Electrochemical Fabrication of Ni–Co Alloy over a Wide pH Range Using Sodium Citrate as a Complexing Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Substrate and Electrolytic Solution
2.2. Experimental Design
2.3. Electrodeposition
2.4. Chemical Composition and Surface Characterization of the Alloy
2.5. Magnetic Analysis
2.6. Corrosion Tests
3. Results and Discussion
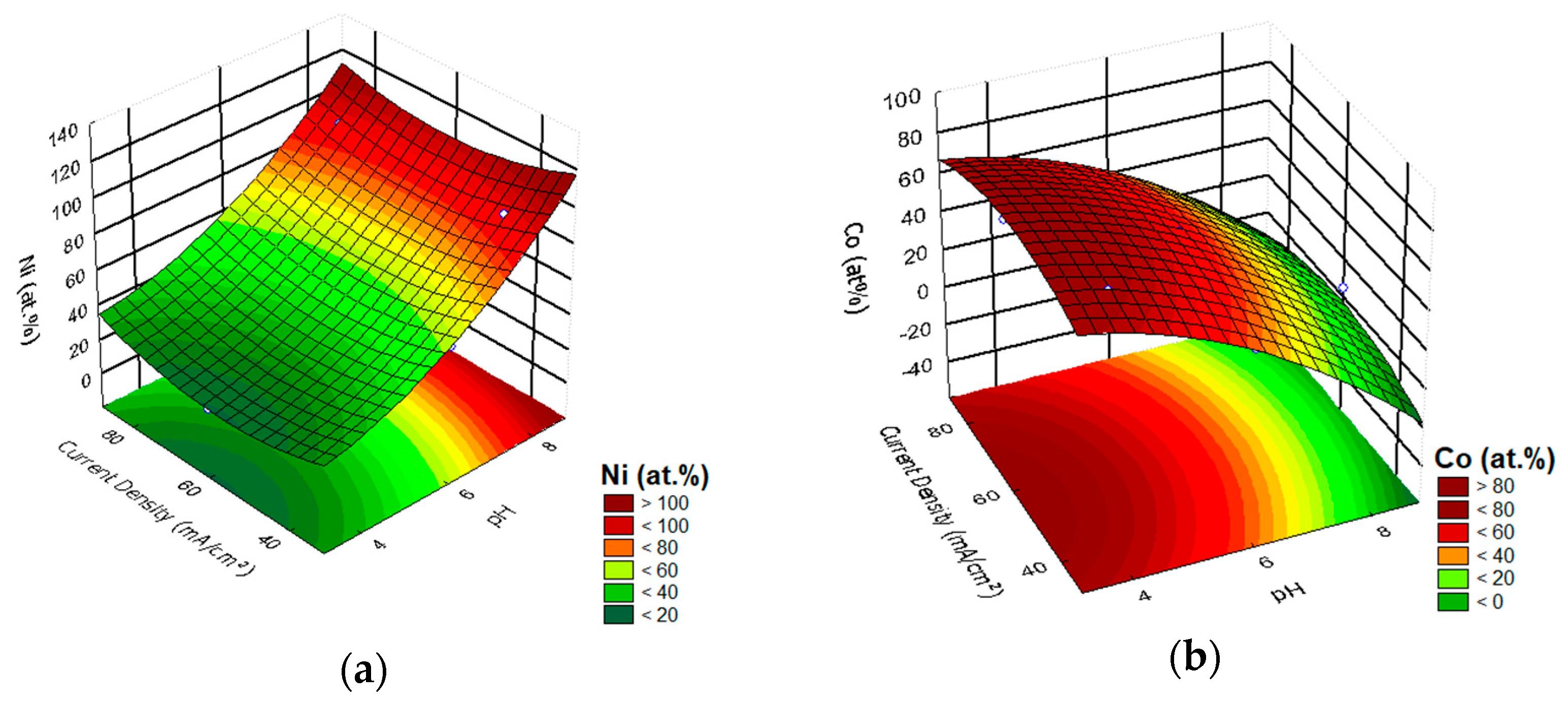
3.1. Effect of Input Variables on the Chemical Composition of the Alloy Ni–Co
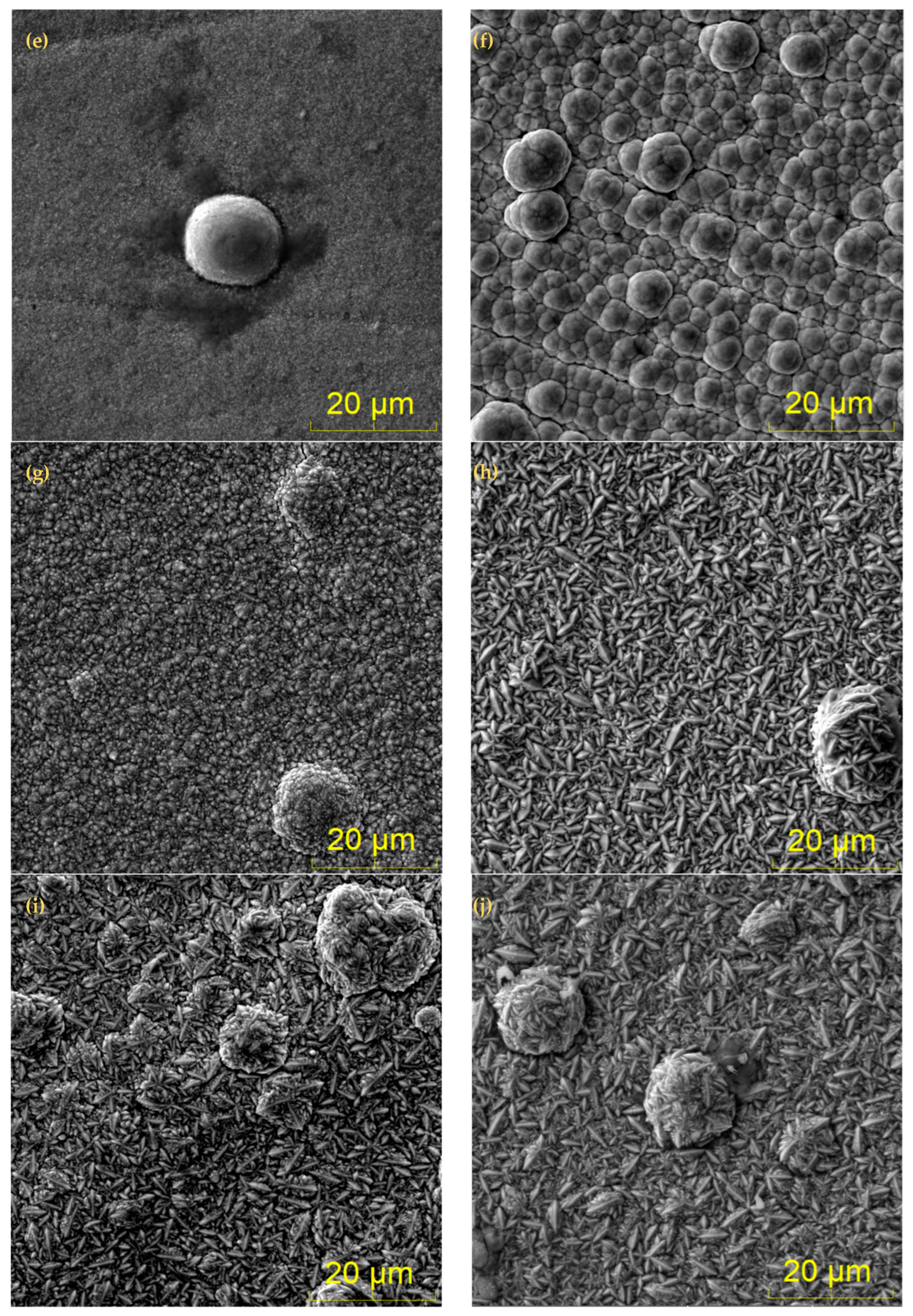
3.2. Surface Morphology
3.3. XRD Analysis
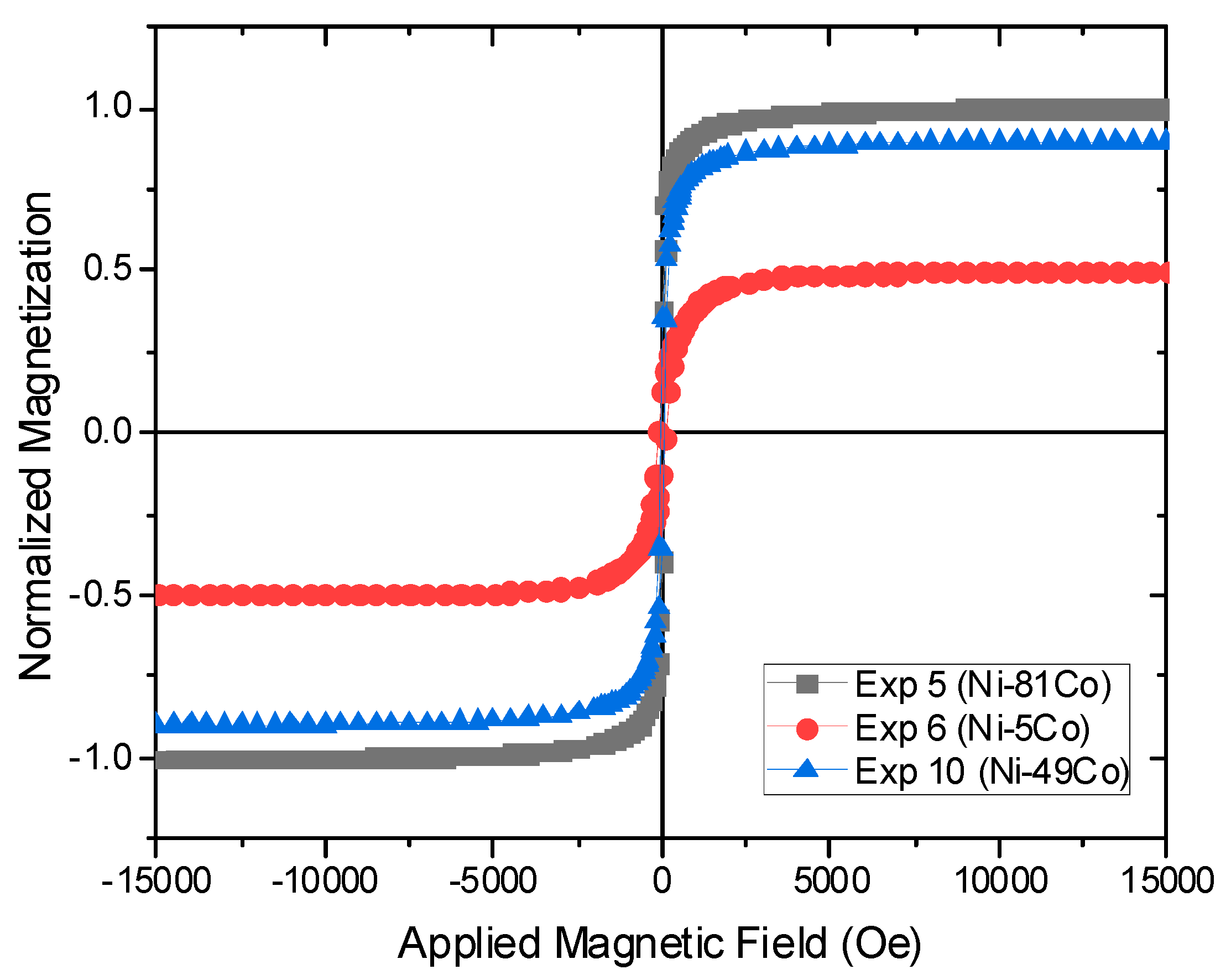
3.4. Magnetic Properties of the Alloy
3.5. Corrosion Resistance Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, X.; Qu, N. Effect of Current Density and Cobalt Concentration on the Characteristics of NiCo Coatings Prepared by Electrodesposition with a Supergravity Field. Thin Solid. Films 2019, 679, 110–119. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, H.; Feng, H.; Yang, P.; Zhang, J.; Shu, B. Electrodeposition and Corrosion Performance of Ni-Co Alloys with Different Cobalt Contents. Mater. Today Commun. 2023, 35, 106058. [Google Scholar] [CrossRef]
- Bakhit, B.; Akbari, A. Nanocrystalline Ni-Co Alloy Coatings: Electrodeposition Using Horizontal Electrodes and Corrosion Resistance. J. Coat. Technol. Res. 2013, 10, 285–295. [Google Scholar] [CrossRef]
- Omar, I.M.; Emran, K.M.; Aziz, M.; Bahru, J. Electrodeposition of Ni-Co Film: A Review. Int. J. Electrochem. Sci. 2021, 16, 150962. [Google Scholar] [CrossRef]
