Chloride Ions Tuning Organic Alkaline Electrolyte for Optimizing MnO2 Cathodes in Aqueous Sodium Batteries
Abstract
1. Introduction
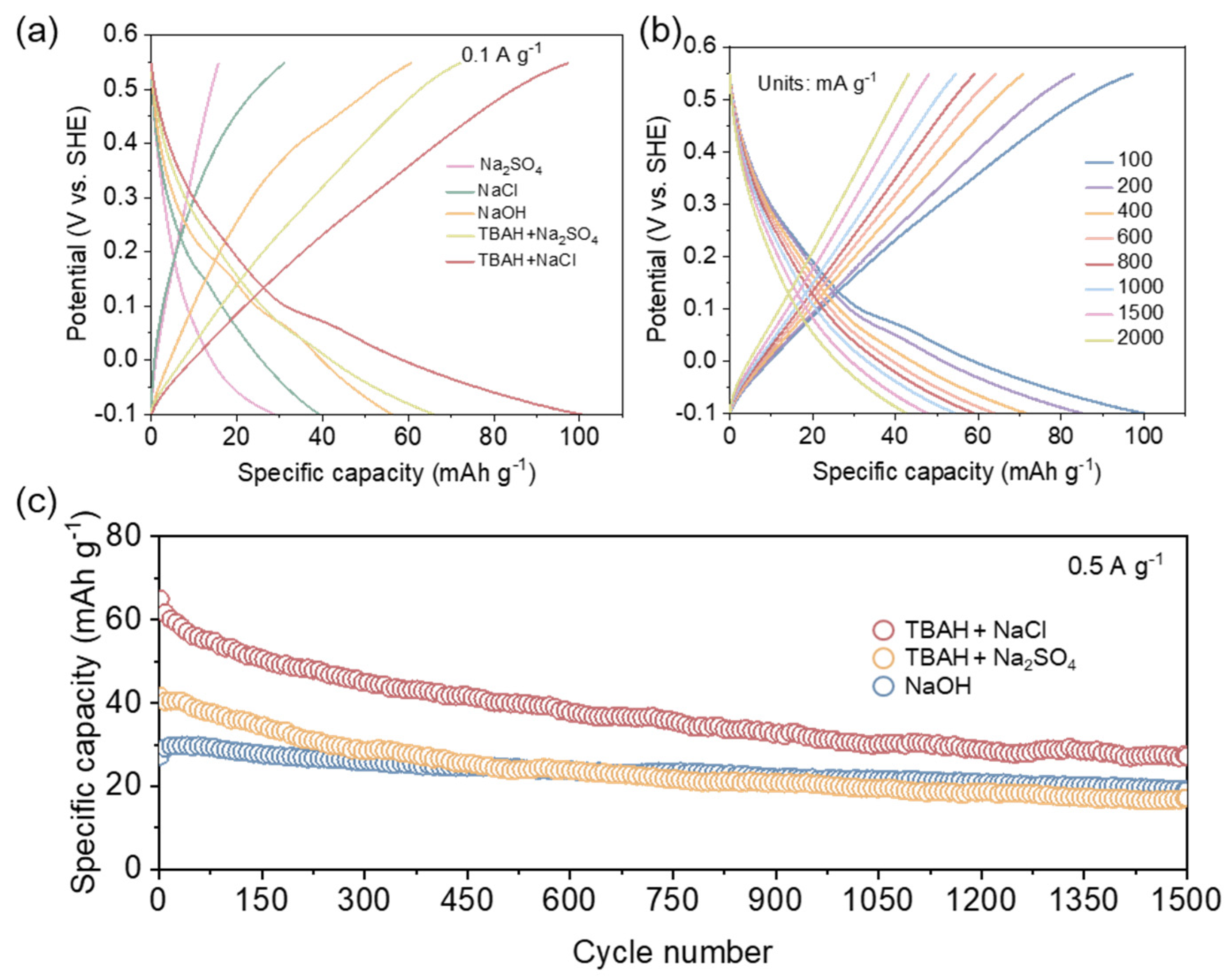
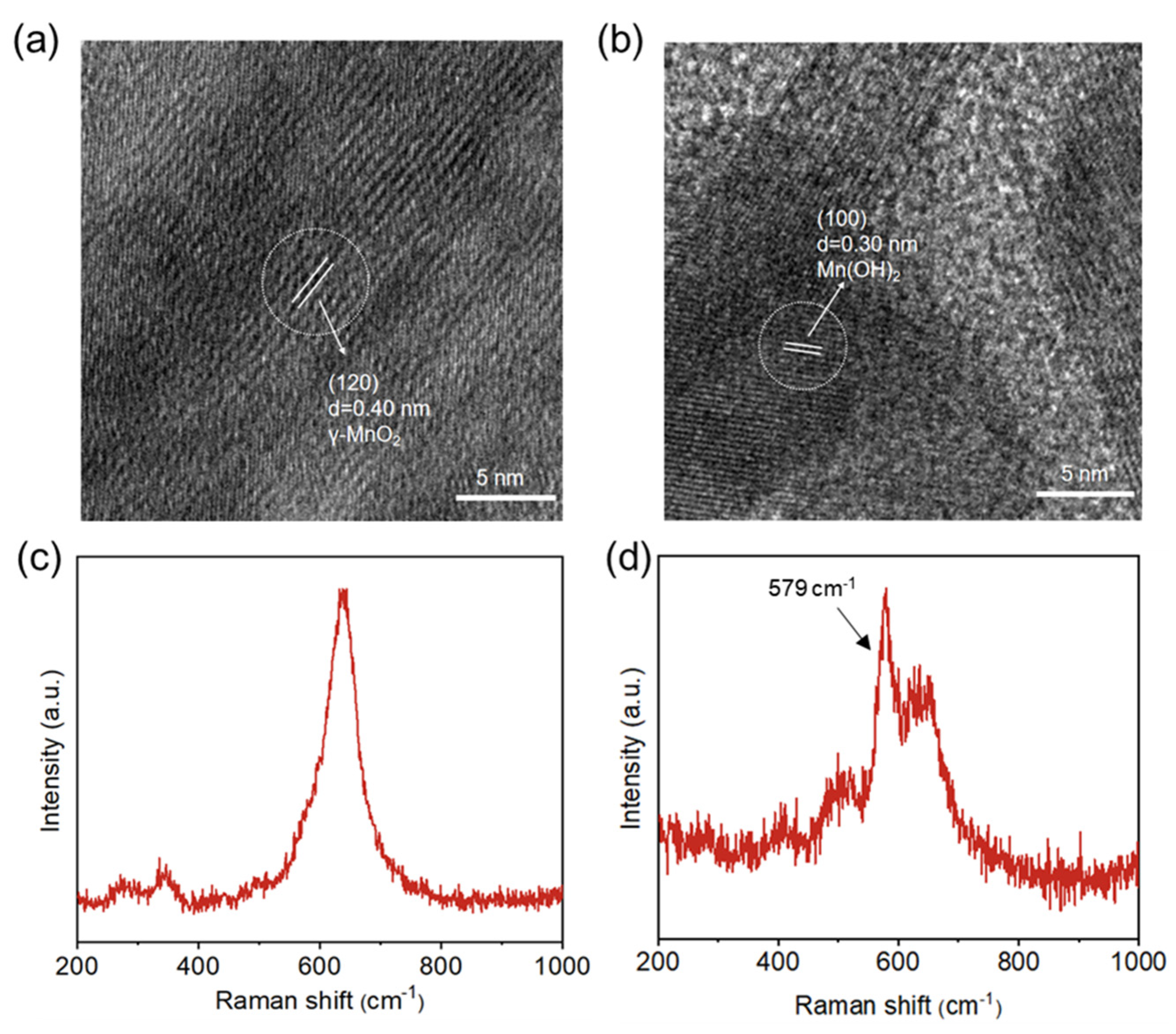
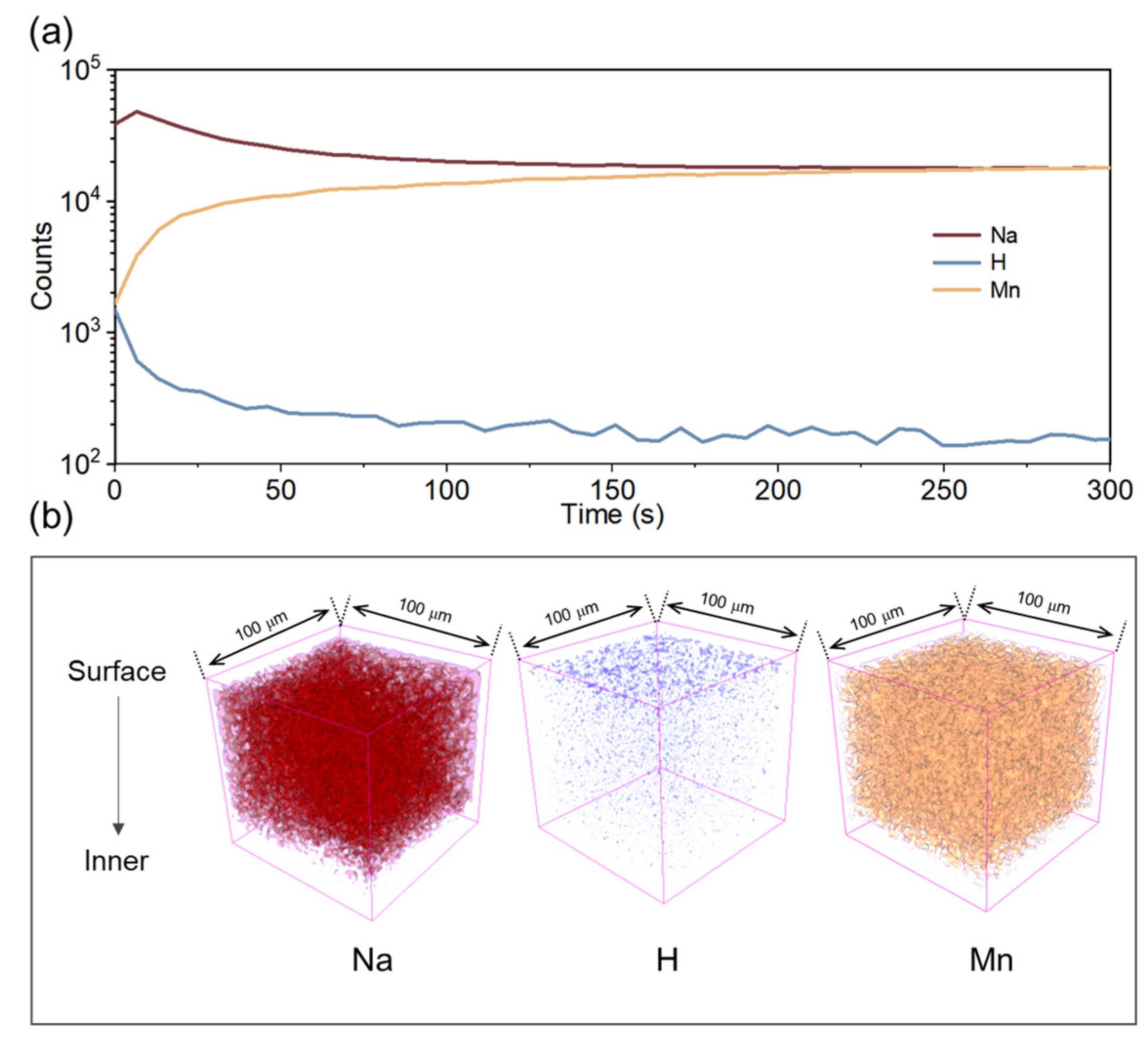
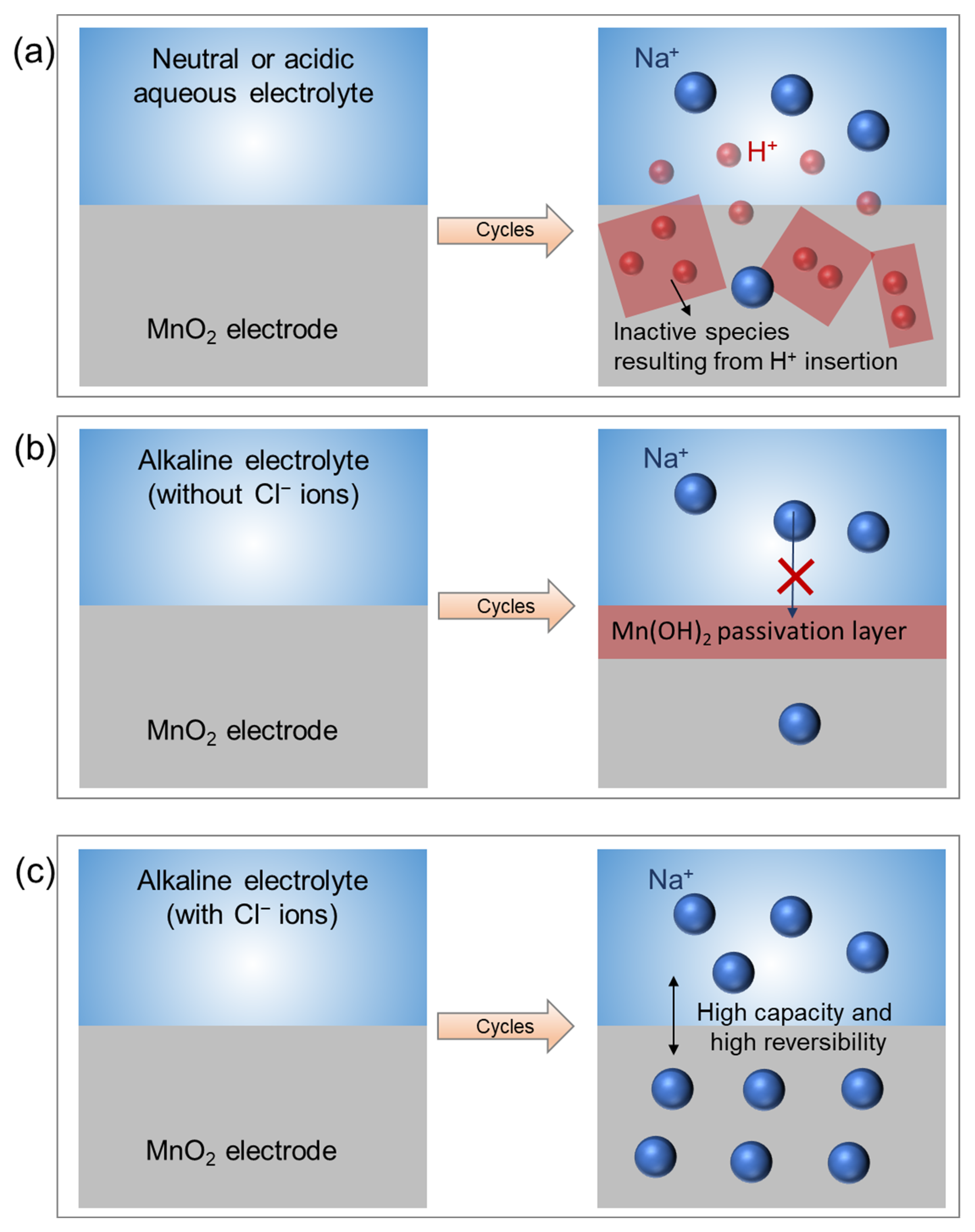
2. Results and Discussion
3. Conclusions
4. Methods
4.1. Synthesis of γ-MnO2 Cathode Materials
4.2. Material and Electrode Characterization
4.3. Electrochemical Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Pohl, O.; Bhatt, A.I.; Collis, G.E.; Mahon, P.J.; Rüther, T.; Hollenkamp, A.F. A Review on Battery Market Trends, Second-Life Reuse, and Recycling. Sustain. Chem. 2021, 2, 167–205. [Google Scholar] [CrossRef]
- Wang, H.; Chen, S.; Fu, C.; Ding, Y.; Liu, G.; Cao, Y.; Chen, Z. Recent Advances in Conversion-Type Electrode Materials for Post Lithium-Ion Batteries. ACS Mater. Lett. 2021, 3, 956–977. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Yang, X.; Zhou, D.; Wang, X.; Jaumaux, P.; Kang, F.; Li, B.; Ji, X.; Wang, G. Rechargeable anion-shuttle batteries for low-cost energy storage. Chem 2021, 7, 1993–2021. [Google Scholar] [CrossRef]
- Liang, Y.; Yao, Y. Designing modern aqueous batteries. Nat. Rev. Mater. 2023, 8, 109–122. [Google Scholar] [CrossRef]
