304-S, OH, USA). Three parallel coupons were tested under each condition to minimize errors. The corrosion rate (CR, mm/y) was calculated by using the following formula [33,34]:
where A is the working area (cm2), ρ is the density of copper (g/cm3), Δm is the weight loss (g), and t is the immersion time (h).
2.5. Electrochemical Measurements
Electrochemical corrosion tests were performed by using a three-electrode system connected to a Gamry Reference 600+ workstation. The reference electrode (RE), working electrode (WE), and counter electrode (CE) were a saturated calomel electrode (SCE), the copper coupon, and a platinum plate, respectively. Electrochemical impedance spectroscopy (EIS) measurements were conducted over a frequency range of 10−2 to 105 Hz after ensuring a stable open-circuit potential (OCP). A sinusoidal voltage perturbation of 10 mV in amplitude was applied. EIS data were analyzed by using ZSimpWin (version 3.21). Potentiodynamic polarization tests were conducted within a potential range of −0.25 V to +0.25 V versus OCP at a scan rate of 0.167 mV/s. All electrochemical tests were performed at room temperature.
2.6. Surface Morphology Characterization
After immersion, the coupons were gently washed with phosphate-buffered saline (PBS) to remove loose surface impurities. The SRB sessile cells on the coupon surfaces were fixed by immersing the samples in PBS containing 4% glutaraldehyde for 2 h. Dehydration was performed by using a graded ethanol series (50%, 75%, and 100% by volume), with each step lasting 10 min. The dehydrated coupons were dried in cold air and stored in a vacuum desiccator before testing.
The surface morphology and elemental composition of the corrosion products and biofilms were analyzed by using a scanning electron microscope (SEM; XL30-FEG, FEI, Netherlands) with energy-dispersive X-ray analysis (EDXA). High-resolution spectra of Fe 2p and S 2p were obtained by using an X-ray photoelectron spectrometer (XPS; ESCALAB 250, Thermo VG, USA), and curve fitting was performed by using XPS PEAK 4.1 software.
After biofilm and corrosion product removal, the surface corrosion morphology was further examined by using a SEM. Additionally, a confocal laser scanning microscope (CLSM; ZEISS LSM700, Germany) was employed to measure the distribution and depth of corrosion pits on the coupon surfaces.
3. Results
3.1. Evaluation of Antibacterial Performance of CPC
The planktonic SRB cell counts and pH changes over a 14-day incubation period in simulated oilfield-produced water with varying CPC concentrations are shown in Figure 2. Table 2 further summarizes the sessile SRB cell counts at the start and end of the incubation period. An increase in CPC concentration led to a gradual rise in the pH of the test solution over the 14-day incubation. Without CPC, the planktonic SRB cell counts initially increased, peaking on the 5th day at 6.4 × 106 cells/mL, before slightly decreasing by the 14th day but remaining above 106 cells/mL. In contrast, the addition of CPC significantly inhibited SRB growth. With 10 mg/L CPC, planktonic SRB cell counts followed a similar initial trend but became undetectable by the 7th day. At 50 mg/L and 80 mg/L CPC, planktonic SRB cells fell below detection limits by the 3rd and 5th days, respectively. Sessile SRB cells followed a similar trend. Without CPC, their numbers increased to 7.5 × 107 cells/cm2 after 14 days. In contrast, sessile SRB cells were entirely undetectable after 14 days when CPC was added at concentrations of 10 mg/L, 50 mg/L, or 80 mg/L. Previous studies have shown that sessile SRB cells are more challenging to eliminate due to biofilm formation, which provides physical protection and enhances their resistance to antimicrobials [35]. However, the ability of CPC to penetrate biofilms and inhibit sessile SRB cells, as demonstrated here, highlights its potency compared with traditional biocides.
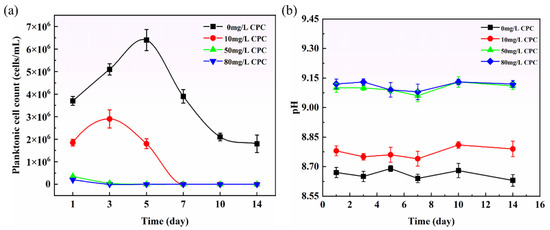
Figure 2.
The change in planktonic SRB cell counts and pH values with different CPC concentrations during the 14-day incubation in the simulated oilfield-produced water: (a) planktonic SRB cell counts; (b) pH values.

Table 2.
Sessile cell counts (cells/cm2) at the beginning and end of the 14–day incubation period with different CPC concentrations in the simulated oilfield-produced water.
The observed increase in pH with rising CPC concentrations may also contribute to its antibacterial effects. Higher pH levels can suppress SRB metabolic activity, as reported by Kielemoes et al. [36], who noted that SRB enzymatic processes, including sulfate reduction, are inhibited under alkaline conditions. This dual mechanism—direct bacterial membrane disruption by CPC and indirect inhibition through pH elevation—provides a comprehensive approach to controlling SRB populations in oilfield environments. These findings demonstrate the significant potential of CPC as a biocide for controlling SRB in simulated oilfield-produced water. Its ability to suppress both planktonic and sessile SRB cells at relatively low concentrations is particularly valuable for mitigating MIC in industrial applications.
