Abstract
This paper studies the coatings deposited on a 65G steel substrate by electric arc metallization using a 30KhGSA wire. The properties of the coatings obtained at 30 V, 40 V and 45 V are discussed, including their microstructure, porosity, microhardness, coefficient of friction and corrosion resistance. The experiments showed that the coatings possess a layered structure formed by sequential deposition of metal microdroplets. It was found that the increase in voltage favors the decrease in porosity, increase in layer density, increase in microhardness and improvement in wear resistance and corrosion resistance. At maximum voltage (45 V), there are optimum performance characteristics, such as minimal porosity (1.36%), high microhardness (305 HV) and improved corrosion resistance. The main defects of the coatings, including pores and oxide inclusions, which are formed during the sputtering process and depend on the kinetic energy of the microdroplets, were identified. These defects affect the mechanical and protective properties of the coatings.
1. Introduction
In recent decades, there has been a significant increase in interest in developing materials with improved performance characteristics such as high wear resistance, corrosion resistance and thermal stability [1,2,3,4]. These properties are particularly important for components operating under extreme conditions, which is relevant for industries such as mechanical engineering, energy, transportation and agricultural machinery. The main operational problems faced by materials in such environments include premature wear, corrosion degradation and reduced mechanical strength, leading to reduced product life and increased maintenance and replacement costs [5,6,7,8]. One of the solutions to this problem is the use of volume-alloyed steels [9,10]. However, in conditions involving the increasing cost of alloying elements, it is advisable to use low-alloy steels with protective coating or modified surface layers [11,12,13,14,15,16]. Protective coatings can significantly increase the wear resistance and durability of parts while reducing production costs.
Steel 65G, due to its high mechanical properties, is widely used for the production of spring parts such as springs, leaf springs and other elements operating under high loads. However, due to its limited corrosion resistance, it requires additional protective measures to increase its durability in hostile environments. In this regard, the application of a protective coating based on 30KhGSA steel, known for its high corrosion and wear resistance, appears to be a promising approach. The combined use of 65G steel as a substrate and 30KhGSA steel as a coating opens up new opportunities for improving the durability and performance characteristics of parts in harsh operation conditions.
Today, there are many technologies that can be used for applying protective metal coatings, among which induction cladding [17,18,19], plasma spraying [20,21,22,23,24,25], arc metallization [26,27,28,29], gas-flame cladding [30,31,32,33] and others are widely used [34,35,36,37]. Among these, electric arc metallization technology has proved to be one of the most effective and economically feasible methods of metal coating application [38,39,40]. This method is characterized by relatively low operating costs, the simple maintenance of the technology and the ability to achieve higher spraying speeds. In addition, electric arc spraying provides significant economic benefits for the treatment of large surfaces, which contributes to its widespread application in industry [41,42,43,44]. The method is based on the melting of wire electrodes by means of an electric arc and the subsequent atomization of the molten metal by a jet of compressed air. This allows the obtainment of high deposition rates and the formation of coatings with a dense structure and good adhesion to the substrate, which significantly improves the mechanical and operational characteristics of surfaces. One of the advantages of electric arc metallization is the ability to flexibly adjust process parameters, such as voltage, current and wire feed rate, allowing the coating to be tailored to specific operating conditions.
The optimization of sputtering modes plays a key role in coating quality assurance. In the literature [45,46], it is noted that varying the process parameters such as voltage, current and electrode wire feed rate significantly affects the structure and properties of the resulting coating. These parameters directly determine the rate of material melting and deposition, which in turn affects the density of the coating and its adhesion to the substrate. The study presented in Ref. [38] examines in detail the effect of different modes of electric arc metallization on the mechanical and physical properties of coatings deposited on metal substrates. It was shown that the proper tuning of technological parameters allows the obtainment of high wear resistance and corrosion resistance of coatings.
In particular, increasing the stress leads to a denser coating, which improves its adhesion and enhances its mechanical properties such as microhardness and wear resistance. However, an excessive increase in stress can cause undesirable effects such as the overheating of the material and formation of microcracks in the coating. These changes in microstructure require further analysis to ensure that the coatings are as reliable as possible.
Previous studies [47,48] have already investigated the influence of process parameters such as stress on the structure and properties of coatings. For example, Rakhadilov et al. [47] showed that increasing the stress decreases the porosity of the coating and increases its microhardness. However, the effect of stress on corrosion resistance has not been sufficiently investigated, which limits the applicability of the results in corrosive environments. In another study by Rakhadilov et al. [48], they examined the properties of coatings from 30KhGSA steel obtained by supersonic arc metallization. It was found that the optimal stress promotes the formation of a dense coating structure with low porosity, but there are no data on the friction coefficient, nor is there a detailed analysis of the mechanical characteristics of the coatings.
The purpose of this study is to investigate the influence of electric arc metallization modes on the structure and properties of coatings from 30KhGSA steel applied on 65G steel. To achieve this goal, experimental work was carried out by varying the voltage parameter at constant values of current, wire feed rate and compressed air pressure. This approach allows for a detailed study of the effect of voltage on the coating structure and its performance properties. Also, despite the significant progress in the study of protective coatings, the application of coatings from 30KhGSA steel on 65G steel to improve its performance characteristics remains insufficiently investigated, which makes this work relevant for further research and industrial implementation.