- Gu, C.; Liu, Z.; Gao, X.; Zhang, Q.; Zhang, Z.; Liu, Z.; Wang, C. Polymerization increasing the capacitive charge storage for better rate performance: A case study of electrodes in aqueous sodium-ion capacitors. Battery Energy 2022, 1, 20220031. [Google Scholar] [CrossRef]
- Liang, Z.; Tian, F.; Yang, G.; Wang, C. Enabling long-cycling aqueous sodium-ion batteries via Mn dissolution inhibition using sodium ferrocyanide electrolyte additive. Nat. Commun. 2023, 14, 3591. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shen, J.; Yao, Y.; Dai, J.; Ling, F.; Li, L.; Jiang, Y.; Wu, X.; Rui, X.; Yu, Y. Inhibiting the Jahn–Teller Effect of Manganese Hexacyanoferrate via Ni and Cu Codoping for Advanced Sodium-Ion Batteries. Adv. Mater. 2024, 36, 2405458. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Kim, D.; Lee, M.; Nam, K.W. Challenges and possibilities for aqueous battery systems. Commun. Mater. 2023, 4, 37. [Google Scholar] [CrossRef]
- Boyd, S.; Dhall, R.; LeBeau, J.M.; Augustyn, V. Charge storage mechanism and degradation of P2-type sodium transition metal oxides in aqueous electrolytes. J. Mater. Chem. A 2018, 6, 22266–22276. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, L.; Yin, J.; Zheng, J.; Zhang, D.; Chen, J.; Archer, L.A. Proton Intercalation/De-Intercalation Dynamics in Vanadium Oxides for Aqueous Aluminum Electrochemical Cells. Angew. Chem. Int. Ed. 2020, 59, 3048–3052. