1. Introduction
Despite the warnings and reports published by the World Health Organization for a long time, tobacco use is continuously increasing, both in the form of traditional cigarette smoking and newer methods, such as electronic cigarettes [
1,
2,
3]. The new methods have a greater impact on the adolescent population, which is much more receptive to novelty, since vaping is presented as a healthier version of tobacco consumption [
4].
Tobacco smoke is a complex mixture of chemical compounds in the form of gases and microscopic droplets suspended in the air [
5]. The composition of tobacco smoke varies depending on the physical conditions in which it was produced. Thus, two types of tobacco smoke are described: mainstream smoke (MS) and side stream smoke (SS) [
1,
2,
6].
Mainstream smoke is emitted at the filter end of a cigarette when a smoker draws air through the burning cigarette to inhale, and the tobacco burns at a high temperature (up to 950 °C), due to the increased supply of oxygen. By contrast, side stream smoke is emitted from the smoldering tip of the cigarette between puffs, at a lower temperature (600–800 °C) [
1,
2,
6]. Side stream smoke contains more toxic chemical compounds than mainstream smoke: 147 times more ammonia; 16 times more pyridine; 15 times more formaldehyde; 12 times more quinolone; 3 times more styrene; and twice as much nicotine [
1,
7,
8,
9].
The tobacco cigarette is composed of a filter, the shredded tobacco leaves, and a thin paper that holds all the components rolled together. Among the cigarette components, only the filter is not involved in the production of smoke. The chemical composition of smoke also includes substances intentionally added to affect appearance, taste, odor, color, uptake, and availability of tobacco smoke constituents, as well as residual substances derived from processing, curing, and storing of tobacco [
7,
8].
Cigarette smoke inhalation exposes the tissues in the oral cavity and respiratory system to the toxic chemicals found in tobacco, causes multiple pathogenic mechanisms that lead to an increased risk of oral diseases (such as oral cancer) and respiratory pathologies, such as heightened susceptibility to viral and bacterial lung infections [
9,
10].
Studies focusing on the individual components in the cigarette smoke demonstrated that TAR (tobacco residue) after smoking, representing the insoluble particles generated in the process of burning the tobacco, exerts tissue-level deleterious effects and alters the air–blood barrier in the lung by disrupting the surfactant [
11]. To comprehend the physiological and physical–chemical processes that are linked to tobacco consumption, it is necessary to comprehend the complicated dynamics of tobacco smoke [
12].
In vitro tobacco smoke assessment has traditionally been centered on the particle phase captured on a Cambridge filter [
13] or bubbling through cell culture media [
14].
Over time, researchers have tried to standardize human behavior during cigarette smoking, in order to create a device that simulates smoking activity [
15]. This paper is the first to present the capabilities of a syringe smoking device to simulate the processes involved in cigarette burning and smoke inhalation. Preclinical models used tobacco particle extracts and cigarette smoke extract (CSE) in order to assess the full potential of cigarette smoke constituents and smokeless tobacco extracts [
16,
17,
18]. CSE is the only aqueous solution that includes all the elements of primary cigarette smoke that is directly inhaled [
19].
In the human oral cavity of the smokers (HOC-S), who had oral rehabilitation prosthetics treatments (ORPTs) and whose remaining tooth has been prepared and received a dental crown (DC), the inhaled smoke comes in contact with saliva (S), the prosthetic reconstructions (PRs) [
20], and the adjacent soft gingival tissues (ASGTs) [
21] at the human body temperature [
22].
The aim of the present study is to assess, by in vitro simulation
using Mesenchymal Stem Cells (MSC) and Cigarette Smoke Extract (CSE) solution, the impact of cigarette smoke (TAR and NICOTINE) [
23] that is absorbed in human saliva comes into contact with the tissues of the oral cavity.
2. Materials and Methods
2.1. Chemicals and Reagent
The experimental component was centered around obtaining a solution with cigarette smoke extract (CSE) and tobacco residues (TAR) through the use of a syringe smoking device (SSD).
2.2. Tobacco Cigarettes: The Precursor Material
The precursor material was represented by Marlboro Red filter tobacco cigarettes (MR), Philip Morris Brands Sarl Neuchatel—Switzerland (PM), sold in packs of 20 pieces. The MR pack was 90 mm long and comprised 83.45 mm long tobacco cigarettes from the PM Flavor category. They had a TAR quantity of 13 mg/cig and a nicotine volume of 9 mg/cig.
MR tobacco cigarettes were extracted one by one from the sealed pack, measured, and weighed. The length of the cigarettes was measured using an electronic caliper Profi scale-Precise PS 7215 (Burg-Wachter KG, Altenhofer Weg 15–58300 Wetter, Germany)—and the weight was assessed using a digital scale with two decimal places.
2.3. Syringe Smoking Device
The smoking device proposed in this research is named SSD. We address the need for specifications and requirements according to the international standard (ISO 3308:2012). This includes considering the typical behavior of real smokers, such as the time it takes to smoke a cigarette and the number of puffs taken with each cigarette [
24].
The MR cigarettes were weighed before being inserted into the SSD. For all 20 cigarettes, the following parameters were assessed: duration, volume, frequency, and number of puffs. Each intake puff lasted for two seconds at a volume of 17.5 mL/s. The smoke was kept inside the syringe for 60 s and then released for two seconds. Six successive intake/release cycles lasted for six minutes and 24 s.
After the cigarette was consumed, the remaining filter was weighed. The weight of the consumed cigarette was calculated as the difference between the initial cigarette weight and the standard weight of the filter (220 mg) before filtering the smoke (BS—before smoking). The length of the consumed cigarette was calculated as the difference between the initial cigarette length and the length of the filter that resulted after burning the cigarette (AS—after smoking) and accumulating tobacco residues.
2.4. Cigarette Smoking Extract (CSE) and Tobacco After Smoking Residues (TAR)
To simulate human saliva in the oral cavity of a smoker exposed to cigarette smoke, Ringer’s saline solution (Stada Hemofarm, Timișoara, Romania) was used in vitro as a saliva replacement, with a volume of 500 mL per bottle. Cigarette smoke extracts (CSE) are prepared by bubbling cigarette smoke through the Ringer’s saline solution. To obtain each CSE solution, a 60 mL single-use syringe is required. Each syringe consists of several components: a plunger with a rubber stopper and thumb rest, a barrel with a plain tip and finger flange, and a cap with a needle.
The syringe was modified for experimental use. The internal threading of the plain tip was removed using a No. 11 surgical blade, leaving a hollow cylinder with a diameter matching that of a tobacco cigarette filter. At the plain tip end of the barrel, a hole was created, and a one-way air valve was installed along with an exhaust hose. The barrel was filled with 20 mL of Ringer’s saline solution, using a single-use 20 mL syringe for extraction, dosing, transport, and filling. After filling the barrel in a vertical position, with the plunger positioned at the 20 mL mark, and the tobacco cigarette was attached with the filter placed in the plain tip.
Cigarette smoke extracts (CSE-5, CSE-10, CSE-15, and CSE-20) were obtained by burning 5, 10, 15, and 20 cigarettes, respectively, and collecting the smoke in a syringe filled with 20 mL of Ringer’s saline solution. When the cigarette smoke comes into contact with the liquid solution, it produces the corresponding CSE solution.
The tobacco smoke residues (TAR) were collected by burning 5, 10, 15, and 20 cigarettes in succession and drawing the smoke into a syringe that contained CoCr dental alloy samples fixed to the head of the plunger.
The contact between the cigarette smoke and the Cobalt-Chromium samples results in a residual deposit forming on their surfaces.
