Novel Omniphobic Teflon/PAI Composite Membrane Prepared by Vacuum-Assisted Dip-Coating Strategy for Dissolved Gases Separation from Transformer Oil
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials and Chemicals
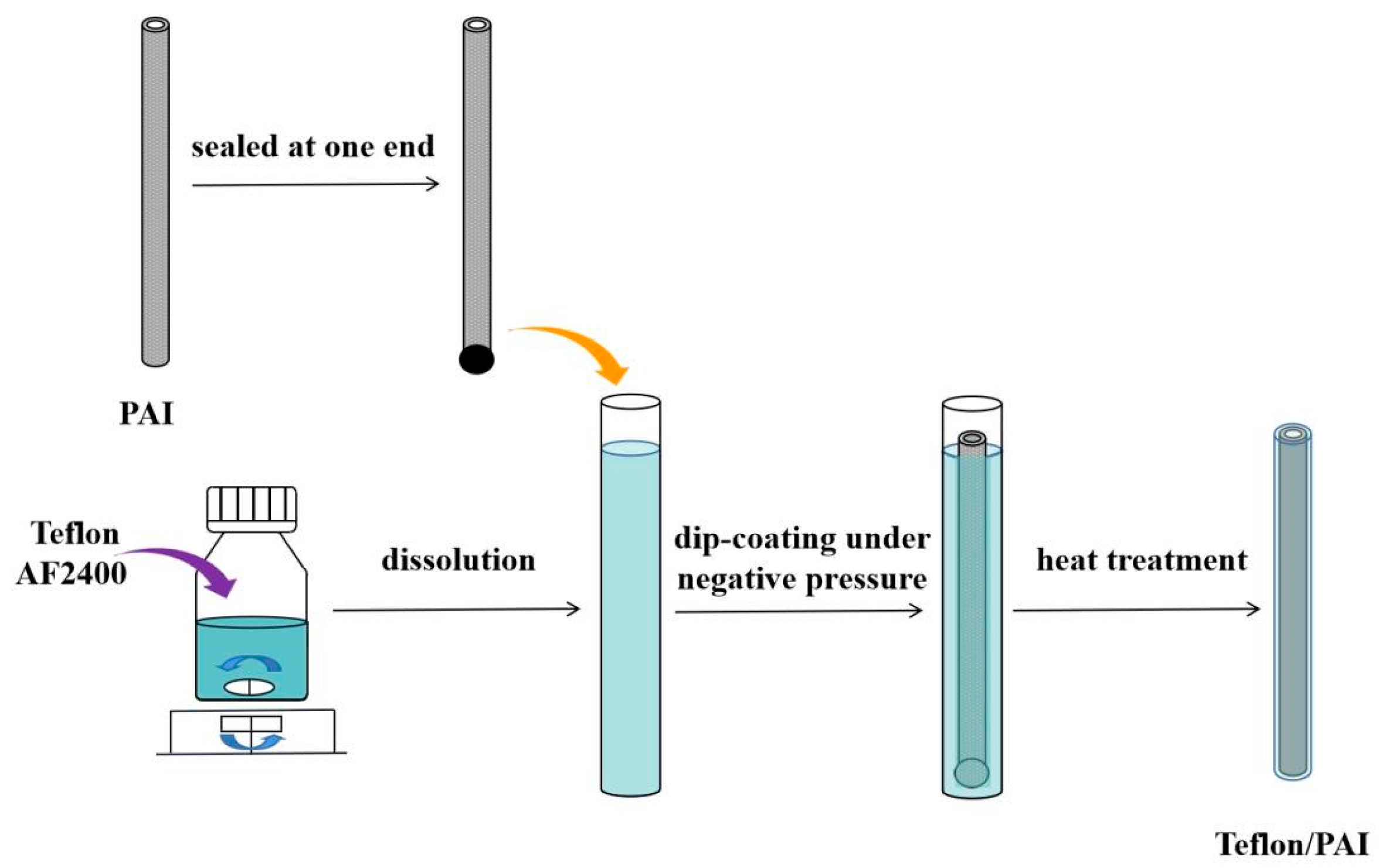
2.2. Fabrication of Teflon/PAI Composite Membrane
- (1)
- PAI hollow fiber membrane prepared by the dry-wet phase inversion method.
- (2)
- Teflon/PAI composite membrane prepared by vacuum-assisted dip-coating method.
2.3. Membrane Characterization and Test
- (1)
- Microscopic characterization of membrane morphology.
- (2)
- Membrane composition analysis.