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Yadav, G.G.; Gallaway, J.W.; Turney, D.E.; Nyce, M.; Huang, J.; Wei, X.; Banerjee, S. Regenerable Cu-intercalated MnO2 layered cathode for highly cyclable energy dense batteries. Nat. Commun. 2017, 8, 14424. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Yao, J.; Xu, Y.; Wan, H.; Zhang, B.; Lv, L.; Li, J.; Wang, N.; Zheng, Z.; Zhang, J.; et al. Promoting Proton Migration Kinetics by Ni2+ Regulating Enables Improved Aqueous Zn-MnO2 Batteries. Energy Environ. Mater. 2023, 6, e12340. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Berg, E.J. Mastering Proton Activities in Aqueous Batteries. Adv. Mater. 2024, 2407852. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Mo, F.; Ji, X.; Zhi, C. Non-metallic charge carriers for aqueous batteries. Nat. Rev. Mater. 2021, 6, 109–123. [Google Scholar] [CrossRef]
- Liu, X.; Euchner, H.; Zarrabeitia, M.; Gao, X.; Elia, G.A.; Groß, A.; Passerini, S. Operando pH Measurements Decipher H+/Zn2+ Intercalation Chemistry in High-Performance Aqueous Zn/δ-V2O5 Batteries. ACS Energy Lett. 2020, 5, 2979–2986. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; et al. Zn/MnO2 Battery Chemistry with H+ and Zn2+ Coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef]
- Chabre, Y.; Pannetier, J. Structural and electrochemical properties of the proton / γ-MnO2 system. Prog. Solid State Chem. 1995, 23, 1–130. [Google Scholar] [CrossRef]
