Abstract
Due to the extremely high hardness of high-entropy monoborides (HEMB), this study successfully prepared a new type of HEMB-(V0.2Cr0.2Ta0.2Mo0.2W0.2)B coating by adjusting the spraying process using atmospheric plasma spraying technology. The high-entropy coating was characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), and energy dispersive spectroscopy (EDS). The change rule concerning the alteration in the phase composition of the coating surface with power and protective atmosphere was explored, indicating that with the decrease in power and the application of a protective atmosphere, the generation of oxide on the coating surface is successfully inhibited. The wear resistance of the coating in a temperature range from room temperature to 800 °C was evaluated in dry sliding wear test conditions. The results indicated that the wear mechanism of (V0.2Cr0.2Ta0.2Mo0.2W0.2)B coating below 400 °C is abrasive wear, and above 400 °C, the wear mechanism is mainly oxidative wear.
1. Introduction
High-temperature wear failure is a major problem for precision components, and developing reliable high-temperature wear-resistant materials is crucial for the development of advanced industries (e.g., the aerospace, wind turbine, and micro and nanoelectromechanical system industries) [1,2,3]. Wear is primarily related to the surface properties of the material, and replacement with newly designed materials is usually less cost-effective, but the development of a wear-resistant coating capable of resisting high temperatures would be one of the most direct and economical solutions to the problems described above [4,5].
It is widely recognized that there is a positive correlation between the hardness of a material and its wear resistance [6]. The concept of high-entropy materials is considered to be the most promising solution to fulfill this need. Inspired by high-entropy alloys (HEAs), the high-entropy concept was extended to ceramic materials in 2015 [7]. High-entropy ceramics (HECs) are solid solutions of inorganic compounds with one or more Wyckoff sites shared by equal or near-equal atomic ratios of multi-principal elements [8]. Up to now, diverse high-entropy ceramics, including high-entropy oxides [9,10], high-entropy diborides [11,12], and high-entropy carbides [13,14], among others, have been synthesized. This unique structural feature has led to a large number of new materials with excellent properties, such as high strength at elevated temperatures [15], superb specific strength [16], good wear and corrosion resistance [17], high cryogenic temperature fracture toughness [18], high hydrogen storage capacity [19], enhanced magnetocaloric effect [20], and superconductivity [21], etc. Our previous studies of novel high-entropy ceramics have shown that high-entropy monoborides (HEMB) tend to have extremely high hardness due to severe lattice distortions that impede dislocation slip and high valence electron concentrations, while transition metals provide high incompressibility [22,23]. However, the current research on HEMB materials mainly focuses on preparation and mechanical property studies, while the research on the preparation of coatings using APS technology has rarely been reported. In addition, the deposition mechanism and high-temperature tribological properties of the coating materials in close to practical application scenarios are still unclear.
Among the potential coating technologies, atmospheric plasma spraying (APS) technology is often considered for the preparation of high-hardness and high-melting-point ceramic coatings due to its high throughput and extremely high plasma plume energy [24]. While HEMB ceramics with ultra-high hardness have great potential for wear-resistant coating applications, exploring whether corresponding HEMB coatings can be deposited by APS remains a challenge. This is due to the fact that APS may negatively affect the physical and mechanical properties of HEMB coatings, such as porosity, oxidation, and splash boundary issues. Therefore, regulating the APS process parameters becomes a top priority for preparing wear-resistant coatings [25,26,27,28].
In this study, the feasibility of depositing (V0.2Cr0.2Ta0.2Mo0.2W0.2)B coatings using the APS technique was investigated, and the effects of plasma spraying parameters on the microstructure and phase composition of the coatings were studied. The tribological properties and wear mechanisms of (V0.2Cr0.2Ta0.2Mo0.2W0.2)B coatings were explored for the first time in the temperature range of 25–800 °C. A new design concept for the deposition of easily oxidized materials by APS technology is explored, and a new candidate material is provided for the development of wear-resistant coatings.
2. Experimental Procedures
2.1. Preparation of High-Entropy Monoboride Coating
The materials used for the synthesis of (V0.2Cr0.2Ta0.2Mo0.2W0.2)B powders are commercially available. Powdered Ta (99.95%, 45 µm), Mo (99.95%, 0.5 µm), W (99.95%, 45 µm), V (99.8%, 45 µm), Cr (99.9%, 75 µm), and amorphous B (99%, 1–5 µm) were purchased from Zhongnuo New Material Technology Co., Ltd., Beijing, China. We mixed the starting powder stoichiometrically at 300 rpm for 12 h, and then the dried slurry was placed in a vacuum sintering furnace and held at 1900 °C for 2 h to produce HEMB powder. Subsequently, the HEMB powder was ball-milled with Polyvinyl Alcohol (PVA) and zirconia grinding balls in deionized water for 4 h. The ball-milled HEMB slurry was granulated by spray dryer (YC-019, Shanghai Ya Cheng Instrument Equipment Co., Ltd., Shanghai, China). The spherical powders were calcined at 650 °C for 6 h under argon protection to completely remove moisture and organic impurities, and calcined at 1400 °C for 2 h after removal of impurities to enhance the bonding of the spherical powders.
