Photocatalytically Induced Degradation of Nano-TiO2-Modified Paint Coatings Under Low-Radiation Conditions
Abstract
1. Introduction
Research Significance
2. Materials and Methods
2.1. Materials
2.2. Methods
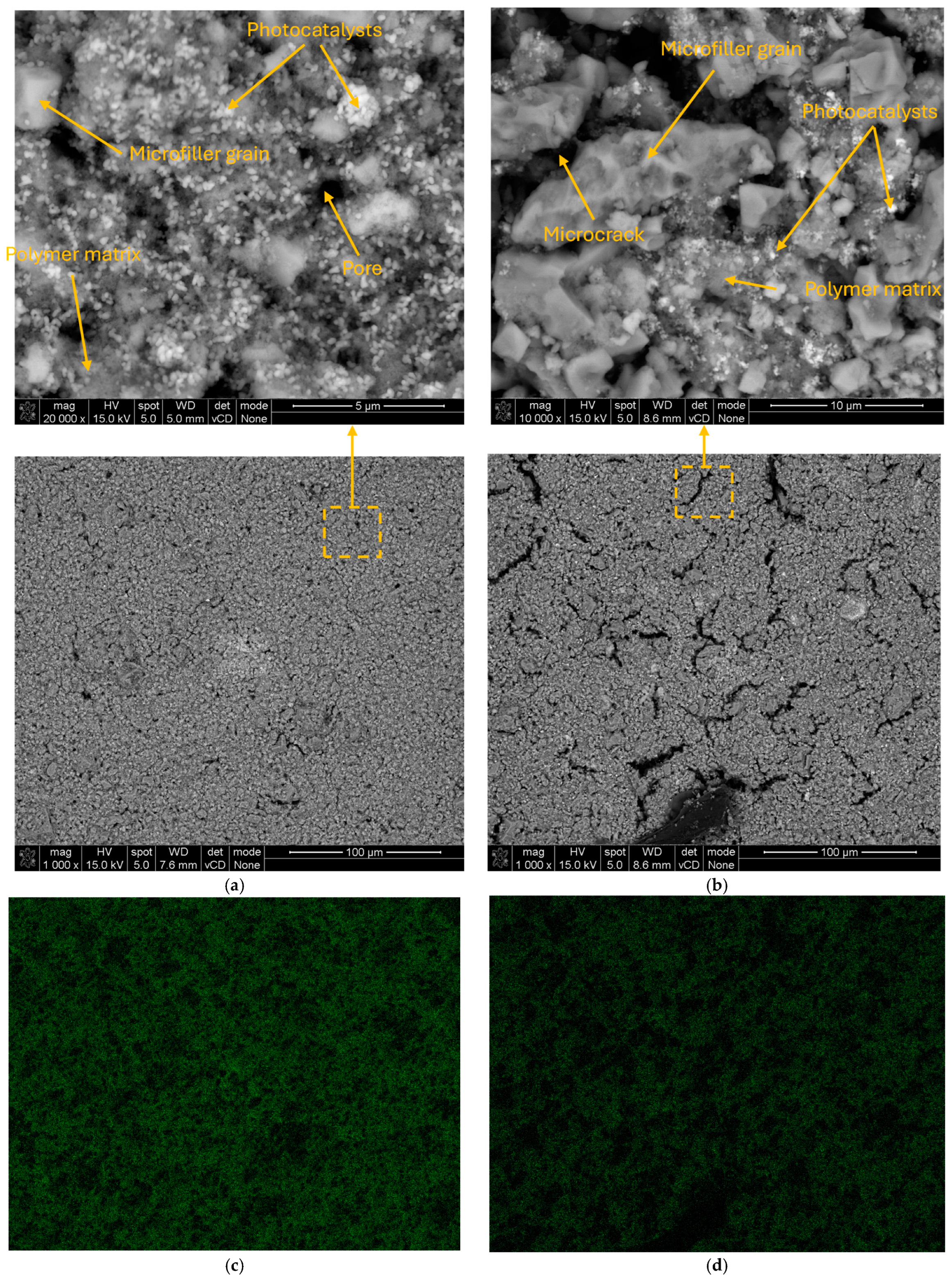
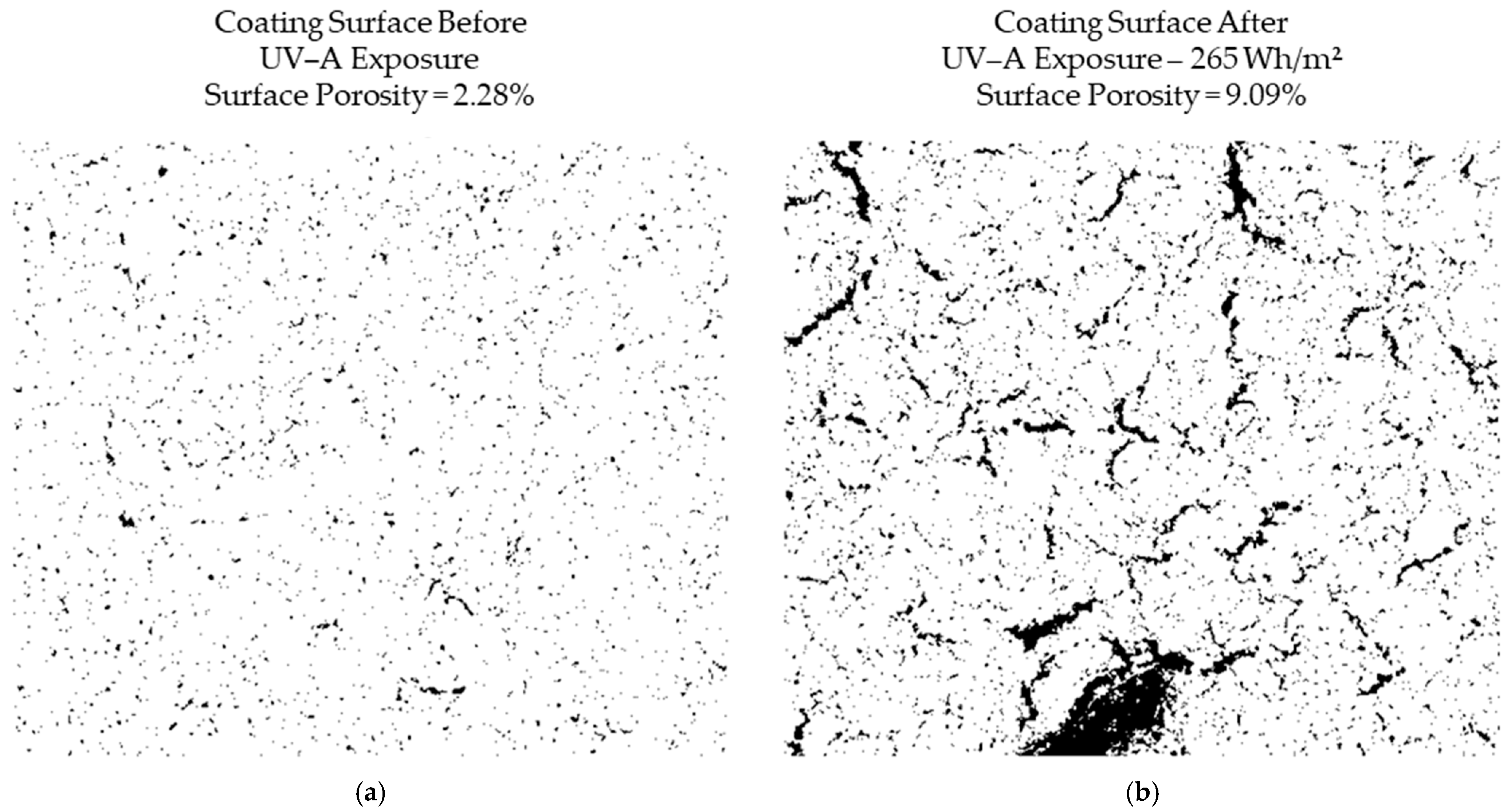
3. Results
4. Discussion
5. Conclusions
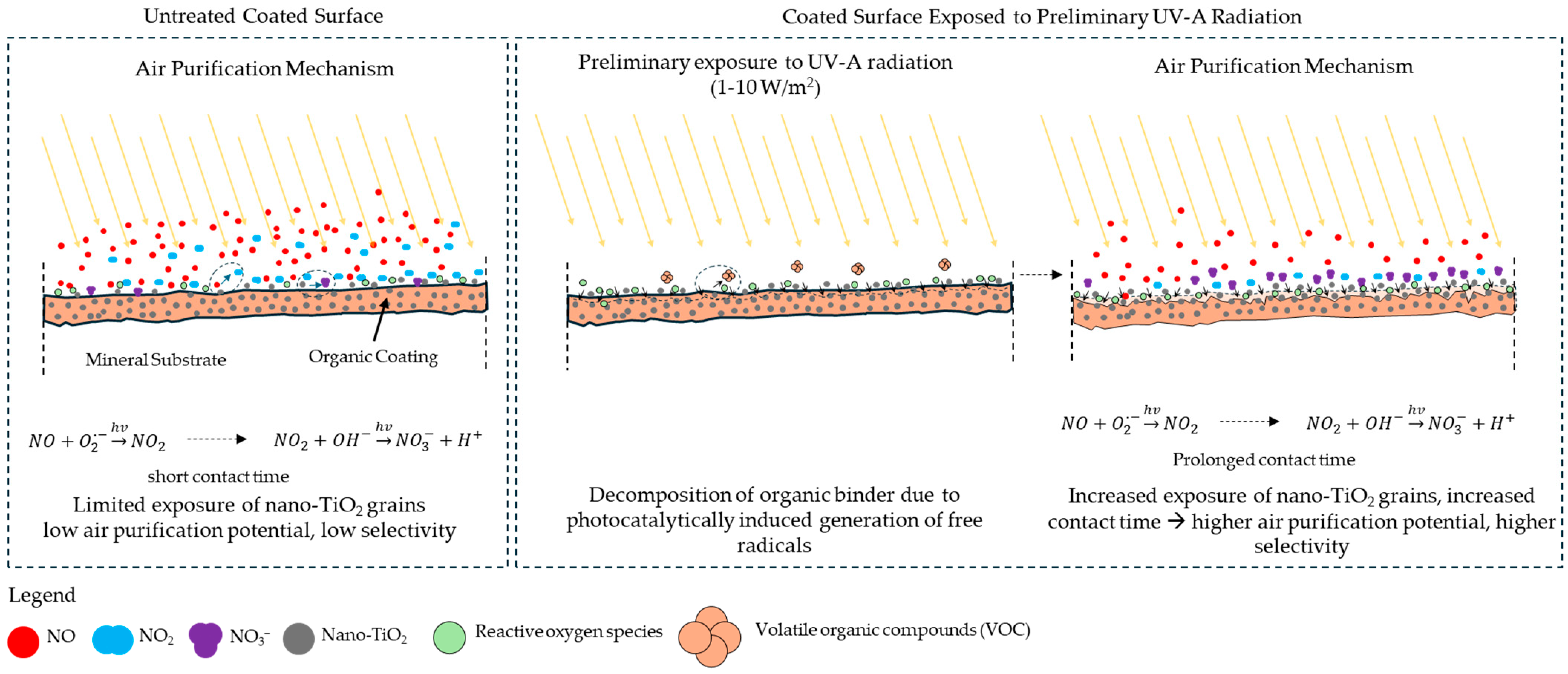
- Significant organic binder decomposition was observed even under low irradiation conditions (UV-A irradiance in the range of 1–10 W/m2), corresponding to light conditions during autumn and winter in regions such as Northern and Central Europe. This finding raises concerns regarding organic-based photocatalytic coatings, as the research demonstrated that acrylic-based coatings could undergo continuous degradation throughout the year. As shown in this study, such degradation may lead to the emission of VOCs into the atmosphere and potentially the release of nano-TiO2 due to the disintegration of the polymer matrix.
