In-Flight Particle Oxidation Evolution in HVAF: A Numerical Study
Abstract
1. Introduction
2. Experimental Set-Up
2.1. Powder Material
2.2. HVAF Process
3. Numerical Model Details
3.1. Computational Domain and Meshing
3.2. Gas Flow Model
3.3. Combustion Model
3.4. Particle Injection-Discrete Phase Model
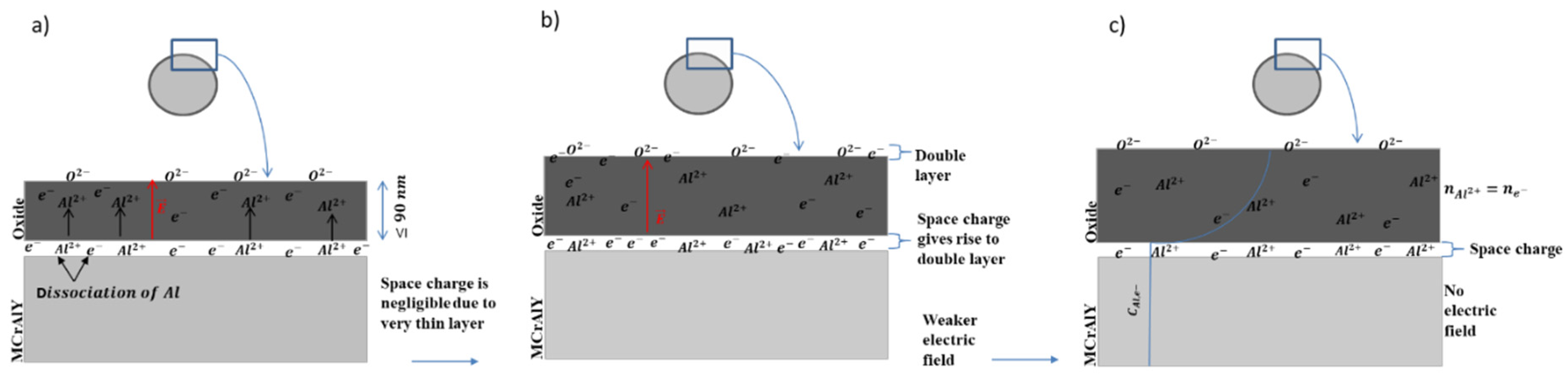
3.5. Oxidation Model
4. Results and Discussions
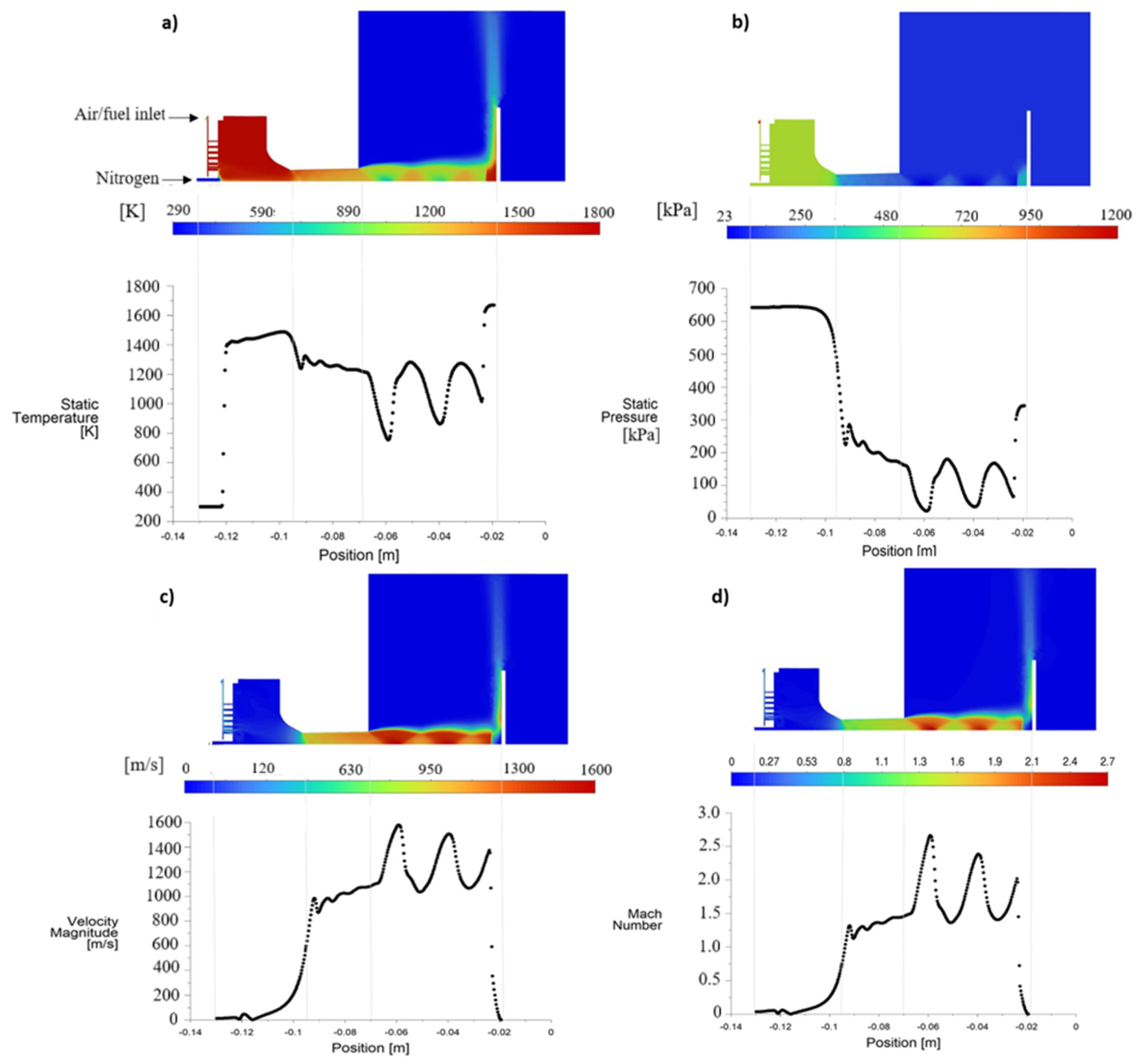
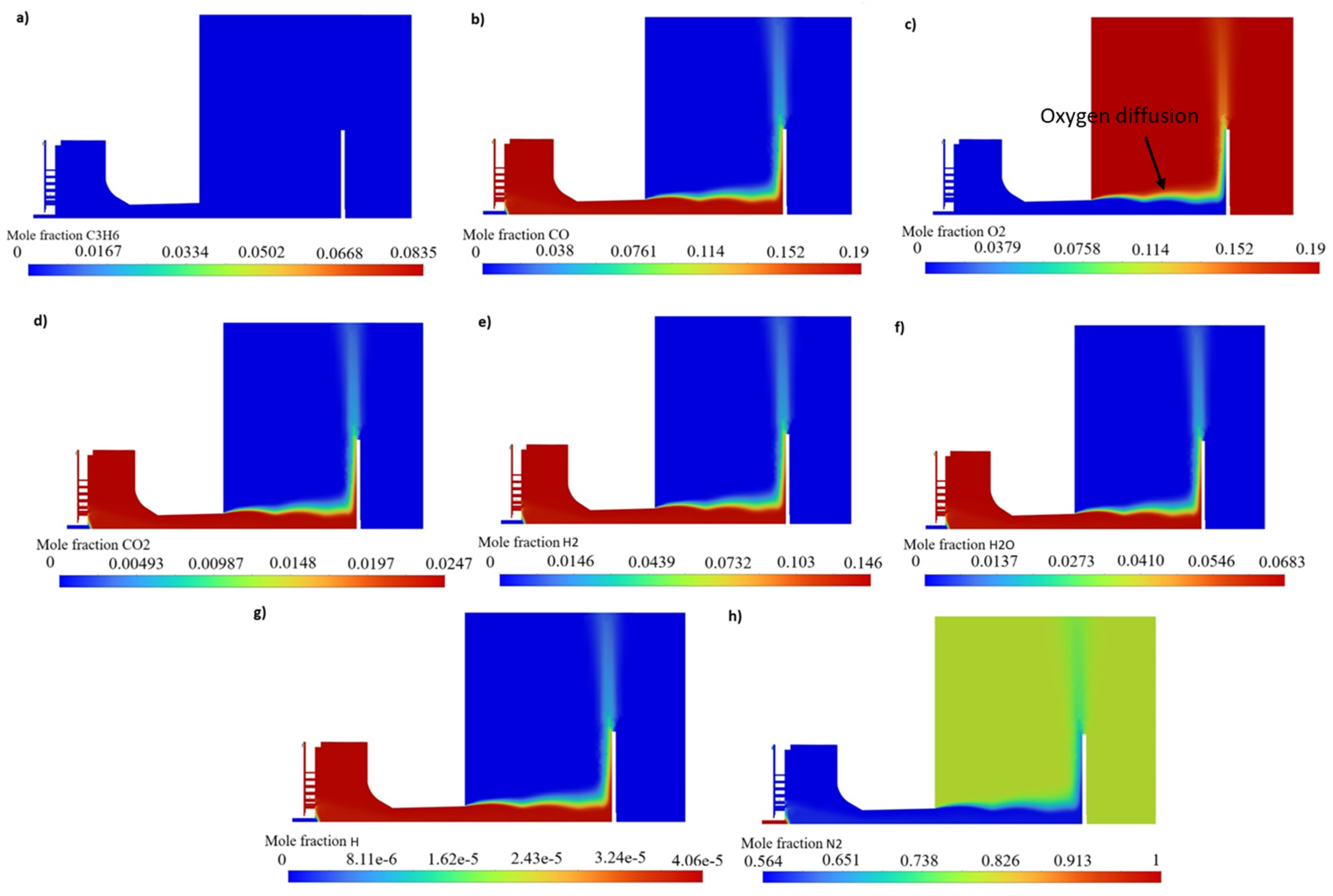
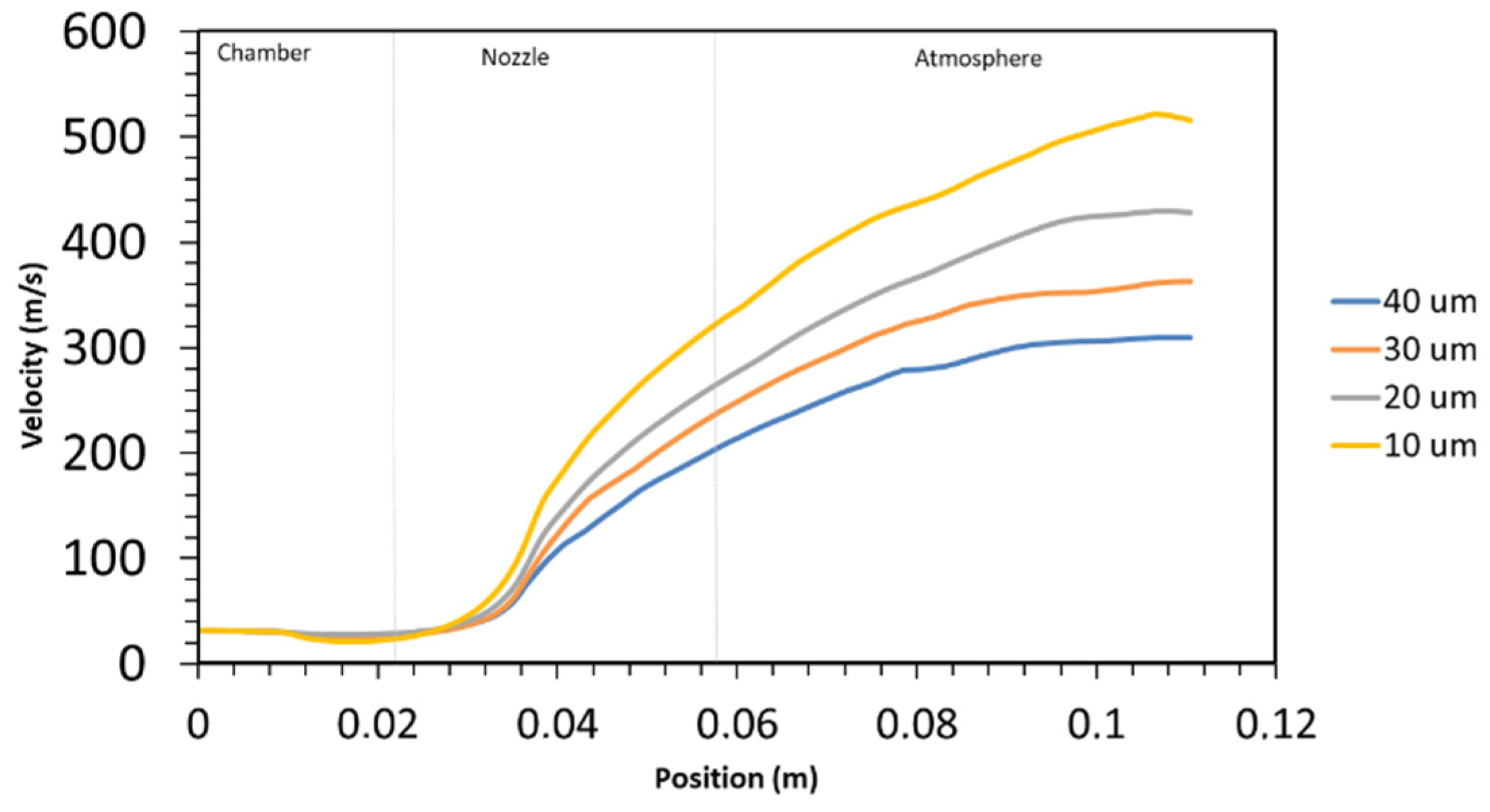
4.1. Gas Characteristics and Gas Components Mole Fraction
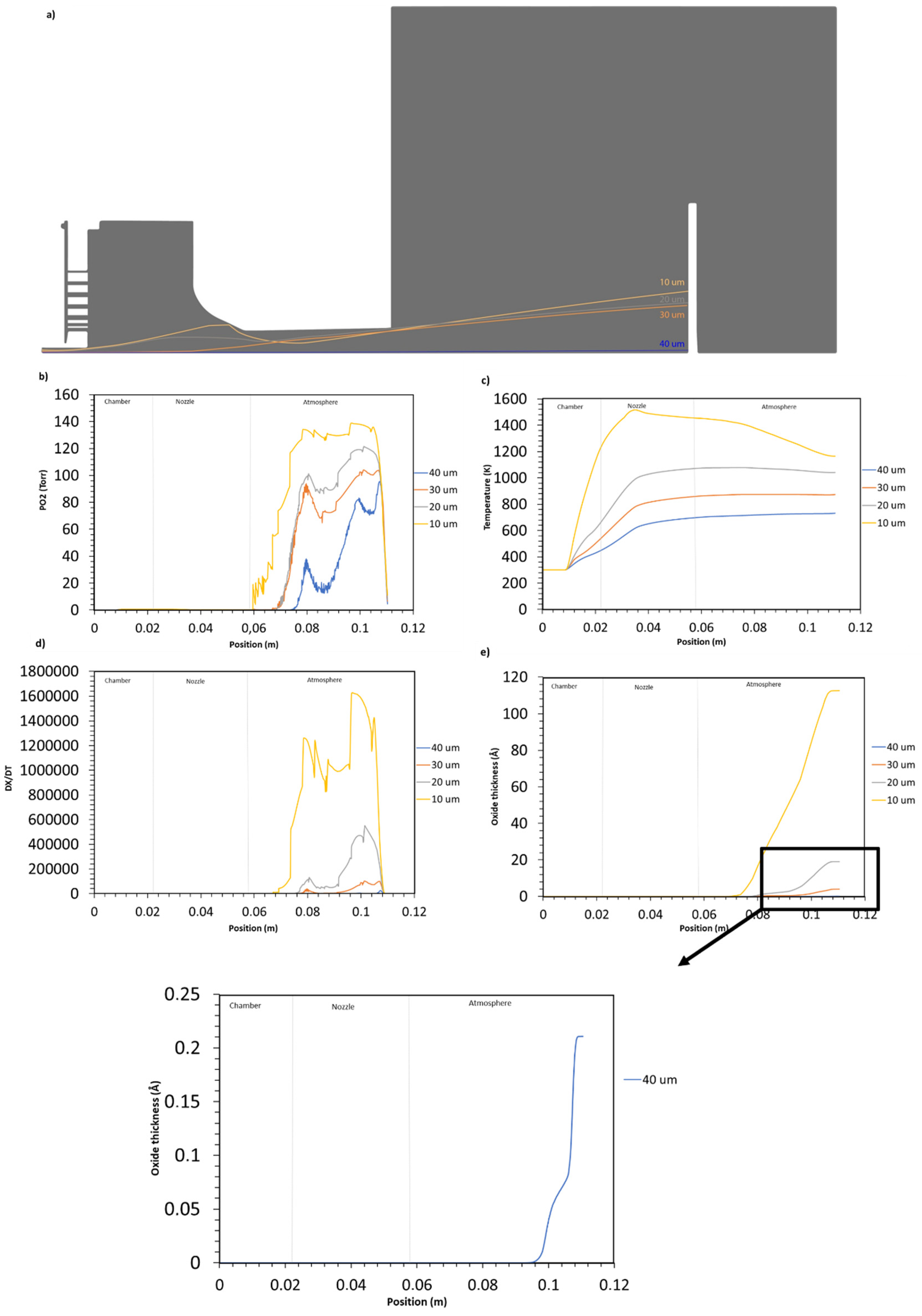
4.2. Particle Oxidation
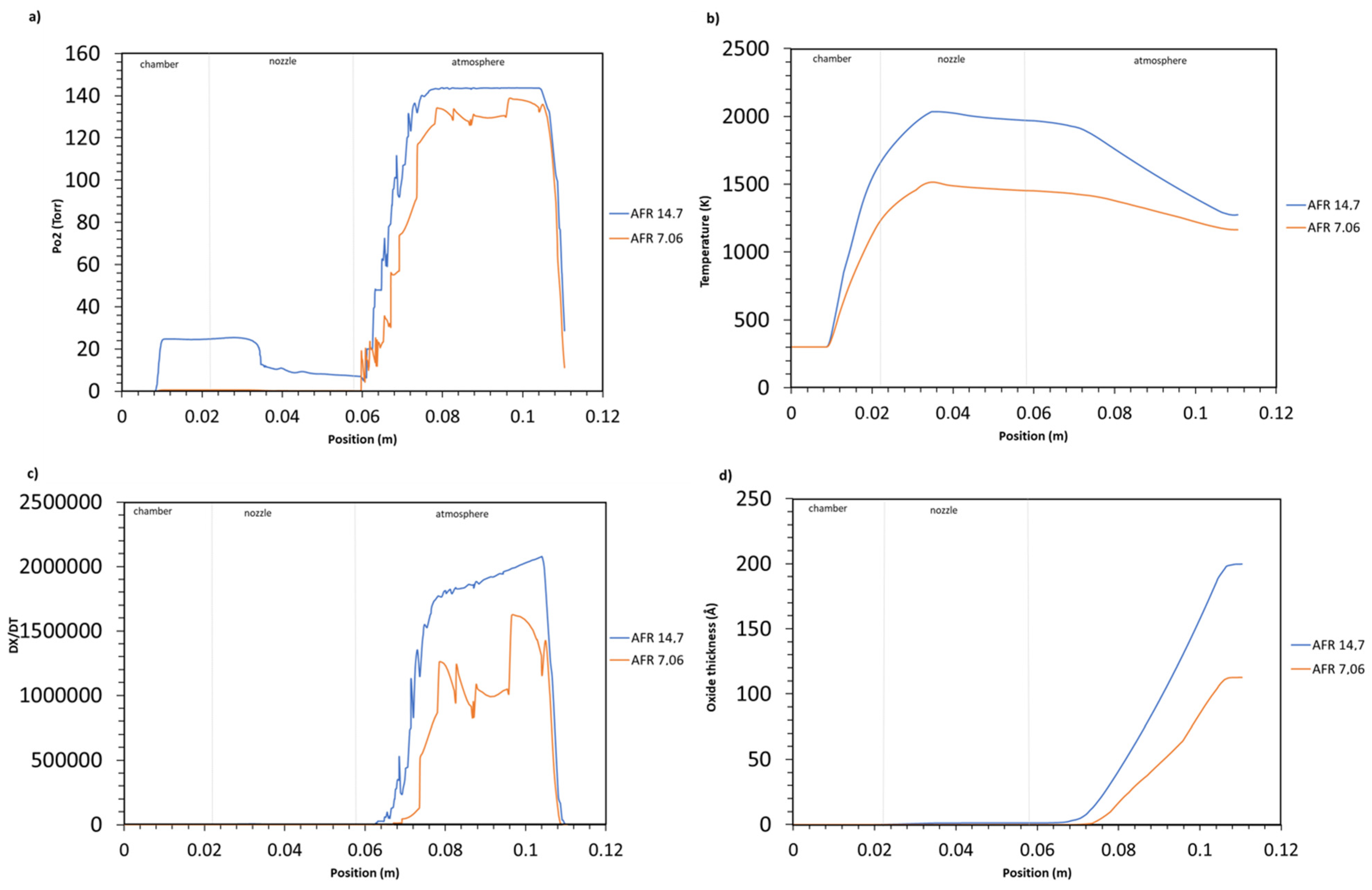
4.2.1. Air/Fuel Ratio Influence on Oxide Growth
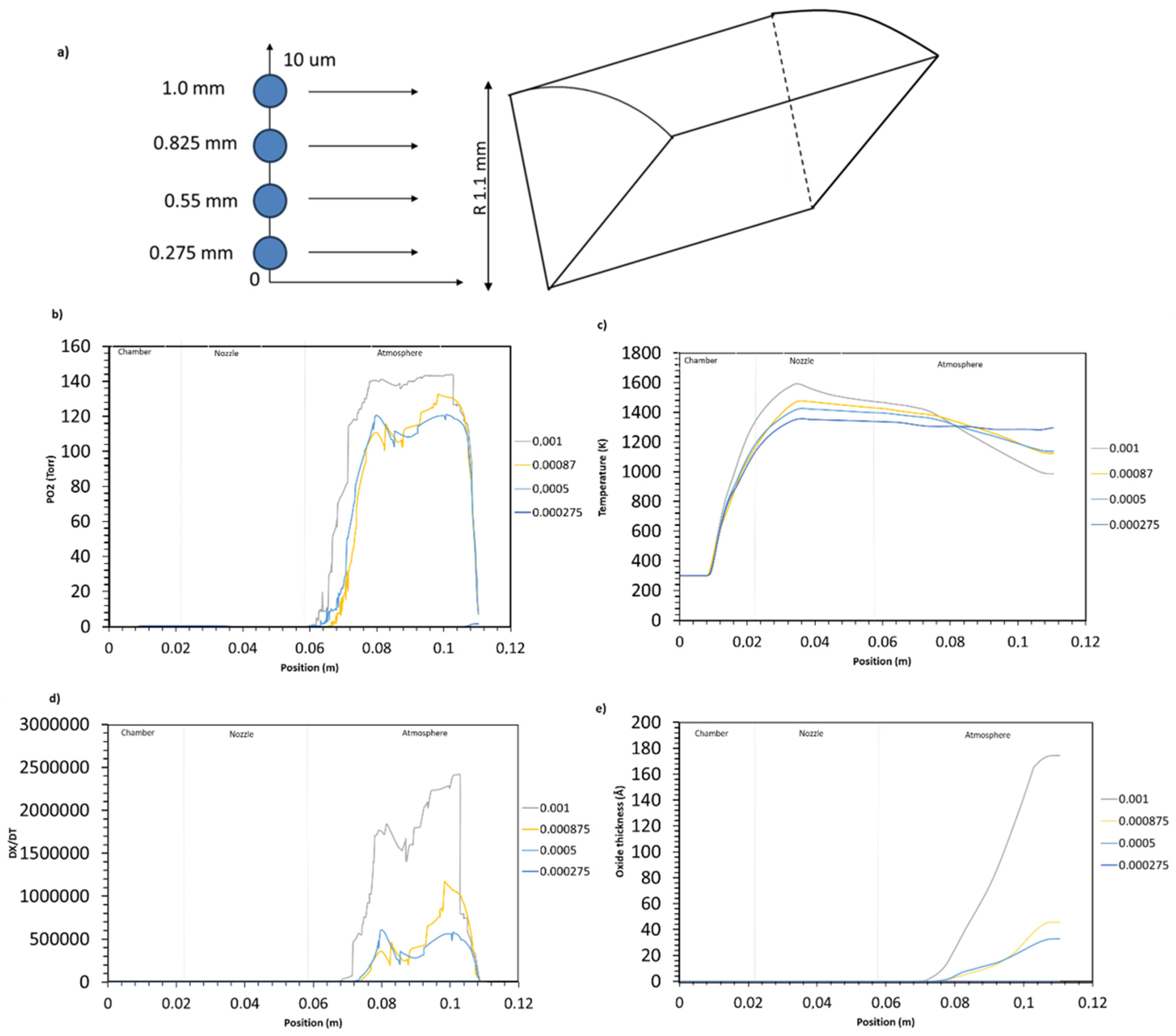
4.2.2. Influence of Particle Injection Location on Oxidation
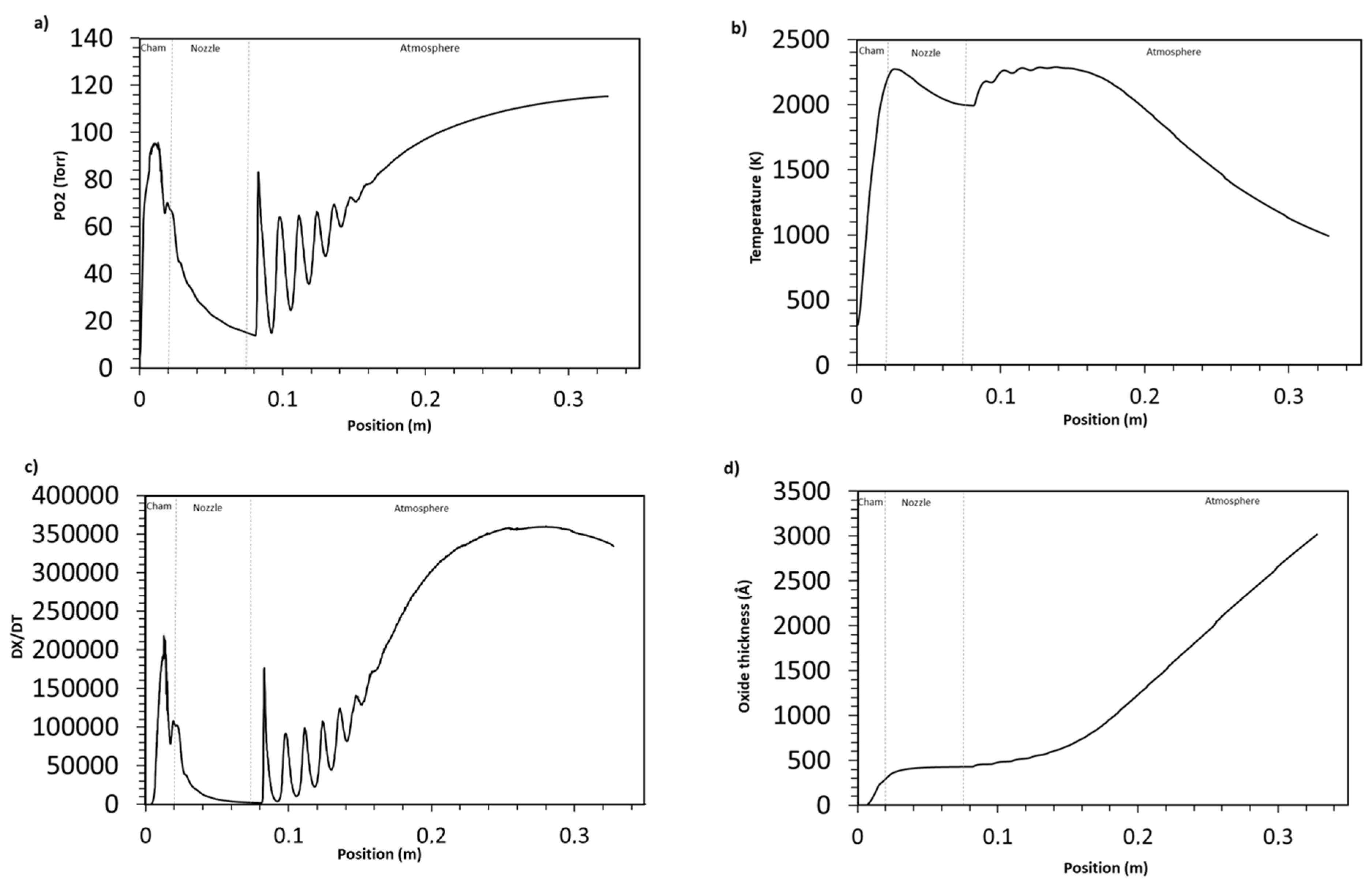
4.2.3. Oxidation in High Velocity Oxy-Fuel Process (HVOF)
Gas Flow Characteristics
Particle Oxidation Process
5. Experimental Validation
6. Conclusions
- -
- It is evident that the degree of oxidation for in-flight particles is contingent upon their size, with smaller particles (10 µm) exhibiting higher rates of oxidation 1.6 Å/s vs. for the largest particle (40 µm).
- -
- In fuel-rich combustion, the oxidation process takes place primarily within the atmospheric domain from the oxygen turbulent diffusion into the main flow.
- -
- A strong correlation has been observed between particle oxidation and spray parameters. The oxide layer thickens as the air-fuel ratio increases. The higher air-fuel ratio increases the oxygen content and the flame temperature.