3.2. Weight Loss
The corrosion rate of pure copper during a 14-day immersion in simulated oilfield-produced water is presented in Figure 3a. The results reveal that the corrosion rate in the biotic group (with SRB) was significantly higher than that in the abiotic group at the same CPC concentrations, demonstrating the pronounced role of SRB in accelerating the corrosion of pure copper. This finding is consistent with prior studies showing that SRB can enhance corrosion through mechanisms such as sulfide production and biofilm formation, which destabilize the protective oxide layer on metal surfaces. Interestingly, the corrosion rates in both the abiotic and biotic groups decreased consistently with the increase in CPC concentrations, demonstrating the effective inhibitory role of CPC. For instance, at 10 mg/L CPC, the corrosion rate in the biotic group decreased from 0.069 to 0.0167 mm/y, marking a nearly fourfold reduction. To quantitatively assess the inhibitory effect of CPC, the corrosion inhibition efficiency (ƞe) was calculated by using the following equation [34]:
where ƞe, (CR)untreated, and (CR)CPC represent the extent of corrosion inhibition, the corrosion rate (mm/y) without CPC under abiotic and biotic conditions, and the corrosion rate (mm/y) after CPC application under the corresponding condition, respectively. Under abiotic conditions, 10 mg/L CPC reduced the corrosion rate by 50%, whereas in the biotic group, the inhibition efficiency reached 76%. At the higher CPC concentrations of 50 mg/L and 80 mg/L, the corrosion rates further decreased to 0.006 mm/y and 0.005 mm/y, with inhibition efficiencies of 68% and 73%, respectively, under abiotic conditions. In the biotic group, the corrosion rates dropped to 0.009 mm/y and 0.008 mm/y, achieving impressive inhibition efficiencies of 87% and 89%, respectively. These results underscore the dual efficacy of CPC: it not only acts as a potent inhibitor against SRB-induced corrosion but also provides corrosion protection under abiotic conditions. The enhanced inhibition efficiency observed in the biotic group suggests that CPC effectively targets SRB-induced corrosion pathways, potentially by disrupting SRB biofilms and metabolic activity. Furthermore, the effectiveness of CPC in reducing the corrosion rate of pure copper under abiotic conditions highlights its ability to interact with copper surfaces, potentially forming a protective adsorption layer that limits further electrochemical reactions.
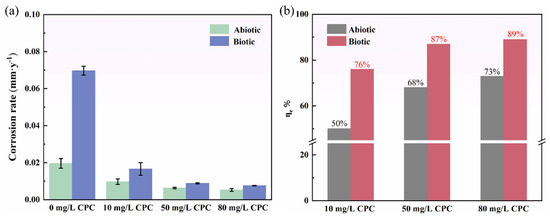
Figure 3.
Corrosion rate based on weight loss data in the absence and presence of SRB after 14-day incubation: (a) corrosion rate; (b) corrosion inhibition efficiency.
3.3. EIS Measurements
The diagrams in Figure 4 and Figure 5 show the EIS data, including Nyquist and Bode information, for different concentrations of CPC in the simulated oilfield-produced water. As shown in Figure 4, the diameter of the impedance semicircle curve significantly decreased after inoculation with SRB compared with the abiotic group, indicating that the corrosion rate of the biotic group was higher than that of the abiotic group. Furthermore, in the same environment, as the concentration of CPC increased, the diameter of the impedance semicircle curve were increased, which represented that the higher the concentration of CPC, the better the corrosion inhibition ability. These results were consistent with the weight loss data.
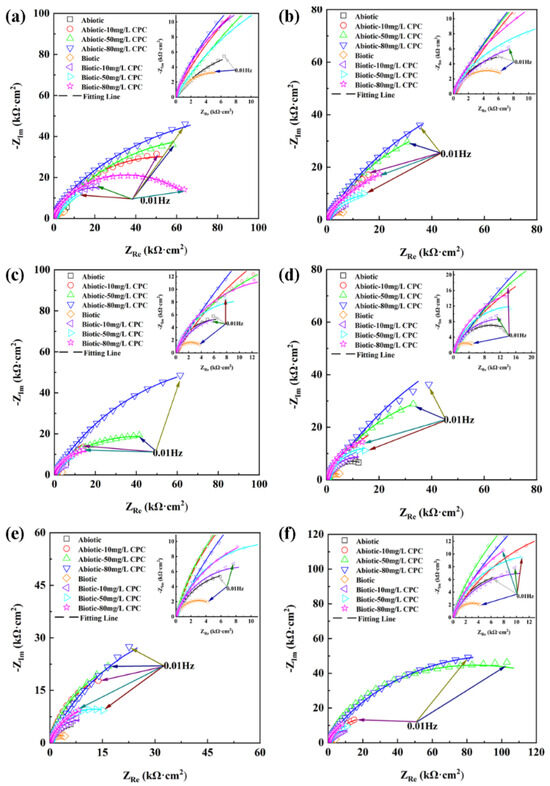
Figure 4.
Nyquist plots for pure copper in the absence and presence of SRB after 14-day incubation with different contents of CPC. (a) After 1 day; (b) 3 days; (c) 5 days; (d) 7 days; (e) 10 days; and (f) 14 days.
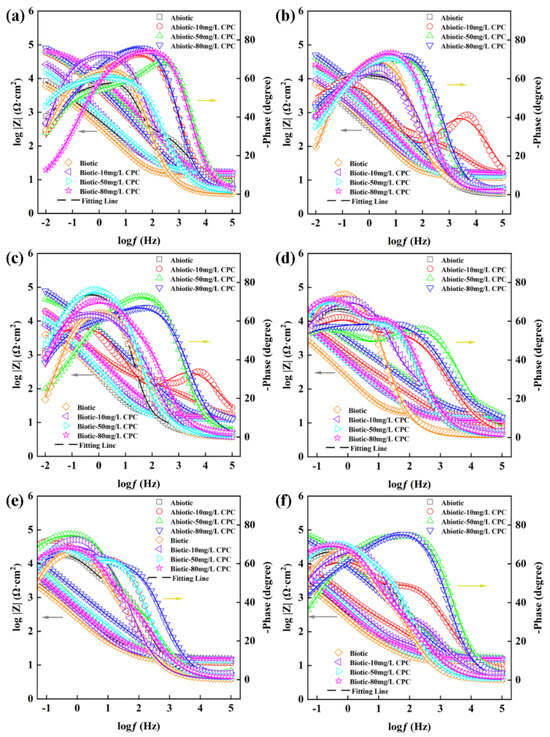
Figure 5.
Bode plots for pure copper in the absence and presence of SRB after 14-day incubation with different contents of CPC. (a) After 1 day; (b) 3 days; (c) 5 days; (d) 7 days; (e) 10 days; and (f) 14 days.