- (3)
- Membrane wetting behavior analysis.
- (4)
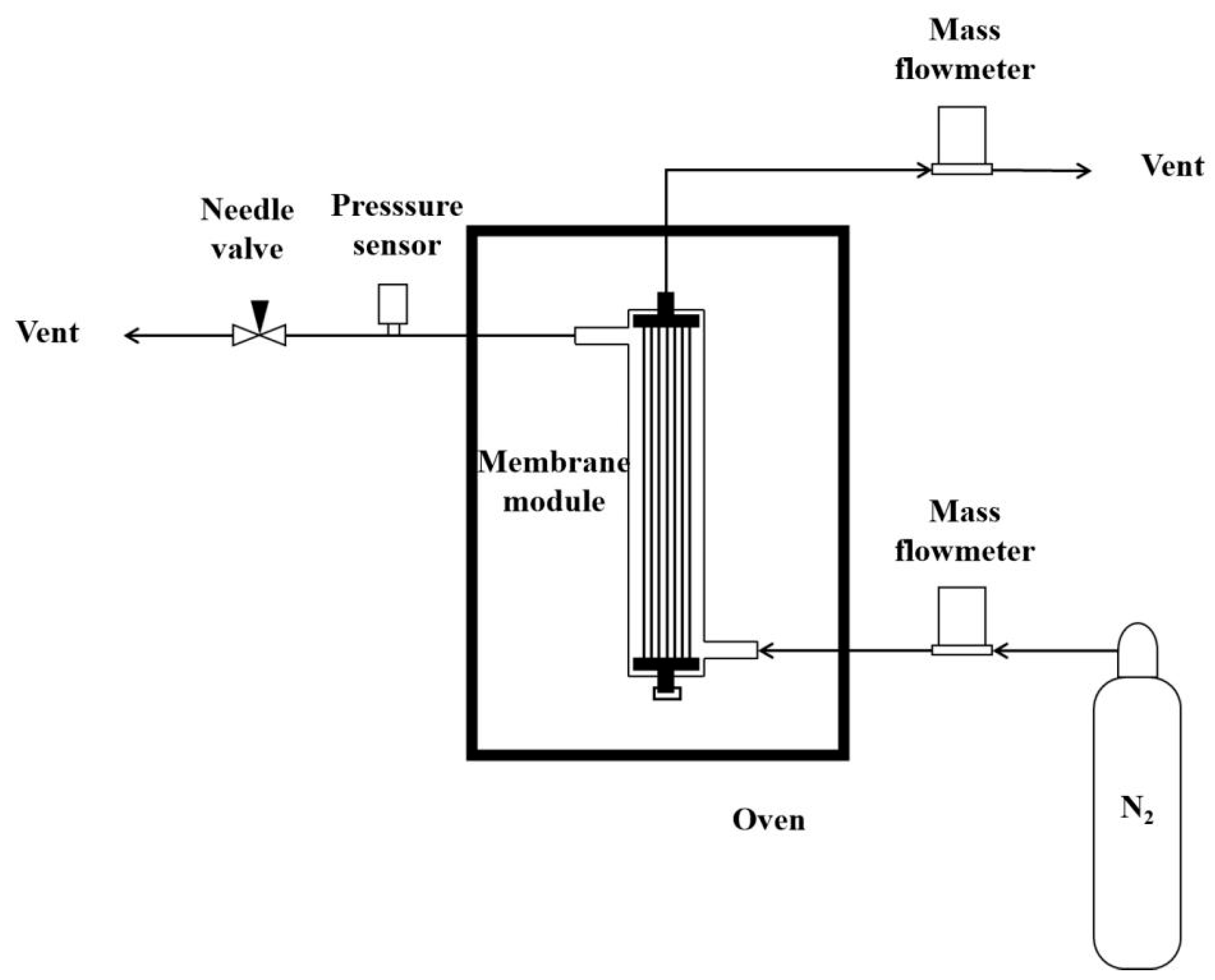
- Membrane permeability test.
- (5)
- Membrane mechanical performance test.
- (6)
- Membrane stability test.