- Prolonged exposure to low-intensity UV-A radiation (1–10 W/m2) led to the progressive degradation of the organic binder, increasing surface porosity from 2.28% to 9.09%. This transformation enhanced the accessibility of nano-TiO2 particles, improving the photocatalytic efficiency of NOx removal by facilitating more significant interaction between pollutants and active sites.
- The organic binder tended to decompose due to the reactive species generated during photocatalytic reactions, with the extent of degradation being strongly linked to the duration of UV-A irradiation exposure.
- The partial decomposition of the binder resulted in increased VOC emissions (up to 5 ppb), which could serve as a precursor to the formation of near-to-surface ozone.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, M.-Z.; Chen, J.; Xia, M.; Wang, T.; Poon, C.S. Pathways of Conversion of Nitrogen Oxides by Nano TiO2 Incorporated in Cement-Based Materials. Build. Environ. 2018, 144, 412–418. [Google Scholar] [CrossRef]
- Seo, D.; Yun, T.S. NOx Removal Rate of Photocatalytic Cementitious Materials with TiO2 in Wet Condition. Build. Environ. 2017, 112, 233–240. [Google Scholar] [CrossRef]
- Staub de Melo, J.V.; Trichês, G. Evaluation of the Influence of Environmental Conditions on the Efficiency of Photocatalytic Coatings in the Degradation of Nitrogen Oxides (NOx). Build. Environ. 2012, 49, 117–123. [Google Scholar] [CrossRef]
- Nahorski, Z.; Holnicki, P.; Kałuszko, A. Towards Air Quality Protection in an Urban Area—Case Study. Atmosphere 2024, 15, 1106. [Google Scholar] [CrossRef]
- Ballari, M.M.; Hunger, M.; Hüsken, G.; Brouwers, H.J.H. NOx Photocatalytic Degradation Employing Concrete Pavement Containing Titanium Dioxide. Appl. Catal. B Environ. 2010, 95, 245–254. [Google Scholar] [CrossRef]
- Holnicki, P.; Kałuszko, A.; Nahorski, Z. A Projection of Environmental Impact of a Low Emission Zone Planned in Warsaw, Poland. Sustainability 2023, 15, 16260. [Google Scholar] [CrossRef]
- Cakaj, A.; Lisiak-Zielińska, M.; Khaniabadi, Y.O.; Sicard, P. Premature Deaths Related to Urban Air Pollution in Poland. Atmosphere 2023, 301, 119723. [Google Scholar] [CrossRef]
- Holnicki, P.; Tainio, M.; Kałuszko, A.; Nahorski, Z. Burden of Mortality and Disease Attributable to Multiple Air Pollutants in Warsaw, Poland. Int. J. Environ. Res. Public Health 2017, 14, 1359. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Calheiros, C.S.C.; Villanueva, F.; Alonso-Cuevilla, N.P.; Gabriel, M.F.; Silva, G.V. Indoor Air Quality: A Review of Cleaning Technologies. Environments 2022, 9, 118. [Google Scholar] [CrossRef]
- European Union. Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on Ambient Air Quality and Cleaner Air for Europe. Off. J. Eur. Union 2008, L 152, 1–44.
- Chilmon, K.; Kalinowski, M.; Jackiewicz-Rek, W. Influence of Coarse Aggregate Exposure on Air Purification Efficiency in Photocatalytic Cement Composites. Buildings 2024, 14, 3639. [Google Scholar] [CrossRef]
- Parasuraman, V.; Sekar, P.P.; Lee, H.; Sheraz, M.; Ly, H.N.; Azizar, G.A.B.; Hong, J.W.; Lee, W.R.; Kim, S. Photocatalytic Self-Cleaning Eco-Friendly Paint: A Unique Approach for Efficient Indoor Air Pollutant Removal and Surface Disinfection. Constr. Build. Mater. 2024, 412, 134671. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Sun, H.; Liu, J.; Pareek, V.K.; Wang, S. A Review on Photocatalysis for Air Treatment: From Catalyst Development to Reactor Design. Chem. Eng. J. 2017, 310 Pt 2, 537–559. [Google Scholar] [CrossRef]
- Chilmon, K.; Kalinowski, M.; Jackiewicz-Rek, W. Effect of Cement Substitution with Mineral Fillers on NOx Air-Purification Efficiency and Photocatalytic Reaction Selectivity of Nano-TiO2-Modified Cementitious Composites. Materials 2024, 17, 5775. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yin, H.; Wang, C.; Wang, R.; Peng, Y.; You, C.; Li, J. Environmental Science & Technology. Environ. Sci. Technol. 2021, 55, 9285–9292. [Google Scholar] [CrossRef] [PubMed]
- Seremak, W.; Jasiorski, M.; Baszczuk, A.; Winnicki, M. Durability Assessment of Low-Pressure Cold-Sprayed TiO2 Photocatalytic Coatings: Photocatalytic and Mechanical Stability. Surf. Coat. Technol. 2025, 497, 131740. [Google Scholar] [CrossRef]
- Coelho, S.; Ferreira, J.; Rodrigues, V.; Lopes, M. Source Apportionment of Air Pollution in European Urban Areas: Lessons from the ClairCity Project. J. Environ. Manag. 2022, 320, 115899. [Google Scholar] [CrossRef]
