Abstract
This paper focuses on the evaluation of the surface pretreatment of glass specimens and its effect on the strength of bonded joints. The experimental portion of this study includes the testing of three types of surface treatments (degreasing, blasting and etching) and the use of three types of adhesives (two-component epoxy, instant cyanoacrylate and two-component acrylate). Surface roughness measurements and bond strength testing using the pull-off test and shear tests were performed on the samples. The results showed that surface etching in conjunction with the Auratech AR 708 acrylic adhesive provided the highest bond strength. These findings contribute to the development of more reliable and stronger bonded joints for industrial applications. Scientific knowledge lies in determining the effect of appropriate glass surface treatment on adhesive adhesion under load with respect to joint aging due to environmental effects (UV, temperature, humidity) for transparent adhesives. This study provides an assessment of the effect of surface cleanliness on adhesion to glass.
1. Introduction
Glass occupies a prominent place in contemporary technical practice. The use of glass bonded joints is becoming a trend in many fields. In the field of glass bonding, the mechanical and construction industries are also interconnected. There are several types of glass, but for the purpose of this research, low-iron glass was used. Less iron results in a reduction in the yellow or green coloration of the glass and increases its transparency. The glass is formed by float technology [1]. In the float technology process, a continuous strip of glass is moved out of the furnace after treatment at a temperature of about 1100 °C and drifted on a bath of molten tin. As the glass flows down the bath, the temperature of the glass gradually decreases, allowing the glass strip to solidify. During glass fabrication, tin ions diffuse from the underlying molten tin bath to the bottom surface of the glass. As a result, the glass has two distinctly different surfaces, with the properties of the tin-containing surface being significantly different from those of the upper surface [2]. Qin Zhang et al. addressed the simulation of tin permeation in the float glass process. They concluded that the maximum tin concentration is a fundamental characteristic of green glass. The maximum occurs due to tin accumulation and diffusion characteristics. The location of the local maximum is determined by the gradient of oxygen activity in the glass [3]. Many experimental and theoretical studies have tried to understand the interaction between tin and molten glass in a tin bath. The diffusion that takes place in molten tin is influenced by several parameters such as glass composition, reduction and oxidation processes, ion exchange, tin bath atmosphere, temperature and float time [4].
Pretreatment of the Surface Before Bonding
The surface pretreatment of bonded materials is the first stage of the bonding process and is crucial because it allows the formation of a bond with ideal properties [5]. Proper surface pretreatment will ensure wetting and adhesion [6]. A well-performed pretreatment is an essential requirement for bonding technology [7]. Surface pretreatment before the bonding of glass joints can be of two types, mechanical or chemical. Mechanical pretreatments may include blasting, grinding and polishing. For chemical pretreatments, alkaline degreasing is widely used [8] and preferred due to several factors over acid degreasing, which has a rather negative effect on bonded glass joints. Alkaline degreasing is often better at removing organic contaminants such as oils and fats without disturbing the material structure too much. Acidic solutions can cause corrosion or degradation. Alkaline cleaners are often less aggressive than acid degreasing. In the case of imperfect acid degreasing, this could negatively affect the ability of the adhesive to form a strong bond [9]. Another surface treatment could be chemical etching [10]; Åsa Lundevall et al. have looked at improving glass bonding using plasma [11] and a laser [12].
The surfaces of all specimens were always treated prior to adhesive with technical gasoline P 6402, which was applied on a paper towel before the application of the adhesive, to finally remove any impurities that might have adhered to the surface after degreasing and rinsing. An important parameter before applying the adhesive is the wettability of the surface. In this context, Takeda et al. investigated the relationship between wettability and the density of surface OH groups of various commercial glasses on the tin side and the tin-free side using X-ray photoelectron spectroscopy (XPS), atomic force microscopy (AFM) and contact angle measurements. The authors found that the density of surface OH groups was dependent on the surface silicon concentration in the glass, except for in the case of the bottom side of soda–lime float glass. On the other hand, the ability to form a post-surface OH group on the underside of float glass was affected by tin, which was also present on the surface due to direct contact with the molten tin bath [13]. Furthermore, Prakash and Prasanth also pointed out that the wettability of the liquid on the surface varies with surface properties such as roughness, surface energy or texture [14,15].
2. Experimental Part
2.1. Methods and Materials
In the experimental portion of this study, 3 different pretreatments (9 samples each) were performed for 27 samples. The first 9 samples were degreased by ultrasonication, the next 9 samples were blasted with crushed corundum and the last 9 samples were etched with etching paste.