- -
- The injection point location along the radial length of the injector influences the oxidation. The oxide thickness increases drastically with the increase in particle deviation from the centerline: , and 170 Å for particles injected at 0.275 mm, 0.55 mm, 0.825 mm, and 1 mm, respectively.
- -
- The degree of oxidation is also altered by different spray processes. The HVOF process, with its hotter jet, produces a thicker particle oxide layer than does the HVAF; an increase of 18.5% is detected.
- -
- Experimental analysis, conducted via FIB/SEM, has revealed that a 10 µm collected particle possesses an oxide thickness of 200 Å with an initial layer of 100 Å, validating the developed numerical model, by confirming a very thin oxide layer growth process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| List of Symbols | |
| Xcr | Critical thickness (Å) |
| A0 | Model constant (Å s−1) |
| LD | Debye length (Å) |
| Cp | Specific heat (J kg K−1) |
| m | Mass (kg) |
| vp | Particle velocity (m s−1) |
| Ap | The surface area of the particle (m2) |
| CD | Drag coefficient |
| Rp | Reynolds number |
| Tp | Particle temperature (K) |
| h | Heat transfer coefficient (W m−2 K−1) |
| Nu | Nusselt number |
| Pr | Prandlt number |
| ΔHox | Heat of oxidation (J kg−1) |
| Tmelting | Melting temperature (K) |
| ΔHmelting | Latent of heat (J kg−1) |
| f | Melting ratio |
| P | Pressure (Pa) |
| Po2 | Partial pressure of oxygen (Torr) |
| Q | Model constant (eV) |
| u | Velocity (m s−1) |
| Δt | Time (sec) |
| k | Thermal conductivity (W m−1 K−1) |