For fitting the EIS data, the equivalent circuit Rs(Qf(Rf(QdlRct))) was adopted to a calculated model, where Rct, Rf, and Rs stand for charge transfer resistance, product resistance, and solution resistance, respectively. In addition, Qf and Qdl represent the capacitance of product film and a constant-phase element (CPE) denoting the capacitance of the electric double layer, respectively. The fitting electrochemical results in the absence and presence of SRB are displayed in Table 3 and Table 4. Additionally, the corrosion resistance (Rp) was calculated according to the following equation [37]:
where ZF, ω, and YF represent Faraday resistance, frequency, and admittance, respectively. Actually, Rp is the sum of Rct and Rf (Rp = Rct + Rf), and Figure 6a displays the variation in Rp values with time for different contents of CPC. In the presence of SRB, it was observed that the Rp value significantly increased when CPC was added to the simulated oilfield-produced water, and the Rp value increased with the increase in CPC content, indicating that CPC could reduce the MIC of pure copper in simulated oilfield-produced water. And as its content increased, the inhibitory effect became more significant. Moreover, the change in Rp value under the abiotic condition also showed the same trend, revealing that CPC also had corrosion inhibition performance in the abiotic environment. It was reported that the value of 1/Rp was directly proportional to the corrosion rate; therefore, the decrease in the 1/Rp value after the introduction of CPC can be applied to represent the corrosion inhibition efficiency, as indicated in Figure 6b. Obviously, on the first day, the corrosion inhibition efficiency reached over 75% at 10 mg/L CPC in the absence of SRB. In addition, the corrosion inhibition efficiency at 80 mg/L CPC remained above 90% during the 14-day corrosion period, indicating that with the increase in CPC content, its corrosion inhibition efficiency was higher. Although the corrosion inhibition efficiency trend of CPC under biotic conditions was consistent with that under the abiotic condition, the overall corrosion inhibition efficiency was slightly lower than that of the abiotic group, which was different from the corrosion inhibition efficiency based on weight loss data. It is noteworthy that the weight loss data indicate the cumulative metal loss during the whole 14-day inoculation period, while Rp data are the electrochemical results measured instantaneously and also affected by physiological changes in SRB [19], so the difference was expected. In summary, CPC possessed a corrosion inhibition effect on pure copper in simulated oilfield-produced water, and the corrosion inhibition efficiency improved with the increase in CPC content.

Table 3.
Fitted EIS parameters of pure copper in the absence of SRB after 14-day incubation with different contents of CPC.

Table 4.
Fitted EIS parameters of the pure copper in the presence of SRB after 14-day incubation with different contents of CPC.
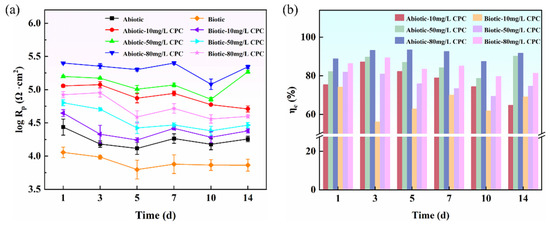
Figure 6.
Changes in corrosion resistance Rp with time calculated from EIS parameters (a) and corresponding efficiency of inhibition via CPC based on the value of 1/Rp (b).
3.4. Potentiodynamic Polarization
The potentiodynamic polarization curves for pure copper in abiotic and biotic simulated oilfield-produced water with varying CPC concentrations after 14 days of immersion are shown in Figure 7, with the corresponding Tafel fitting results summarized in Table 5. The trends observed under both abiotic and biotic conditions were consistent, revealing that the addition of CPC significantly influenced the electrochemical behavior of pure copper. Specifically, the incorporation of CPC caused a positive shift in the corrosion potential (Ecorr) and a noticeable leftward shift in the polarization curves, indicating reduced corrosion activity.
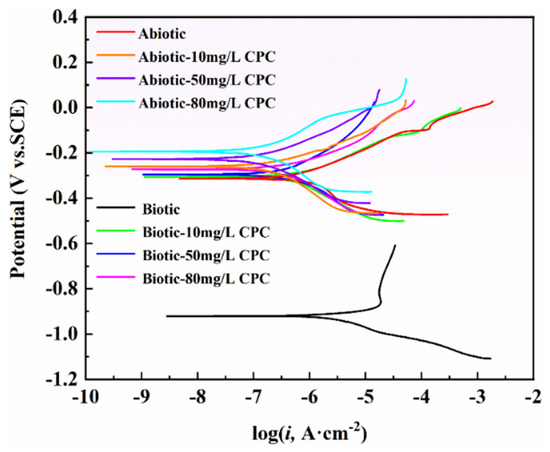
Figure 7.
Potentiodynamic polarization curves of the samples tested in SRB-inoculated solution with different CPC concentrations.

Table 5.
Tafel parameters fitted from the potentiodynamic polarization curves in Figure 7.
For example, the introduction of 10 mg/L CPC increased the corrosion potential from −0.920 V to −0.307 V, while the corresponding corrosion current density (icorr) decreased substantially from 3.47 μA/cm2 to 0.550 μA/cm2. These results highlight the strong inhibitory effect of CPC on the corrosion process. In addition, the corrosion efficiency was calculated by the following equation, relying on the icorr data of CPC with different contents [38]:
where (icorr)untreated and (icorr)CPC represent the corrosion current density in the absence and presence of CPC under abiotic and biotic conditions, respectively. As shown in Table 5, the corrosion inhibition efficiencies of CPC at concentrations of 10 mg/L, 50 mg/L, and 80 mg/L in the biotic group were 84%, 87%, and 91%, respectively. These values are in close agreement with the inhibition efficiencies derived from the weight loss data, further confirming the reliability and effectiveness of CPC as a corrosion inhibitor. The significant reduction in icorr and the associated positive shift in Ecorr suggest that CPC effectively suppresses both anodic and cathodic reactions, acting as a mixed-type inhibitor. Additionally, the increase in inhibition efficiency with higher CPC concentrations indicates a dose-dependent protective effect, likely due to enhanced adsorption of CPC molecules on the copper surface. This adsorption forms a protective barrier, reducing the access of corrosive agents such as SRB metabolites or chloride ions to the copper surface. Overall, the potentiodynamic polarization results confirm that CPC exhibits excellent corrosion inhibition performance against both abiotic and SRB-induced corrosion in simulated oilfield environments, with efficacy improving as the concentration of CPC increases.