2.4.1. CSE Solution pH Investigation
The pH of the five CSE solutions was measured using a Consort C931 pH-meter (Consort, Turnhout, Belgium) equipped with a built-in Ag/AgCl, KCl 3M reference pH electrode. Before the tests, the electrode was calibrated at 25 °C using calibration solutions of pH 4, 7, and 10 (pH buffer solutions were purchased from Hanna Instruments).
The pH for the initial standard CSE-0 solution was assessed by using the pH-meter and 20 mL of Ringer’s saline solution. The same method was used to determine the pH of the CSE-0, CSE-5, CSE-10, CSE-15, and CSE-20 solutions. All tests were run at least 3 times, and the average of the individual measurements was calculated.
2.4.2. Characterization by IR Spectroscopy
The CSE solutions and the residues deposited on Cobalt-Chromium dental alloy samples were characterized by FR-IR spectroscopy using an ATR FT-IR spectrometer (Jasco FT-IR 4100) equipped with a ZnSe ATR crystal.
Spectra were recorded for the liquid sample after the evaporation of the solvent. The spectra were collected using the attenuated total reflection technique in the 4000–550 cm−1 spectral range with a resolution of 4 cm−1: averaged over 32 scans, and analyzed using a Jasco Manager 2 software.
2.4.3. Characterization by LC-MS
The LC-MS analyses were performed using an I-Class LC system coupled to a Synapt G2-Si high-resolution mass spectrometer (Waters, Milford, MA, USA). The chromatographic separation was achieved using a BEH C18 stationary phase (50 × 2 mm) (Waters, Milford, MA, USA) and mobile phases formed of 0.1% formic acid in water (A) and acetonitrile (B), delivered at a flow rate of 0.4 mL/min at a temperature of 40 °C. The gradient elution used the following profile: 0 min 3% B–5 min 95% B.
The mass spectrometer was set in resolution mode using positive electrospray ionization. Data was acquired independently (DIA) in the range of 50 to 1200 m/z using two functions.
The first function had collision energies set to 4 eV (trap) and 2 eV (transfer), while the second fragmentation was a ramp between 20–45 eV (trap). Leucine-enkephalin was used for the mass correction as a single-point reference (m/z 554.2615). All samples were analyzed in triplicate after a 20-fold dilution with water.
All data processing was performed using Progenesis QI software version 3.0.76 (Waters, Milford, MA, USA), consisting of alignment, peak picking, deconvolution, compound identification, and statistical analysis.
The compound identification was performed using the ChemSpider addon of the Progenesis QI software, using a precursor tolerance of 10 ppm, theoretical fragmentation with a tolerance of also 10 ppm, and the results needed to have an isotope similarity of more than 95%.
The databases used for identification were KEGG, PlantCyc, Royal Society of Chemistry, Phenol-Explorer, NIST, MassBank, LipidMAPS, and BioCyc.
2.5. Cell Culturing and Citotoxyc Evaluations
Stromal mesenchymal stem cells (MSCs) were generously provided by Dr. Emoke Pall from the University of Agricultural Sciences and Veterinary Medicine in Cluj-Napoca, Romania. The characterization of these MSCs was conducted as described in previously published studies [
25,
26,
27]. The MSCs were cultured in Dulbecco’s Modified Eagle’s Medium/Nutrient F-12 Ham (DMEM/F12), supplemented with 10% Fetal Bovine Serum (FBS), 1% Non-essential Amino Acids (NEAAs), 1% Penicillin/Streptomycin, and 1% Glutamine. After each subculture, the cells were washed with D-PBS that lacked Ca
2+ and Mg
2+. All reagents used for cell culture were obtained from Gibco (Dublin, Ireland). Throughout the experiments, the cells were maintained in a humidified chamber at 37 °C and 5% CO
2.
2.5.1. Cell Viability Testing by MTT Assay
Cells were cultured at a density of 1 × 104 cells per well in a flat-bottom 96-well plate, with three replicates for each experimental group across three independent experiments. After 24 h of incubation, the cell culture media were removed, and the cells were washed with Dulbecco’s phosphate-buffered saline (D-PBS) without calcium (Ca2+) and magnesium (Mg2+).
Following this, four concentrations of Cigarette Smoke Extract (CSE) were applied to each well according to the experimental design, with incubation times of 24 h and 72 h. After the incubation period, another wash step with D-PBS was carried out to remove any residual extracts and phenol red that could interfere with the MTT assay. Subsequently, 100 μL of a 1 mg/mL MTT working solution was added to each well, and the plate was incubated for 4 h at 37 °C in a 5% CO2 atmosphere, protected from light.
After the incubation, the MTT solution was carefully removed using a multichannel pipette, and 150 μL of dimethyl sulfoxide (DMSO) was added to each well to dissolve the formazan crystals.
The absorbance corresponding to the viable cells was measured at a wavelength of 570 nm using a SPARK10M multi-plate reader (Tecan, Männedorf, Switzerland), as previously documented. Each plate also included a blank control (medium only) and a positive control (100% DMSO).
2.5.2. Statistical Analysis
The experiment was conducted three times. Statistical analysis was carried out using GraphPad Prism software (version 8) from GraphPad Software in San Diego, CA, USA. Cell viability results were expressed as a percentage of the control group, which was set at 100%, and are presented as the mean along with the relative standard deviation (RSD).
The quantitative data were analyzed using one-way ANOVA, followed by Dunnett’s multiple-comparison test. A significance level of p < 0.05 was accepted for the statistical analyses.
2.5.3. Morphological Analysis
In a 24-well plate, 20,000 cells were seeded and incubated at 37 °C with 5% CO2 for 24 h. Once the cells had adhered to the surface, solutions containing 10%, 5%, 2.5%, and 1% of each CSE (cigarette smoke extract) were diluted in culture media and added to the respective groups of cells.
The plate was then incubated again at 37 °C with 5% CO2 for 72 h. After this incubation period, the cells were evaluated morphologically using a Zeiss Axio Vert 10 inverted microscope (Zeiss, Jena, Germany) with a 10× objective. A scale bar was automatically included in the images using ZEN 3.9 software.
The evaluation focused on morphological changes, cell population density, and debris resulting from the addition of the CSE solutions.
2.5.4. Tobacco After Smoking Residues (TAR)
To simulate the surface of metal prosthetic dental work, samples (M) of Cobalt–Chromium alloy (Heraenium PW-Mitsui Co., Ltd., Saitama, Japan) were obtained by sectioning Co-Cr alloy cylinders in the horizontal plane using a rotary instrument with a silicon carbide (SiC) disc (15,000 rpm) mounted on a dental micro-motor (Forte 200α—Saeshin Precision Co., Ltd., Daegu, Republic of Korea).
Some of the M samples were mechanically machined with rotary abrasive tools, rubber, and fibers (cotton wool) to achieve a mirror-like surface, representing the metal-polished samples group (MP). The remaining M samples were sandblasted with Al2O3 particles (175 μm granulation) at a pressure of 3.5 bar, using an Easyblast blaster (Bego Goldschlagerei GmbH, Bremen, Germany), to create a rough surface, which represented the metal-sandblasted samples group (MS).
The two types of metal samples, each with differently processed surfaces, were inserted into the piston head of a 60 mL disposable syringe and secured with adhesive.
The syringe was then placed into an SSD smoking device, exposing the samples to smoke produced by burning 5, 10, 15, and 20 cigarettes.
2.6. SEM—Scanning Electron Microscopy
Metallic samples and MSCs were analyzed by scanning electron microscopy (SEM) and EDS analysis, using a Hitachi SU8230 microscope with a 30 kV, 10 μA, and 8 mm working distance and an EDX spectrometer (Oxford Instruments, Abingdon, UK).