High-temperature alloy disks (ø 25 mm × 10 mm) and strips (40 mm × 10 mm) were used as substrates. The substrate was sandblasted by a box sandblasting machine (HC-9080, Shijiazhuang Hongchen Machinery Co., Ltd., Shijiazhuang, China) to increase its surface roughness to >4 μm and placed in an acetone solution for ultrasonic cleaning for 10 min. The APS equipment (DH-2080, Shanghai Ruifa Spraying Machinery Co., Ltd., Shanghai, China) was used to prepare the bond coating (NiCrCoAlY) and HEMB ceramic coating with a specific thickness on the surface of the substrate. Figure 1 is a flowchart for the preparation of the coating in order to show more clearly the details of the experimental part.
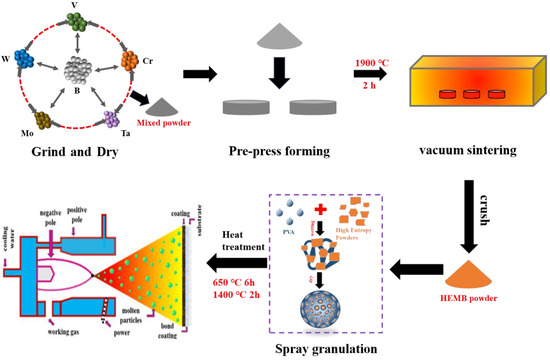
Figure 1.
Synthesis procedure of high-entropy (V0.2Cr0.2Ta0.2Mo0.2W0.2) B coating.
2.2. Tribological Tests
The dry sliding wear test was tested on a high-temperature reciprocating friction wear tester (Lanzhou Zhongke Kaihua Technology Development Co., Ltd., GF-I, Lanzhou, China). The relative humidity of the environment was 30 ± 10%, and the operating temperatures were RT to 800 °C with a temperature interval of 200 °C. The diameter of the friction pairs (Si3N4) was 6 mm. The tribological tests were run at loads of 3 N. The constant sliding time was 30 min. During the tests, the coefficient of friction (COF) was automatically and digitally recorded. The wear rate of the samples was measured by a surface abrasion measuring instrument (Lanzhou Zhongke Kaihua Technology Development Co., Ltd., MT-500, China).
2.3. Characterization
The phase structure of the prepared high-entropy powder and coatings was verified by X-ray diffraction (XRD) using an Empyrean (PANalytical B.V., Almelo, The Netherlands). The microstructure of the worn surface was observed and the energy dispersion X-ray spectrometer (EDS) results were obtained by scanning electron microscopy (SEM, FEI NOVA NANO450, Hillsboro, OR, USA). The particle size distribution curves of the spray granulation were measured using a laser particle size analyzer (MS3000E + EV, Malvern Instruments, Malvern, UK).
3. Results and Discussion
3.1. Phase Composition of High-Entropy Powders
Figure 2a demonstrates the XRD patterns of high-entropy (V0.2Cr0.2Ta0.2Mo0.2W0.2)B powders prepared at different sintering temperatures. From the figure, it can be seen that the preparation of single-phase (V0.2Cr0.2Ta0.2Mo0.2W0.2)B is a process of slowly occurring solid solution changes. The intermediate reaction product (TaB) and some unreacted W metal monomers were present in the powder prepared at a temperature of 1800 °C. This might be due to the relatively low temperature used for synthesis, resulting in an incomplete solid solution of TaB and W with large lattice parameters. When the temperature is increased to 1900 °C, W monomers and B elements react to form WB, and WB and TaB are gradually solid-solved into the main lattice, which shows that a single orthorhombic phase has been synthesized without other heterogeneous phases. As the temperature is further increased to 1950 °C, no phase change occurs compared to the diffraction peaks at 1900 °C. The synthesis of high-entropy (V0.2Cr0.2Ta0.2Mo0.2W0.2)B powders shows that a homogeneous single-phase high-entropy powder can be successfully prepared by holding the temperature at 1900 °C for 2 h. In addition, the elemental distributions show that the five transition metal metallic elements (V, Cr, Ta, Mo, and W) have no obvious bias aggregation on the micrometer scale and exhibit a uniform distribution, which more accurately proves the successful synthesis of high-entropy powders (Figure 2b).
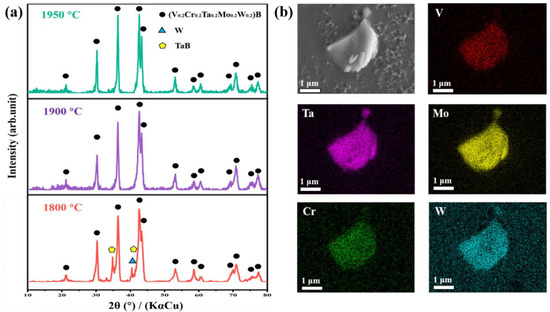
Figure 2.