3.5. Characterization of Surface Films and Corrosion Products
The SEM morphologies of surface films and corrosion products on pure copper coupons after a 14-day immersion period under abiotic and biotic conditions with varying CPC concentrations are presented in Figure 8, while the corresponding energy-dispersive X-ray spectroscopy (EDXA) results are summarized in Table 6. In the absence of CPC, the biotic group exhibited significantly higher amounts of corrosion products compared with the abiotic group. The surface of the coupons under biotic conditions was characterized by dense layers of corrosion products and visible clusters of SRB cells, indicating an aggressive MIC process. Notably, sulfur (S), a key element associated with SRB activity, was detected in high concentrations, confirming the metabolic activity of SRB and the formation of sulfide-based corrosion products. Upon the introduction of CPC, a dramatic reduction in surface corrosion products was observed. For instance, at a CPC concentration of 10 mg/L, no large clusters of corrosion products or SRB cells were visible, and the sulfur content on the surface was reduced to undetectable levels, as indicated by the EDXA results. This suggests that CPC effectively disrupts the metabolic activity of SRB and inhibits the formation of sulfide-rich corrosion products.

Figure 8.
The SEM morphologies at 5000× and 1000× of the surface films and corrosion products after the 14-day inoculation period in the (a,c,e,g) absence and (b,d,f,h) presence of SRB with different concentrations of CPC: (a,b) 0 mg/L, (c,d) 10 mg/L, (e,f) 50 mg/L, and (g,h) 80 mg/L.

Table 6.
EDXA mapping results of the films and corrosion products on the coupon surface in the absence and presence of SRB with different contents of CPC in simulated oilfield-produced water.
Figure 9 presents the cross-sectional images of the surface films on the coupons under different conditions. The results revealed that in the biotic group without CPC, the corrosion product films were significantly thicker compared with the abiotic group, indicating deeper corrosion penetration into the substrate due to MIC. However, with the addition of CPC, the thickness of the corrosion product films was markedly reduced. At the CPC concentrations of 50 mg/L and 80 mg/L, the corrosion product films were almost absent, demonstrating the strong inhibitory effect of CPC on the formation and accumulation of corrosion products. These results highlight the dual role of CPC in combating MIC: (1) inhibiting the activity of SRB, thereby reducing the biogenic sulfide production that accelerates corrosion, and (2) limiting the accumulation of corrosion products on the copper surface.
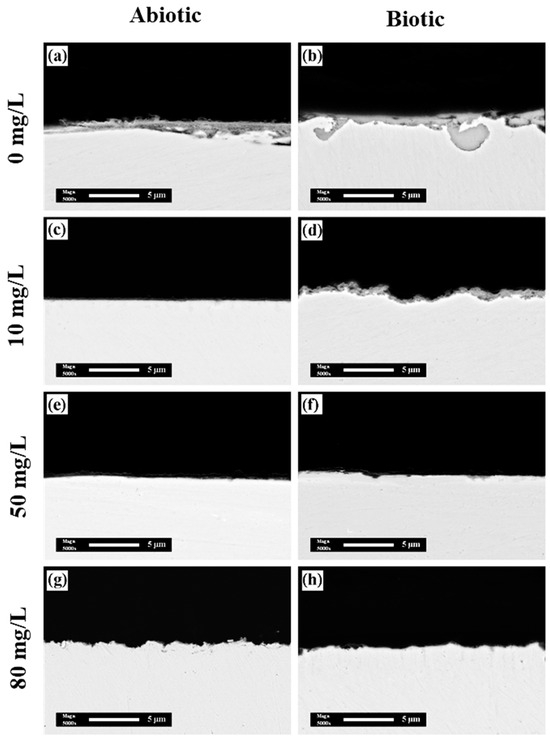
Figure 9.
Sectional images of films on the coupons’ surface with different contents of CPC in the (a,c,e,g) absence and (b,d,f,h) presence of SRB with different concentrations of CPC: (a,b) 0 mg/L, (c,d) 10 mg/L, (e,f) 50 mg/L, and (g,h) 80 mg/L.
3.6. Pit Analysis
Figure 10 illustrates the SEM morphologies of the pure copper coupons after the removal of surface films, under both abiotic and biotic conditions, with varying concentrations of CPC. In the absence of CPC, the coupons immersed in simulated oilfield-produced water exhibited significant pitting corrosion. Notably, the biotic group showed higher density and size of corrosion pits compared with the abiotic group, reflecting the aggressive nature of MIC caused by SRB.
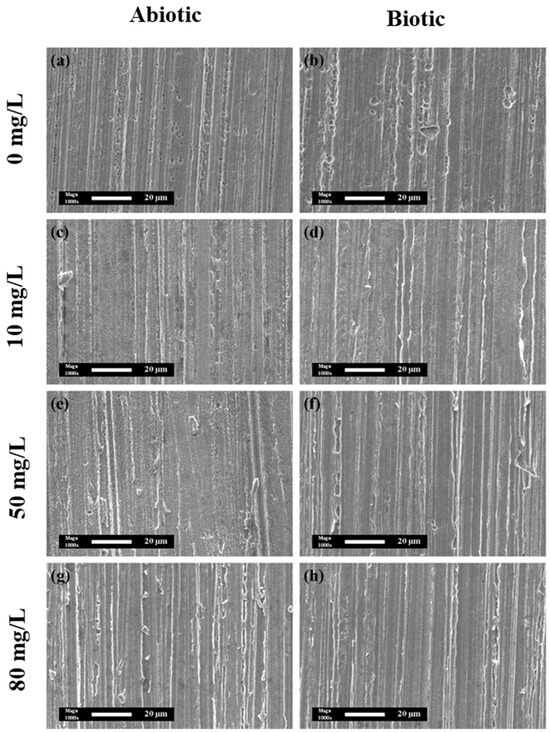
Figure 10.