3. Results
3.1. The Results of Consumption by Burning Tobacco Cigarettes
Although the packs of MR tobacco cigarettes appeared to have identical packaging and contained 20 cigarettes each, they were not the same. The weight of the tobacco component varied between them, even though the cigarettes had a similar total length of 82.93 mm, a filter weight of 220 mg, and a filter length of 27.03 mm.
3.2. In Vitro Changes in the pH Measurement of Cigarette Smoke Extracts
The pH levels of the standard Ringer solution and the four cigarette smoke extract (CSE) solutions obtained after smoking 5, 10, 15, and 20 cigarettes were measured. All tests were repeated at least three times, and the results presented represent the average of the individual measurements (see
Table 1). The average pH values indicate that CSE-5 and CSE-20 exhibit the highest pH levels, while CSE-15 shows the lowest. Additionally, the minimum relative standard deviation (RSD) was observed for CSE-10, indicating that this measurement was the most precise. In contrast, CSE-20 had the highest RSD, suggesting greater variability in its measurements.
The standard Ringer’s saline solution (CSE-0) and four variants of CSE solutions were used for further studies: CSE-5 (after five cigarettes), CSE-10 (after 10 cigarettes), CSE-15 (after 15 cigarettes), and CSE-20 (after 20 cigarettes) (see
Figure 1b–i).
The standard Ringer’s saline solution (CSE-0) and four variants of CSE were used for further studies: CSE-5 (after five cigarettes), CSE-10 (after ten cigarettes), CSE-15 (after fifteen cigarettes), and CSE-20 (after twenty cigarettes) (see
Figure 1b–i).
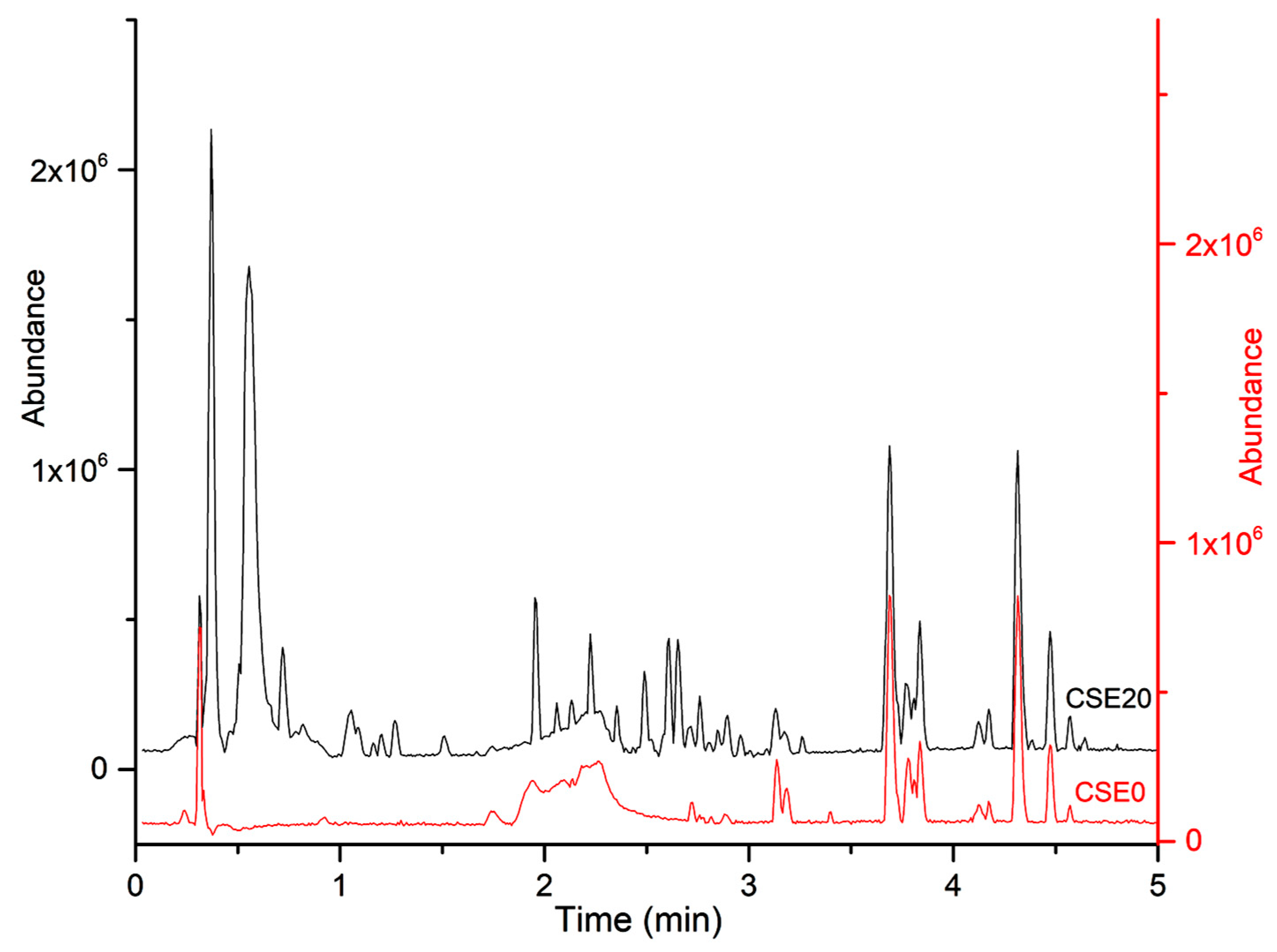
3.3. LC-MS Characterization
The smoke extracts were characterized using an untargeted approach. To determine the optimal separation conditions, several elution gradients were tested. Initially, a broad gradient profile ranging from 10% to 95% B over 5 min was applied. However, it was found that most compounds extracted from cigarette smoke were polar and eluted in the first half of the chromatogram. As a result, a more suitable gradient was developed, varying the composition of mobile phase B from 5% to 65% over 5 min. This adjustment improved the retention of highly polar compounds (see
Figure 2).
Following alignment, peak picking, and deconvolution, the data matrix comprised 1302 features corresponding to 1243 compounds. Of these, 1011 showed significant differences compared to the control group (ANOVA p < 0.05). Identification attempts were made on the top 100 most abundant compounds using the ChemSpider add-on of the Progenesis QI software.
The identities of 2 compounds were confirmed as nicotine and cotinine, while for 42 other compounds, chemical formulas could be proposed. No database matches were found for the remaining analytes. Most of the detected compounds exhibited the highest abundance in the CSE15 group, with an average fold-change of 2.79 compared to CSE5, which corresponds closely to the number of cigarettes.
Interestingly, a significant decrease in abundance was observed in the CSE20 extract for most compounds when compared to CSE15, CSE10, or even CSE5. These findings correlate with the pH measurements of the solutions.