(a) XRD patterns of synthetic powders at different temperature conditions. (b) SEM image and elemental distribution of synthesized (V0.2Cr0.2Ta0.2Mo0.2W0.2)B powders.
3.2. Regulation of HEMB Agglomerated Powder Properties by Spray Granulation Process
The use of spray granulation technology improves the particle size dispersion and flowability of HEMB powders and facilitates the continuous delivery of raw materials in the APS process [29]. Figure 3a shows the mechanism of spray granulation, which is a granulation method in which the powder slurry or solution is sprayed into the granulation tower, and the powder slurry or solution is dried and agglomerated under the action of the spraying hot air, so as to obtain the spherical agglomerates. Figure 3b investigates the morphology of agglomerated powders prepared with different binder (PVA) contents and solid contents. It can be seen that both the solid content and the binder content of the slurry have a great influence on the microscopic morphology of the granulated powder. At the same solid content, the sphericity of the powder gets better and better as the binder content increases from 3 wt% to 5 wt% step by step. This is because when the binder content is insufficient, the bonding strength between the powders in the granulation process is not high, and fragmentation occurs when it is subjected to the action of the inner wall of the spray dryer or other objects. However, when the binder content is 6 wt%, the viscosity of the slurry is too high, causing the spherical powder formed to stick together, which adversely affects the flowability of the spherical powder and is not conducive to subsequent spraying. Therefore, the optimal binder content for this experiment is 5 wt%. When the binder content is 5 wt% and the solid content is 50 wt%, the agglomerated powder shows a round cake shape and relatively more broken particles, while when the solid content is 60 wt%, the powders are agglomerated into a regular spherical shape, and the agglomeration effect is better. Therefore, in order to obtain granulation powder with a good agglomeration effect and uniform balling, the optimal granulation process explored is 5 wt% PVA and 60 wt% solid content. Under this process, the surface of the spherical powder after heat treatment is smooth and still has good sphericity, and the five transition metal elements are evenly distributed, indicating that it has good thermal stability and meets the spraying requirements (Figure 3c).
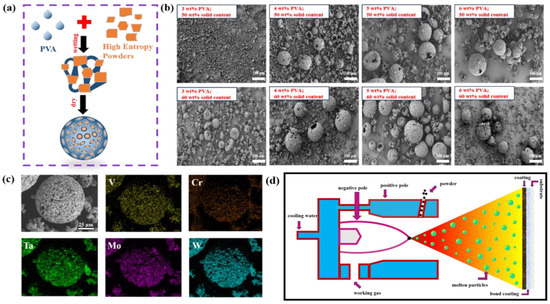
Figure 3.
(a) Spray granulation mechanism. (b) Microscopic morphology of agglomerated powders with a different PVA content and solid phase content. (c) EDS maps of HEMB powders after spray granulation and heat treatment. (d) Schematic diagram of plasma spraying process.
3.3. Structural and Phase Control of Coatings Deposited by APS
Due to the extremely high flame temperature during spraying, it is easy for some physical and chemical changes to occur during the spraying process, resulting in changes in the phase composition of the coating. Therefore, it is necessary to explore the effect of spraying parameters on the phase composition of the coating in order to determine the optimal spraying process and obtain an ideal HEMB coatings. The key technological parameters of APS are shown in Table 1.

Table 1.
Key technological parameters of bonding layer and HEMB coating prepared by APS.
Samples with a spraying power of 46 kW (unprotected gas), 44 kW (protective gas), 42 kW (protective gas), and 42 kW (unprotected gas) are defined as HEMB-1, HEMB-2, HEMB-3, and HEMB-4, respectively. Figure 4 shows the XRD patterns of each coating. It can be seen that due to the absence of protective atmosphere during the spraying process of sample HEMB-1, the main peak of the XRD diffraction peak of the coating is no longer HEMB, but various oxide diffraction peaks (WO3, Ta2O5, V2O3) and elemental W diffraction peaks (Figure 4a). The appearance of oxides is partly due to the fact that HEMB powders themselves undergo oxidation at high temperatures. Although the spraying process is rapid during APS, the high-entropy powder melted by the high-temperature flame flow inevitably entrains some of the surrounding air when sprayed at high speed onto the substrate surface, causing oxidation of the coating. On the other hand, after completing the spraying process, the instantaneous temperature of the coating surface can reach over 800 °C. Natural cooling under normal atmospheric conditions will inevitably cause oxidation on the coating surface.
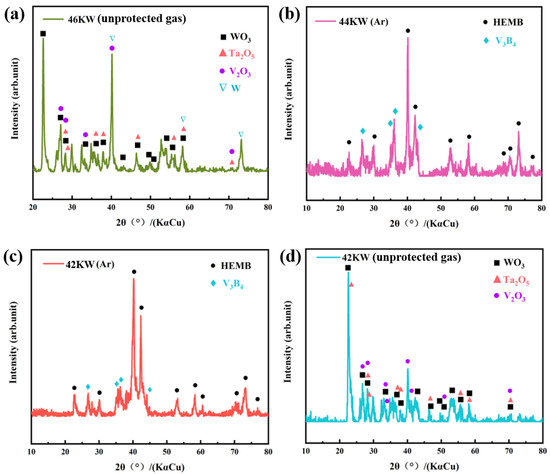
Figure 4.