The scientific novelty of the work comes from the fact that this is a detailed study of the influence of technological parameters of electric arc metallization on the properties of the coating, which allows us to propose new approaches to the development of highly effective protective coatings. The practical significance of the results lies in the possibility of their use for the optimization of coating technologies used in mechanical engineering, power engineering and other industries requiring high wear resistance and material strength.
2. Materials and Methods
Steel 65G, which is a high-carbon low-alloy steel with a carbon content of about 0.65%, was chosen as the substrate material. The substrate samples were made in the form of rectangular plates with dimensions of 25 × 25 × 10 mm. Before coating, the surface of the samples was thoroughly prepared. For this purpose, they were sanded with sandpaper with a sequential change in grit from 100 to 360 to achieve the necessary smoothness and removal of oxide films and contaminants. Then, sandblasting was carried out on Nordberg NS3 machine using electrocorundum to create surface roughness and increase the adhesion of the coating to the substrate. The chemical composition of 65G steel is presented in Table 1.

Table 1.
Chemical composition of 65G steel (by mass, %).
For conducting the experiments with electrodes, a steel wire of 30KhGSA grade (analog AISI 1330) with diameter of 1.4 mm, manufactured in accordance with the requirements of GOST 10543-98, was used. The chemical composition of 30KhGSA steel is given in Table 2.

Table 2.
Chemical composition of 30KhGSA steel (by mass, %).
Coating was carried out using supersonic arc metallizer model SX-600 (Guangzhou, China), which is a complex piece of equipment including a power supply, arc atomizer, control system and compressed air supply system (Figure 1). The pri nciple of operation of the metalizer is based on the melting of wire electrodes with an electric arc followed by atomization of the molten metal with a jet of compressed air [47]. Molten metal particles, deposited on the surface, form a continuous coating, while the thickness of the coating is controlled by the number of passes of the metalizer and the speed of its movement relative to the treated surface. The design of the arc metalizer provides for the presence of guides through which a constant supply of two metal wires. An electric arc is generated between their ends, and compressed air is fed through the nozzle of the metalizer. Compressed air with a velocity of more than 100 m/s tears off molten metal particles from the ends of the wires and transfers them to the surface of the workpiece, forming a coating with high adhesion and low porosity.
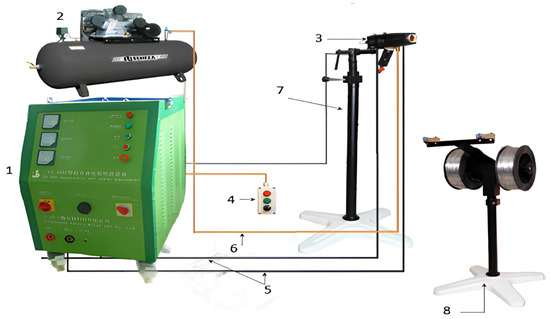
Figure 1.
General view of the complex for high-speed electric arc metallization SX-600: 1—welding rectifier, 2—piston compressor, 3—spray gun, 4—remote control panel, 5—power cables, 6—air hose, 7—manipulator, 8—wire spools (electrodes).
Coating experiments were carried out on 65G steel samples. Several sputtering modes were selected for the study by varying the voltage, while the current, wire feed rate, compressed air pressure and sputtering distance remained unchanged for all samples. The details of the sputtering modes are summarized in Table 3.

Table 3.
Spraying parameters for high-speed arc metallization on the SX600 machine.
The phase composition of the samples after the metallization process was analyzed using an X’Pert Pro X-ray diffractometer (Philips Corporation, Amsterdam, The Netherlands ), which allowed a qualitative and quantitative assessment of the crystalline phases in the coating. Measurements were performed at 45 kV and 40 mA current, using Cu-Kα radiation, over an angular range of 2θ from 20° to 90° in steps of 0.02°.
Scanning electron microscopy (SEM) was used to investigate the microstructure of the cross sections of the coatings after metallization. The analysis was performed on a Tescan Vega 4 scanning electron microscope (Tescan, Brno, Czech Republic) equipped with an energy dispersive spectroscopy (EDS) system for detailed analysis of elemental composition.
The microhardness of the investigated samples was measured by Vickers method on Metolab 502 device (Metolab, Moscow, Russia). Measurements were carried out at an indenter load of 0.2 N with a dwell time of 10 s. A pyramid-shaped diamond tip with an angle of 136° between the faces was used as an indenter.
Tribological tests were performed on a tribometer TRB3 (Anton Paar GmbH, Graz, Austria) using the “ball-disk” method. A steel ball made of ShX15 material with a diameter of 3 mm was used as a counterbody. The experiments were conducted at a normal load of 10 N, with a linear sliding velocity of 3 cm/s and a radius of wear curvature of 1.50 mm. The total friction path was 70 m.