| ko | Model constant (eV Torr−0.5) |
| Bi | Biot number |
| ρ | Density (kg m3) |
| λ | Thermal conductivity (W m−1 K−1) |
| Γ | Total energy (internal + kinetic) |
| µ | Coefficient of viscosity (N m−2 s−1) |
| τ | Deviator stress tensor |
| X | Oxide thickness (Å) |
| List of Abbreviations | |
| AFR | Air-fuel ratio |
| FIB | Focus ion beam |
| TEM | Transmission electron microscope |
| SEM | Scanning electron microscope |
| EDS | Dispersive x-ray spectroscopy |
| DPM | Discrete phase model |
| List of Subscripts | |
| p | Particle |
| g | Gas |
| i, j | Co-ordinate indices |
| ox | Oxide |
References
- Khamsepour, P.; Moreau, C.; Dolatabadi, A. Numerical Simulation of the Effect of Particle and Substrate Preheating on Porosity Level and Residual Stress of As-sprayed Ti6Al4V Components. J. Therm. Spray Technol. 2022, 31, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Cavaliere, P.; Aldwell, B.; Jenkins, R.; Liao, H.; Li, W.; Lupoi, R. Cold spray additive manufacturing and repair: Fundamentals and applications. Addit. Manuf. 2018, 21, 628–650. [Google Scholar] [CrossRef]
- Molak, R.M.; Araki, H.; Watanabe, M.; Katanoda, H.; Ohno, N.; Kuroda, S. Effects of Spray Parameters and Post-spray Heat Treatment on Microstructure and Mechanical Properties of Warm-Sprayed Ti-6Al-4V Coatings. J. Therm. Spray Technol. 2017, 26, 627–647. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, M.; Singh, R. An overview of various applications of cold spray coating process. Mater. Today Proc. 2022, 56, 2826–2830. [Google Scholar] [CrossRef]