- Karimzadeh, A.; Aliofkhazraei, M.; Walsh, F.C. A Review of Electrodeposited Ni-Co Alloy and Composite Coatings: Microstructure, Properties and Applications. Surf. Coat. Technol. 2019, 372, 463–498. [Google Scholar] [CrossRef]
- Yang, X.; Lu, X.; Zhang, W.; Guo, X.; Ren, J.; Xue, H.; Tang, F. Preparation and Application of Nano-Ni–Co Alloy. J. Nanoparticle Res. 2023, 25, 152. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, C.-Y.; Kurioka, T.; Luo, X.; Yamane, D.; Sone, M.; Chang, T.-F.M. Effects of Bromide Ions in Anomalous Codeposition of Ni-Co Alloys with a Sulfamate Based Electrolyte. J. Electrochem. Soc. 2023, 170, 072507. [Google Scholar] [CrossRef]
- Shetty, A.R.; Hegde, A.C. Effect of Magnetic Field on Corrosion Performance of Ni–Co Alloy Coatings. J. Bio Tribocorros 2023, 9, 16. [Google Scholar] [CrossRef]
- Raveendran, M.N.; Hegde, A.C. Anomalous Codeposition of NiCo Alloy Coatings and Their Corrosion Behaviour. Mater. Today Proc. 2022, 62, 5047–5052. [Google Scholar] [CrossRef]
- Boulegane, A.; Guittoum, A.; Laggoun, A.; Boudissa, M.; Hemmous, M. Structural, Morphological, and Magnetic Properties of Electrodeposited CoNi Thin Films on the FTO Substrate. J. Supercond. Nov. Magn. 2022, 35, 2583–2593. [Google Scholar] [CrossRef]