2.1.1. Preparation of Samples Before Bonding
Two chemical and one mechanical pretreatments were selected for the purpose of experimental research. The pretreatments were selected based on commonly used methods in industrial applications.
The samples were degreased in three pieces in the ultrasonic cleaner Kraintek K-2LE (Hradec Králové, Czechia). The cleaning agent used was STAR 75PN at a concentration of 10% per 2 L of demineralized water + SurTec 086 tenside 0.6% (12 mL). Degreasing was carried out at an interval of 5 min at a bath temperature of 50 °C. After the end of the degreasing process, a two-step rinse in demineralized water followed, and the length of each rinse was 1 min. The samples were then dried with a Bosch GHG 660 LCD Professional (Gerlingen, Germany) hot air gun to allow the bonding process to proceed immediately after degreasing.
The blasting of the samples was carried out on a manual pressure blasting machine PTZ 100 T from S.A.F. PRAHA s.r.o (Prague, Czech Republic). The samples were placed in the blast chamber where they were blasted with brown corundum F60 from a distance of approximately 20 cm for 30 s on each side by continuous manual movement of the blast head.
Etching was performed using commercially available Glass Etching Paste from Penta color [16]. The samples were cleaned with a dry cloth, as recommended by the manufacturer. The paste was stirred in the glass using a wooden spatula and then applied to the glass surface to form a continuous layer, completely covering the surface. The same process was carried out on both the tin and the reverse side without a tin coating. After 45 min of etching (manufacturer-recommended minimum: 30 min), the specimens were individually rinsed thoroughly with running water to remove any residual etching paste. Rinsing with running water was followed by drying with a dry cloth and ultrasonic degreasing, followed by two rinses in demineralized water, followed by drying with a Bosh heat gun.
2.1.2. Selection of Adhesives
Important requirements for adhesives in glass–glass joints were transparency and clarity. The following three adhesives were selected according to these requirements: Loctite EA 9445 is a two-component, fast-curing, low-viscosity epoxy adhesive designed for industrial applications [17]. The epoxy adhesive was selected based on the properties of the epoxy polymer such as high strength, excellent surface adhesion, reasonable viscosity and a suitable curing time [18]. Furthermore, the Auratech AR 708 clear and colorless adhesive is suitable for bonding transparent objects [19]. The last type of adhesive selected was the Auratech AR 011 acrylic, a fast-curing standard type of adhesive with various applications [20]. All three types of adhesives are mainly designed for glass and metal bonding applications. The adhesives were selected on the basis of their transparent color and transparency as per the requirements of GACR project 23-06016S. All three adhesives have applications in the construction, automotive and electrical industries, where the emphasis is on the quality and reliability of joints. Experimental research often concerns glass-to-glass bonding. Meanwhile, research on glass-to-glass bonded joints with transparent epoxy adhesives is still rare [21].
2.1.3. Bonding of Samples
The Loctite EA 9455 and Auratech AR 708 adhesives were applied using an application gun and mixing tip. The workability of both adhesives was within approximately two minutes of pushing the adhesive into the mixing tip, so it was necessary to proceed without breaks and after fewer samples. The AR 011 instant adhesive was applied with the dispensing tip on the product packaging.
2.2. Test Methods and Samples
In the practical portion of this study, “clear vision” glass samples, shown in Figure 1, were used. These are cuboid-shaped samples with the dimensions 50 × 50 × 19 mm. The side marked with a sticker is the sensitized side. The other side of the sample is the non-tinned side. If the samples were placed side by side without the label, they would not be visually different from each other.
Figure 1.
Sample of clear vision glass.
Clear vision is a low-iron extra-clear glass containing less than <200 ppm of iron oxide (Fe203) [22].
2.3. Surface Structure Measurement
The surface roughness was measured using a Mitutoyo SURFTEST SJ-210 (Kawasaki, Japan)roughness tester, which is a roughness tester that is sensitive to the sensing of small irregularities due to its small peak angle sensing tip. Two parameters, Ra and Rz, were measured. The cut-off wavelength chosen was λc = 0.8 μm. The actual measurement was performed on each sample 6 times from each side. The measured roughness values were tabulated from the instrument, and then the average roughness values for the tinned and non-tinned sides of all samples were calculated. A complete summary of the results is shown in Figure 2.