- Wang, H.-T.; Li, C.-J.; Yang, G.-J.; Li, C.-X. Cold spraying of Fe/Al powder mixture: Coating characteristics and influence of heat treatment on the phase structure. Appl. Surf. Sci. 2008, 255, 2538–2544. [Google Scholar] [CrossRef]
- Varis, T.; Lagerbom, J.; Suhonen, T.; Raami, L.; Terho, S.; Laurila, J.; Peura, P.; Vuoristo, P. Effect of heat treatments on the wear resistance of HVAF and HVOF sprayed tool steel coatings. Surf. Coatings Technol. 2023, 462, 129508. [Google Scholar] [CrossRef]
- Saltykov, P.; Fabrichnaya, O.; Golczewski, J.; Aldinger, F. Thermodynamic modeling of oxidation of Al–Cr–Ni alloys. J. Alloys Compd. 2004, 381, 99–113. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, H.; Chen, Y.; Zhao, X.; Guo, F.; Xiao, P. Effect of microstructure of a NiCoCrAlY coating fabricated by high-velocity air fuel on the isothermal oxidation. Corros. Sci. 2019, 159, 108126. [Google Scholar] [CrossRef]
- Zakeri, A.; Bahmani, E.; Aghdam, A.S.R.; Saeedi, B. A comparative study on the microstructure evolution of conventional and nanostructured MCrAlY powders at high-temperature. Surf. Coatings Technol. 2020, 389, 125629. [Google Scholar] [CrossRef]
- Dai, S.L.; Delplanque, J.-P.; Lavernia, E.J. Microstructural Characteristics of 5083 Al Alloys Processed by Reactive Spray Deposition for Net-Shape Manufacturing. Met. Mater. Trans. A 1998, 29, 2597–2611. [Google Scholar] [CrossRef]
- Zeoli, N.; Gu, S.; Kamnis, S. Numerical simulation of in-flight particle oxidation during thermal spraying. Comput. Chem. Eng. 2008, 32, 1661–1668. [Google Scholar] [CrossRef]