- Zamani, M.; Amadeh, A.; Lari Baghal, S.M. Effect of Co Content on Electrodeposition Mechanism and Mechanical Properties of Electrodeposited Ni-Co Alloy. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2016, 26, 484–491. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, H.; Wang, D.; Ge, H. Effects of Saccharin and Cobalt Concentration in Electrolytic Solution on Microhardness of Nanocrystalline Ni–Co Alloys. Surf. Coat. Technol. 2008, 202, 4952–4956. [Google Scholar] [CrossRef]
- Lupi, C.; Dell’Era, A.; Pasquali, M.; Imperatori, P. Composition, Morphology, Structural Aspects and Electrochemical Properties of Ni–Co Alloy Coatings. Surf. Coat. Technol. 2011, 205, 5394–5399. [Google Scholar] [CrossRef]
- Sarac, U.; Baykul, M.C.; Uguz, Y. The Influence of Applied Current Density on Microstructural, Magnetic, and Morphological Properties of Electrodeposited Nanocrystalline Ni–Co Thin Films. J. Supercond. Nov. Magn. 2015, 28, 1041–1045. [Google Scholar] [CrossRef]
- Tian, L.; Xu, J.; Xiao, S. The Influence of PH and Bath Composition on the Properties of Ni–Co Coatings Synthesized by Electrodeposition. Vacuum 2011, 86, 27–33. [Google Scholar] [CrossRef]
- Oriňáková, R.; Oriňák, A.; Vering, G.; Talian, I.; Smith, R.M.; Arlinghaus, H.F. Influence of PH on the Electrolytic Deposition of Ni–Co Films. Thin Solid. Films 2008, 516, 3045–3050. [Google Scholar] [CrossRef]
- Karpuz, A.; Kockar, H.; Alper, M. Electrodeposited Co-Ni Films: Electrolyte PH—Property Relationships. J. Supercond. Nov. Magn. 2013, 26, 651–655. [Google Scholar] [CrossRef]