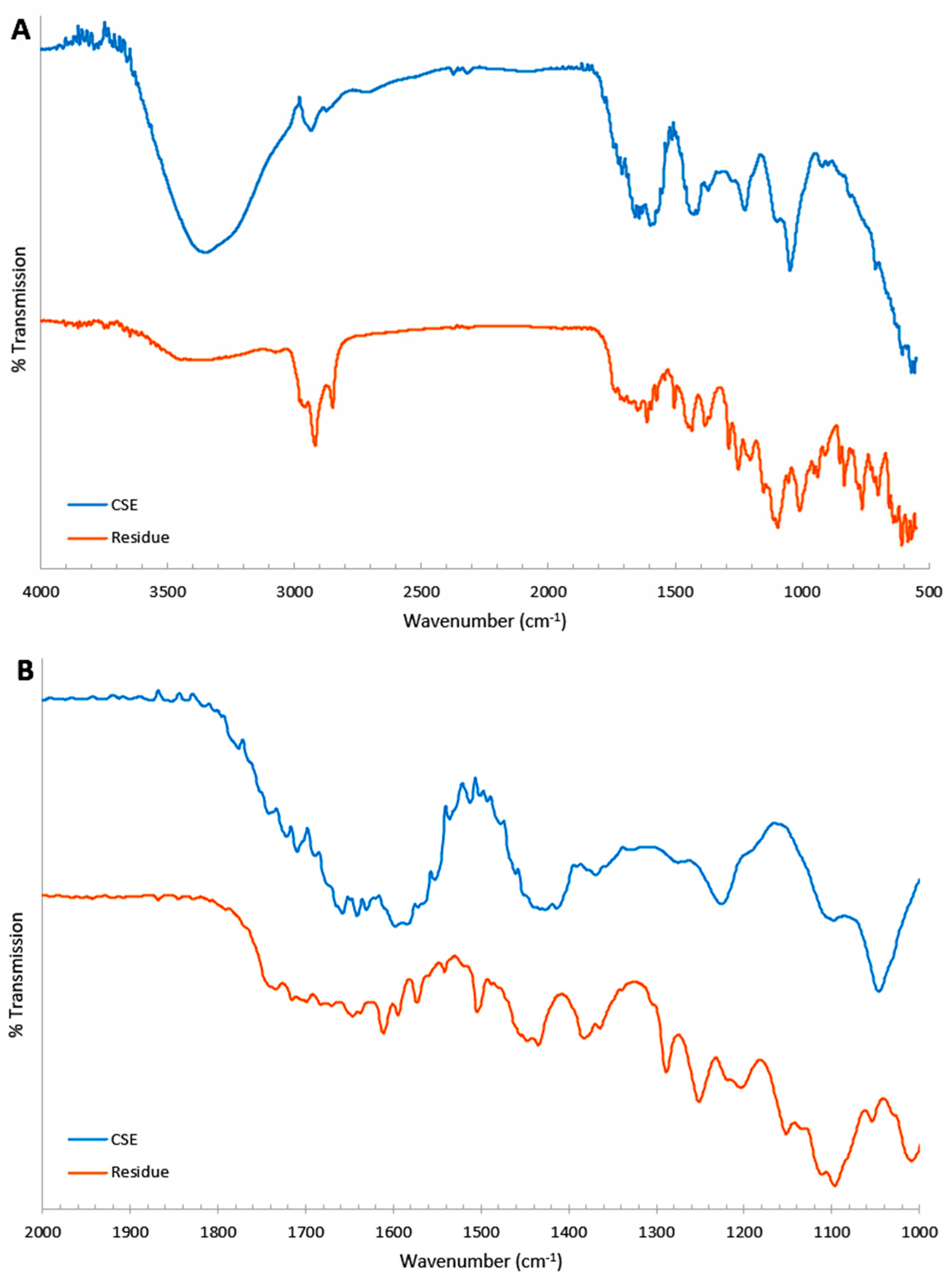
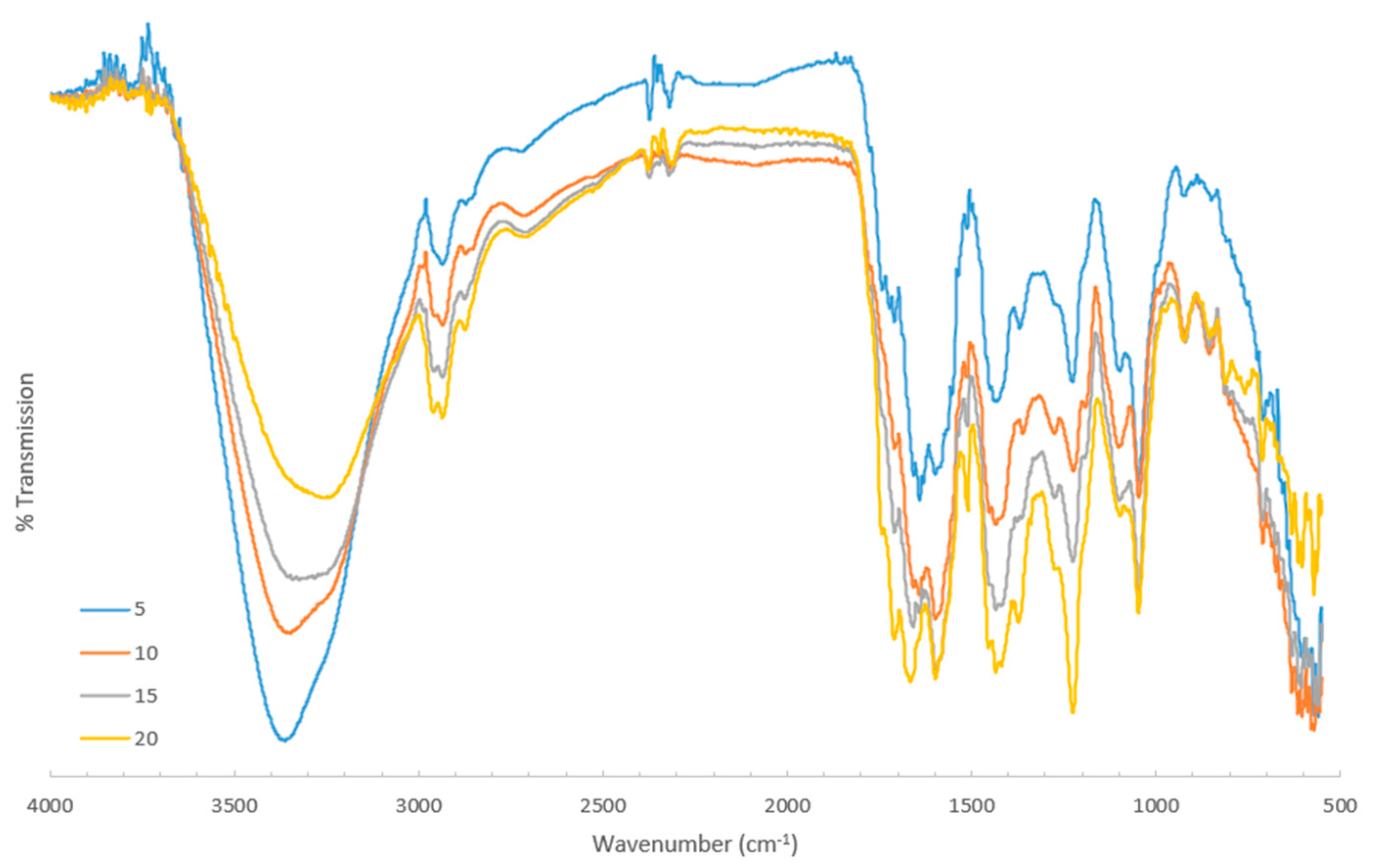
3.4. IR Spectroscopy
The FT-IR spectra for the CSE solutions and the residue samples are presented in
Figure 3, with 1276–1251 cm
−1, and 1153 cm
−1 attributed to N–H and C–N stretching vibrations. Bands centered around 2987–2850 cm
−1 indicated the presence of C–H stretching from aliphatic groups.
The spectra also show a distinct summary of the observed peaks listed in
Table 2 (see
Figure 3). The samples displayed broad bands in the range of 3300–3400 cm
−1. Peaks were within the ranges of 3024–3073 cm
−1, 1440 cm
−1, and 1367 cm
−1, and bands were at 1722 cm
−1 and 1737 cm
−1.
Additionally, well-defined peaks were observed at 1658–1630 cm−1, 1646–1609 cm−1, 1597–1551 cm−1, and 1575–1540 cm−1. The bands at 1367 cm−1 and 1361 cm−1 were consistent with the O–H bending vibrations typical of phenols. Both spectra displayed sharp, strong bands at 1046–1095 cm−1.