- Wu, N.-C.; Yang, F.; Sun, W.-H.; Yang, G.; Liang, Y.; Zhang, S.-D.; Wang, J.-Q. In-Flight Oxidation of Fe-Based Amorphous Particle During HVAF Spraying: Numerical Simulation and Experiment. J. Therm. Spray Technol. 2023, 32, 2187–2201. [Google Scholar] [CrossRef]
- Fauchais, P.; Vardelle, A. Thermal Sprayed Coatings Used Against Corrosion and Corrosive Wear. In Advanced Plasma Spray Applications; Jazi, H.S., Ed.; IntechOpen: Rijeka, Croatia, 2012; Chapter 1. [Google Scholar] [CrossRef]
- Analysis, I.; Gordon, S.; Cleveland, A.; Mcbride, B.J. NASA Reference Computer Program for Calculation Complex Chemical Equilibrium Compositions and Applications. Available online: https://ntrs.nasa.gov/citations/19950013764 (accessed on 4 February 2023).
- Gao, X.; Li, C.; Xu, Y.; Chen, X.; Han, X. Effects of Fuel Types and Process Parameters on the Performance of an Activated Combustion High Velocity Air-Fuel (AC-HVAF) Thermal Spray System. J. Therm. Spray Technol. 2021, 30, 1875–1890. [Google Scholar] [CrossRef]
- Clift, R.; Grace, J.R.; Weber, M.E. Bubbles, drops, and particles. Dry. Technol. 1978, 11, 263–264. [Google Scholar]
- Wu, N.; Li, T.; Lian, J. Numerical Investigation of Hvaf-Sprayed Fe-BASED Amorphous Coatings. Mater. Technol. 2022, 56, 677–688. [Google Scholar] [CrossRef]
- Crowe, C.T.; Schwarzkopf, J.D.; Sommerfeld, M.; Tsuji, Y. Multiphase Flows with Droplets and Particles; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Cabrera, N.; Mott, N.F. Theory of the oxidation of metals. Rep. Prog. Phys. 1949, 12, 163–184. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, Y.; Lavernia, E.J. A Numerical Study of Oxidation Behavior During Reactive Atomization and Deposition. Met. Mater. Trans. B 2004, 35, 1173–1185. [Google Scholar] [CrossRef]