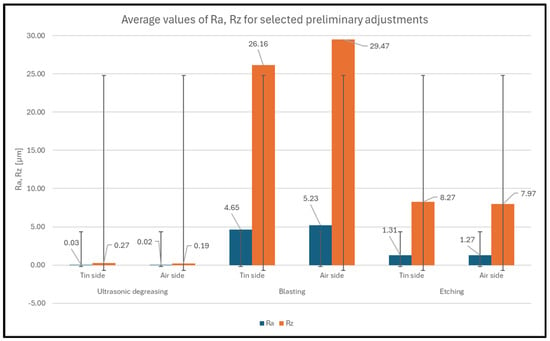
Figure 2.
Average values of Ra and Rz for selected pretreatments with standard deviations.
Based on the analysis of both graphs, it can be concluded that the surface roughness of the sample is not affected by whether the tinned or non-tinned side is assessed. In the case of degreased samples, the difference between the two sides, based on the data analysis, was negligible. In the case of samples that were blasted and etched, there was a significant change in surface morphology, and the effect of the tin side or the non-tin side was no longer relevant here. As expected, the highest roughness values were measured for the blasted samples, followed by the etched samples, and the lowest roughness values were measured for the degreased samples. Based on EN ISO 21920-2, which deals with surface structure, the following figure under number 3 is described. They are described by the parameter SDR (surface development ratio), which is the total interfacial area ratio, and the parameter is given in % [23].
The SDR parameter is calculated according to Equation (1) as follows:
The SDR parameter can be expressed as a dimensionless positive number or as a percentage, with values typically between 0 and 100%. A perfectly smooth and flat surface has a value of SDR = 0% [24].
For the evaluation of glass samples based on surface texture analysis and the SDR parameter, a MarSurf CM expert confocal microscope was used. Based on the parameter measurements, the following values were found:
Pure glass without treatment: 0.02%.
- -
- This value indicates a smooth surface with no significant texture.
Blasted glass: 63.07%.
- -
- This is the highest SDR value, meaning that the surface is significantly coarse, with micro- and macro-structures increasing adhesion properties.
Etched glass: 14.45%.
- -
- Etched glass has a finer texture than blasted glass but still produces some roughness.
The results show that a higher SDR value is associated with rougher glass surfaces, while smoother surfaces are presented by glass samples with lower SDR values, which can also be seen in Figure 3. There is some correlation between the parameters Ra, Rz and SDR. Larger values of Ra and Rz usually lead to higher SDR values because rougher surfaces have a larger actual contact area due to microscopic depressions. Lower Ra and Rz values usually result in smoother surfaces, with lower values of these parameters tending to co-occur with a lower SDR. However, it is important to note that the relationship between these parameters is not always linear and can be influenced by various factors (material properties, application specifications, etc.).

Figure 3.
Pure glass without treatment, blasted glass and etched glass.
2.4. Pull-Off Test
The pull-off test is suitable for testing the adhesion [25] between a test body and a specimen. The adhesive must not induce changes in the coating and must have a higher strength than the joint itself. The test evaluates the stress required to break the bond between the coating and the substrate. When the dummy is removed from the coating, the nature of the fracture is observed, which either takes place at the interface between the two components (adhesion fracture) or breaks the internal bonds of the components (cohesive fracture). In many cases, a combination of both fractures can be observed [26,27]. The pull-off test was performed using an Elcometer 510 (MCFC, UK) automatic tear gauge, which has a range of 0–30 MPa (0–4000 psi), a resolution capability of 0.01 MPa (1 psi) and an accuracy of ±0.1 MPa (±14.5 psi) [28]. In the experiment, 27 samples were tested by the pull-off test.
On the basis of the measured values, it can be concluded that the highest stress was required to pull off the dolls glued with the two-component acrylate adhesive AR 708. This is followed by the two-component epoxy adhesive Loctite EA 9455, and the lowest tension was required to avoid tearing the dolls bonded with the AR 011 instant adhesive. In the case of the AR 011 instant adhesive, cohesive fracture in the substrate was also by far the most frequent occurrence, with the doll being torn out of the sample with part of the glass. For the adhesives EA 9455 and AR 708, etching appears to be the most suitable pretreatment to achieve maximum pull-off resistance. In the case of the AR 708 adhesive, the values for the blasted and degreased surfaces are comparable and at the same time noticeably lower than for the etched surface. In the case of the non-tinned side, the values for the blasting and degreasing technology are between 8% and 10%, and for the tinned side, the values are between 23% and 25% compared to the casting technology (Table 1).

Table 1.
Average stress values at break-off.
An explanation of the different types of tear test fractures shown in the graph that occurred is provided below.