Potentiodynamic polarization methods were used to analyze the corrosion behavior of the samples. The measurements were carried out in a three-electrode electrolytic cell, which provided the separation of cathodic and anodic spaces. The experiments were carried out at a temperature of 25 ± 10 °C in an atmosphere with air access and in NaCl solution with a concentration of 3.5 wt.%. A platinum electrode was used as an auxiliary electrode, and a chlorosilver electrode (Ag/AgCl in 1 M KCl) was used for comparison. The potentiodynamic polarization process was started at −0.1 V and terminated at 0.1 V relative to open circuit potential, with a potential sweep of 0.5 mV/s. A CS300M potentiostat/galvanostat (Wuhan Corrtest Instruments Co., Ltd., located in Wuhan, China) was used for polarization and related measurements.
3. Results and Discussion
The coatings obtained by electric arc metallization are characterized by a formation process consisting of the rapid deposition of molten metal droplets on the substrate. These droplets, striking the surface, flatten and solidify to form successive layers, which can be seen in micrographs obtained using a scanning electron microscope (Figure 2). Regardless of the selected mode, the coatings exhibit a layered structure that contributes to improved wear and corrosion resistance due to the uniform distribution of microstructural elements. The layered structure enhances the barrier properties of the protective layer by creating a tortuous pathway for corrosive media. This configuration significantly reduces the permeability of harmful substances such as moisture, oxygen and chemical compounds by forcing them through multiple interfaces. Each layer acts as a partial barrier, slowing the diffusion process and effectively limiting the depth of penetration. As a result, the protective layer exhibits increased durability as it better resists prolonged exposure to aggressive environments.
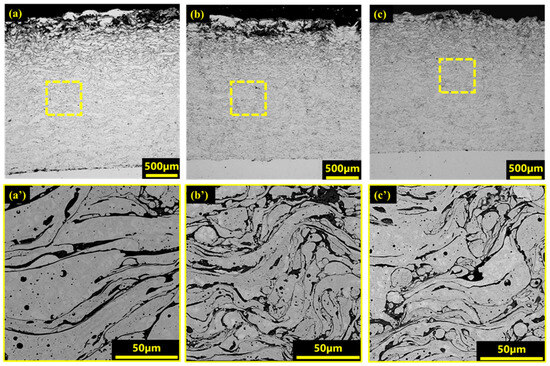
Figure 2.
Microstructure of coatings obtained using scanning electron microscope: (a,a’) V1; (b,b’) V2; (c,c’) V3.
However, along with positive characteristics, the coatings contain defects including pores and oxide inclusions. As noted by Li et al. [49], pores are formed due to air entrapment by molten particles during their solidification process. Porosity significantly affects corrosion resistance because pores can serve as channels for corrosive media to penetrate the substrate, leading to damage to both the coating and the substrate. According to the SEM images (Figure 2), the porosity of the coating varies with stress.
At low voltage (30 V), the coating (sample V1) shows porosity at 3.58% and a less homogeneous layer distribution compared to coatings obtained at higher voltages. The SEM images show a layer structure with varying thickness and density. This phenomenon is probably due to the low kinetic energy of the microdroplets, which leads to a decrease in the adhesive force between them and the formation of pores. In the literature [47], it has been observed that at low stress values, microdroplets are deposited less intensively and with lower energy, which increases the probability of pore formation and reduces the homogeneity. This also limits the adhesion between layers, resulting in increased porosity and weaker layer cohesion [50].
In the second mode, at 40 V (sample V2), a decrease in coating porosity to 2.72% and a more homogeneous structure compared to sample V1 are observed. SEM images demonstrate that in this regime, the deposition of metal microdroplets is more uniform, which ensures a tight fit of the layers to each other. In the article [45], it is noted that increasing the voltage improves the density and uniformity of the coating structure by reducing porosity, as the microdroplets are deposited with higher energy, filling micro voids and creating a denser coating. This also has a positive effect on the corrosion resistance of the coating, as fewer pores limit the penetration of aggressive media inside the material.
In the third mode, at a maximum voltage of 45 V, sample V3 showed the lowest porosity among all the samples studied, 1.36%, with the most homogeneous layer distribution. The high voltage at this mode increases the kinetic energy of microdroplets to a maximum, which allows them to firmly connect with the substrate surface and with each other, forming a dense coating. According to the literature [45], this dense coating effectively prevents corrosion processes and improves wear resistance. SEM images show that the layers form an almost continuous coating without pronounced interlayer boundaries, which also confirms the high degree of adhesion between the layers.
Oxide inclusions are a common defect formed during electric arc metallization. They can form in the air during particle flight, during contact with the substrate or during cooling. During the experiments, it was found that oxide inclusions in the coatings were predominantly concentrated in areas with high porosity and at interlayer boundaries, which indicates the dependence of the oxidation degree on the kinetic parameters of the sputtering process. The literature sources [51] indicate that excess oxides can reduce the quality of the coating, but their small content can have a positive effect on wear resistance. For example, a study [52,53] showed that the presence of oxides in the FeAl/Cr3C2 structure improves the abrasion resistance and increases the bonding strength of the layers.