- Perry, E.J. Triphenylmethyl Radical Formation on Silver. Ph.D. Thesis, McGill University, Montreal, QC, Canada, 1951. [Google Scholar]
- Zeng, Z.; Sakoda, N.; Tajiri, T.; Kuroda, S. Structure and corrosion behavior of 316L stainless steel coatings formed by HVAF spraying with and without sealing. Surf. Coatings Technol. 2008, 203, 284–290. [Google Scholar] [CrossRef]
- Dignam, M.J.; Fawcett, W.R.; Böhni, H. The Kinetics and Mechanism of Oxidation of Superpurity Aluminum in Dry Oxygen I. Apparatus Description and the Growth of ‘Amorphous’ Oxide. J. Electrochem. Society 1966, 113, 656. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Rangel, R.H.; Sobolev, V.V.; Guilemany, J.M. In-fight oxidation of composite powder particles during thermal spraying. Int. J. Heat Mass Transf. 2001, 44, 4667–4677. [Google Scholar] [CrossRef]
- Li, M.; Christofides, P.D. Modeling and control of high-velocity oxygen-fuel (HVOF) thermal spray: A tutorial review. J. Therm. Spray Technol. 2009, 18, 753–768. [Google Scholar] [CrossRef]
- Baik, J.-S.; Kim, Y.-J. Effect of nozzle shape on the performance of high velocity oxygen-fuel thermal spray system. Surf. Coatings Technol. 2008, 202, 5457–5462. [Google Scholar] [CrossRef]
- Srivatsan, V.R.; Dolatabadi, A. Simulation of particle-shock interaction in a high velocity oxygen fuel process. J. Therm. Spray Technol. 2006, 15, 481–487. [Google Scholar] [CrossRef]