- (7)
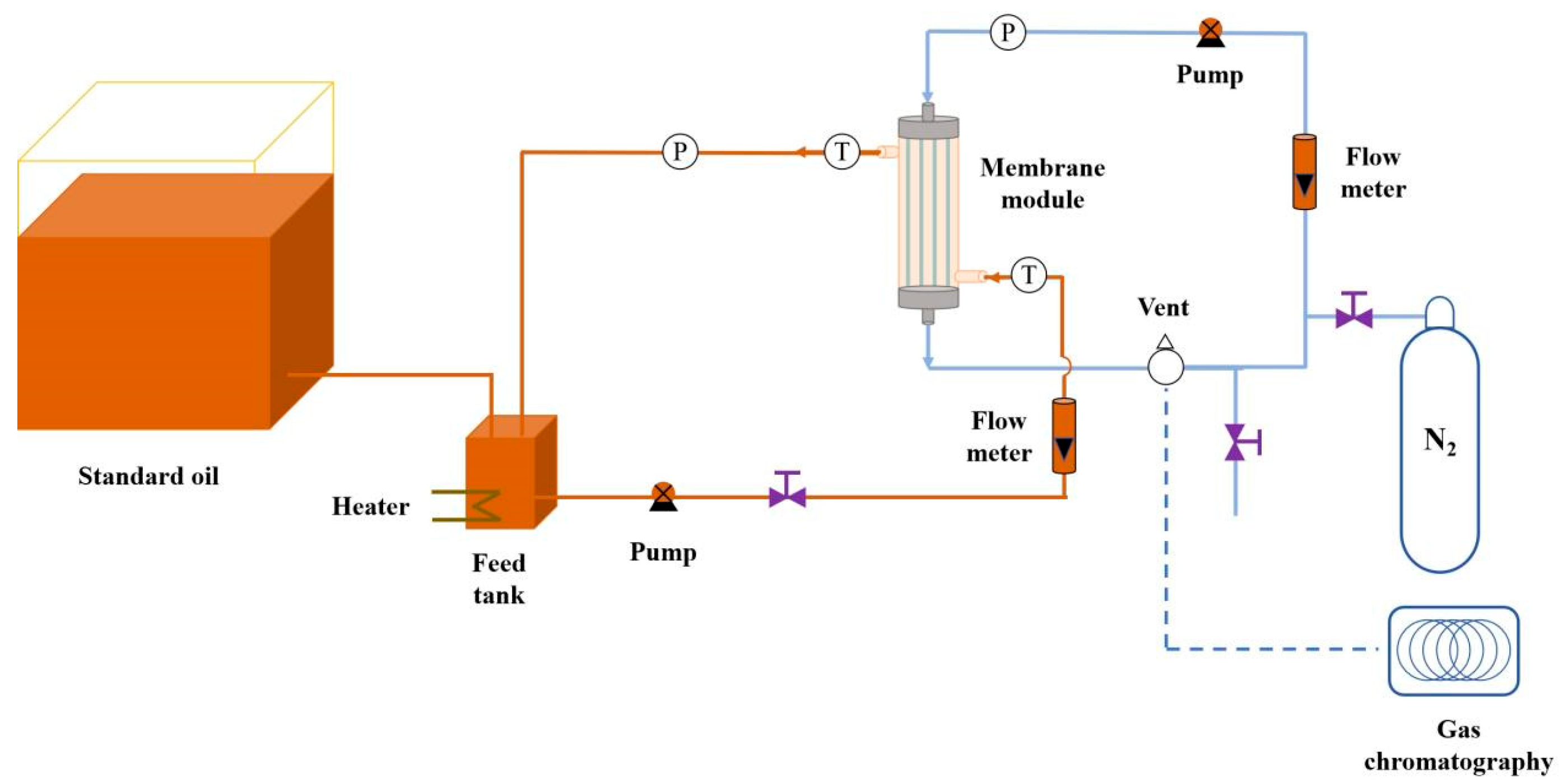
- Membrane oil-gas separation performance test.