SEM morphologies at 5000× and 1000× with corrosion products removed on the surface of pure copper in the (a,c,e,g) absence and (b,d,f,h) presence of SRB with different concentrations of CPC: (a,b) 0 mg/L, (c,d) 10 mg/L, (e,f) 50 mg/L, and (g,h) 80 mg/L.
To quantify the severity of pitting corrosion, the 3D morphologies of the maximum pits are presented in Figure 11, and the corresponding depths of the 10 largest pits are summarized in Figure 12. In the biotic group without CPC, the maximum pit depth reached 7.5397 μm, approximately 1.83 times greater than that of the abiotic group, where the maximum pit depth was 4.1259 μm. This significant difference underscores the role of SRB in enhancing localized corrosion through the production of sulfide species and biofilms that concentrate corrosive agents. The introduction of CPC markedly reduced the severity of pitting corrosion. At CPC concentrations of 10 mg/L, 50 mg/L, and 80 mg/L, the maximum pit depths were significantly decreased to 2.9739 μm, 1.1094 μm, and 1.0204 μm, respectively. This reduction demonstrates CPC’s efficacy in mitigating localized MIC on pure copper in simulated oilfield-produced water.
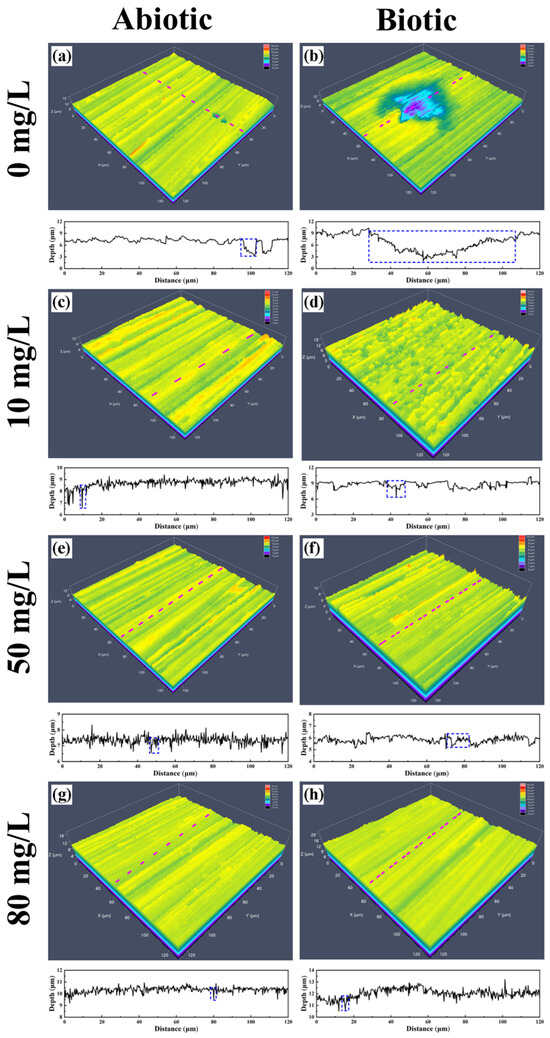
Figure 11.
CLSM images of pits on the surface of pure copper in the (a,c,e,g) absence and (b,d,f,h) presence of SRB with different concentrations of CPC: (a,b) 0 mg/L, (c,d) 10 mg/L, (e,f) 50 mg/L, and (g,h) 80 mg/L.
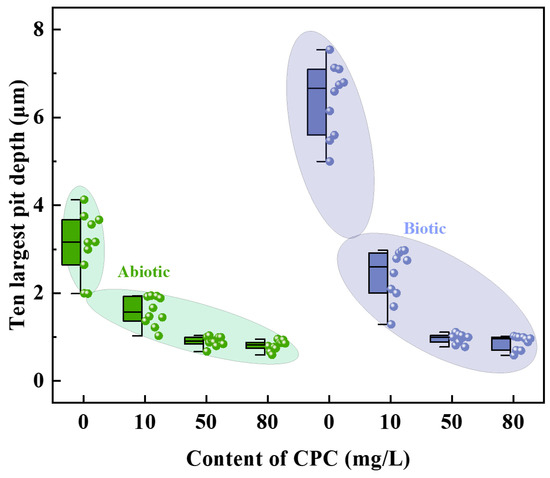
Figure 12.
Three-dimensional morphologies of pits with corrosion products removed on the surface of pure copper for different contents of CPC in the absence and presence of SRB.
4. Discussion
The weight loss and electrochemical results demonstrated that SRB significantly accelerated the corrosion of pure copper, a phenomenon widely attributed to biofilm formation and metabolic by-products such as hydrogen sulfide and sulfides. These findings align with earlier studies [39,40], which identified biofilm-facilitated sulfide production as a primary driver of MIC in copper and its alloys. H2S generated by SRB can react with copper to form Cu2S, a porous and poorly protective film that exacerbates localized corrosion.
CPC, as a quaternary ammonium compound, showed significant corrosion inhibition in both abiotic and biotic environments. Its inhibition efficiency increased with the concentration, reaching over 89% at 80 mg/L in SRB-containing environments. The positive shift in corrosion potential and reduction in corrosion current density observed in the polarization curves suggest that CPC acts as a mixed-type inhibitor, influencing both anodic and cathodic reactions. One of the key mechanisms by which CPC mitigates MIC is through its antimicrobial properties. Quaternary ammonium compounds (QACs) such as CPC are well documented for their ability to disrupt bacterial membranes, leading to cell lysis and biofilm disruption [34]. Biofilm formation by SRB creates localized anaerobic environments that promote sulfide production and electrochemical activity. CPC interferes with biofilm development by reducing bacterial adhesion and quorum sensing, thereby suppressing SRB metabolic activity and sulfide generation. The SEM and EDX results in this study confirmed a drastic reduction in surface sulfur content and biofilm coverage in the presence of CPC. This suggests that CPC not only inhibits SRB growth but also prevents the accumulation of sulfide corrosion products such as Cu2S, which are known to act as cathodic depolarizers, accelerating the corrosion process [41]. By disrupting the biofilm and sulfide pathways, CPC reduces the MIC-driving mechanisms at their source.