- Amakiri, K.T.; Angelis-Dimakis, A.; Chatzisymeon, E. Photocatalytic Degradation of Phenol and Polycyclic Aromatic Hydrocarbons in Water by Novel Acid Soluble Collagen-Polyvinylpyrrolidone Polymer Embedded in Nitrogen-TiO2. Chem. Phys. 2025, 589, 112485. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 Photocatalysis: Design and Applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium Dioxide Photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Kalinowski, M.; Chilmon, K.; Jackiewicz-Rek, W.; Rakowski, B. The Influence of Selected Material Variables of Photocatalytic Cementitious Composites on the Self-Cleaning Properties and Air Purification Efficiency from NOx Pollutants. Sustainability 2023, 15, 853. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Guo, H.; Ding, S. Low Temperature Synthesis of Polyaniline–Crystalline TiO2–Halloysite Composite Nanotubes with Enhanced Visible Light Photocatalytic Activity. J. Colloid Interface Sci. 2015, 458, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Relinque, E.; Hingorani, R.; Rubiano, F.; Grande, M.; Castillo, Á.; Castellote, M. In Situ Evaluation of the NOx Removal Efficiency of Photocatalytic Pavements: Statistical Analysis of the Relevance of Exposure Time and Environmental Variables. Environ. Sci. Pollut. Res. 2019, 26, 36088–36095. [Google Scholar] [CrossRef] [PubMed]
- Folli, A.; Strøm, M.; Madsen, T.P.; Henriksen, T.; Lang, J.; Emenius, J.; Klevebrant, T.; Nilsson, Å. Field Study of Air Purifying Paving Elements Containing TiO2. Atmos. Environ. 2015, 107, 44–51. [Google Scholar] [CrossRef]
- Park, H.-J.; Hossain, S.M.; Choi, K.; Shon, H.-K.; Kim, J.-H. A Study on the Evaluation Methods of Nitrogen Oxide Removal Performance of Photocatalytic Concrete for Outdoor Applications. Catalysts 2022, 12, 846. [Google Scholar] [CrossRef]
- Russell, H.S.; Frederickson, L.B.; Hertel, O.; Ellermann, T.; Jensen, S.S. A Review of Photocatalytic Materials for Urban NOx Remediation. Catalysts 2021, 11, 675. [Google Scholar] [CrossRef]
- Kalinowski, M.; Chilmon, K.; Jackiewicz-Rek, W. Carbon-Negative Nano-TiO2-Modified Photocatalytic Cementitious Composites: Removal of Airborne Pollutants (NOx and O3) and Its Impact on CO2 Footprint. Coatings 2024, 14, 1607. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Nguyen, T.N.; Tran, V.V.; Hur, J.; Kim, I.T.; Park, D.; Lee, Y.-C. Photocatalytic Materials for Indoor Air Purification Systems: An Updated Mini-Review. Environ. Technol. Innov. 2021, 22, 101471. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M.; Ortega, A.; Sandoval, G.; Liauw, C.M.; Verran, J.; McIntyre, R.B. Degradation and stabilisation of polymers and coatings: Nano versus pigmentary titania particles. Polym. Degrad. Stab. 2004, 85, 927–946. [Google Scholar] [CrossRef]
- Dao, P.H.; Nguyen, T.V.; Nguyen, T.A.; Doan, T.Y.O.; Hoang, T.H.; Le, T.T.; Nguyen-Tri, P. Acrylic polymer/TiO2 nanocomposite coatings: Mechanism for photo-degradation and solar heat reflective recovery. Mater. Chem. Phys. 2021, 272, 124984. [Google Scholar] [CrossRef]
- ISO 16474-2:2013; Paints and Varnishes—Methods of Exposure to Laboratory Light Sources—Part 2: Xenon-Arc Lamps. International Organization for Standardization (ISO): Geneva, Switzerland, 2013.
- Wang, A.; Li, Y.; Zhang, D.; Li, X. A comparative study on UV degradation of organic coatings for concrete: Structure, adhesion, and protection performance. Prog. Org. Coat. 2020, 149, 105892. [Google Scholar] [CrossRef]
- Mizielińska, M.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. The Influence of Accelerated UV-A and Q-SUN Irradiation on the Antimicrobial Properties of Coatings Containing ZnO Nanoparticles. Molecules 2017, 22, 1556. [Google Scholar] [CrossRef] [PubMed]
- Pill, D.; Wiesen, P.; Kleffmann, J. Temperature Dependencies of the Degradation of NO, NO2, and HONO on a Photocatalytic Dispersion Paint. Phys. Chem. Chem. Phys. 2021, 23, 9418–9427. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Van Bui, H.; Valdesueiro, D.; Yuan, S.; Liang, B.; Van Ommen, J.R. Suppressing the Photocatalytic Activity of TiO2 Nanoparticles by Extremely Thin Al2O3 Films Grown by Gas-Phase Deposition at Ambient Conditions. Nanomaterials 2018, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Bonil, L.; Rojas, S.; Buesa, J.; Pérez-Rodríguez, F.J.; Domingo-Calap, P.; Sánchez, G. Virucidal Properties of Photocatalytic Coating on Glass against a Human Coronavirus. Microbiol. Spectr. 2022, 10, e00269-22. [Google Scholar] [CrossRef]
- Han, R.; Coey, J.; O’Rourke, C.; Mills, A. Flexible, disposable photocatalytic plastic films for the destruction of viruses. J. Photochem. Photobiol. B 2022, 234, 112551. [Google Scholar] [CrossRef] [PubMed]
- PN-EN 196-1:2016-07; Methods of Testing Cement—Part 1: Determination of Strength. PKN—The Polish Committee for Standardization: Warsaw, Poland, 2016.
- PN-EN 196-3:2016; Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness. PKN—The Polish Committee for Standardization: Warsaw, Poland, 2016.