3. Results and Discussion
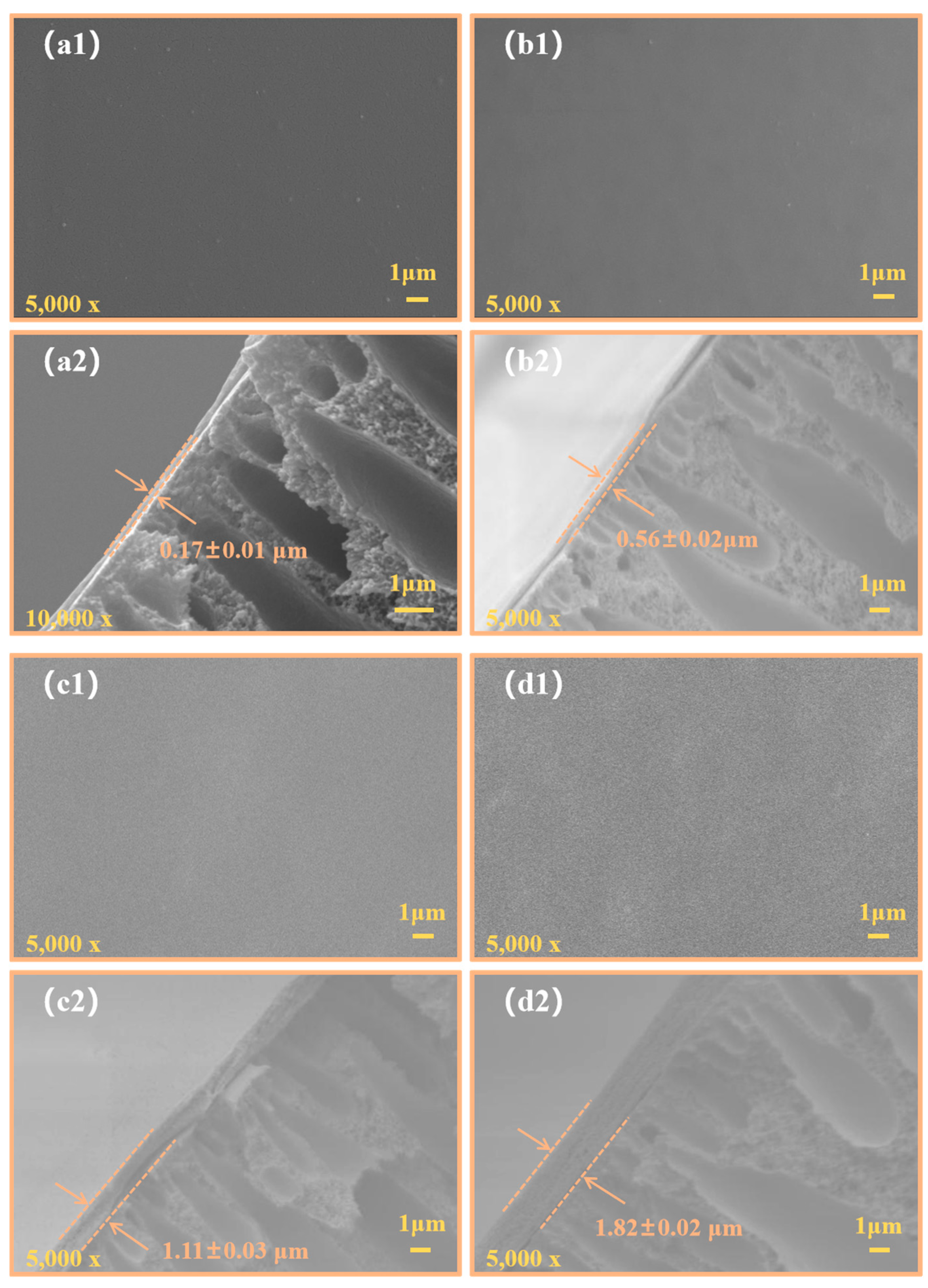
3.1. Membrane Morphology