Cohesive fracture in adhesive = %Y
Adhesive fracture between adhesive and test body = %Y/Z
Adhesive fracture between last layer (glass specimen) and adhesive = %A/Y
Cohesive fracture in substrate = %A
On the basis of the results shown in Figure 4, Figure 5 and Figure 6, which show the percentage representation of individual types of failure (see the classification of fractures according to ISO 4624), the following can be stated:
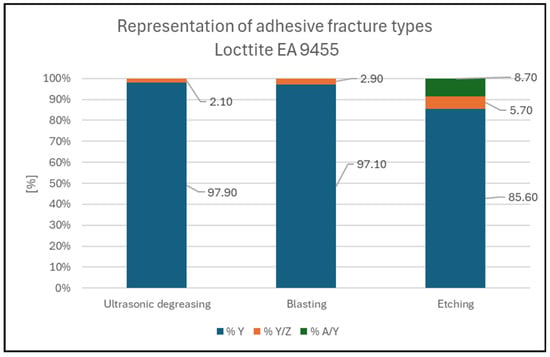
Figure 4.
Representation of fracture types for Loctite EA 9455.
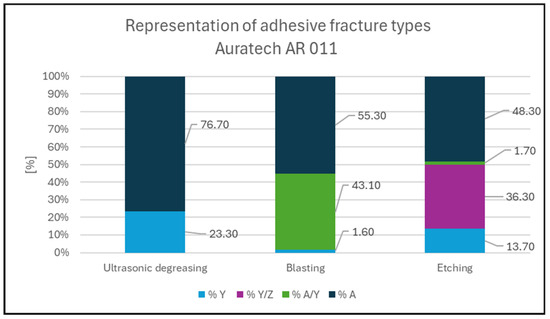
Figure 5.
Representation of fracture types for Auratech AR 011.
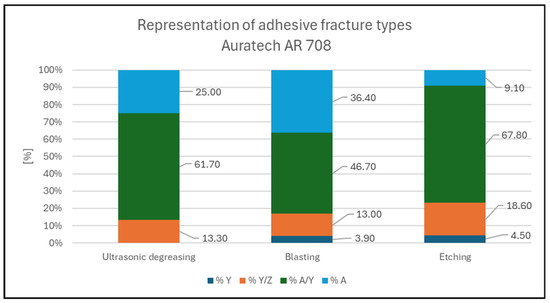
Figure 6.
Representation of fracture types for Auratech AR 708.
- The Loctite EA 9455 adhesive broke in the most similar manner across all three pretreatments, with over 93% of all pull-off trials ending in cohesive fracture in the adhesive (%Y), as shown in Figure 7, and even over 97% of pull-off trials in the degreasing and blasting pretreatments;
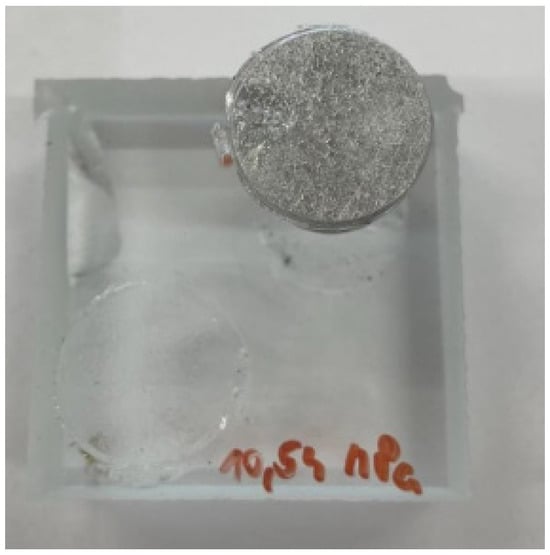 Figure 7. Adhesive fracture in Loctite EA 9455; degreasing pretreatment. 97.9% Y; 2.1% Y/Z.