- Gómez, E.; Pané, S.; Vallés, E. Electrodeposition of Co–Ni and Co–Ni–Cu Systems in Sulphate–Citrate Medium. Electrochim. Acta 2005, 51, 146–153. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Mu, Y.; Yang, L.; Yang, J.; Feng, S.; Shi, M.; Yang, W.; Fu, W.; Yang, H. Sodium Citrate Complexing Agent-Dependent Growth of n- and p-Type CdTe Thin Films for Applications in CdTe/CdS Based Photovoltaic Devices. J. Alloys Compd. 2018, 748, 515–521. [Google Scholar] [CrossRef]
- Filgueira de Almeida, A.; Venceslau de Souto, J.I.; Lima dos Santos, M.; Costa de Santana, R.A.; Alves, J.J.N.; Nascimento Campos, A.R.; Prasad, S. Establishing Relationships between Bath Composition and the Properties of Amorphous Ni–Mo Alloys Obtained by Electrodeposition. J. Alloys Compd. 2021, 888, 161595. [Google Scholar] [CrossRef]
- da Silva, C.R.P.; Costa, J.D.; de Almeida, A.F.; de Santana, R.A.C.; Campos, A.R.N.; Alves, J.J.N.; de Abreu Santos, T.F. Chemical Composition Variation of the Ni–W Alloy as a Function of Parameters Used in the Electrodeposition Process. J. Appl. Electrochem. 2023, 54, 611–623. [Google Scholar] [CrossRef]
- Costa, J.D.; Sousa, M.B.; Almeida, A.F.; Oliveira, J.A.M.; Silva, P.C.S.; Alves, J.J.N.; Campos, A.R.N.; Araújo, C.J.; Santana, R.A.C.; Delgado, J.M.P.Q.; et al. Thermal, Mechanical, and Electrochemical Characterization of Ti50Ni50−XMox Alloys Obtained by Plasma Arc Melting. Metals 2023, 13, 1637. [Google Scholar] [CrossRef]