Structure Analysis
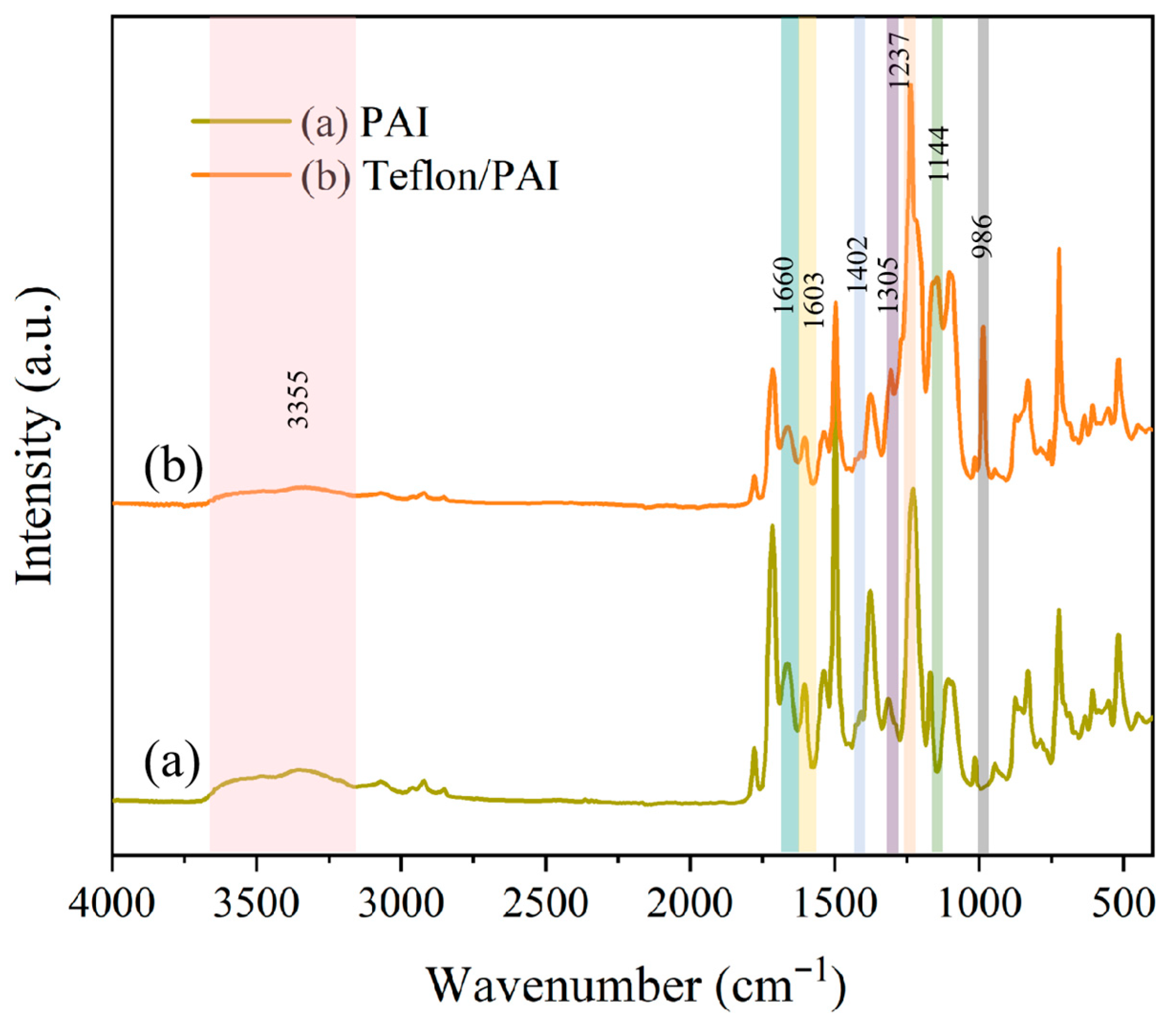
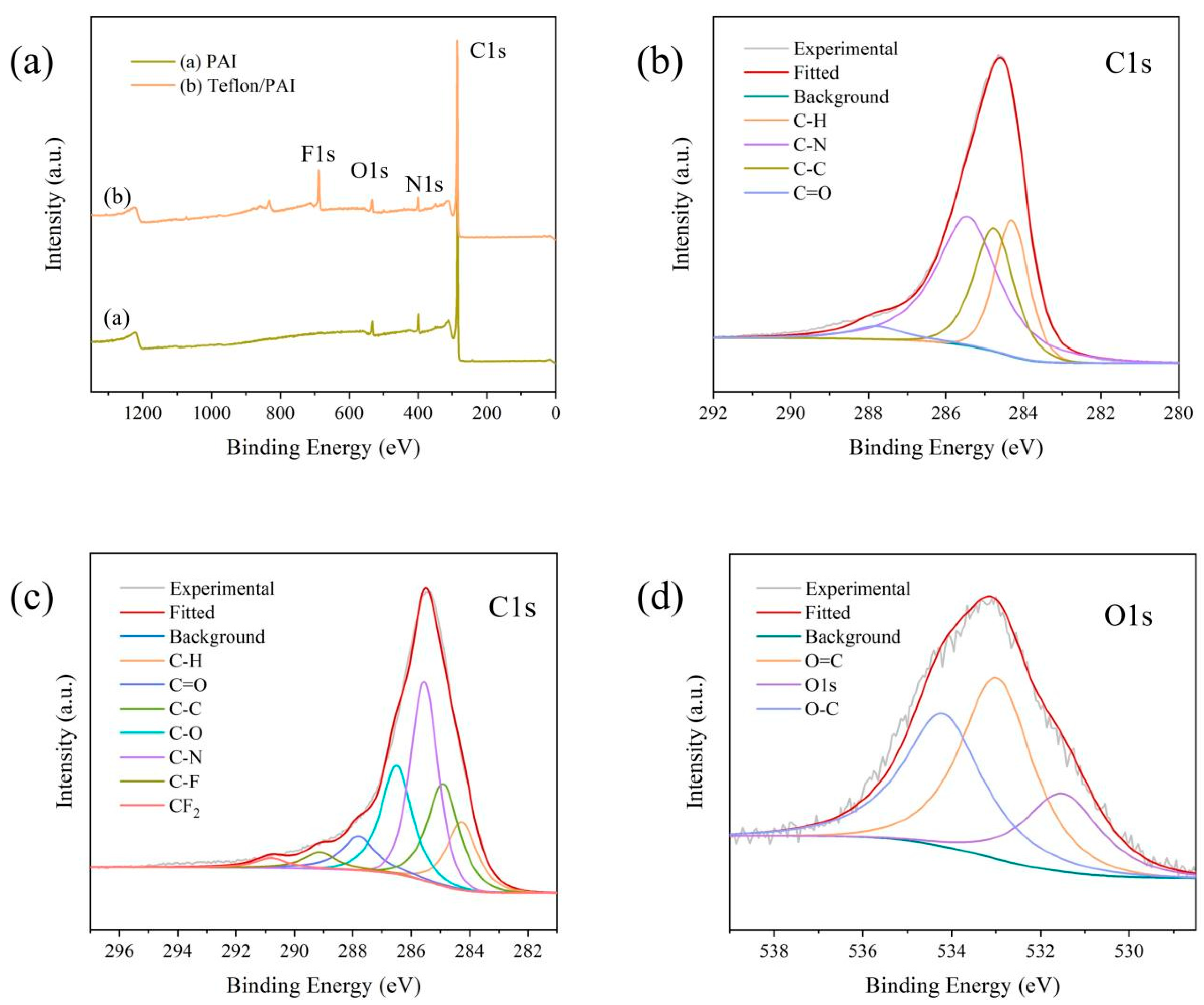