According to the results of X-ray phase analysis, the phases and oxides presented in Figure 3 were detected on the studied samples. The highest number of oxides (Fe3O4, Fe2O3) was found in sample V1 at low voltage level. The oxide fraction was significantly higher in samples V2 and V1 compared to sample V3, where the voltage was highest. The lowest oxide intensity was observed in sample V3, which is attributed to the minimal development of oxidative processes with increasing voltage.
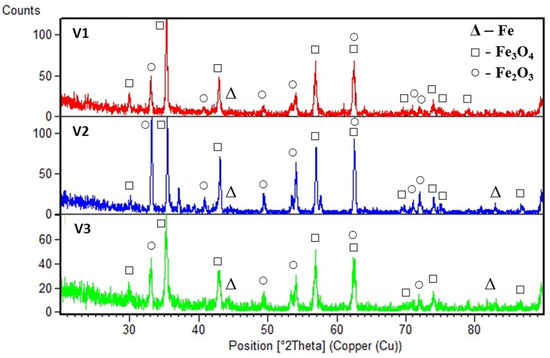
Figure 3.
Results of X-ray phase analysis.
The presence of the phases shown in Figure 3 confirms the occurrence of oxidative processes on the surface of the coatings. This is explained by the fact that the arc spraying process is carried out in an atmospheric environment, which inevitably leads to a partial oxidation of all coatings. The intensity of oxidation varies depending on the level of applied voltage: the increase in voltage contributes to a decrease in oxide content, which may be due to changes in the kinetics of layer formation during sputtering.
Figure 4 shows the microhardness values (HV0.1/10) for each sample. The graph shows that the microhardness of the coating increases as the voltage at which it was obtained increases. Sample V1, deposited at a low voltage of 30 V, shows a minimum microhardness of 258 HV. This value indicates a relatively less dense coating structure, which is attributed to the insufficient kinetic energy of the deposited microdroplets leading to pore formation and less adhesion between layers. When the voltage is increased to 45 V (sample V3), the microhardness of the coating increases to 305 HV, which is attributed to the improved density of the structure due to the increased deposition energy providing a tighter fit of the microdroplets to each other.
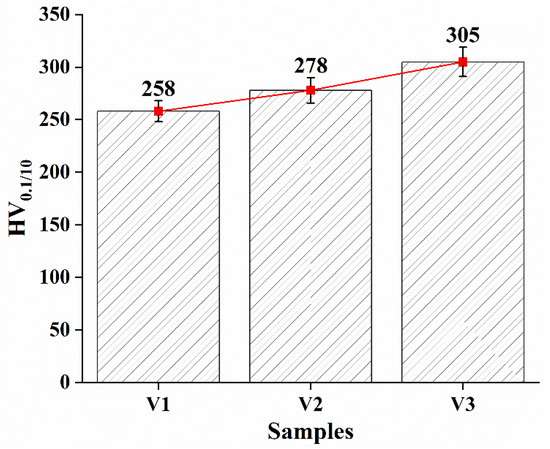
Figure 4.
Microhardness of the coating.
Figure 5 shows the dependence of the friction coefficient on the traveled distance for three investigated coating samples (V1, V2 and V3) obtained at different voltages (30 V, 40 V and 45 V, respectively). Figure 5 shows that the change in the friction coefficient for each sample passes through two distinct stages: an initial stage with a sharp increase in the friction coefficient and a subsequent stabilization stage where the friction coefficient remains almost constant. At the first 20 m of traveled distance, all three specimens show a sharp increase in the friction coefficient, indicating active processes of adaptation of the coating surface to the friction conditions. For specimen V1 (30 V voltage), the coefficient of friction increases from an initial value of about 0.1 to a stabilized level of about 0.4. A similar behavior is observed for sample V2 (voltage 40 V), where the friction coefficient increases to 0.4. Sample V3, formed at a maximum voltage of 45 V, shows the highest final coefficient of friction of about 0.5 after the adaptation stage.
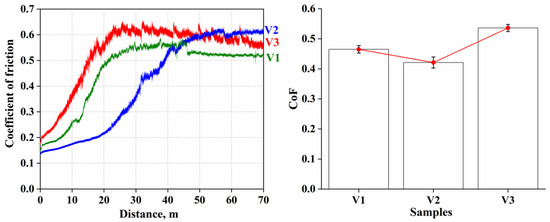
Figure 5.
Results of tribological tests of coatings: friction coefficient.
At this stage of friction, the coating surfaces are not yet fully adapted to mechanical action and have micro-irregularities and defects formed during the spraying process. During the initial contact between the surfaces of the wear body and the coating, these irregularities are microscopically broken, which causes friction and leads to a rapid increase in the coefficient of friction. These microscopic irregularities gradually break down and compact, creating a denser and flatter contact layer on the coating surface. In addition, during the friction process, material particles can move and even form localized adhesive bonds between the contacting surfaces, which also increases the friction resistance. The formation of such a stable layer on the coating surface explains why the coefficient of friction stabilizes after an initial sharp increase. It is important to note that the V3 sample, with a denser and harder coating structure, has a higher coefficient of friction because dense layers better resist wear but also create more resistance to contact.