Figure 7. Adhesive fracture in Loctite EA 9455; degreasing pretreatment. 97.9% Y; 2.1% Y/Z. - In the case of the Auratech AR 011 adhesive, the most frequent failure that occurred was the cohesive fracture of the glass, as shown in Figure 8 (sample); in total, over 60% of the samples were broken, and the cohesive fracture (%Y) occurred most frequently in degreased samples (76.7%). In the case of blasted samples, 43% of the samples had adhesive fracture between the last layer (glass) and the adhesive (%A/Y). On the other hand, adhesion fracture between the adhesive and the test body (36.3%) was more frequent in the blasted samples (%Y/Z);
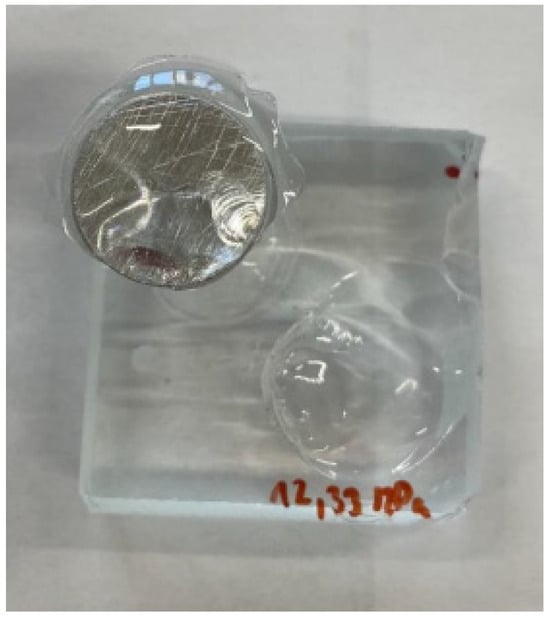 Figure 8. Cohesive fracture in glass sample with Auratech AR 011 adhesive; pretreatment by degreasing. 76.7% A; 23.3% Y.
Figure 8. Cohesive fracture in glass sample with Auratech AR 011 adhesive; pretreatment by degreasing. 76.7% A; 23.3% Y. - In the case of the Auratech 708 adhesive, adhesive fracture between the sample and the adhesive (% A/Y), shown in Figure 9, occurred most frequently (altogether representing almost 59% of all pull-off events), followed by glass cohesive fracture (% A) (23.5% in total, mostly in the blasted samples), which occurred mainly in the second series of measurements. Adhesive fracture between the adhesive and the test body (%Y/Z) occurred in less than 15% of the pull-off samples. Cohesive fracture (%Y) occurred in less than 3% of cases and was not observed at all in the degreased samples.
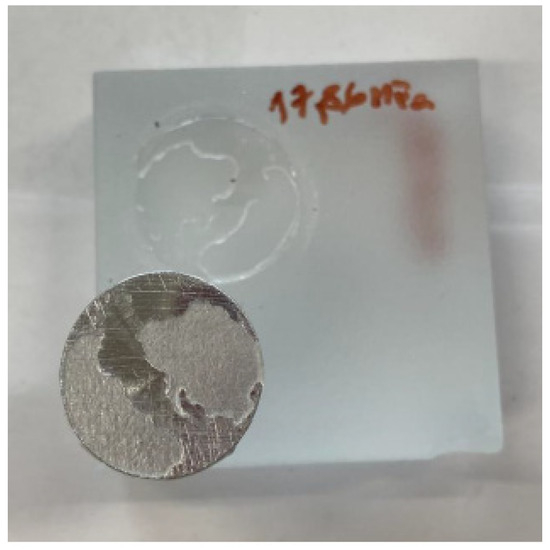 Figure 9. Combination of adhesive fracture between specimen and adhesive and adhesive fracture between adhesive and test body; Auratech AR 708 adhesive; pretreatment by blasting. 46.7% A/Y; 36.4% A; 13% Y/Z; 3.9%Y.
Figure 9. Combination of adhesive fracture between specimen and adhesive and adhesive fracture between adhesive and test body; Auratech AR 708 adhesive; pretreatment by blasting. 46.7% A/Y; 36.4% A; 13% Y/Z; 3.9%Y.
2.5. Shear Test
The experiment also included an attempt to develop and test a new methodology for the shear testing of two bonded glass samples. The motivation here was primarily to create a fixture that would allow measurements to be repeated on a larger scale in the future. According to the bonded specimen scheme [6], the first step was to design the fixture for bonding the specimens so that the bonded area was 50 × 12 mm2 and the adhesive layer was 1 mm, as shown in Figure 10. For the purpose of bonding the two samples, a simple fixture was designed in Autodesk Inventor and subsequently fabricated on a 3D printer to positionally secure the two samples while allowing for the required adhesive thickness of 1 mm. The production was carried out on a Creality Ender 5 3D printer, and the material used was PLA, with 20% fill (higher fill values were not required as the product was not designed to be stressed).
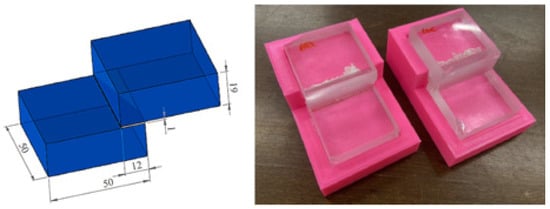
Figure 10.
Schematic of bonding samples for shear test [22]; bonded samples in the manufactured product.