- Boukhouiete, A.; Boumendjel, S.; Sobhi, N.-E.-H. Effect of Current Density on the Microstructure and Morphology of the Electrodeposited Nickel Coatings. Turk. J. Chem. 2021, 45, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Khormali, A. Optimization of the Corrosion Inhibition Performance of 2-Mercaptobenzothiazole for Carbon Steel in HCl Media Using Response Surface Methodology. Fuel 2024, 357, 129783. [Google Scholar] [CrossRef]
- Golodnitsky, D.; Rosenberg, Y.; Ulus, A. The Role of Anion Additives in the Electrodeposition of Nickel–Cobalt Alloys from Sulfamate Electrolyte. Electrochim. Acta 2002, 47, 2707–2714. [Google Scholar] [CrossRef]
- Bai, A.; Hu, C.C. Effects of Electroplating Variables on the Composition and Morphology of Nickel–Cobalt Deposits Plated through Means of Cyclic Voltammetry. Electrochim. Acta 2002, 47, 3447–3456. [Google Scholar] [CrossRef]
- Oliveira, J.A.M.; de Almeida, A.F.; Campos, A.R.N.; Prasad, S.; Alves, J.J.N.; de Santana, R.A.C. Effect of Current Density, Temperature and Bath PH on Properties of Ni–W–Co Alloys Obtained by Electrodeposition. J. Alloys Compd. 2021, 853, 157104. [Google Scholar] [CrossRef]
- Deepatana, A.; Valix, M. Adsorption of Metals from Metal-Organic Complexes Derived from Bioleaching of Nickel Laterite Ores. In Separations Technology VI: New Perspectives on Very Large-Scale Operations; Fell, C., University of New South Wales, Keller, G.E., II, MATRIC, Eds.; ECI Symposium Series; 2004; Available online: https://dc.engconfintl.org/separations_technology_vi/4 (accessed on 30 December 2024).