3.2. Membrane Chemical Composition Analysis
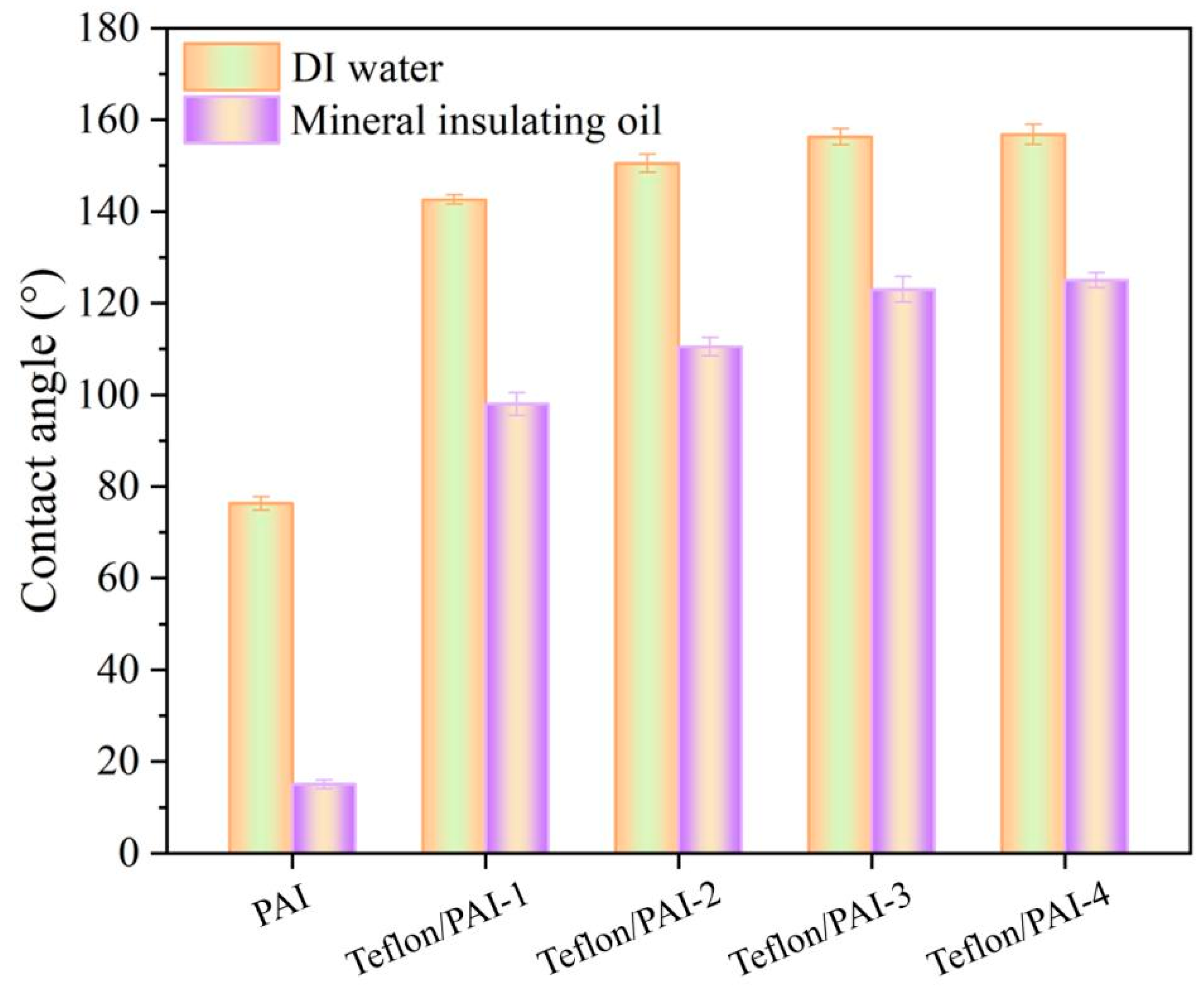