- EN 1008:2002; Mixing Water for Concrete-Specification for Sampling, Testing and Assessing the Suitability of Water, Including Water Recovered from Processes in the Concrete Industry, as Mixing Water for Concrete. European Committee for Standardization: Brussels, Belgium, 2002.
- ISO 22197-1:2016; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Air-Purification Performance of Semiconducting Photocatalytic Materials. Part 1: Removal of Nitric Oxide. ISO (The International Organization for Standardization): Geneva, Switzerland, 2016.
- Yoo, S.-W.; Lee, J.-W.; Park, B.; Choi, Y.C. Photocatalytic NOx Degradation Performance of TiO2-Nanofiber-Spray-Coated Foam Composite According to Saturated Conditions. Constr. Build. Mater. 2022, 358, 129414. [Google Scholar] [CrossRef]
- Casagrande, E.A.; Repette, W.L.; Hotza, D. Effect of Environmental Conditions on Degradation of NOx Gases by Photocatalytic Nanotitania-Based Cement Mortars after Long-Term Hydration. J. Clean. Prod. 2020, 274, 123067. [Google Scholar] [CrossRef]
- Xu, M.; Clack, H.; Xia, T.; Bao, Y.; Wu, K.; Shi, H.; Li, V. Effect of TiO2 and Fly Ash on Photocatalytic NOx Abatement of Engineered Cementitious Composites. Constr. Build. Mater. 2020, 236, 117559. [Google Scholar] [CrossRef]
- Şahin, O. Assessment of the Long-Term Photocatalytic Performance of Cementitious Systems Incorporating Different Mineral Admixtures. Constr. Build. Mater. 2022, 354, 129215. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, Z.; Khan, A.U.; Mastoi, N.R.; Aslam, M.; Kim, J. Photocatalytic Systems as an Advanced Environmental Remediation: Recent Developments, Limitations and New Avenues for Applications. J. Environ. Chem. Eng. 2016, 4 Pt A, 4143–4164. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Z.; Zhao, W.; Guan, B. Photocatalytic Oxidation of Nitrogen Oxides Using TiO2 Loading on Woven Glass Fabric. Chemosphere 2007, 66, 185–190. [Google Scholar] [CrossRef]