After a sharp increase, the friction coefficient for all samples reaches its stabilized values and remains almost unchanged for the rest of the experiment. At this stage, the coefficient of friction for sample V1 stabilizes at about 0.46, for V2 at about 0.42 and for V3 at about 0.53. This transition to steady state indicates that the contacting surfaces of the coating and the wear body have reached a state of equilibrium in which the mechanical properties of the coating and the friction force remain stable. The stabilization stage is due to the completion of the adaptation and compaction processes of the coating surface. After the initial period of destruction and the equalization of irregularities, a stable contact layer is formed on the surface. This layer plays the role of a barrier that prevents the further significant wear of the coating and provides constant friction resistance. The formed contact layer has a high density and cohesion between particles, which makes it resistant to further destruction under the action of the wear body. As a result, the coating retains its tribological properties throughout the remainder of the test. Specimen V3, obtained at the highest voltage (45 V), has the highest stable coefficient of friction (0.536), indicating its higher density and wear resistance. A higher coating density increases the friction resistance force because the surface is harder and less susceptible to deformation, resulting in greater resistance when interacting with the wear body.
The results of the corrosion test are shown in Table 4. The analysis of the data shows that with increasing voltage, there is a decrease in the corrosion potential (Ecorr) and a simultaneous increase in the current density (Icorr). Special attention should be paid to sample V3, which, according to Table 4, has the lowest corrosion rate among the studied samples. This can be explained by the presence of a dense and homogeneous coating structure of sample V3, which leads to a reduced penetration of moisture and aggressive chemical media. As a consequence, this circumstance contributes significantly to its corrosion resistance, while sample V1, due to the low density of the structure and the presence of defects, has a lower corrosion resistance. Such pores and cracks can become points of penetration of moisture and corrosive agents, deteriorating the corrosion resistance properties of the coating.

Table 4.
Comparative characteristics of coatings obtained under different stress conditions (V1, V2, V3).
4. Conclusions
As the voltage increases from 30 V to 45 V, the following trend is observed:
- The coatings obtained at 45 V have the lowest porosity (1.36%) and the most homogeneous structure, which is due to the increase in the kinetic energy of the molten particles. At 30 V, the porosity was 3.58%, which is explained by the insufficient energy of the particles for dense deposition on the substrate.
- The microhardness of the coating increases with increasing voltage. Sample V3 (45 V) shows a maximum microhardness of 305 HV, which is due to the improved density of the coating structure. At 30 V (sample V1), the microhardness was 258 HV, which corresponds to a less dense coating structure.
- Sample V3 (45 V) showed the lowest corrosion rate (0.12859 mm/yr) and the most favorable corrosion potential (−0.45 V). This is due to the minimal porosity and dense structure of the coating. Samples V1 and V2 showed higher values of corrosion rate (0.4087 mm/yr and 0.25178 mm/yr, respectively) due to the presence of defects and oxide inclusions.
- The coefficient of friction stabilizes at a higher level for specimen V3 (0.53) compared to specimens V1 and V2 (0.46 and 0.42, respectively). This is due to the higher coating density and better wear resistance, although the higher density also results in higher contact friction force.
The optimum voltage for electric arc metallization, which ensures minimum porosity, high microhardness, low corrosion rate and stable tribological characteristics, is 45 V. This mode is suitable for applications requiring high wear and corrosion resistance. It should be noted that the application of arc spraying in combination with other methods, like hydrophobization or/and laser texturing, will notably enhance the potential of the arc spraying treatment of materials.