- To, D.T.; Park, S.H.; Kim, M.J.; Cho, H.S.; Myung, N.V. Effects of NH4+/Citrate Complexing Agent Ratio on Ni–Mo and Ni–Mo–O Electrodeposits from Ammonium Citrate Baths. Front. Chem. 2022, 10, 942423. [Google Scholar] [CrossRef]
- Wyrzykowski, D.; Chmurzyński, L. Thermodynamics of Citrate Complexation with Mn2+, Co2+, Ni2+ and Zn2+ Ions. J. Therm. Anal. Calorim. 2010, 102, 61–64. [Google Scholar] [CrossRef]
- Bigos, A.; Wolowicz, M.; Janusz-Skuza, M.; Starowicz, Z.; Szczerba, M.J.; Bogucki, R.; Beltowska-Lehman, E. Citrate-Based Baths for Electrodeposition of Nanocrystalline Nickel Coatings with Enhanced Hardness. J. Alloys Compd. 2021, 850, 156857. [Google Scholar] [CrossRef]
- Xinmei, L.; Shuai, S.; Tianxiang, X.; Wen, L.; Yanjiang, B.; Dongting, W. Influence of Heat Treatment on Corrosion Resistance of NiTi Shape Memory Alloys in NaCl Solution. Int. J. Electrochem. Sci. 2025, 100949. [Google Scholar] [CrossRef]
- Ghaferi, Z.; Sharafi, S.; Bahrololoom, M.E. The Role of Electrolyte PH on Phase Evolution and Magnetic Properties of CoFeW Codeposited Films. Appl. Surf. Sci. 2016, 375, 35–41. [Google Scholar] [CrossRef]
- Radadi, R.M.A.; Ibrahim, M.A.M. Nickel-Cobalt Alloy Coatings Prepared by Electrodeposition Part II: Morphology, Structure, Microhardness, and Electrochemical Studies. Korean J. Chem. Eng. 2021, 38, 152–162. [Google Scholar] [CrossRef]
- Kamel, M.M.; Alzahrani, E.; Ibrahim, I.S.; Rashwan, S.M. Electrodeposition of Well-Crystalline Ni-Co Alloy Thin Films on Steel Substrates from Aqueous Solutions Containing Citrate Anions. Int. J. Electrochem. Sci. 2021, 16, 210942. [Google Scholar] [CrossRef]
- Lupi, C.; Dell’Era, A.; Pasquali, M. Effectiveness of Sodium Citrate on Electrodeposition Process of NiCoW Alloys for Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2017, 42, 28766–28776. [Google Scholar] [CrossRef]
- Hajizadeh, K.; Chianeh, V.A. Influence of Mechanical Attrition on Protective Properties of Anomalous Electrodeposited Ni-Co Alloys. Trans. Indian Inst. Met. 2023, 1, 1–8. [Google Scholar] [CrossRef]
- Sharma, A.; Chhangani, S.; Madhavan, R.; Suwas, S. Correlation between Crystallographic Texture, Microstructure and Magnetic Properties of Pulse Electrodeposited Nanocrystalline Nickel–Cobalt Alloys. J. Magn. Magn. Mater. 2017, 434, 68–77. [Google Scholar] [CrossRef]
- Ling, W.; Wang, H. Study on Electrochemical Properties of Cobalt-Nickel Alloy Prepared by Pulsed Electrodeposition. Int. J. Electrochem. Sci. 2023, 18, 100053. [Google Scholar] [CrossRef]
- Callister, W.D., Jr.; Rethwisch, D.G. Ciência e Engenharia de Materiais-Uma Introdução; LTC Ltda, Ed.; 10a Edição: Rio de Janeiro, Brazil, 2021; Volume 1, ISBN 978-85-216-3728-8. [Google Scholar]