The bands near 1509–1505 cm−1 and 850 cm−1 (in the residue spectrum) were associated with N–O stretching, while peaks in the range of 700–712 cm−1 corresponded to C–Cl stretching vibrations.
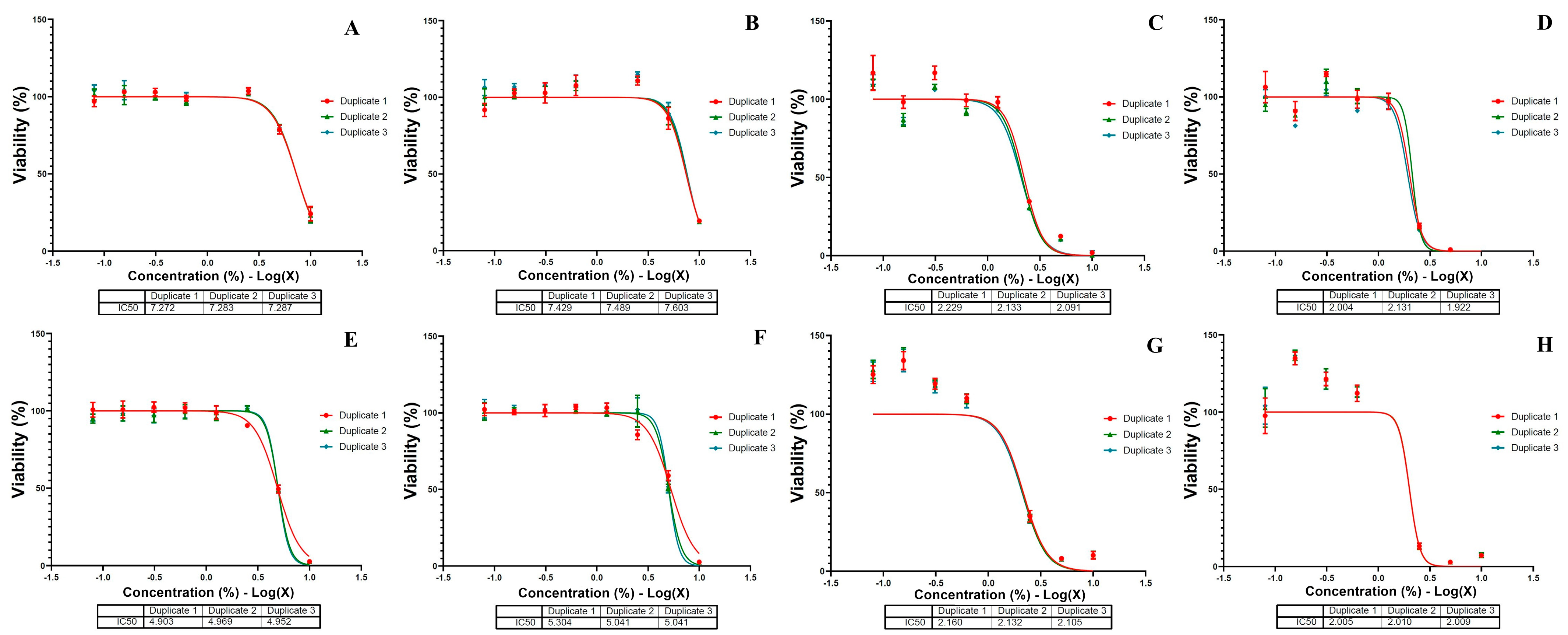
3.5. Morphological Evaluation and Cellular Viability of MSCs
After 72 h of exposure to CSE solutions, mesenchymal stem cells (MSCs) exhibited morphological changes that varied with the number of cigarettes and the concentration of the CSE solution in the culture media (see
Figure 4). The CSE-5 solution caused isolated cellular damage and created larger gaps between the cells at concentrations above 5% (see
Figure 4(A2,A3)). Additionally, high concentrations of CSE-10, CSE-15, and CSE-20 solutions led to cell shrinkage, the formation of large intercellular spaces (see
Figure 4(B2, B3,C2,C3,D2,D3)), and the presence of debris precipitates (see
Figure 4(C2,D2,D3)).
Based on
Figure 4 and