Author Contributions
Conceptualization, B.R. and D.B.; methodology, D.K. and N.M.; formal analysis, D.B. and D.K.; investigation, A.A., A.N. and N.M.; writing—original draft preparation, A.A. and A.N.; writing—review and editing, B.R. and D.B.; supervision, B.R. and D.B.; project administration, B.R. and N.M.; funding acquisition, B.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Committee of Science of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. BR21882370).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Bauyrzhan Rakhadilov and Dauir Kakimzhanov were employed by the company PlasmaScience LLP. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Rakhadilov, B.; Zhurerova, L.; Sagdoldina, Z.; Kenesbekov, A.; Bayatanova, L. Morphological Changes in the Dislocation Structure of Structural Steel 20GL after Electrolytic-Plasma Hardening of the Surface. J. Surf. Investig. X-Ray Synchrotron Neutron Tech. 2021, 15, 408–413. [Google Scholar] [CrossRef]
- Wielage, B.; Wank, A.; Rupprecht, C. Tailoring of Wire Feedstock and Processing Conditions in High Velocity Combustion Wire Spraying. In Proceedings of the ITSC2006—ASM International, Seattle, WA, USA, 15–18 May 2006; pp. 1009–1014. [Google Scholar]
- Gornik, M.; Jonda, E.; Latka, L.; Nowakowska, M.; Godzierz, M. Influence of spray distance on mechanical and tribological properties of HVOF sprayed WC-Co-Cr coatings. Mater. Sci.-Pol. 2021, 39, 545–554. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Domantovsky, A.G.; Emelyanenko, A.M.; Emelyanenko, K.A. Synergism of Arc Spraying, Laser Processing, and Hydrophobization for Long-Lasting Corrosion Protection of MA8 Magnesium Alloy. Adv. Eng. Mater. 2024; Early View. [Google Scholar] [CrossRef]
- Qiang, W.; Kang, M.; Liu, J.; Ndumia, J.N. Microstructure and Wear Performance of High-Velocity Arc Sprayed FeMnCrNiBNbAl Coating. Coatings 2024, 14, 428. [Google Scholar] [CrossRef]
- Szymański, K.; Hernas, A.; Moskal, G.; Myalska, H. Thermally Sprayed Coatings Resistant to Erosion and Corrosion for Power Plant Boilers—A Review. Surf. Coat. Technol. 2015, 268, 153–164. [Google Scholar] [CrossRef]
- Wielage, B.; Pokhmurska, H.; Student, M.; Gvozdeckii, V.; Stupnyckyj, T.; Pokhmurskii, V. Iron-Based Coatings Arc-Sprayed with Cored Wires for Applications at Elevated Temperatures. Surf. Coat. Technol. 2013, 220, 27–35. [Google Scholar] [CrossRef]
- Romankov, S.E.; Sagdoldina, Z.B.; Kaloshkin, S.D.; Kaevitser, E.V. Fabrication of Ti-Al composite coatings by the mechanical alloying method. Phys. Met. Metallogr. 2008, 106, 67–75. [Google Scholar] [CrossRef]
- Tabieva, E.E.; Zhurerova, L.G.; Baizhan, D. Influence of electrolyte-plasma hardening technological parameters on the structure and properties of banding steel 2. Key Eng. Mater. 2020, 839, 57–62. [Google Scholar] [CrossRef]
- Popova, N.A.; Zhurerova, L.G.; Nikonenko, E.L.; Skakov, M.K. Effect of plasma electrolytic nitrocarburizing on phase composition of 0.3 C-1Mn-1Si-fe steel. Inorg. Mater. Appl. Res. 2017, 8, 130–135. [Google Scholar] [CrossRef]
- Kakimzhanov, D.N.; Rakhadilov, B.K.; Tyurin, Y.N.; Kolisnichenko, O.V.; Zhurerova, L.G.; Dautbekov, M.K. Influence of pulsed plasma treatment on phase composition and hardness of Cr 3 C 2-NiCr coatings. Eurasian J. Phys. Funct. Mater. 2021, 5, 45–51. [Google Scholar] [CrossRef]
- Ramezani, M.; Mohd Ripin, Z.; Pasang, T.; Jiang, C.P. Surface engineering of metals: Techniques, characterizations and applications. Metals 2023, 13, 1299. [Google Scholar] [CrossRef]
- Kengesbekov, A.; Rakhadilov, B.; Sagdoldina, Z.; Buitkenov, D.; Dosymov, Y.; Kylyshkanov, M. Improving the efficiency of air plasma spraying of titanium nitride powder. Coatings 2022, 12, 1644. [Google Scholar] [CrossRef]
- Bauyrzhan, R.; Alexander, P.; Zhuldyz, S.; Dastan, B.; Vyacheslav, B.; Mukhamedova, A. Effect of Bilayer Thickness and Bias Potential on the Structure and Properties of (TiZr/Nb) N Multilayer Coatings as a Result of Arc-PVD Deposition. Materials 2022, 15, 7696. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Baizhan, D.R.; Sagdoldina, Z.B.; Buitkenov, D.B.; Maulet, M. Phase composition and structure of composite Ti/HA coatings synthesized by detonation spraying. AIP Conf. Proc. 2020, 2297, 010001. [Google Scholar]