- Karpuz, A.; Kockar, H.; Alper, M.; Karaagac, O.; Haciismailoglu, M. Electrodeposited Ni–Co Films from Electrolytes with Different Co Contents. Appl. Surf. Sci. 2012, 258, 4005–4010. [Google Scholar] [CrossRef]
- Özdemir, R.; Korkmaz, C.A. Investigation of Structural and Magnetic Properties of Co, Ni and CoNi Alloy Thin Films by Fabricated with Electrodeposition. El-Cezeri 2022, 9, 1122–1135. [Google Scholar] [CrossRef]
- dos Santos, É.S.; Costa, J.D.; de Almeida, A.F.; de Sousa, M.B.; Oliveira, J.A.M.; da Costa Neto, H.; dos Santos, A.X.; de Santana, R.A.C.; Campos, A.R.N. Magnetic Properties of Nickel-Cobalt Alloys: A Systematic Review of Alloys Obtained by Electrodeposition. Rev. Virtual De Química 2024, 16, 612–619. [Google Scholar] [CrossRef]
- Ergeneman, O.; Sivaraman, K.M.; Pané, S.; Pellicer, E.; Teleki, A.; Hirt, A.M.; Baró, M.D.; Nelson, B.J. Morphology, Structure and Magnetic Properties of Cobalt–Nickel Films Obtained from Acidic Electrolytes Containing Glycine. Electrochim. Acta 2011, 56, 1399–1408. [Google Scholar] [CrossRef]
- Bakhit, B. The Influence of Electrolyte Composition on the Properties of Ni–Co Alloy Coatings Reinforced by SiC Nano-Particles. Surf. Coat. Technol. 2015, 275, 324–331. [Google Scholar] [CrossRef]
- You, Y.H.; Gu, C.D.; Wang, X.L.; Tu, J.P. Electrodeposition of Ni–Co Alloys from a Deep Eutectic Solvent. Surf. Coat. Technol. 2012, 206, 3632–3638. [Google Scholar] [CrossRef]
- Liu, B.; Yan, S.; He, Y.; He, T.; He, Y.; Song, R.; Zhang, Z.; Li, H.; Song, J.; Li, Z. Study on Wear Resistance and Corrosion Resistance of Zirconium Phenylphosphonate Reinforced Ni–W Composite Coating. Appl. Surf. Sci. 2022, 603, 154483. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; Liu, B.; Fan, Y.; Lei, C.; Zhou, H.; Zhong, J.; Yan, L.; Cheng, X.; Song, J.; et al. Study on Mechanical and Corrosion Resistance of Ni-W-P Composite Coating Reinforced by Cationic Cubic Boron Nitride. Surf. Coat. Technol. 2023, 468, 129754. [Google Scholar] [CrossRef]




| Factors | Levels | ||||
|---|---|---|---|---|---|
| −1.414 | −1 | 0 | +1 | +1.414 | |
| pH | 3.17 | 4 | 6 | 8 | 8.83 |
| Current density (mA/cm2) | 31.72 | 40 | 60 | 80 | 88.28 |
| Exp. | pH | I (mA/cm2) | Ni (at.%) | Co (at.%) | CCE (%) | Thickness (µm) |
|---|---|---|---|---|---|---|
| 1 | 4 (−1) | 40 (−1) | 36 | 64 | 66 | 16 |
| 2 | 4 (−1) | 80 (+1) | 41 | 59 | 45 | 15 |
| 3 | 8 (+1) | 40 (−1) | 93 | 7 | 65 | 17 |
| 4 | 8 (+1) | 80 (+1) | 89 | 11 | 58 | 15 |
| 5 | 3.17 (−1.414) | 60 (0) | 19 | 81 | 52 | 11 |