The specimens were always bonded on the tin-free side, and a total of 3 bonded specimens (from 6 original glass specimens) were made in the fixture, using all 3 previously mentioned adhesives (EA 9455, AR 011, AR 708). In the second phase, a fixture had to be designed that would allow shear testing by fixing the specimens against non-vertical stresses. The designed model had to meet the conditions for clamping in the LabTest Model 5.100SP1 (OP, Czech Republic) tear tester and at the same time had to be durable enough to prevent failure before the bonded joint was broken. The resulting model of the fixture half is shown in Figure 11. The model then had to be printed in two pieces that could be slipped onto the bonded specimen after subsequent fabrication.
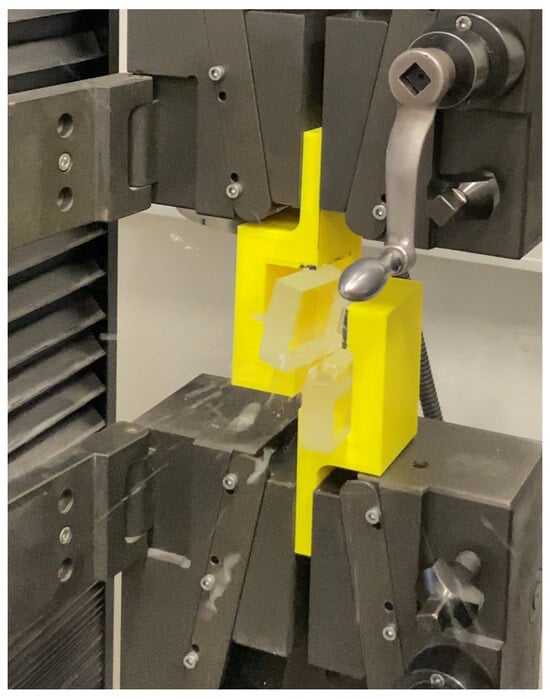
Figure 11.
3D Failure moment in shear test on LabTest 5.100SP1, 306 glue EA 9455
The printing of the product was conducted on a Creality Ender 5 3D (Shenzhen, China)printer; the chosen material was PLA, the fill rate was set to 40% (due to the requirement for stress resistance), the layer was 0.3 mm and the printing speed was 60 mm·s−1. The test was carried out on a LabTest Model 5.100SP1 machine. The bonded samples were carefully slid into the fabricated fixtures, and everything was clamped into the LabTest machine. The test was then run, and the results were recorded by software on the PC. The specimens bonded with the AR 011 instant adhesive separated when clamped into the machine, before the actual start of the test, so no data could be obtained, but it can be stated that the bond of the two glass specimens with the AR 011 adhesive did not reach high strength values (Figure 12). An analysis of the separated samples revealed that the separation was due to adhesion failure in the AR 011 adhesive. This was due to the failure of the adhesive to achieve the required strength through curing, which in this type of adhesive is achieved by pressure bonding.
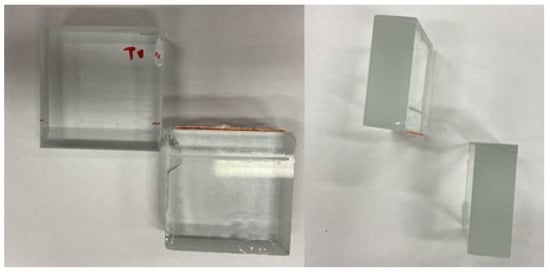
Figure 12.
Failure of specimens bonded with AR 011 adhesive.
In the case of the EA 9455 and AR 708 adhesives, the same problem did not occur, and the test could be performed to the intended extent. The test runs are shown in the following graph in Figure 13.
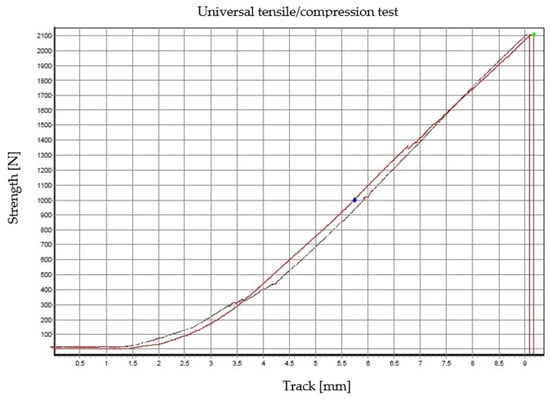
Figure 13.
Dependence of force on path for specimens bonded with Locttite EA 9455 and Auratech AR 708 adhesives.