- Rakhadilov, B.K.; Kenesbekov, A.B.; Kowalevski, P.; Ocheredko, Y.A.; Sagdoldina, Z.B. Development of air-plasma technology for hardening cutting tools by applying wear-resistant coatings. News Natl. Acad. Sci. Repub. Kazakhstan 2020, 3, 54–62. [Google Scholar] [CrossRef]
- Yu, H.L.; Zhang, W.; Wang, H.M.; Ji, X.C.; Song, Z.Y.; Li, X.Y.; Xu, B.S. In-situ synthesis of TiC/Ti composite coating by high frequency induction cladding. J. Alloys Compd. 2017, 701, 244–255. [Google Scholar] [CrossRef]
- Sidorov, S.A.; Mironov, D.A.; Khoroshenkov, V.K.; Khlusova, E.I. Surfacing methods for increasing the service life of rapidly wearing working tools of agricultural machines. Weld. Int. 2016, 30, 808–812. [Google Scholar] [CrossRef]
- Sun, R.; Shi, Y.; Pei, Z.; Li, Q.; Wang, R. Heat transfer and temperature distribution during high-frequency induction cladding of 45 steel plate. Appl. Therm. Eng. 2018, 139, 1–10. [Google Scholar] [CrossRef]
- Hui, R.; Wang, Z.; Kesler, O.; Rose, L.; Jankovic, J.; Yick, S.; Ghosh, D. Thermal plasma spraying for SOFCs: Applications, potential advantages, and challenges. J. Power Sources 2007, 170, 308–323. [Google Scholar] [CrossRef]
- Chahal, H.K.; Matthews, S.; Jones, M.I. Fabrication of calcium phosphate coatings by the inflight reaction of precursor feedstocks using plasma spraying. J. Therm. Spray Technol. 2023, 32, 1465–1481. [Google Scholar] [CrossRef]
- Qadir, D.; Sharif, R.; Nasir, R.; Awad, A.; Mannan, H.A. A review on coatings through thermal spraying. Chem. Pap. 2024, 78, 71–91. [Google Scholar] [CrossRef]
- Liao, X.J.; Zhang, L.; Sun, Y.Q.; Luo, X.T.; Li, C.X.; Yang, G.J.; Li, C.J. Effect of Inter-Splat Bonding Quality on the Dependence of Wear Behavior of Plasma-Sprayed Stainless Steel Coating on Applied Load. J. Therm. Spray Technol. 2024, 33, 1–11. [Google Scholar] [CrossRef]
- Abdivakhidov, K.; Sharipov, K. Corrosion-resistant protective coatings for metals: A review of metallic and non-metallic coatings. AIP Conf. Proc. 2024, 3045, 060011. [Google Scholar]
- Liu, M.; Peng, Q.; Huang, Y.; Li, P.; Tan, G.; Luo, X.; Qiao, Q.; Wang, H.; Lang, W. Influencing Factors and Process Optimization of Al/SiC Powder-Cored Wires by Plasma Transferred Wire Arc Spraying. J. Therm. Spray Technol. 2024, 33, 2167–2183. [Google Scholar] [CrossRef]
- Yao, H.H.; Zhou, Z.; Wang, Y.M.; He, D.Y.; Bobzin, K.; Zhao, L.; Öte, M.; Königstein, T. Microstructure and Properties of FeCrB Alloy Coatings Prepared by Wire-Arc Spraying. J. Therm. Spray Tech. 2017, 26, 483–491. [Google Scholar] [CrossRef]
- Arizmendi-Morquecho, A.; Campa-Castilla, A.; Leyva-Porras, C.; Aguilar Martinez, J.A.; Vargas Gutiérrez, G.; Moreno Bello, K.J.; López López, L. Microstructural Characterization and Wear Properties of Fe-Based Amorphous-Crystalline Coating Deposited by Twin Wire Arc Spraying. Adv. Mater. Sci. Eng. 2014, 2014, 836739. [Google Scholar] [CrossRef]
- Kumar, D.; Murtaza, Q.; Singh, R.C. Sliding Wear Behavior of Aluminum Alloy Coating Prepared by Two-Wire Electric Arc Spray Process. Int. J. Adv. Manuf. Technol. 2016, 85, 237–252. [Google Scholar] [CrossRef]
- Johnston, A.L.; Hall, A.C.; McCloskey, J.F. Effect of Process Inputs on Coating Properties in the Twin-Wire Arc Zinc Process. J. Therm. Spray Tech. 2013, 22, 856–863. [Google Scholar] [CrossRef]
- Czupryński, A. Flame spraying of aluminum coatings reinforced with particles of carbonaceous materials as an alternative for laser cladding technologies. Materials 2019, 12, 3467. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Neisius, N.M.; Gaan, S. Recent developments in flame retardant polymeric coatings. Prog. Org. Coat. 2013, 76, 1642–1665. [Google Scholar] [CrossRef]
- Xie, X.; Guo, H.; Gong, S.; Xu, H. Thermal cycling behavior and failure mechanism of LaTi2Al9O19/YSZ thermal barrier coatings exposed to gas flame. Surf. Coat. Technol. 2011, 205, 4291–4298. [Google Scholar] [CrossRef]
- Nakamura, M.; Akamatsu, F.; Kurose, R.; Katsuki, M. Combustion mechanism of liquid fuel spray in a gaseous flame. Phys. Fluids 2005, 17, 123301. [Google Scholar] [CrossRef]
- Pokhmursky, V.I.; Student, M.M.; Ryabtsev, I.A.; Sidorak, I.I.; Dzioba, Y.V.; Dovgunyk, V.M.; Formanek, B. Influence of electric arc metallizing modes and compositions of applied flux-cored wires on structure and abrasive wear resistance of coatings. Paton Weld. J. 2006, 7, 26. [Google Scholar]