- Li, C.; Xu, H.; Ni, L.; Qin, B.; Ma, Y.; Jiang, H.; Xu, G.; Zhao, J.; Cui, G. Nonaqueous Liquid Electrolytes for Sodium-Ion Batteries: Fundamentals, Progress and Perspectives. Adv. Energy Mater. 2023, 13, 2301758. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Ye, J.; Zhang, T.; Hou, Z. Revealing the Competitive Intercalation between Na+ and H+ into Na0.44MnO2 in Aqueous Sodium Ion Batteries. Adv. Energy Mater. 2023, 13, 2204413. [Google Scholar] [CrossRef]
- Hertzberg, B.J.; Huang, A.; Hsieh, A.; Chamoun, M.; Davies, G.; Seo, J.K.; Zhong, Z.; Croft, M.; Erdonmez, C.; Meng, Y.S.; et al. Effect of Multiple Cation Electrolyte Mixtures on Rechargeable Zn–MnO2 Alkaline Battery. Chem. Mater. 2016, 28, 4536–4545. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, S.; Fu, W.; Cui, Y.; Wang, J.; Zhao, D.; Yang, C.; Wang, X.; Cao, B. Plasma-induced ε-MnO2 based aqueous zinc-ion batteries and their dissolution-deposition mechanism. J. Mater. Sci. Technol. 2022, 127, 206–213. [Google Scholar] [CrossRef]
- Wang, M.; Meng, Y.; Gao, P.; Li, K.; Liu, Z.; Zhu, Z.; Ali, M.; Ahmad, T.; Chen, N.; Yuan, Y.; et al. Anions Regulation Engineering Enables a Highly Reversible and Dendrite-Free Nickel-Metal Anode with Ultrahigh Capacities. Adv. Mater. 2023, 35, 2305368. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Ponraj, R.; Kannan, A.G.; Lee, H.