- Liu, F.; Li, Z.; Fang, M.; Hou, H. Numerical analysis of the activated combustion high-velocity air-fuel spraying process: A three-dimensional simulation with improved gas mixing and combustion mode. Materials 2021, 14, 657. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Eidelman, S. Numerical analysis of a high-velocity oxygen-fuel thermal spray system. J. Therm. Spray Technol. 1996, 5, 175–184. [Google Scholar] [CrossRef]
- Joshi, S.V.; Sivakumar, R. Particle behaviour during high velocity oxy-fuel spraying. Surf. Coatings Technol. 1991, 50, 67–74. [Google Scholar] [CrossRef]
- Cheng, D.; Xu, Q.; Lavernia, E.J.; Trapaga, G. The effect of particle size and morphology on the in-flight behavior of particles during high-velocity oxyfuel thermal spraying. Met. Mater. Trans. B 2001, 32, 525–535. [Google Scholar] [CrossRef]
- Li, M.; Christofides, P.D. Computational study of particle in-flight behavior in the HVOF thermal spray process. Chem. Eng. Sci. 2006, 61, 6540–6552. [Google Scholar] [CrossRef]
- Li, M.; Shi, D.; Christofides, P.D. Diamond Jet Hybrid HVOF Thermal Spray: Gas-Phase and Particle Behavior Modeling and Feedback Control Design. Ind. Eng. Chem. Res. 2004, 43, 3632–3652. [Google Scholar] [CrossRef]
- Jiang, H.-R.; Li, M.-L.; Wei, X.-S.; Ma, T.-C.; Dong, Y.; Ying, C.-X.; Liao, Z.-Y.; Shen, J. Numerical Investigation of In-Flight Behavior of Fe-Based Amorphous Alloy Particles in AC-HVAF Thermal Spray Process. J. Therm. Spray Technol. 2019, 28, 1146–1159. [Google Scholar] [CrossRef]
- Jadidi, M.; Moghtadernejad, S.; Dolatabadi, A. Numerical Modeling of Suspension HVOF Spray. J. Therm. Spray Technol. 2016, 25, 451–464. [Google Scholar] [CrossRef]