- Jia, Z.-M.; Zhao, Y.-R.; Shi, J.-N. Adsorption Kinetics of the Photocatalytic Reaction of Nano-TiO2 Cement-Based Materials: A Review. Constr. Build. Mater. 2023, 370, 130462. [Google Scholar] [CrossRef]
- Lakshminarasimhan, N.; Kim, W.; Choi, W. Effect of the Agglomerated State on the Photocatalytic Hydrogen Production with in Situ Agglomeration of Colloidal TiO2 Nanoparticles. J. Phys. Chem. C 2008, 112, 20451–20457. [Google Scholar] [CrossRef]
- Folli, A.; Pochard, I.; Nonat, A.; Jakobsen, U.H.; Shepherd, A.M.; Macphee, D.E. Engineering Photocatalytic Cements: Understanding TiO2 Surface Chemistry to Control and Modulate Photocatalytic Performances. J. Am. Ceram. Soc. 2010, 93, 3360–3369. [Google Scholar] [CrossRef]
- Kanayankottupoyil, J.; John, K. Characterization and Source Apportionment of Ambient VOC Concentrations: Assessing Ozone Formation Potential in the Barnett Shale Oil and Gas Region. Atmos. Pollut. Res. 2025, 16, 102327. [Google Scholar] [CrossRef]
- Derwent, R.G.; Utembe, S.R.; Jenkin, M.E.; Khan, M.A.H.; Shallcross, D.E. Investigating the Role of Organic Compounds in Intercontinental Ozone Transport: Reactivity Scales and Global Warming Potentials (GWPs). Atmos. Environ. 2023, 306, 119817. [Google Scholar] [CrossRef]
- Badia, A.; Vidal, V.; Ventura, S.; Curcoll, R.; Segura, R.; Villalba, G. Modelling the Impacts of Emission Changes on O3 Sensitivity, Atmospheric Oxidation Capacity, and Pollution Transport over the Catalonia Region. Atmos. Chem. Phys. 2023, 23, 10751–10774. [Google Scholar] [CrossRef]