-W.; Fathi, R.; Ruffo, R.; Mari, C.M.; Kim, D.K. Diffusion behavior of sodium ions in Na0.44MnO2 in aqueous and non-aqueous electrolytes. J. Power Sources 2013, 244, 758–763. [Google Scholar] [CrossRef]
- Hou, Z.; Li, X.; Liang, J.; Zhu, Y.; Qian, Y. An aqueous rechargeable sodium ion battery based on a NaMnO2–NaTi2(PO4)3 hybrid system for stationary energy storage. J. Mater. Chem. A 2015, 3, 1400–1404. [Google Scholar] [CrossRef]
- Zhang, F.; Li, W.; Xiang, X.; Sun, M. Highly stable Na-storage performance of Na0.5Mn0.5Ti0.5O2 microrods as cathode for aqueous sodium-ion batteries. J. Electroanal. Chem. 2017, 802, 22–26. [Google Scholar] [CrossRef]
- Zhang, B.H.; Liu, Y.; Chang, Z.; Yang, Y.Q.; Wen, Z.B.; Wu, Y.P.; Holze, R. Nanowire Na0.35MnO2 from a hydrothermal method as a cathode material for aqueous asymmetric supercapacitors. J. Power Sources 2014, 253, 98–103. [Google Scholar] [CrossRef]
- Zhao, A.; Zhong, F.; Feng, X.; Chen, W.; Ai, X.; Yang, H.; Cao, Y. A Membrane-Free and Energy-Efficient Three-Step Chlor-Alkali Electrolysis with Higher-Purity NaOH Production. ACS Appl. Mater. Interfaces 2019, 11, 45126–45132. [Google Scholar] [CrossRef]
- Shan, X.; Guo, F.; Page, K.; Neuefeind, J.C.; Ravel, B.; Abeykoon, A.M.M.; Kwon, G.; Olds, D.; Su, D.; Teng, X. Framework Doping of Ni Enhances Pseudocapacitive Na-Ion Storage of (Ni)MnO2 Layered Birnessite. Chem. Mater. 2019, 31, 8774–8786. [Google Scholar] [CrossRef]