The phase composition of coatings under different spraying process parameters: (a) 46 kW, unprotected gas; (b) 44 kW, adequate protective gas; (c) 42 kW, adequate protective gas; and (d) 42 kW, unprotected gas.
In order to avoid the occurrence of oxidation phenomenon, we took two measures: one was to reduce the spraying power, and the other was to apply a protective atmosphere (Ar gas) around the plasma flame during the spraying process. The results show that the coatings HEMB-2 and HEMB-3 have diffraction peaks, similar to those of the HEMB powder, indicating successful deposition of the coating (Figure 4b,c). However, both coatings contain diffraction peaks of the second phase V3B4, and the relative intensity of the V3B4 diffraction peak slightly increased with the increase of spraying power. This is due to the influence of the plasma beam, which creates a huge thermal gradient and rapid cooling rate in the coating, creating a new dynamic process that makes it difficult to achieve ideal atomic diffusion during the coating deposition process [30]. Therefore, in the non-equilibrium cooling process, borides with higher melting points containing W, Mo, and Ta preferentially crystallize over borides with lower melting points of V, ultimately forming V3B4.
In order to further investigate the effects of protective atmosphere and spraying power on the coating phase, coating HEMB-4 was prepared using the same process as coating HEMB-3, except that no protective atmosphere was applied. It was found that its phase composition is almost the same as coating HEMB-1, consisting of multiple oxides (Figure 4d). However, the relative intensity of the diffraction peak of the oxide in coating HEMB-4 is significantly lower than that in coating HEMB-1, indicating that the selection of the protective atmosphere and spraying power is crucial for the preparation of HEMB coatings.
Under different spraying parameters, there are significant differences in the microstructure of the coating surface. Due to the absence of a protective atmosphere, the surface roughness of coating HEMB-4 is significantly higher (Figure 5a), and oxidation products such as flakes, rods, and spheres can be observed in the enlarged image. This is consistent with the phase characterization results of the coating (Figure 4d).
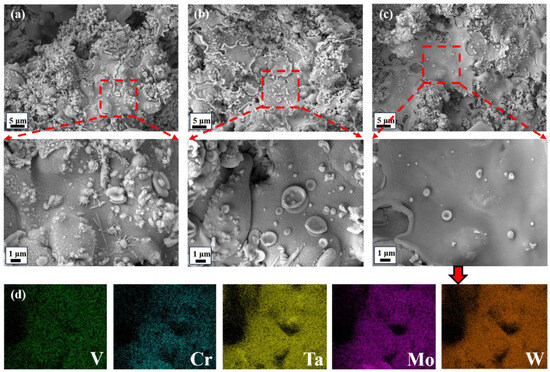
Figure 5.
The surface morphology of ceramic coatings under different spraying process conditions: (a) coating HEMB-4; (b) coating HEMB-3; (c) coating HEMB-2; and (d) element distribution corresponding to HEMB-2.
By applying a protective atmosphere, the surface roughness of the coating is improved and the surface oxidation phenomenon is significantly suppressed at the same power (Figure 5b). This may be due to the low power of APS and the insufficient temperature of the plasma flame to melt the HEMB spherical powder, resulting in poor coating spreading. The unmelted spherical powder mainly relies on the initial force to adsorb onto the coating, which will lead to relatively poor adhesion between the coating layers and is not conducive to the subsequent exploration of the frictional properties of the coating. As shown in Figure 5c, it can be seen that with the increase in spraying power to 44 kW, the spreading and surface roughness of the coating have been significantly improved. This is because the increase of APS power heats the spherical powder to a higher temperature in the plasma flame, resulting in a higher degree of powder melting. This enables HEMB droplets to diffuse more fully when in contact with the adhesive layer, resulting in a denser and smoother coating surface. It can be clearly seen from Figure 5d that V, Cr, Ta, Mo, and W elements in the HEMB-2 coating prepared by plasma spraying are evenly distributed without significant segregation and aggregation.
When observing the cross-section of the coating, the coating shall be inlaid first. The coating sample is placed into a special abrasive tool, then the acrylic adhesive poured into it; it is taken out after the acrylic adhesive has completely cured, and then the coating section is polished. The backscattered images of the cross-section of HEMB coatings under different spraying parameters are shown in Figure 6. The thickness of the coating is measured using a ruler and marks with a red arrow in the figure. The coating cross-section shows a clear three-layer structure, but due to the close relative molecular weight between the bonding layer and the substrate, the boundary between the two is not very clear. The average thicknesses of coatings HEMB-4, HEMB-3, and HEMB-2 are 45.50 μm, 84.85 μm, and 82.06 μm, respectively. It can be seen that coating HEMB-3 prepared at a power of 42 kW has a large number of pores in the area near the bonding layer, and the overall structure is relatively loose. This is because the low spraying power leads to insufficient temperature of the plasma flame, and the spherical powder delivered to the flame only partially melts. The particles are only connected by the melted parts, forming a loose coating. When the power is increased to 44 kW, the melting degree of the spherical powder is improved, and the spreading degree of coating HEMB-2 is higher, meaning that almost no pores can be observed in the coating cross-section.