| 6 | 8.83 (+1.414) | 60 (0) | 95 | 5 | 66 | 18 |
| 7 | 6 (0) | 31.72 (−1.414) | 57 | 43 | 71 | 18 |
| 8 | 6 (0) | 88.28 (+1.414) | 56 | 44 | 86 | 14 |
| 9 | 6 (0) | 60 (0) | 51 | 49 | 65 | 16 |
| 10 | 6 (0) | 60 (0) | 51 | 49 | 65 | 16 |
| Factors | SS | df | MS | F-Value | p-Value |
|---|---|---|---|---|---|
| pH (L) | 5643.471 | 1 | 5643.471 | 174.4325 | 0.000190 |
| pH (S) | 114.346 | 1 | 114.346 | 3.5343 | 0.133286 |
| Current density (L) | 0.021 | 1 | 0.021 | 0.0007 | 0.980701 |
| Current density (Q) | 103.199 | 1 | 103.199 | 3.1897 | 0.148644 |
| Interaction | 20.250 | 1 | 20.250 | 0.6259 | 0.473130 |
| Error | 129.413 | 4 | 32.353 | ||
| Total sum of squares | 5945.600 | 9 |
| Exp. | pH | i (mA/cm2) | ECorr (V) | icorr (µA/cm2) | ba (mV/dec) | bc (mV/dec) |
|---|---|---|---|---|---|---|
| 5 | 3.17 (−1.414) | 60 (0) | −0.405 | 2.58 | 169 | 267 |
| 6 | 8.83 (+1.414) | 60 (0) | −0.338 | 0.77 | 100 | 63 |
| 10 | 6 (0) | 60 (0) | −0.418 | 3.89 | 334 | 247 |
| Exp. | Rs (Ω.cm2) | CPE1 (μMho.sN) | n1 | CPE2 (μMho.sN) | n2 | Rp (R1+R2) (kΩ.cm2) | X2 |
|---|---|---|---|---|---|---|---|
| 5 | 47.8 | 24.0 | 0.86 | 4.17 | 0.94 | 22.05 | 0.02 |
| 6 | 44.6 | 18.1 | 0.91 | 11.5 | 0.52 | 74.70 | 0.005 |
| 10 | 45.7 | 38.4 | 0.87 | 2.70 | 0.90 | 29.59 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, É.S.d.; Costa, J.D.; Filgueira de Almeida, A.; Santos, A.X.d.; Araújo, L.R.S.d.; Santana, R.A.C.d.; Campos, A.R.N. Electrochemical Fabrication of Ni–Co Alloy over a Wide pH Range Using Sodium Citrate as a Complexing Agent. Coatings 2025, 15, 138. https://doi.org/10.3390/coatings15020138
Santos ÉSd, Costa JD, Filgueira de Almeida A, Santos AXd, Araújo LRSd, Santana RACd, Campos ARN. Electrochemical Fabrication of Ni–Co Alloy over a Wide pH Range Using Sodium Citrate as a Complexing Agent. Coatings. 2025; 15(2):138. https://doi.org/10.3390/coatings15020138
Chicago/Turabian StyleSantos, Évany Silva dos, Josiane Dantas Costa, Arthur Filgueira de Almeida, Aureliano Xavier dos Santos, Lincoln Rodrigues Sampaio de Araújo, Renato Alexandre Costa de Santana, and Ana Regina Nascimento Campos. 2025. "Electrochemical Fabrication of Ni–Co Alloy over a Wide pH Range Using Sodium Citrate as a Complexing Agent" Coatings 15, no. 2: 138. https://doi.org/10.3390/coatings15020138
APA StyleSantos, É. S. d., Costa, J. D., Filgueira de Almeida, A., Santos, A. X. d., Araújo, L. R. S. d., Santana, R. A. C. d., & Campos, A. R. N. (2025). Electrochemical Fabrication of Ni–Co Alloy over a Wide pH Range Using Sodium Citrate as a Complexing Agent. Coatings, 15(2), 138. https://doi.org/10.3390/coatings15020138