- Witkowski, H.; Jarosławski, J.; Szkop, A.; Chilmon, K.; Kalinowski, M.; Jackiewicz-Rek, W. The Potential Risk of Nanoparticulate Release from Photocatalytic Pavement Concrete Surface Due to a Simulated Abrasion Load—An Experimental Study. Materials 2024, 17, 3022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, J.; Li, Z.; Shen, Y.; Wang, P.; Wang, D.; Hu, X. Preparation and properties of UV-curable waterborne silicon-containing polyurethane acrylate emulsion. Prog. Org. Coat. 2021, 160, 106503. [Google Scholar] [CrossRef]
- Malshe, V.C.; Sangaj, N.S. Fluorinated acrylic copolymers: Part I: Study of clear coatings. Prog. Org. Coat. 2005, 53, 207–211. [Google Scholar] [CrossRef]
- Hodgson, J.L.; Coote, M.L. Clarifying the Mechanism of the Denisov Cycle: How Do Hindered Amine Light Stabilizers Protect Polymer Coatings from Photo-oxidative Degradation? Macromolecules 2010, 43, 4573–4583. [Google Scholar] [CrossRef]

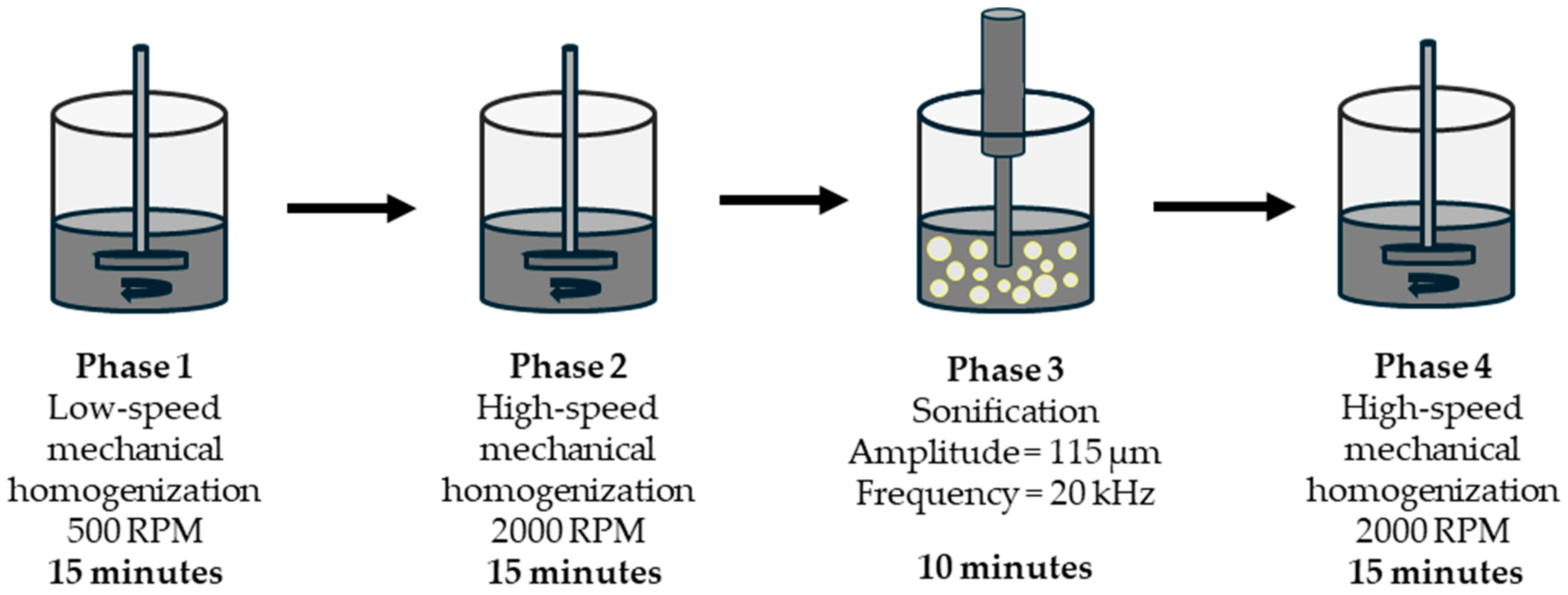
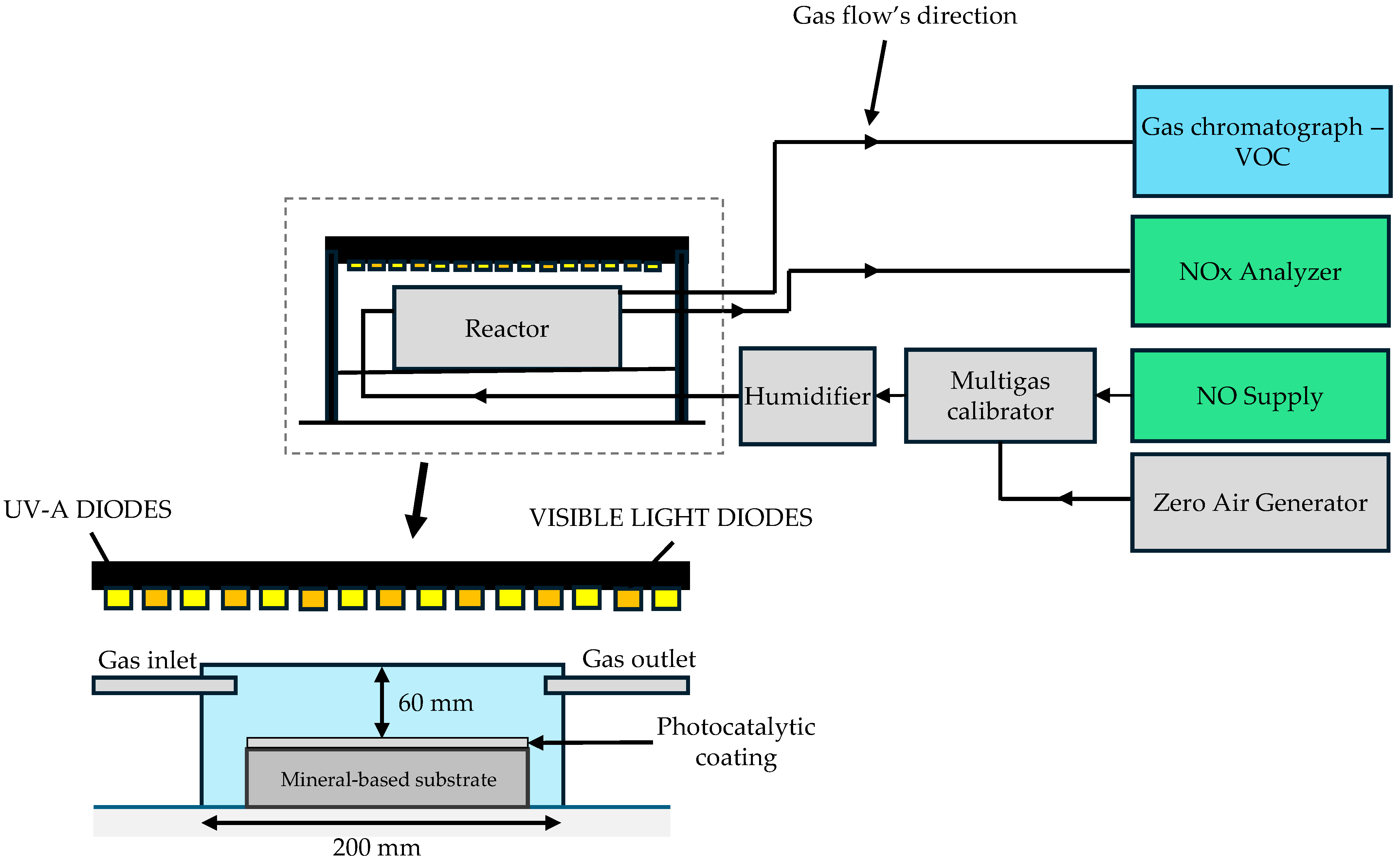
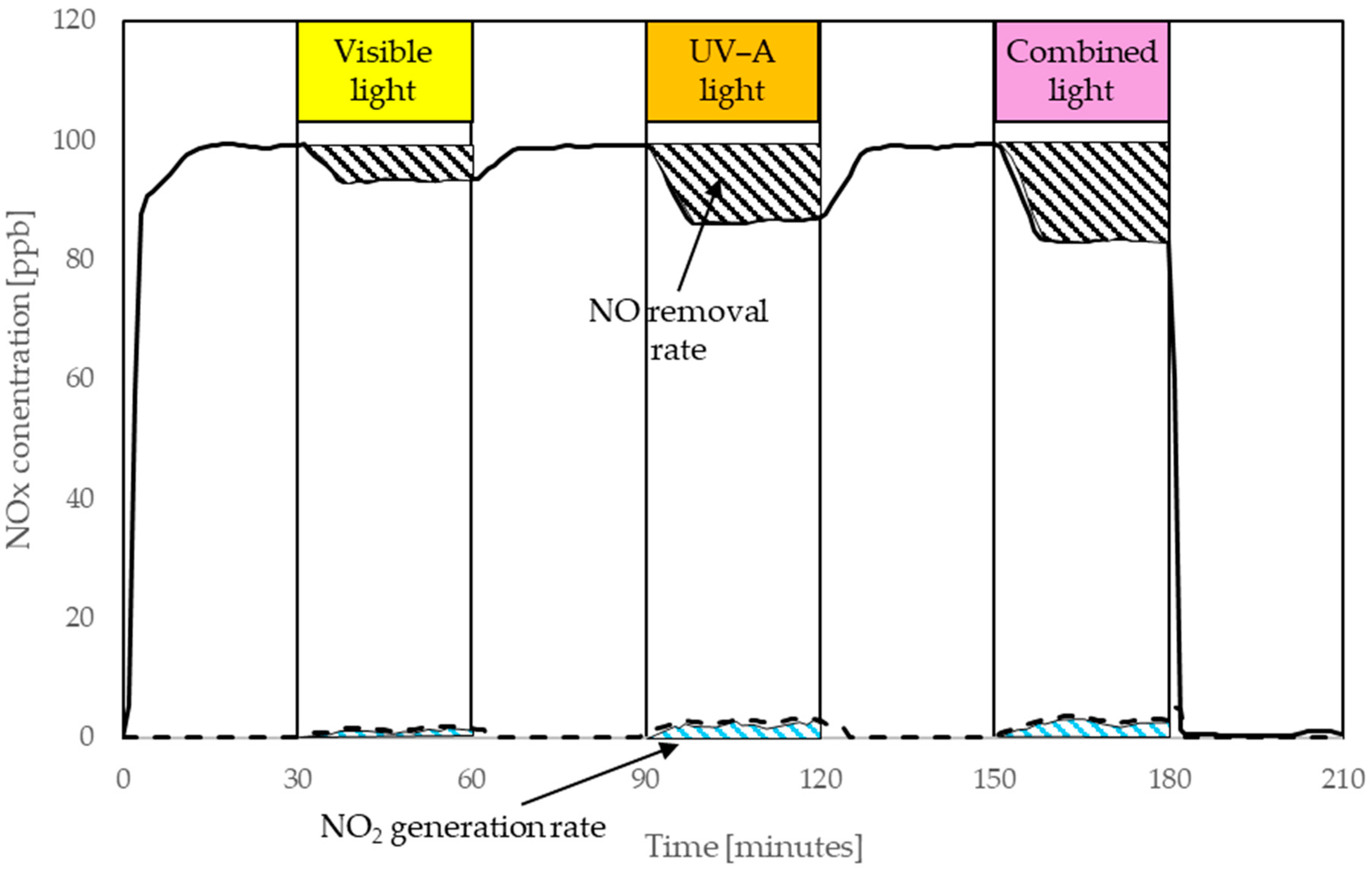
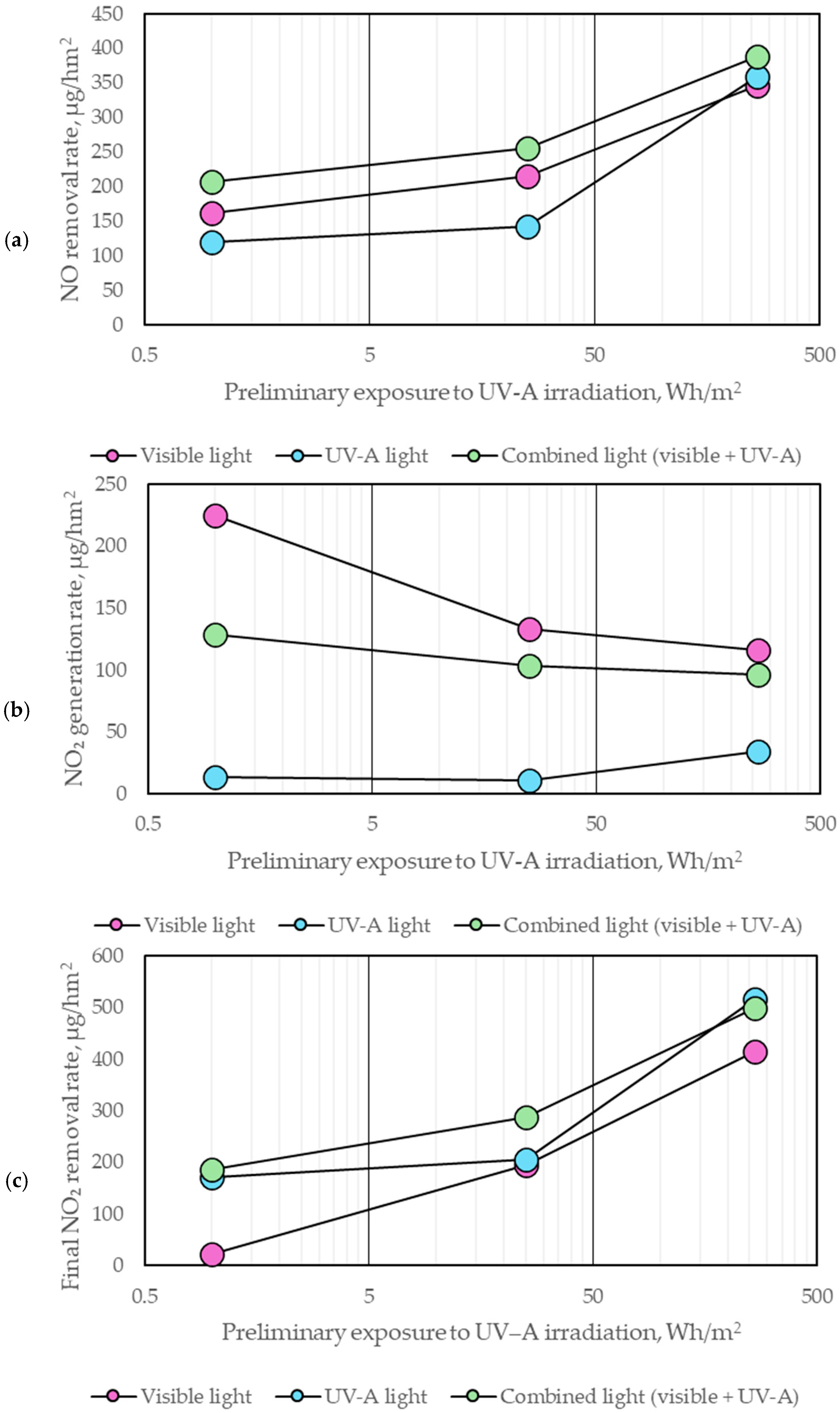

| Components of Photocatalytic Paint | Type | Component Content, % m.c. * |
|---|---|---|
| Nano-TiO2 photocatalyst (A) | first-generation nano-TiO2 | 5.00 |
| Nano-TiO2 photocatalyst (B) | carbon-doped second-generation nano-TiO2 | 5.00 |
| Distilled water | - | 10.30 |
| Dispersing agent | sodium metaphosphate, 1% | 10.00 |
| Defoamer | silicone oil | 1.00 |
| Organic viscosity-modifying agent | methylhydroxycellulose, 2% | 13.52 |
| Stabilizing agent | polypropylene glycol | 4.00 |
| Organic binder | mix of acrylic polymer, water-soluble polycarboxylate, and polyvinyl alcohol | 17.20 |
| Inorganic micro filler | fine marble powder < 32 µm | 27.00 |
| Inorganic pigment | titanium (IV) oxide and iron (II) oxide | 5.48 |