Figure 6.
The cross-sectional morphology of ceramic coatings under different spraying process conditions: (a) coating HEMB-4; (b) coating HEMB-3; and (c) coating HEMB-2.
Based on the exploration of the phases, surfaces, and cross-sections of coatings prepared under different spraying parameters, and considering the application environment of wear-resistant coatings, coating HEMB-2 prepared under a protective atmosphere and a spraying power of 44 kW is selected as the research object for the subsequent friction and wear experiments.
3.4. Tribological Behavior and Wear Mechanism of HEMB-2 Coatings at Different Temperatures
Figure 7 shows the curve of the COF versus the time for the HEMB-2 coatings from RT to 800 °C. It can be seen that the temperature has a significant impact on the COF of HEMB-2 coatings. The COF at RT is 0.60. As the temperature rises to 200 °C, the COF rises to 0.78. With the further increase in temperature, the COF reaches 0.59 and 0.84 at 400 °C and 600 °C, respectively. At 800 °C, the COF decreases to 0.27, which is the lowest value for all the temperatures tested. It is noteworthy that the curves at different temperatures show a distinct jagged shape, which may be due to the periodic accumulation and exfoliation of wear debris on the worn surface [31].
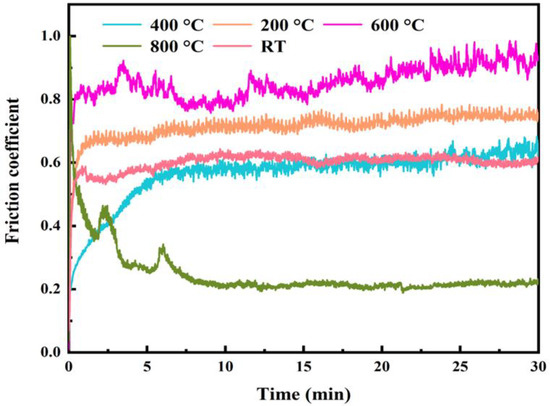
Figure 7.
Evolution of the friction coefficient with respect to the sliding time and temperature.
The wear rate is usually used to assess the wear resistance of a material: the smaller the wear rate, the higher the wear resistance, as more energy is required to remove the same volume. Figure 8a–e shows the corresponding two-dimensional cross-sectional profiles of the worn surface. It can be observed that the width and depth of the worn surface increase gradually from RT to 800 °C. The effect of temperature on the wear rate of HEMB-2 coatings is shown in Figure 8f. From RT to 800 °C, the wear rate increases with the increase in experimental temperature. Among them, the wear rate of the coating at 800 °C increases rapidly, which is eight times higher than that at 600 °C, indicating that destructive wear of the coating occurred at 800 °C and the coating has failed.
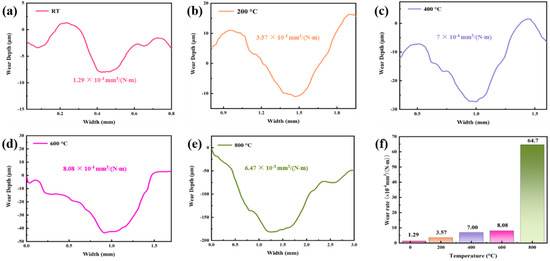
Figure 8.
(a–e) Corresponding two-dimensional cross-sectional profiles of the worn surface of the coating at different temperatures, (f) Wear rate of the coating at different temperatures.
To further investigate the relationship between the operating temperature and tribological behavior of HEMB-2 coatings, the morphology of the worn surface is analyzed at different temperatures. At RT, typical debris and groove structures can be observed, which are typical signs of abrasive wear (Figure 9a). At the same time, under high magnification, cracks can be observed on the surface of the coating, and many hard particles can be observed at the cracks. These hard particles will slide between the friction pair surfaces during the test, leading to severe three-body wear. At 200 °C, the width of the worn surface increases significantly, and the amount of debris and cracks also increase substantially relative to RT (Figure 9b). This is due to the fact that the plastic deformation of the coating increases progressively with increasing softening at high temperatures. During friction, shear plastic deformation accumulates on the softened surface while cracks develop and expand until they peel off in the form of fragments.