Figure 5, exposure to the CSE solutions inhibited cell growth in a dose-dependent manner. The IC50 values for the CSE-20 and CSE-15 solutions remained relatively stable at around 2% after both 24 h and 72 h. In contrast, the IC50 values for the CSE-5 and CSE-10 solutions were significantly lower, indicating that prolonged exposure of the MSC to these solutions resulted in increased cells death.
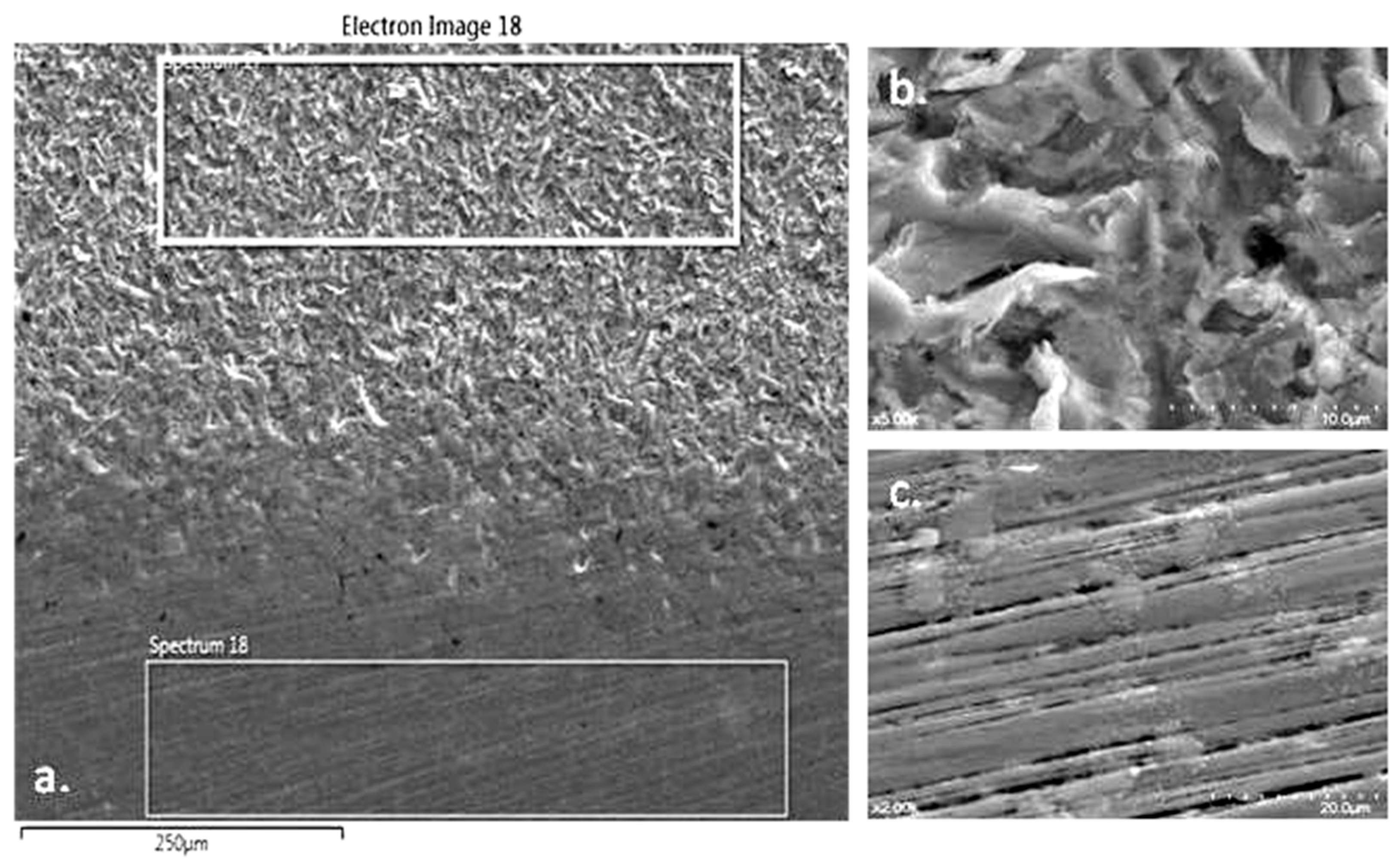
3.6. SEM—Scanning Electron Microscopy
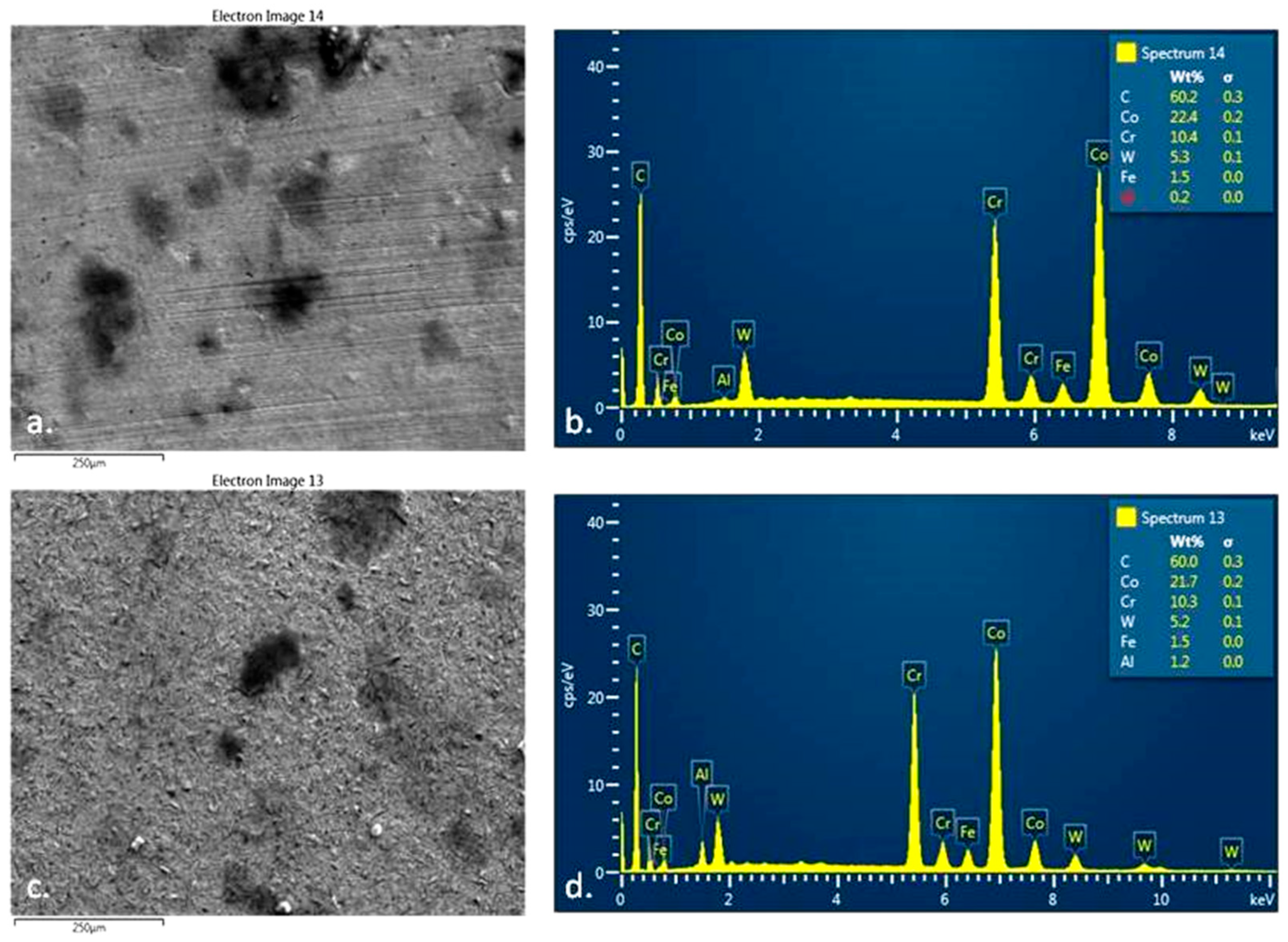
3.6.1. Deposition of TAR on the Surface of Metal M Samples
The surface analysis of the metal samples M from the Cobalt-Chromium dental alloy was performed using Scanning Electron Microscopy (SEM) (see
Figure 6a). The samples were processed using two methods: sandblasting (
Figure 6b) and polishing (
Figure 6c). This comparison emphasizes the significance of finishing and polishing dental prosthetics before their application in the oral cavity. Additionally, the quality of finishing is directly related to the amount and adhesion of TAR deposits on dental prosthetic works.
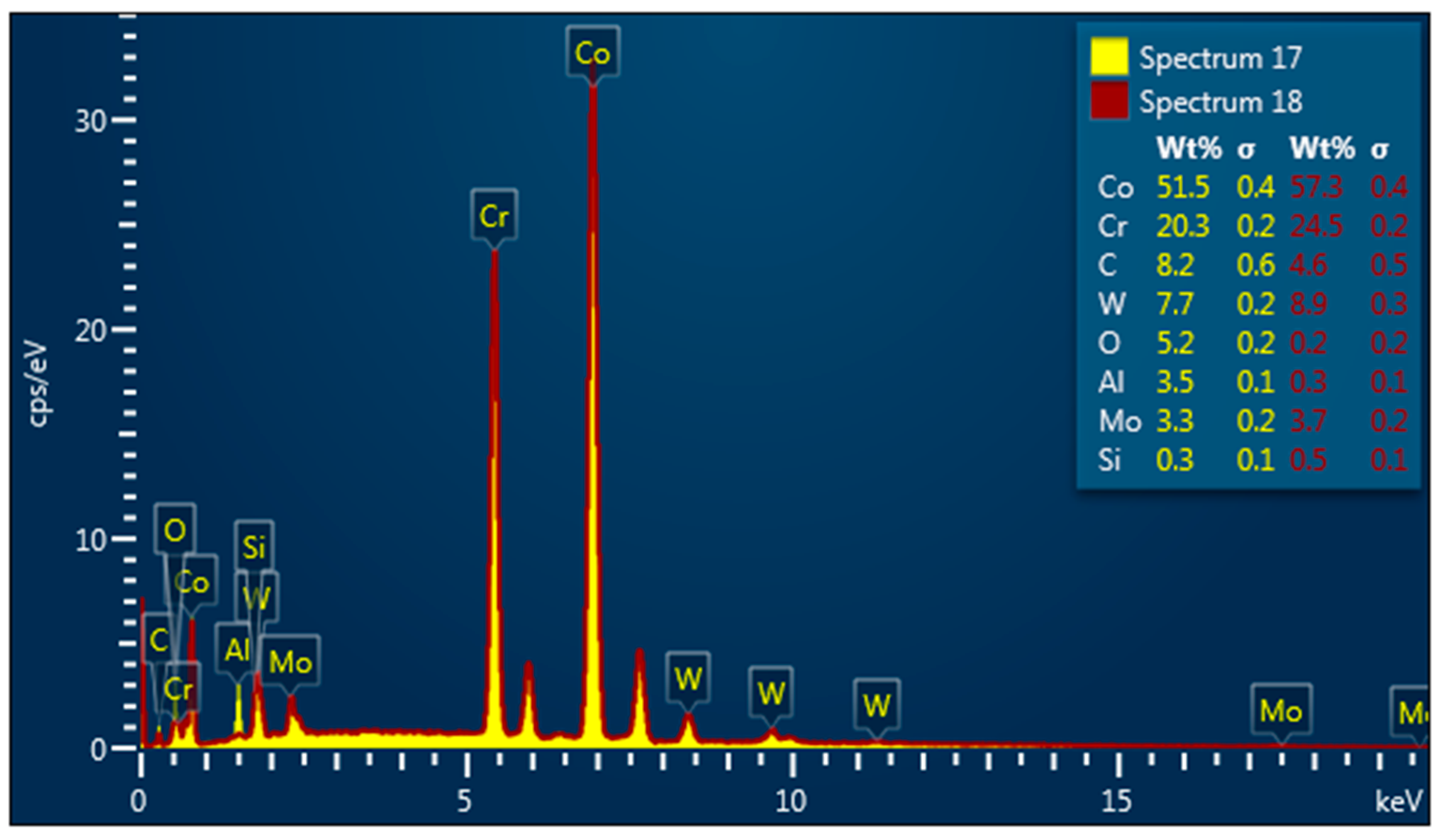
EDX Spectroscopy was made at the surface of sample M (
Figure 7) before smoking.