CPC also plays a crucial role in forming a protective layer on the copper surface. The adsorption of CPC molecules on the metal surface occurs via electrostatic interactions between the positively charged ammonium head group and the negatively charged copper surface [42]. This adsorption creates a hydrophobic barrier that impedes the ingress of aggressive ions such as chloride and sulfide, thereby reducing electrochemical activity and corrosion rates. Polarization data and corrosion inhibition efficiencies calculated from weight loss and electrochemical methods corroborate CPC’s surface-blocking behavior. The inhibition efficiency increased with CPC concentration, reflecting enhanced surface coverage and adsorption strength. A previous study by Noor [43] similarly highlighted the importance of surface adsorption in the corrosion inhibition mechanisms of QACs.
Localized pitting, a main corrosion form of SRB MIC, was mitigated by CPC in this work. In the absence of CPC, the maximum pit depth in the SRB-containing group was nearly twice that in the abiotic group, driven by localized sulfide accumulation and biofilm activity. However, the introduction of CPC reduced pit depth by up to 86%, as evidenced by 3D profiling and SEM analysis. The reduction in pitting severity can be attributed to CPC’s dual action. First, by disrupting biofilms, CPC minimizes the localized concentration of metabolic by-products such as H2S that create differential aeration cells and drive pit nucleation. Second, the CPC adsorption layer acts as a physical barrier, preventing chloride ions and other aggressive species from penetrating the copper surface. Moreover, the hydrophobicity imparted by CPC adsorption reduces water retention on the copper surface, further mitigating pit initiation and propagation. This behavior has been observed in other studies on MIC inhibitors, such as the work by Kong et al. [44], which demonstrated the importance of hydrophobic surface modifications in reducing pitting severity in microbial environments.
The superior antibacterial properties of CPC offer a promising alternative to traditional biocides, particularly for mitigating MIC in industrial applications. However, practical considerations, including the cost-effectiveness, scalability, and application techniques of CPC in real-world oilfield environments, must be addressed for widespread implementation. Future research should focus on exploring CPC’s molecular interactions with SRB, as well as its long-term environmental impacts and its integration into existing corrosion management strategies in the oil and gas industry.
5. Conclusions
This study systematically investigated the MIC of pure copper caused by D. desulfuricans in simulated oilfield-produced water. This research study evaluated the effectiveness of CPC as a corrosion inhibitor, focusing on its impact on SRB biofilm activity, corrosion rates, electrochemical behavior, and pitting corrosion. CPC demonstrated significant inhibition of MIC through biofilm disruption and protective surface film formation, making it a highly effective solution for mitigating SRB-induced corrosion in industrial environments. The findings are summarized as follows:
- (1).
- SRB biofilms accelerated the corrosion of pure copper by producing metabolites such as hydrogen sulfide, which induced severe pitting and localized corrosion. Surface analysis revealed that corrosion products were thicker and more extensive in the presence of SRB compared with abiotic conditions.
- (2).
- CPC effectively inhibited SRB-induced corrosion, with an inhibition efficiency of up to 89% at 80 mg/L. Weight loss measurements and potentiodynamic polarization curves confirmed that CPC reduced corrosion rates under both biotic and abiotic conditions.
- (3).
- CPC disrupted SRB biofilm formation and metabolic activity, reducing the production of corrosive metabolites such as H2S. CPC adsorbed on the copper surface, forming a protective barrier that minimized metal dissolution and slowed cathodic and anodic reactions.
Author Contributions
Methodology, Y.H.; Investigation, Y.H., B.L., L.C. and B.W.; Resources, L.C.; Writing—original draft, Y.H.; Writing—review & editing, B.L., L.C., B.W., J.X. and C.S.; Supervision, J.X. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially funded by National Natural Science Foundation of China (52261022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Author Yong Hu was employed by the company Anhui Hailuo Environmental Protection Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Li, X.G.; Zhang, D.W.; Liu, Z.Y.; Li, Z.; Du, C.W.; Dong, C.F. Share corrosion data. Nature 2015, 527, 441–442. [Google Scholar] [CrossRef]
- Gu, T.Y.; Wang, D.; Lekbach, Y.; Xu, D.K. Extracellular electron transfer in microbial biocorrosion. Curr. Opin. Electrochem. 2021, 29, 100763. [Google Scholar] [CrossRef]
- Javaherdashti, R. A review of some characteristics of MIC caused by sulfate-reducing bacteria: Past, present and future. Anti Corros. Methods Mater. 1999, 46, 173–180. [Google Scholar] [CrossRef]