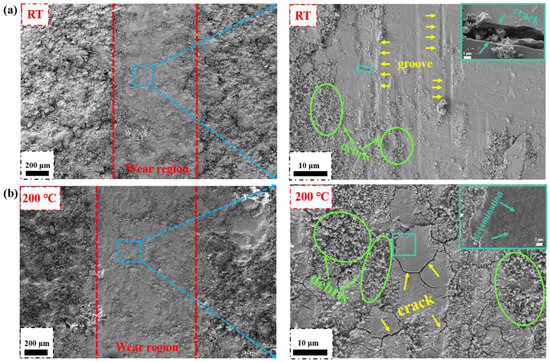
Figure 9.
Microscopic morphology of HEMB-2 coatings’ worn surfaces: (a) RT, (b) 200 °C.
With further rises in the operating temperature to 400 °C, the COF of the coating decreases, which may be related to the observation of a large area of flaky structure on the worn surface (Figure 10). Through an enlarged image of the worn surfaces and in the XRD patterns, it is found that this is an oxide with a regular hexagonal morphology, rich in B and O elements, indicating that boric acid is produced on the worn surface. Firstly, the boric acid molecule has the characteristic of polarity, which can form physical adsorption or chemical adsorption on the surface of the coating, including the Si3N4, and this adsorption film can play the role of reducing direct contact and friction between the friction surfaces [32]. Secondly, boric acid is a small molecule with polarity that penetrates into defective cracks on the coating surface and reduces the point contact area between the coating and friction pairs, thus reducing the friction and wear between the surfaces [33]. It is worth noting that by characterizing the morphology of the non-wear zone at this temperature, no boric acid appears, indicating that under the combined effect of ambient temperature and frictional heat, the temperature at the worn surfaces in the actual friction test exceeds the ambient temperature set for the experiment. Therefore, the wear mechanism at 400 °C is mainly abrasive wear and slight oxidative wear.
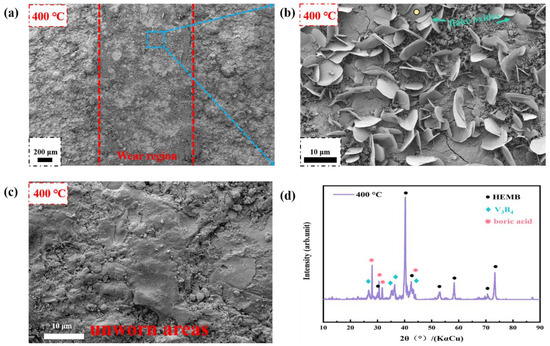
Figure 10.
Microscopic morphology and phase composition of HEMB-2 coatings’ worn surfaces at 400 °C: (a) Worn area; (b) Enlarged image of worn area; (c) Unworn area; (d) XRD pattern of worn area.
At 600 °C, the roughness of the worn surface increases significantly, and a large number of loose oxidation products appear on the surface of the coating in the non-wear area (Figure 11), which suggests that the large area of debris appear at the worn surfaces should be formed by the breakage of the loose oxidation products under the action of the friction load. Meanwhile, no lubricating boric acid generated at 400 °C is detected in the enlarged image of the worn surfaces and in the XRD patterns, which is replaced by a variety of transition metal oxides, so the COF at 600 °C is the maximum value under all test conditions.
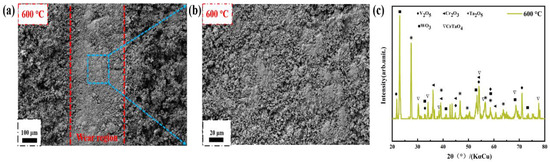
Figure 11.
Microscopic morphology and phase composition of HEMB-2 coatings’ worn surfaces at 600 °C: (a) Worn area; (b) Enlarged image of worn area; (c) XRD pattern of worn area.
When the experimental temperature reaches 800 °C, the worn surface is significantly different from the previously mentioned morphology, and the surface is noticeably smoother, which is mainly related to the formation of oxide film on the worn surface (Figure 12). The width of the wear scar significantly increases. According to the growth mechanism of the oxide film, when the oxidation temperature is too high, the generated oxide film will peel off during the film formation process due to excessive internal stress [34]. Therefore, the wear rate at 800 °C reaches the maximum value in this experiment (Figure 8f). In the magnified image, it is observed that the surface of the oxide film contains a large number of groove structures, indicating that the generated oxide film has a certain toughness and density. At the same time, it is found that the surface of the oxide film is composed of nanoscale columnar particles. The reason for this phenomenon may be that under high-temperature and cyclic tensile loads, the oxide film is broken into small particles, which are then compacted under pressure to fill the surface defects caused by sliding during friction. Therefore, the COF of the HEMB-2 coatings reaches its minimum value at 800 °C.
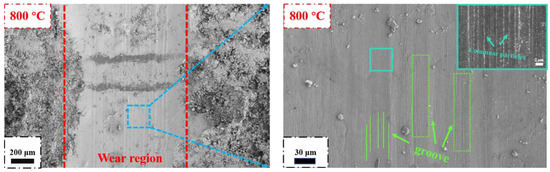
Figure 12.
Microscopic morphology of HEMB-2 coatings’ worn surfaces at 800 °C.