- Gu, F.; Sun, T.; Yao, X.; Shui, M.; Shu, J. Studies on the improved electro-chemical performance and the sodium ion migration mechanism of Na0·44MnO2-CNT electrodes for aqueous sodium batteries. J. Phys. Chem. Solids 2021, 149, 109771. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Bao, W.; Cai, H.; Chen, R.; Fu, K.; Luo, W. Chloride Ions Tuning Organic Alkaline Electrolyte for Optimizing MnO2 Cathodes in Aqueous Sodium Batteries. Coatings 2025, 15, 298. https://doi.org/10.3390/coatings15030298
Zhang X, Bao W, Cai H, Chen R, Fu K, Luo W. Chloride Ions Tuning Organic Alkaline Electrolyte for Optimizing MnO2 Cathodes in Aqueous Sodium Batteries. Coatings. 2025; 15(3):298. https://doi.org/10.3390/coatings15030298
Chicago/Turabian StyleZhang, Xiangchen, Wenyuan Bao, Hongwei Cai, Ruixi Chen, Kai Fu, and Wen Luo. 2025. "Chloride Ions Tuning Organic Alkaline Electrolyte for Optimizing MnO2 Cathodes in Aqueous Sodium Batteries" Coatings 15, no. 3: 298. https://doi.org/10.3390/coatings15030298
APA StyleZhang, X., Bao, W., Cai, H., Chen, R., Fu, K., & Luo, W. (2025). Chloride Ions Tuning Organic Alkaline Electrolyte for Optimizing MnO2 Cathodes in Aqueous Sodium Batteries. Coatings, 15(3), 298. https://doi.org/10.3390/coatings15030298