The measured values are shown in Table 2.

Table 2.
Force values at failure of bonded specimens.
The failure force values for both adhesives were very similar, and the failure mode was almost identical, with cohesive fracture in the glass, while the bonded joint remained intact. Thus, it appears that the limit of the glass sample was reached before the bonded joint itself could be broken. In Figure 14 and Figure 15, the moment of failure of the specimens bonded with the EA 9455 adhesive can be seen; due to the cohesive failure in the glass, flying off shards can be observed.

Figure 14.
Failure of samples bonded with Loctite EA 9455.
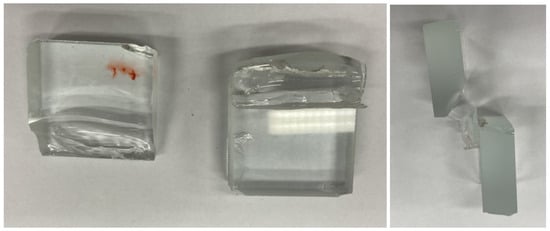
Figure 15.
Failure of specimens bonded with AR 708 adhesive.
3. Conclusions
This research focused on evaluating the effect of different surface pretreatment methods and the use of three types of adhesives on the strength of bonded joints of glass samples. The main objective was to determine the most appropriate combination of pretreatment and adhesive to achieve maximum joint strength and to design an effective methodology for testing these joints. The results show that the highest strength was achieved when the Auratech AR 708 acrylic adhesive was used on the etch-treated specimens. This combination showed 13% higher strength compared to the second-best adhesive, Loctite EA 9455. In contrast, the AR 011 adhesive resulted in more frequent cohesive fracture in glass, indicating the need for further investigation. Pretreatments by etching and blasting had a significant effect on the surface texture of the surface and thus on the strength of the joints. Degreasing proved to be less effective, but due to the low cost and transparency of the glass, it may be preferred in applications where aesthetics play a key role. This research contributes to a deeper understanding of the effect of pretreatments on the strength of bonded glass joints and suggests a new preparation methodology for in-sheet testing.
Author Contributions
Conceptualization, N.B.; methodology, N.B., J.K. (Jan Kudláček) and J.K. (Jiří Kuchař); investigation, N.B., J.K. (Jan Kudláček) and J.K. (Jiří Kuchař); sample preparation N.B. and J.Č.; writing—original draft preparation, N.B., J.K. (Jan Kudláček), J.K. (Jiří Kuchař) and J.Č.; writing—review and editing, N.B. and J.K. (Jan Kudláček); visualization, N.B.; supervision, J.K. (Jan Kudláček). All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the GACR project 23-06016S (Czech Science Foundation).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This article was written with the support of the GACR project 23-06016S (Surface treatment of glass and its effect on the reliability of bonded joints for glass structures at elevated temperatures).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fernández Oro, J.M.; Argüelles Díaz, K.M.; Santolaria Morros, C.; Cobo Hedilla, A.F.; Lemaille, M. Multiphase modelling of pouring glass over the spout lip of an industrial float in the flat glass forming process. Int. J. Numer. Methods Fluids 2008, 58, 1147–1177. [Google Scholar] [CrossRef]
- Takeda, S. Oxygen and silver diffusion into float glass. J. Non-Cryst. Solids 2006, 352, 3910–3913. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Z.; Li, Z. Simulation of tin penetration in the float glass process (float glass tin penetration). Appl. Therm. Eng. 2011, 31, 1272–1278. [Google Scholar] [CrossRef]
- Krohna, M.H.; Hellmanna, J.R.; Mahieub, B.; Pantano, C.G. Effect of tin-oxide on the hysical properties of soda-lime-silica glass. J. Non-Cryst. Solids 2005, 351, 455–465. [Google Scholar] [CrossRef]
- Ebnesajjad, S. 5-Material Surface Preparation Techniques. In Plastics Design Library; Handbook of Adhesives and Surface Preparation; William Andrew Publishing: Norwich, NY, USA, 2011; pp. 49–81. [Google Scholar] [CrossRef]
- Ebnesajjad, S. Surface Treatments of Materials for Adhesive Bonding, 2nd ed.; Elsevier: Oxford, UK, 2014; ISBN 978-0-323-26435-8. [Google Scholar]