EDX spectroscopy and SEM instrumentation were made on the surface of sample M (see
Figure 8) after smoking.
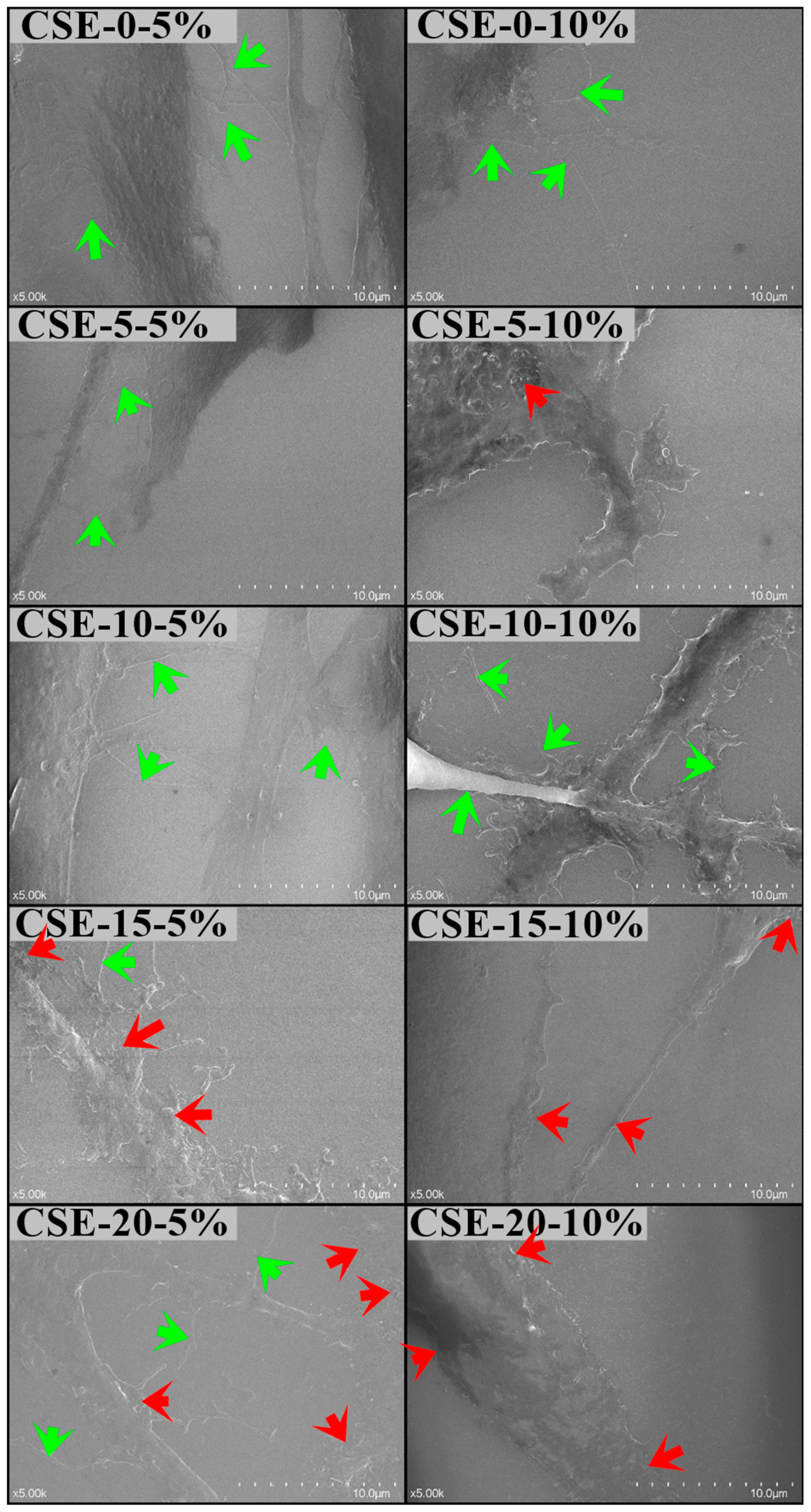
3.6.2. Gingival Mesenchymal Stem Cells (MSCs) Cultures
The Scanning Electron Microscopy (SEM) analysis of gingival mesenchymal stem cell (MSC) cultures exposed to 5% and 10% concentrations of CSE-0, CSE-5, CSE-10, CSE-15, and CSE-20 solutions in the culture media revealed notable morphological changes in the cell membranes.
The CSE-0 solution at both concentrations, as well as the 5% concentrations of CSE-5 and CSE-10, did not affect the cell morphology.
This was evidenced by the presence of cytoplasmic processes and membrane pseudopods, which are important for cell-to-cell contact and normal signaling.
However, the higher concentrations of CSE-15 and CSE-20 induced cellular ruffling, characterized by an irregular cell surface, indicating membrane stress (see
Figure 9).
4. Discussion
The pH of the CSE solutions significantly decreased, and this acidity was correlated with the number of cigarettes consumed. The differences in filter weight before smoking (BS) and after smoking (AS) were attributed to the accumulation of filtered residues in the material.
Despite the limitations of the study, the results indicated that, after each cigarette was smoked, the color of the artificial saliva solution changed from transparent to yellowish and then to brown. Furthermore, a correlation could be established between the degree of solubility of the post-smoking residues, the acidity of the solution, and the observed color change. The color modification was assessed through spectrum-photometric analysis.
Three replicates of the CSE solutions were placed in a 96-well plate with a flat and transparent bottom. The plate was then analyzed using the TECAN SPARK 10M spectrophotometer, employing the absorbance scan module.