- Dong, X.C.; Zhai, X.F.; Yang, J.; Guan, F.; Zhang, Y.M.; Duan, J.Z.; Hou, B.R. Two metabolic stages of SRB strain Desulfovibrio bizertensis affecting corrosion mechanism of carbon steel Q235. Corros. Commun. 2023, 10, 56–68. [Google Scholar] [CrossRef]
- Li, Y.C.; Ru, J.; Al-Mahamedh, H.H.; Xu, D.K.; Gu, T.Y. Enhanced biocide mitigation of field biofilm consortia by a mixture of d-amino acids. Front. Microbiol. 2016, 7, 896. [Google Scholar] [CrossRef]
- Ilhan-Sungur, E.; Cotuk, A. Microbial corrosion of galvanized steel in a simulated recirculating cooling tower system. Corros. Sci. 2010, 52, 161–171. [Google Scholar] [CrossRef]
- Zhao, J.L.; Sun, D.; Arroussi, M.; Lian, T.Y.; Zhang, X.R.; Yang, C.G.; Yang, K. Effect of anodic polarization treatment on microbiologically influenced corrosion resistance of Cu-bearing stainless steel against marine Pseudomonas aeruginosa. Corros. Sci. 2022, 207, 110592. [Google Scholar] [CrossRef]
- Chen, S.Q.; Zhang, D. Study of corrosion behavior of copper in 3.5 wt.% NaCl solution containing extracellular polymeric substances of an aerotolerant sulphate-reducing bacteria. Corros. Sci. 2018, 136, 275–284. [Google Scholar] [CrossRef]
- Liu, H.Y.; Chen, C.Y.; Asif, M.; Zhao, T.; Lei, B.; Meng, G.; Liu, H.F. Mechanistic investigations of corrosion and localized corrosion of X80 steel in seawater comprising sulfate-reducing bacteria under continuous carbon starvation. Corros. Commun. 2022, 8, 70–80. [Google Scholar] [CrossRef]
- Smith, M.; Shibulal, B.; Burgess, H.; Cooper, I.; Moles, N.; Willows, A. Reaction mechanisms and diagnostic mineralogy of intertidal steel corrosion: An X-ray photoelectron spectroscopy study. Corros. Commun. 2024, 13, 68–82. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Z.P.; Zhang, Z.; Zhang, B.W.; Ma, G.J.; Yao, Q.; Gan, Z.H.; Wu, J.; Li, X.G. Influence of crevice width on sulfate-reducing bacteria (SRB)-induced corrosion of stainless steel 316L. Corros. Commun. 2021, 4, 33–44. [Google Scholar] [CrossRef]
- Dou, W.W.; Ru, J.; Peng, J.; Liu, J.L.; Chen, S.G.; Gu, T.Y. Investigation of the mechanism and characteristics of copper corrosion by sulfate reducing bacteria. Corros. Sci. 2018, 144, 237–248. [Google Scholar] [CrossRef]
- Tian, J.; Kim, T.; Lu, T.J.; Hodson, H.P.; Queheillalt, D.T.; Sypeck, D.J.; Wadley, H. The effects of topology upon fluid-flow and heat-transfer within cellular copper structures. Int. J. Heat Mass Transf. 2004, 47, 3171–3186. [Google Scholar] [CrossRef]
- Wang, Q.H.; Zhao, C.K.; Wang, R.Z.; Aslam, R.; Zhou, X.; Zhang, Q.; Yan, Z.T.; Li, Y.S.X.M.; Zheng, H.H. Rhododendron simsii leaf extract as a corrosion inhibitor for Cu in 0.5 M H2SO4: Experimental and theoretical studies. Colloids Surf. A Physicochem. Eng. Asp. 2024, 682, 132904. [Google Scholar] [CrossRef]
- Petrisor, I.G.; Kanner, A. Chinese Drywall—Environmental Forensic Opportunities. Environ. Forensics 2010, 11, 6–16. [Google Scholar] [CrossRef]
- Huang, G.; Chan, K.Y.; Fang, H.H.P. Microbiologically Induced Corrosion of 70Cu-30Ni Alloy in Anaerobic Seawater. J. Electrochem. Soc. 2004, 151, B434–B439. [Google Scholar] [CrossRef]
- Huttunen-Saarivirta, E.; Rajala, P.; Carpén, L. Corrosion behaviour of copper under biotic and abiotic conditions in anoxic ground water: Electrochemical study. Electrochim. Acta 2016, 203, 350–365. [Google Scholar] [CrossRef]
- Kahrilas, G.A.; Blotevogel, J.; Stewart, P.S.; Borch, T. Biocides in Hydraulic Fracturing Fluids: A Critical Review of Their Usage, Mobility, Degradation, and Toxicity. Environ. Sci. Technol. 2015, 49, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Hou, B.S.; Xiang, J.; Chen, X.D.; Gu, T.Y.; Liu, H.F. The performance and mechanism of bifunctional biocide sodium pyrithione against sulfate reducing bacteria in X80 carbon steel corrosion. Corros. Sci. 2019, 150, 296–308. [Google Scholar] [CrossRef]
- Xu, J.W.; Yin, Y.G.; Tan, Z.Q.; Wang, B.W.; Guo, X.R.; Li, X.; Liu, J.F. Enhanced removal of Cr(VI) by biochar with Fe as electron shuttles. J. Environ. Sci. 2019, 78, 109–117. [Google Scholar] [CrossRef]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of Water Treatment Membranes: A Review of the Underlying Causes, Monitoring Techniques and Control Measures. Membranes 2012, 2, 804–840. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Jabbar, K.A.; Rasool, K.; Pandey, R.P.; Sliem, M.H.; Helal, M.; Samara, A.; Abdullah, A.M.; Mahmoud, K.A. Controlling the Biocorrosion of Sulfate-Reducing Bacteria (SRB) on Carbon Steel using ZnO/Chitosan Nanocomposite as an Eco-Friendly Biocide. Corros. Sci. 2019, 148, 397–406. [Google Scholar] [CrossRef]
- Ubong, E.; Omar, F.; Jerzy, S. Effect of benzothiazole biocide on SRB-induced biocorrosion of hot-dip galvanized steel. Eng. Fail. Anal. 2018, 93, 111–121. [Google Scholar]
- Seter, M.; Thomson, M.J.; Chong, A.; Macfarlane, D.R.; Forsyth, M. Cetrimonium Nalidixate as a Multifunctional Inhibitor to Combat Biofilm Formation and Microbiologically Influenced Corrosion. Aust. J. Chem. 2013, 66, 921–929. [Google Scholar] [CrossRef]
- Parthipan, P.; Narenkumar, J.; Elumalai, P.; Preethi, P.S.; Usha, R.; Agrawal, A.; Rajasekar, A. Neem extract as a green inhibitor for microbiologically influenced corrosion of carbon steel API 5LX in a hypersaline environments. J. Mol. Liq. 2017, 240, 121–127. [Google Scholar] [CrossRef]