- Mirski, Z.; Wojdat, T.; Zimniak, Z.; Łącka, I.; Pawełko, A. Effect of the Preparation of Aluminium, Magnesium and Titanium Alloys Surface on Properties of Adhesive Bonded Joints. Biul. Inst. Spaw 2017, 5, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-B.; Son, B.-K.; Choi, J.-W.; Son, I. Degreasing Efficiency of Electroplating Pretreatment Process Using Secondary Alcohol Ethoxylate as Nonionic Surfactant. Appl. Sci. 2022, 12, 11285. [Google Scholar] [CrossRef]
- Petrka, J. Lepení Konstrukčních Materiálů ve Strohírensví; SNTL: Rabat, Maroc, 1980. [Google Scholar]
- Khaskhoussi, A.; Calabrese, L.; Patané, S.; Proverbio, E. Effect of Chemical Surface Texturing on the Superhydrophobic Behavior of Micro–Nano-Roughened AA6082 Surfaces. Materials 2021, 14, 7161. [Google Scholar] [CrossRef] [PubMed]
- Lundevall, Å.; Sundberg, P.; Mattsson, L. Improved glass bonding with plasma treatment. Appl. Adhes. Sci. 2018, 6, 9. [Google Scholar] [CrossRef]
- Naat, N.; Boutar, Y.; Naïmi, S.; Mezlini, S.; Da Silva, L.F.M. Effect of surface texture on the mechanical performance of bonded joints: A review. J. Adhes. 2021, 99, 166–258. [Google Scholar] [CrossRef]
- Takeda, S.; Yamamoto, K.; Hayasaka, Y.; Matsumoto, K. Surface OH group governing wettability of commercial glasses. J. Non-Cryst. Solids 1999, 249, 41–46. [Google Scholar] [CrossRef]
- Prakash, C.G.J.; Prasanth, R. Approaches to design a Surface with tunable wettability: A review on surface properties. J. Mat. Sci. 2021, 56, 108–135. [Google Scholar] [CrossRef]
- Rudawska, A.; Miturska-Barańska, I.; Doluk, E.; Olewnik-Kruszkowska, E. Assessment of Surface Treatment Degree of Steel Sheets in the Bonding Process. Materials 2022, 15, 5158. [Google Scholar] [CrossRef] [PubMed]
- Penar. Leptací Pasta. Available online: https://www.pentart.cz/leptaci-pasta-50ml-glass-etching-paste (accessed on 20 January 2022).
- Henkel, LOCTITE-EA-9455. Available online: https://datasheets.tdx.henkel.com/LOCTITE-EA-9455-cs_CZ.pdf (accessed on 5 June 2015).
- Comyn, J. Introduction to adhesion and adhesives. In Adhesion Science; The Royal Society of Chemistry: London, UK, 1997. [Google Scholar] [CrossRef]
- Auratech, Auratech708. Available online: http://www.auratech.cz/Auratech/media/auratech/Technicke-listy/Konstrukcni%20lepidla/AR-708_technicky-list.pdf (accessed on 21 August 2015).
- Auratech, AR001. Available online: http://www.auratech.cz/Auratech/media/auratech/Technicke-listy/Vterinova%20lepidla/AR-011_technicky-list.pdf (accessed on 3 October 2012).
- Boutar, Y.; Eliášová, M.; Tichá, P.; Zikmundová, M. Assessment of the mechanical behavior of bonded glass-to-glass transparent epoxy adhesive joint at elevated temperatures for load-bearing elements. Int. J. Adhes. Adhes. 2023, 127, 103526. [Google Scholar] [CrossRef]
- AGC. Available online: https://www.agc-yourglass.com/sites/default/files/brochures/original/yg_pocket_2015_cz.pdf (accessed on 6 June 2015).
- ČSN EN ISO 21920-2; Geometrical Product Specification (GPS)—Surface Structure: Profile—Part 2: Terms, definitions and parameters of the surface structure. ISO: Geneva, Switzerland, 2022.
- Leach, R. (Ed.) Characterisation of Areal Surface Texture; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-36458-7. [Google Scholar]
- Lacombe, R. Adhesion Measurement Methods: Theory and Practice, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- ČSN EN ISO 4624:2016; Nátěrové Hmoty—Odtrhová Zkouška Přilnavosti. ISO: Geneva, Switzerland, 2016.
- ATRYX, Praktická Příručka—Hodnocení Přilnavosti Nátěru|ATRYX s.r.o. Nátěrové Hmoty|ATRYX s.r.o. 2018. Available online: https://atryx.cz/prakticka-prirucka/16-hodnoceni-prilnavosti (accessed on 5 March 2024).
- Gamin, Elcometer 510 Automatický Odtrhoměr. Available online: https://www.elcometer.cz/fileadmin/user_upload/Automaticky_odtrhomer_Elcometer_510.pdf (accessed on 11 July 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).