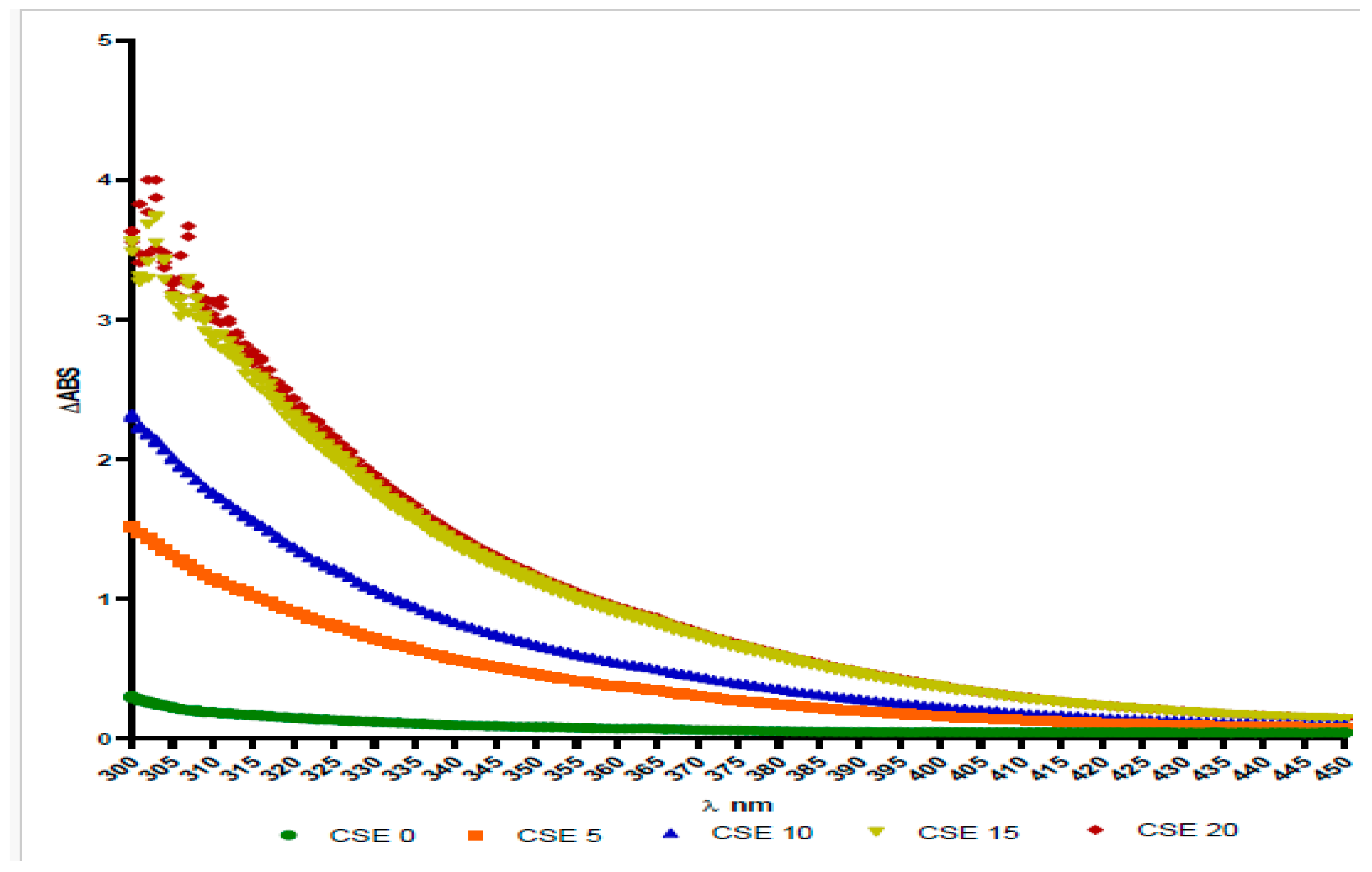
The absorbance of the solution was evaluated in the range of 300 to 450 nm, with measured absorbance values between 0.05 and 0.06 absorbance units. We chose to include only a limited range that was deemed highly relevant.
CSE0 showed a slightly increased absorbance in the 300–350 nm region. In contrast, CSE samples labeled 5, 10, 15, and 20 exhibited absorbance that increased in a dose-dependent manner, particularly for CSE15 and CSE20, which demonstrated very similar absorbance levels, suggesting a plateau.
The differences in absorbance indicate that the color of the solutions varied, which is related to their pH (see
Figure 10).
The most abundant compound identified was nicotine. An interesting observation is that the nicotine concentration likely reached saturation starting from the CSE5 sample, as no significant fold change was detected in the subsequent extracts compared to CSE5 (fold-change between 0.97 and 1.02).
In contrast, a similar saturation effect was not noted for cotinine, which is a closely related metabolite of nicotine, likely due to the much lower concentration of cotinine in cigarette smoke compared to nicotine.
The spectra of the CSEs obtained after smoking 5, 10, 15, and 20 cigarettes were similar, displaying more quantitative than qualitative variations (see
Figure 11). The only significant difference observed was in the 3200–3400 cm
−1 region, corresponding to the O–H stretching vibration.
The broadening and shift towards lower frequencies of this peak may indicate an increase in the carboxylic acid content of the sample, which aligns with a decrease in pH.
The broad bands observed in the samples between 3300 and 3400 cm
−1 indicated the presence of O–H stretching typical of alcohols, phenols, or carboxylates. Peaks attributed to N–H and C–N stretching vibrations suggest the presence of both aliphatic and aromatic amines [
31].
Bands located around 2987 to 2850 cm
−1 indicate C–H stretching from aliphatic groups. Additionally, compounds containing the C=O group, such as aldehydes, ketones, and carboxylates, were identified in both samples, as the spectra displayed distinct bands at 1722 cm
−1 and 1737 cm
−1 [
31].
The peaks within the ranges of 1658 to 1630 cm
−1, 1646 to 1609 cm
−1, 1597 to 1551 cm
−1, and 1575 to 1540 cm
−1 are typically associated with the C=N stretching vibrations of heterocyclic amines and/or the C=C and N–H scissoring vibrations of amines [
31].
The bands observed at 1367 cm
−1 and 1361 cm
−1 are consistent with the O–H bending vibrations characteristic of phenols. The sharp bands in the range of 1046 to 1095 cm
−1 in both spectra point to the presence of aliphatic ethers, as they correspond to C–O stretching vibrations [
31].
Nitro compounds (R–NO
2) were also detected in both samples, indicated by bands associated with N–O stretching. Furthermore, peaks representing C–Cl stretching vibrations may suggest the presence of chloro compounds [
31].
Overall, the main peaks observed were quite similar across the two spectra and align with previous reports on the chemical composition of CSEs [
31,
32,
33].
The morphological changes and cytotoxicity demonstrated by the effect of CSE on MSCs are consistent with a previous in vitro study using various concentrations of nicotine on human oral MSCs [
34]. Oral MSCs could be influenced by a variety of exogenous factors, which can be accurately reproduced in experiments, providing insights for clinical research in tissue regeneration [
35].
5. Conclusions
Both the dry residues deposited after smoking on the metal surface and the solubility of the same residues in Ringer’s solution lead to the conclusion that tobacco residues after smoking (TAR) end up in the human body:
by mixing the residual solid deposits detached from dental materials and natural teeth by the flow of saliva and embedded in the food bowl (in digestive system),
as smoke in the respiratory system.
The quality of finishes in dental prosthetic work is crucial, especially because these prosthetics come into contact with the soft gingival tissues in the oral cavity. A rough, porous surface on dental materials can develop deposits from cigarette smoke and CSE solutions, which negatively impacts gingival health.
In contrast, a glossy surface—whether from a natural tooth or a well-finished prosthetic—promotes optimal self-cleaning thanks to the continuous flow of saliva. While it is unhealthy for saliva to mix with post-smoking residues in the mouth, regular tooth brushing can help eliminate these harmful substances.
Based on the results presented, it appears that cigarette extract solutions decrease the viability of mesenchymal stem cells (MSCs), with cell death becoming more pronounced at higher doses of the extract added to the culture media.
Additionally, exposure to high doses of the extract caused morphological changes in the cells. Taken together, these findings suggest that short-term exposure to cigarette extract can damage cell integrity, leading to cell death.
It is important to note that this study only examined the in vitro effects of cigarette smoke extract, and further research is necessary to determine the effects in in vivo experimental models.
In conclusion, despite its limitations, this study highlights the negative effects of tobacco cigarette smoking and its impact on oral health.