- Degnan, C.C.; Shipway, P.H. A comparison of the reciprocating sliding wear behaviour of steel-based metal matrix composites processed from self-propagating high-temperature synthesised Fe–TiC and Fe–TiB₂ masteralloys. Wear 2002, 252, 832–841. [Google Scholar] [CrossRef]
- Kumar, D.; Pandey, K.N. Optimization of the process parameters in generic thermal barrier coatings using the Taguchi method and grey relational analysis. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2017, 231, 600–610. [Google Scholar] [CrossRef]
- Zhao, W.; Lu, Y.; Fu, H.; He, D.; Tan, Z.; Yao, H.; Yang, Y.; Zhou, Z. Developing Rotary Swaging Process for Improving the Performance of Wire-Arc Sprayed Al/Al2O3 Composite Coating. Surf. Coat. Technol. 2024, 484, 130840. [Google Scholar] [CrossRef]
- Lopata, A.; Lopata, V.; Kachynska, I.; Zaboykina, N. Influence of Factors of the Electric Arc Spraying Process on the Properties of Coatings. Probl. Tribol. 2024, 29, 79–86. [Google Scholar] [CrossRef]
- Steffens, H.-D.; Babiak, Z.; Wewel, M. Recent Developments in Arc Spraying. IEEE Trans. Plasma Sci. 1990, 18, 974–979. [Google Scholar] [CrossRef]
- Abedini, A.; Pourmousa, A.; Chandra, S.; Mostaghimi, J. Effect of Substrate Temperature on the Properties of Coatings and Splats Deposited by Wire Arc Spraying. Surf. Coat. Technol. 2006, 201, 3350–3358. [Google Scholar] [CrossRef]
- Ndumia, J.N.; Kang, M.; Gbenontin, B.V.; Lin, J.; Nyambura, S.M. A Review on the Wear, Corrosion and High-Temperature Resistant Properties of Wire Arc-Sprayed Fe-Based Coatings. Nanomaterials 2021, 11, 2527. [Google Scholar] [CrossRef]
- Planche, M.P.; Liao, H.; Coddet, C. Relationships between In-Flight Particle Characteristics and Coating Microstructure with a Twin Wire Arc Spray Process and Different Working Conditions. Surf. Coat. Technol. 2004, 182, 215–226. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Le, T.D.; Le, Q.T.; Pham, H.T.; Nguyen, A.T.; Pham, L.T.; Dao, T.B.; Ly, C.Q. Characterization and Corrosion Resistance of the Twin-Wire Arc Spray Al-5Mg Alloy Coating Applied on a Carbon Steel Substrate. J. Therm. Spray Technol. 2024, 33, 381–397. [Google Scholar] [CrossRef]
- Wagner, N. Effect of Process Parameters on Twin Wire Arc Sprayed Steel Coatings. J. Mater. Eng. Perform. 2021, 30, 6650–6655. [Google Scholar] [CrossRef]
- Ndumia, J.N.; Kang, M.; Gbenontin, B.V.; Lin, J.; Liu, J.; Li, H.; Nyambura, S.M. Optimizing Parameters of Arc-Sprayed Fe-Based Coatings Using the Response Surface Methodology. J. Therm. Spray Technol. 2023, 32, 2202–2220. [Google Scholar] [CrossRef]
- Mykhailo, S.; Volodymyr, G.; Oleksandra, S.; Olegas, P.; Pavlo, M.; Olena, O.; Liudmyla, T. The Effect of Increasing the Air Flow Pressure on the Properties of Coatings During the Arc Spraying of Cored Wires. Stroj. Časopis—J. Mech. Eng. 2019, 69, 133–146. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Magazov, N.; Kakimzhanov, D.; Apsezhanova, A.; Molbossynov, Y.; Kengesbekov, A. Influence of Spraying Process Parameters on the Characteristics of Steel Coatings Produced by Arc Spraying Method. Coatings 2024, 14, 1145. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Shynarbek, A.; Kakimzhanov, D.; Kusainov, R.; Zhassulan, A.; Ormanbekov, K. Effect of Voltage on Properties of 30HGSA Steel Coatings by Supersonic Supersonic Arc Metallization Method. Adv. Sci. Technol. Res. J. 2024, 18, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.R.; Li, D.Y.; Zhang, N.N.; Huang, H.; Wang, X. Wear mechanism of iron-based alloy coating by arc spraying. J. Iron Steel Res. Int. 2016, 23, 834–841. [Google Scholar] [CrossRef]
- Yao, H.; Zhou, Z.; Wang, G.; He, D.; Bobzin, K.; Zhao, L.; Öte, M.; Königstein, T. Influence of Feedstock Materials and Spray Parameters on Thermal Conductivity of Wire-Arc-sprayed Coatings. J. Mater. Eng. Perform. 2017, 26, 1108–1113. [Google Scholar] [CrossRef]
- Salavati, S.; Coyle, T.; Mostaghimi, J. Twin Wire Arc Spray Process Modification for Production of Porous Metallic Coatings. Surf. Coat. Technol. 2016, 286, 16–24. [Google Scholar] [CrossRef]
- Xu, W.-P.; Xu, B.-S.; Zhang, W.; Wu, Y.-X. High temperature behaviors of high velocity arc sprayed Fe-Al/Cr3C2 composite coatings. Int. J. Miner. Metall. Mater. 2005, 12, 340–346. [Google Scholar]
- Li, R.; Zhou, Z.; He, D.; Wang, Y.; Wu, X.; Song, X. Microstructure and high temperature corrosion behavior of wire-arc sprayed FeCrSiB coating. J. Therm. Spray Technol. 2015, 24, 857–864. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).