4. Conclusions
This study provides the first detailed investigation into the friction and wear performance of HEMB ceramic coatings over a wide temperature range, which has great potential for application in the field of wear-resistant coatings.
(1) By selecting the spraying power and applying a protective atmosphere, the occurrence of HEMB coating oxidation was successfully suppressed, achieving effective deposition of HEMB ceramic coatings using APS technology.
(2) When the temperature is below 400 °C, the wear mechanism of the coating is mainly abrasive wear. When the temperature is above 400 °C, the wear mechanism of the coating is the coexistence of abrasive wear and oxidative wear.
(3) The oxide film on the surface of the coating undergoes a series of evolutions with the increase in temperature: the boric acid with lubricating effect formed at 400 °C disappears when the temperature rises to 600 °C, and a composite oxide film is generated. At 800 °C, the oxide film breaks into nanoscale oxide particles under the action of cyclic loading.
Author Contributions
Validation, M.L.; Investigation, C.G., J.Z. and H.W.; Resources, K.Y.; Data curation, Y.L.; Writing—original draft, C.G.; Writing—review & editing, J.Z.; Supervision, H.W. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (No. 52172075), the Project of Zhongyuan Critical Metals Laboratory (No. GJJSGFYQ202317, GJJSGFJQ202303).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, W.; Pu, J.; Yu, Q.; Zeng, Z.; Wu, X.; Xue, Q. A novel strategy to enhance micro/nano-tribological properties of DLC film by combining micro-pattern and thin ionic liquids film. Colloids Surf. A. 2013, 428, 70–78. [Google Scholar] [CrossRef]
- Teran, L.; Roa, C.; Muñoz-Cubillos, J.; Aponte, R.; Valdes, J.; Larrahondo, F.; Rodríguez, S.; Coronado, J. Failure analysis of a run-of-the-river hydroelectric power plant. Eng. Fail. Anal. 2016, 68, 87–100. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Hu, J. Recent advances in the development of aerospace materials. Prog. Aerosp. Sci. 2018, 97, 22–34. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Rajagopal, G.; Palaniswamy, N.; Raghavan, M. Corrosion protection by chemical vapor deposition: A review. Corros. Rev. 2005, 23, 355–370. [Google Scholar] [CrossRef]
- Carr, G.E.; Conde, R.H. Tribology of hard coating alloys deposited by thermal methods: Applications to industrial components. Surf. Coat. Technol. 2008, 203, 685–690. [Google Scholar] [CrossRef]
- Archard, J. Contact and rubbing of flat surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.-P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Xing, Y.; Dai, F.-Z.; Wang, H.; Su, L.; Miao, L.; Zhang, G.; Wang, Y.; Qi, X.; Yao, L. High-entropy ceramics: Present status, challenges, and a look forward. J. Adv. Ceram. 2021, 10, 385–441. [Google Scholar] [CrossRef]
- Sarkar, A.; Velasco, L.; Wang, D.; Wang, Q.; Talasila, G.; de Biasi, L.; Kübel, C.; Brezesinski, T.; Bhattacharya, S.S.; Hahn, H. High entropy oxides for reversible energy storage. Nat. Commun. 2018, 9, 3400. [Google Scholar] [CrossRef]
- Ma, M.; Han, Y.; Zhao, Z.; Feng, J.; Chu, Y. Ultrafine-grained high-entropy zirconates with superior mechanical and thermal properties. J. Mater. 2023, 9, 370–377. [Google Scholar] [CrossRef]
- Tallarita, G.; Licheri, R.; Garroni, S.; Orrù, R.; Cao, G. Novel processing route for the fabrication of bulk high-entropy metal diborides. Scr. Mater. 2019, 158, 100–104. [Google Scholar] [CrossRef]
- Liu, D.; Wen, T.; Ye, B.; Chu, Y. Synthesis of superfine high-entropy metal diboride powders. Scr. Mater. 2019, 167, 110–114. [Google Scholar] [CrossRef]
- Wang, F.; Yan, X.; Wang, T.; Wu, Y.; Shao, L.; Nastasi, M.; Lu, Y.; Cui, B. Irradiation damage in (Zr0.25Ta0.25Nb0.25Ti0.25) C high-entropy carbide ceramics. Acta Mater. 2020, 195, 739–749. [Google Scholar] [CrossRef]
- Castle, E.; Csanádi, T.; Grasso, S.; Dusza, J.; Reece, M. Processing and properties of high-entropy ultra-high temperature carbides. Sci. Rep. 2018, 8, 8609. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Wang, Y.; Chen, G. Solid solution alloys of AlCoCrFeNiTix with excellent room-temperature mechanical properties. Appl. Phys. Lett. 2007, 90, 181904. [Google Scholar] [CrossRef]