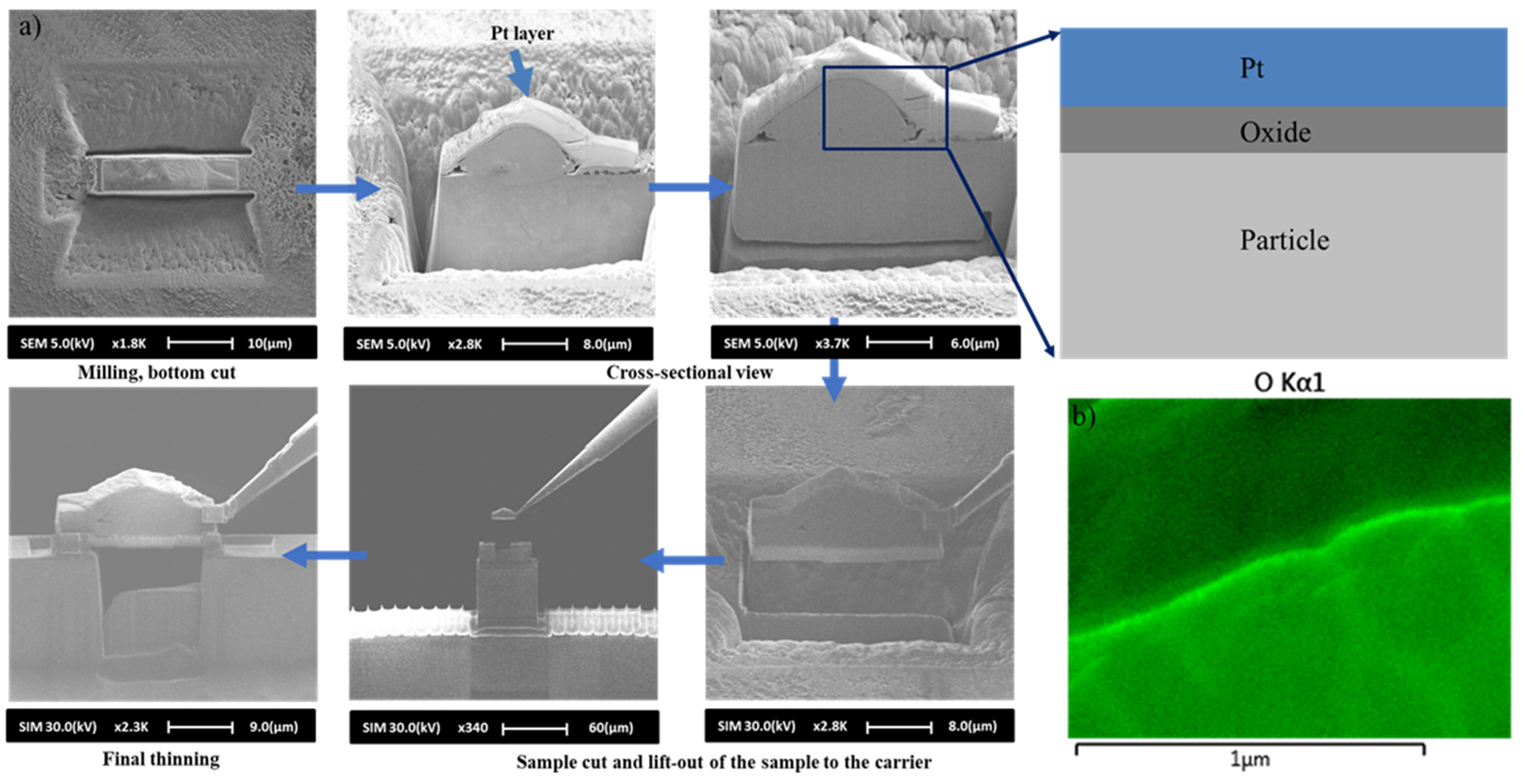
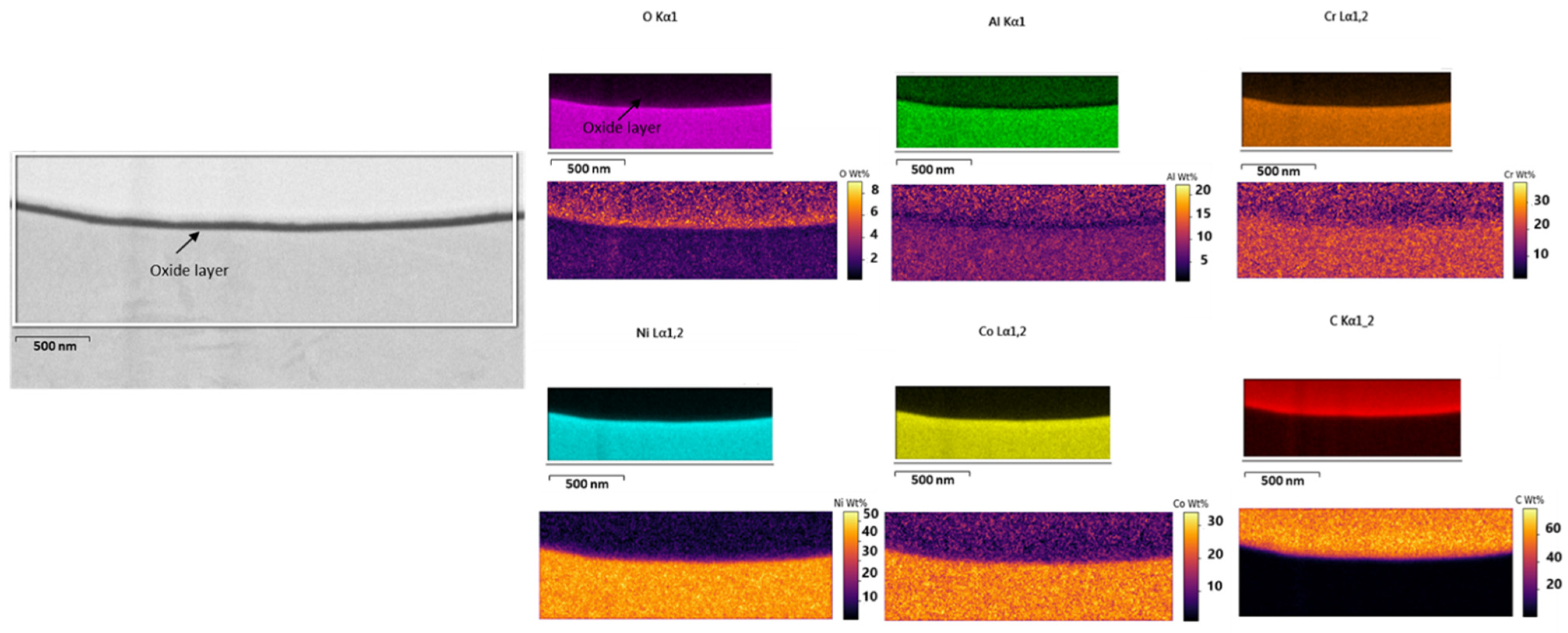
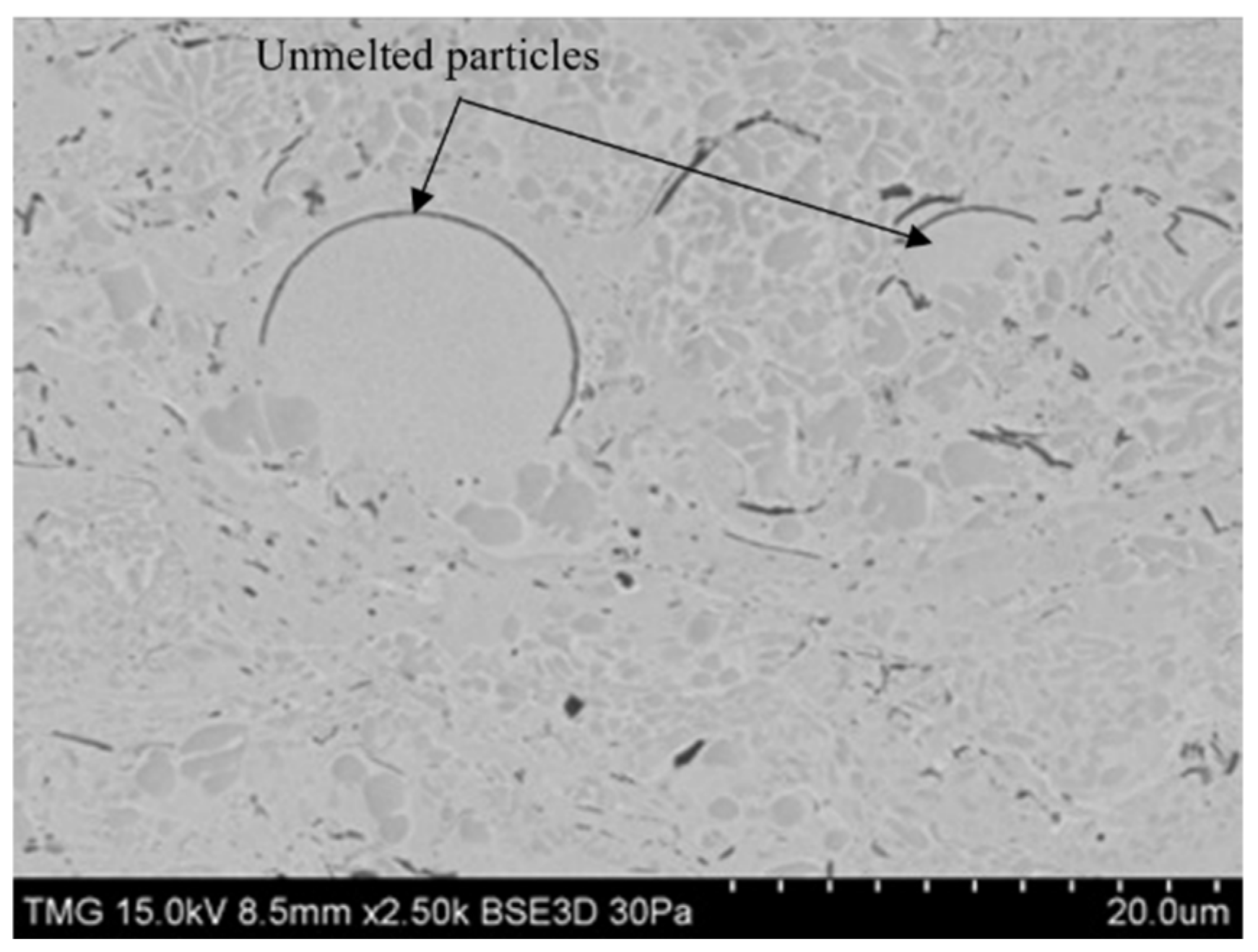
| Parameters | (kg/s) | (kg/s) | Nitrogen Flow Rate (L/min) | SOD (cm) | Air-Fuel Ratio (AFR) | Outlet Pressure (atm) |
|---|---|---|---|---|---|---|
| Values | 0.02332 | 0.0033 | 18 | 5.08 | 7.06 | 1 |
| Temperature °K | CP J/kg.K |
|---|---|
| 366 | 469 |
| 533 | 536 |
| 644 | 574 |
| 755 | 607 |
| 866 | 640 |
| 1042 | 703 |
| 1134 | 749 |
| 1255 | 804 |
| 1366 | 856 |
| Parameters | Fuel Flow Rate (g/s) | Oxygen Flow Rate (g/s) | Nitrogen Flow Rate (g/s) | Particle Initial Velocity (m/s) |
|---|---|---|---|---|
| Values | 2.66 | 6.64 | 0.313 | 6.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diop, S.A.B.; Nastic, A.; Dolatabadi, A.; Attarzadeh, R.; Moreau, C. In-Flight Particle Oxidation Evolution in HVAF: A Numerical Study. Coatings 2025, 15, 215. https://doi.org/10.3390/coatings15020215
Diop SAB, Nastic A, Dolatabadi A, Attarzadeh R, Moreau C. In-Flight Particle Oxidation Evolution in HVAF: A Numerical Study. Coatings. 2025; 15(2):215. https://doi.org/10.3390/coatings15020215
Chicago/Turabian StyleDiop, Sokhna Awa Bousso, Aleksandra Nastic, Ali Dolatabadi, Reza Attarzadeh, and Christian Moreau. 2025. "In-Flight Particle Oxidation Evolution in HVAF: A Numerical Study" Coatings 15, no. 2: 215. https://doi.org/10.3390/coatings15020215
APA StyleDiop, S. A. B., Nastic, A., Dolatabadi, A., Attarzadeh, R., & Moreau, C. (2025). In-Flight Particle Oxidation Evolution in HVAF: A Numerical Study. Coatings, 15(2), 215. https://doi.org/10.3390/coatings15020215