- Punniyakotti, P.; Punniyakotti, E.; Jayaraman, N.; Machuca, L.L.; Kadarkarai, M.; Karthikeyan, O.P.; Aruliah, R. Allium sativum (garlic extract) as a green corrosion inhibitor with biocidal properties for the control of MIC in carbon steel and stainless steel in oilfield environments. Int. Biodeterior. Biodegrad. 2018, 132, 66–73. [Google Scholar]
- Yamamoto, M.; Inokoshi, M.; Tamura, M.; Shimizubata, M.; Nozaki, K.; Takahashi, R.; Yoshihara, K.; Minakuchi, S. Development of 4-META/MMA-TBB resin with added benzalkonium chloride or cetylpyridinium chloride as antimicrobial restorative materials for root caries. J. Mech. Behav. Biomed. 2021, 124, 104838. [Google Scholar] [CrossRef]
- Raut, P.; Weller, S.R.R.; Obeng, B.; Soos, B.L.L.; West, B.E.E.; Potts, C.M.M.; Sangroula, S.; Kinney, M.S.S.; Burnell, J.E.E.; King, B.L.L.; et al. Cetylpyridinium chloride (CPC) reduces zebrafish mortality from influenza infection: Super-resolution microscopy reveals CPC interference with multiple protein interactions with phosphatidylinositol 4,5-bisphosphate in immune function. Toxicol. Appl. Pharm. 2022, 440, 115913. [Google Scholar] [CrossRef]
- Naoe, T.; Hasebe, A.; Horiuchi, R.; Makita, Y.; Okazaki, Y.; Yasuda, K.; Matsuo, K.; Yoshida, Y.; Tsuga, K.; Abe, Y.; et al. Development of tissue conditioner containing cetylpyridinium chloride montmorillonite as new antimicrobial agent: Pilot study on antimicrobial activity and biocompatibility. J. Prosthodont. Res. 2020, 64, 436–443. [Google Scholar] [CrossRef]
- Khamis, A.; Saleh, M.M.; Awad, M.I. Synergistic inhibitor effect of cetylpyridinium chloride and other halides on the corrosion of mild steel in 0.5 M H2SO4. Corros. Sci. 2013, 66, 343–349. [Google Scholar] [CrossRef]
- Xu, D.; Li, Y.; Gu, T. A synergistic D-tyrosine and tetrakis hydroxymethyl phosphonium sulfate biocide combination for the mitigation of an SRB biofilm. World J. Microbiol. Biotechnol. 2012, 28, 3067–3074. [Google Scholar] [CrossRef]
- ASTM G1-03(2017)e1; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens, G01.05. ASTM International: West Conshohocken, PA, USA, 2017.
- Wei, B.; Qin, Q.; Bai, Y.; Yu, C.; Xu, J.; Sun, C.; Ke, W. Short-period corrosion of X80 pipeline steel induced by AC current in acidic red soil. Eng. Fail. Anal. 2019, 105, 156–175. [Google Scholar] [CrossRef]
- Cai, Z.; Wei, B.; Xu, J.; Yan, M.; Ren, Y.; Sun, C. Antibacterial and anticorrosion effects of Cetylpyridinium chloride against Desulfovibrio desulfuricans biofilm and X80 steel corrosion. Constr. Build. Mater. 2024, 414, 134932. [Google Scholar] [CrossRef]
- Videla, H.A.; Herrera, L.K. Microbiologically influenced corrosion: Looking to the future. Int. Microbiol. 2005, 8, 169–180. [Google Scholar]
- Moloantoa, K.; Khetsha, Z.; Mochane, M.; Unuofin, J.; Atangana, A.; Cason, E.; van Heerden, E.; Castillo, J. Evaluating the effects of pH and temperature on sulphate-reducing bacteria and modelling of their effects in stirred bioreactors. Environ. Pollut. Bioavailab. 2023, 35, 2257388. [Google Scholar] [CrossRef]
- Xu, J.; Wang, K.X.; Sun, C.; Wang, F.H.; Li, X.M.; Yang, J.X.; Yu, C.K. The effects of sulfate reducing bacteria on corrosion of carbon steel Q235 under simulated disbonded coating by using electrochemical impedance spectroscopy. Corros. Sci. 2011, 53, 1554–1562. [Google Scholar] [CrossRef]
- Liu, H.W.; Gu, T.Y.; Lv, Y.L.; Asif, M.; Xiong, F.P.; Zhang, G.A.; Liu, H.F. Corrosion inhibition and anti-bacterial efficacy of benzalkonium chloride in artificial CO-saturated oilfield produced water. Corros. Sci. 2017, 117, 24–34. [Google Scholar] [CrossRef]
- Wei, B.X.; Xu, J.; Gao, L.Q.; Feng, H.; Wu, J.J.; Sun, C.; Wang, Z.Y.; Ke, W. Nanosecond pulsed laser-assisted modified copper surface structure: Enhanced surface microhardness and microbial corrosion resistance. J. Mater. Sci. Technol. 2022, 107, 111–123. [Google Scholar] [CrossRef]
- Chen, L.J.; Li, J.J.; Wei, B.; Xu, J.; Sun, C. Accelerated microbiologically influenced corrosion of copper by sulfate-reducing bacterium Desulfovibrio desulfovibrio. Mater. Corros. 2024, 75, 1495–1505. [Google Scholar] [CrossRef]
- Hamilton, W.A. Microbially influenced corrosion as a model system for the study of metal microbe interactions: A unifying electron transfer hypothesis. Biofouling 2003, 19, 65–76. [Google Scholar] [CrossRef]
- Yadav, C.K.; Shahi, N.; Adhikari, M.K.; Neupane, S.; Rakesh, B.; Yadav, A.P.; Bhattarai, A. Effect of cetyl pyridinium chloride on corrosion inhibition of mild steel in acidic medium. Int. J. Electrochem. Sci. 2024, 19, 100776. [Google Scholar] [CrossRef]
- Noor, E.A. Comparative Study on the Corrosion Inhibition of Mild Steel by Aqueous Extract of Fenugreek Seeds and Leaves in Acidic Solutions. J. Eng. Appl. Sci. 2008, 3, 23–30. [Google Scholar]
- Kong, L.; Zhang, Z.; Jiao, H.; Liu, Y.; Yang, H. Effect of hydrophobic modification on the anti-microbial induced corrosion of alkali-activated mortar. Constr. Build. Mater. 2005, 458, 139576. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).