| Organic film-forming agent | texanol, 3-hydroxy-2,2,4-trimethylpentyl isobutyrate | 1.50 |
| Nano-TiO2 | Specific Surface Area, m2/g | Phase, % | Crystallite Size, nm | ||
|---|---|---|---|---|---|
| Anatase | Rutile | Anatase | Rutile | ||
| A—first generation | 53.8 | 87 | 13 | 33 | 54 |
| B—second generation | 246.8 | 100 | 0 | 10 | - |
| Preliminary Exposure to UV-A Irradiation Before Air Purification Test, Wh/m2 | Average Selectivity, - | ||
|---|---|---|---|
| Visible Light | UV-A Light | Combined Light (Visible and UV-A) | |
| 1 | −0.40 | 0.89 | 0.37 |
| 25 | 0.38 | 0.92 | 0.59 |
| 265 | 0.66 | 0.90 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinowski, M.; Chilmon, K.; Kuziak, J.; Łukowski, P.; Jackiewicz-Rek, W. Photocatalytically Induced Degradation of Nano-TiO2-Modified Paint Coatings Under Low-Radiation Conditions. Coatings 2025, 15, 281. https://doi.org/10.3390/coatings15030281
Kalinowski M, Chilmon K, Kuziak J, Łukowski P, Jackiewicz-Rek W. Photocatalytically Induced Degradation of Nano-TiO2-Modified Paint Coatings Under Low-Radiation Conditions. Coatings. 2025; 15(3):281. https://doi.org/10.3390/coatings15030281
Chicago/Turabian StyleKalinowski, Maciej, Karol Chilmon, Justyna Kuziak, Paweł Łukowski, and Wioletta Jackiewicz-Rek. 2025. "Photocatalytically Induced Degradation of Nano-TiO2-Modified Paint Coatings Under Low-Radiation Conditions" Coatings 15, no. 3: 281. https://doi.org/10.3390/coatings15030281
APA StyleKalinowski, M., Chilmon, K., Kuziak, J., Łukowski, P., & Jackiewicz-Rek, W. (2025). Photocatalytically Induced Degradation of Nano-TiO2-Modified Paint Coatings Under Low-Radiation Conditions. Coatings, 15(3), 281. https://doi.org/10.3390/coatings15030281