- Chuang, M.-H.; Tsai, M.-H.; Wang, W.-R.; Lin, S.-J.; Yeh, J.-W. Microstructure and wear behavior of AlxCo1. 5CrFeNi1. 5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Li, Z.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength–ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-F.; Chen, S.-K.; Sheu, J.-H.; Lin, J.-T.; Lin, W.-E.; Yeh, J.-W.; Lin, S.-J.; Liou, T.-H.; Wang, C.-W. Hydrogen storage properties of multi-principal-component CoFeMnTixVyZrz alloys. Int. J. Hydrogen Energy 2010, 35, 9046–9059. [Google Scholar] [CrossRef]
- Perrin, A.; Sorescu, M.; Burton, M.-T.; Laughlin, D.E.; McHenry, M. The role of compositional tuning of the distributed exchange on magnetocaloric properties of high-entropy alloys. JOM 2017, 69, 2125–2129. [Google Scholar] [CrossRef]
- Koželj, P.; Vrtnik, S.; Jelen, A.; Jazbec, S.; Jagličić, Z.; Maiti, S.; Feuerbacher, M.; Steurer, W.; Dolinšek, J. Discovery of a superconducting high-entropy alloy. Phys. Rev. Lett. 2014, 113, 107001. [Google Scholar] [CrossRef]
- Gu, Q.; Krauss, G.; Steurer, W. Transition metal borides: Superhard versus ultra-incompressible. Adv. Mater. 2008, 20, 3620–3626. [Google Scholar] [CrossRef]
- Zhao, P.; Zhu, J.; Zhang, Y.; Shao, G.; Wang, H.; Li, M.; Liu, W.; Fan, B.; Xu, H.; Lu, H. A novel high-entropy monoboride (Mo0.2Ta0.2Ni0.2Cr0.2W0.2) B with superhardness and low thermal conductivity. Ceram. Int. 2020, 46, 26626–26631. [Google Scholar] [CrossRef]
- Zhu, J.; Ma, Z.; Gao, Y.; Gao, L.; Pervak, V.; Wang, L.; Wei, C.; Wang, F. Ablation behavior of plasma-sprayed La1–xSrxTiO3+δ coating irradiated by high-intensity continuous laser. ACS Appl. Mater. 2017, 9, 35444–35452. [Google Scholar] [CrossRef] [PubMed]
- Yahiro, Y.; Mitsuhara, M.; Tokunakga, K.; Yoshida, N.; Hirai, T.; Ezato, K.; Suzuki, S.; Akiba, M.; Nakashima, H. Characterization of thick plasma spray tungsten coating on ferritic/martensitic steel F82H for high heat flux armor. J. Nucl. Mater. 2009, 386, 784–788. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Jun, C.; Ying, L.; Bing, Q.; Jiang, S.; Wang, X.; Luo, G. Vacuum annealing enhances the properties of a tungsten coating deposited on copper by atmospheric plasma spray. J. Nucl. Mater. 2013, 432, 16–19. [Google Scholar]
- Park, J.Y.; Yang, S.J.; Jin, Y.G.; Park, C.R.; Kim, G.H.; Han, H.N. Effect of annealing with pressure on tungsten film properties fabricated by atmospheric plasma spray. Met. Mater. Int. 2014, 20, 1037–1042. [Google Scholar] [CrossRef]
- Hu, D.; Zheng, X.; Niu, Y.; Ji, H.; Chong, F.; Chen, J. Effect of oxidation behavior on the mechanical and thermal properties of plasma sprayed tungsten coatings. J. Therm. Spray Techn. 2008, 17, 377–384. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, C.; Zheng, W.; Jiang, C.; Song, X.; Zeng, Y. A high-entropy (Yb0.2Y0.2Lu0.2Ho0.2Er0.2)2Si2O7 environmental barrier coating prepared by atmospheric plasma-spray. Ceram. Int. 2023, 49, 11323–11333. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, X.-N.; Chen, J.; Li, H.-P.; Ostrikov, K. Non-equilibrium synergistic effects in atmospheric pressure plasmas. Sci. Rep. 2018, 8, 4783. [Google Scholar] [CrossRef]
- Geng, Y.; Chen, J.; Tan, H.; Cheng, J.; Zhu, S.; Yang, J. Tribological performances of CoCrFeNiAl high entropy alloy matrix solid-lubricating composites over a wide temperature range. Tribol. Int. 2021, 157, 106912. [Google Scholar] [CrossRef]
- Sawyer, W.G.; Ziegert, J.C.; Schmitz, T.L.; Barton, T. In situ lubrication with boric acid: Powder delivery of an environmentally benign solid lubricant. Tribol. Trans. 2006, 49, 284–290. [Google Scholar] [CrossRef][Green Version]
- Kilincarslan, S.K.; Cetin, M.H. Improvement of the milling process performance by using cutting fluids prepared with nano-silver and boric acid. J. Manuf. Process. 2020, 56, 707–717. [Google Scholar] [CrossRef]
- Kumar, S.; Narayanan, T.S.; Raman, S.G.S.; Seshadri, S.K. Thermal oxidation of Ti6Al4V alloy: Microstructural and electrochemical characterization. Mater. Chem. Phys. 2010, 119, 337–346. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).