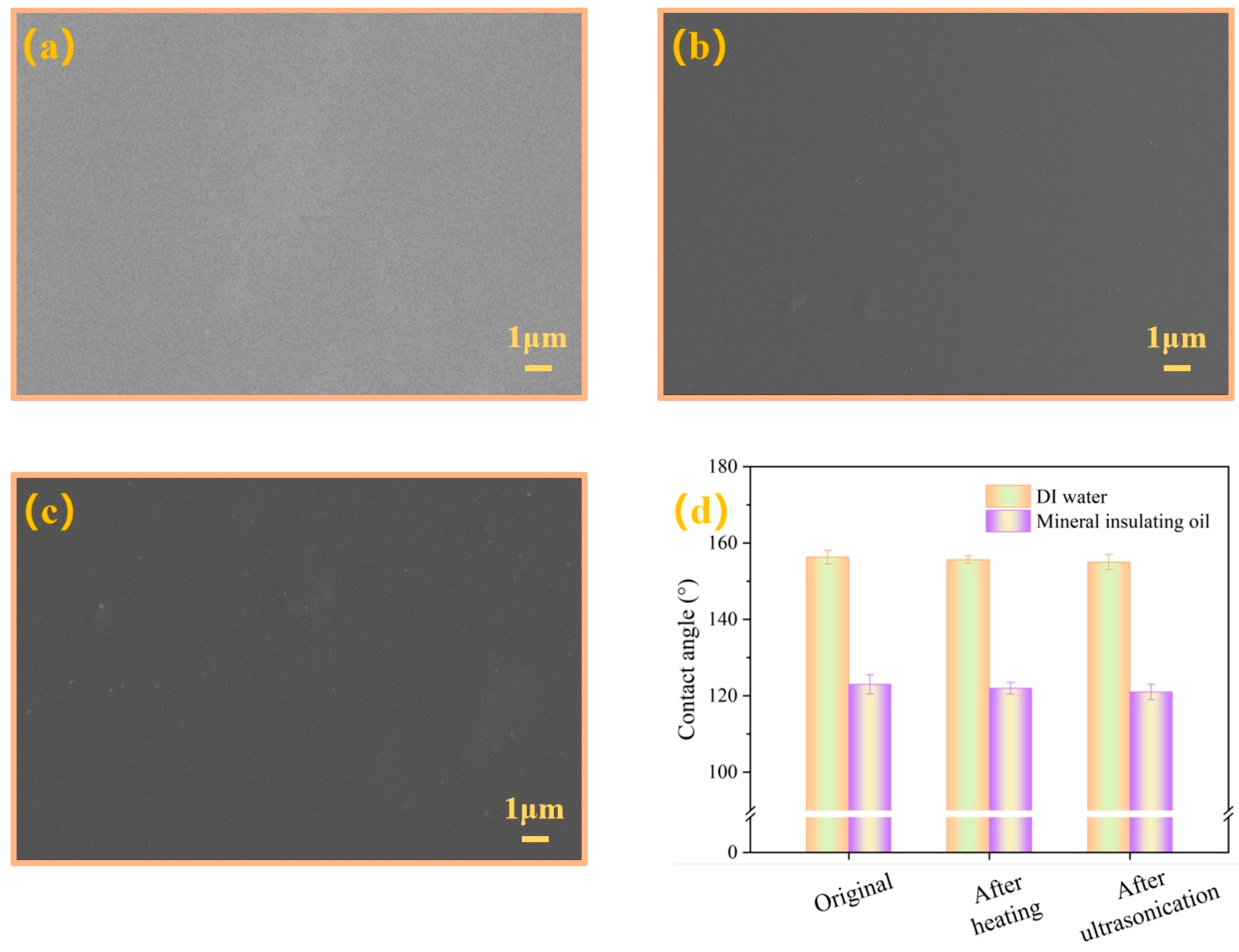
3.3. Membrane Wettability
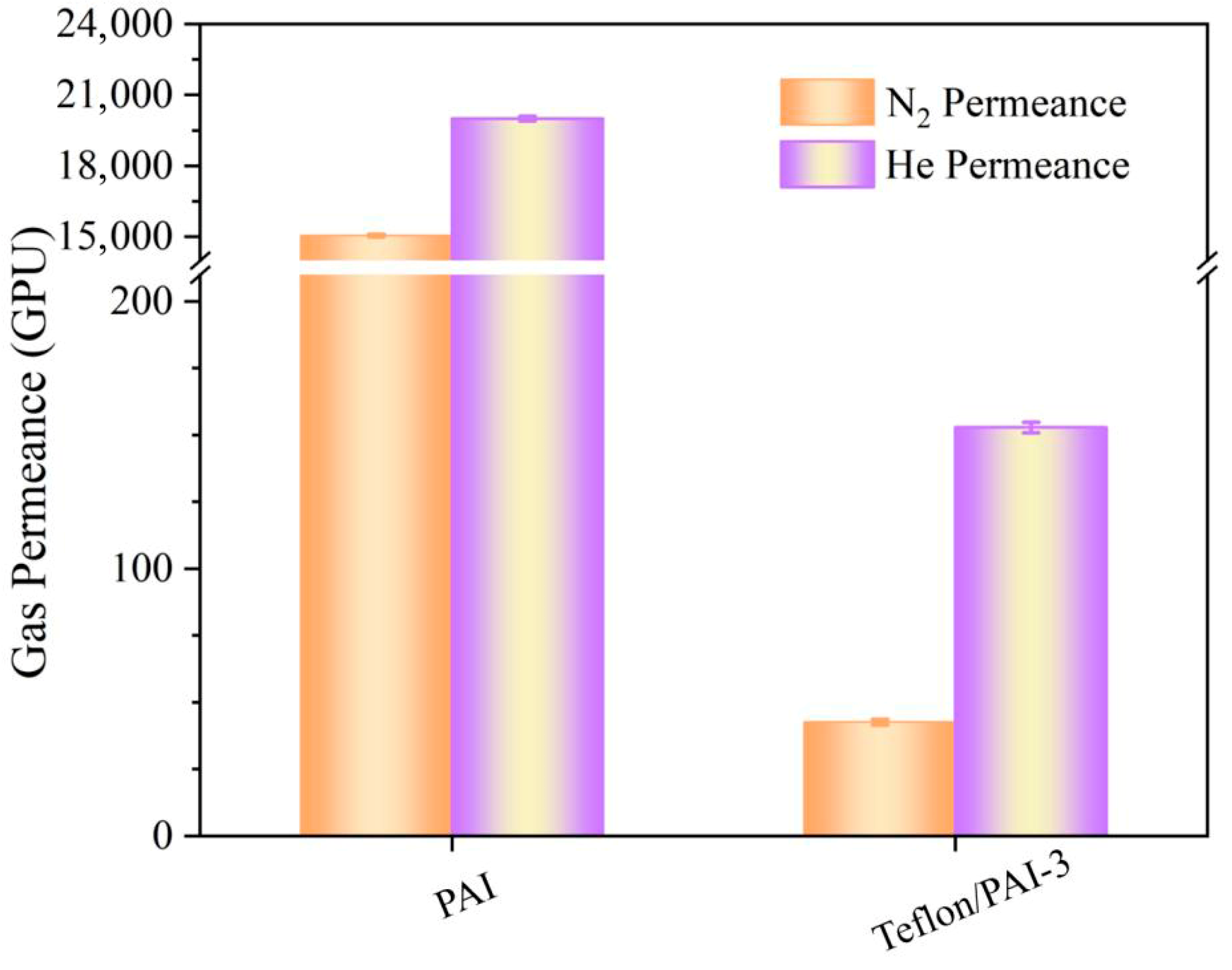
3.4. Membrane Permeability
3.5. Membrane Mechanical Performance and Stability
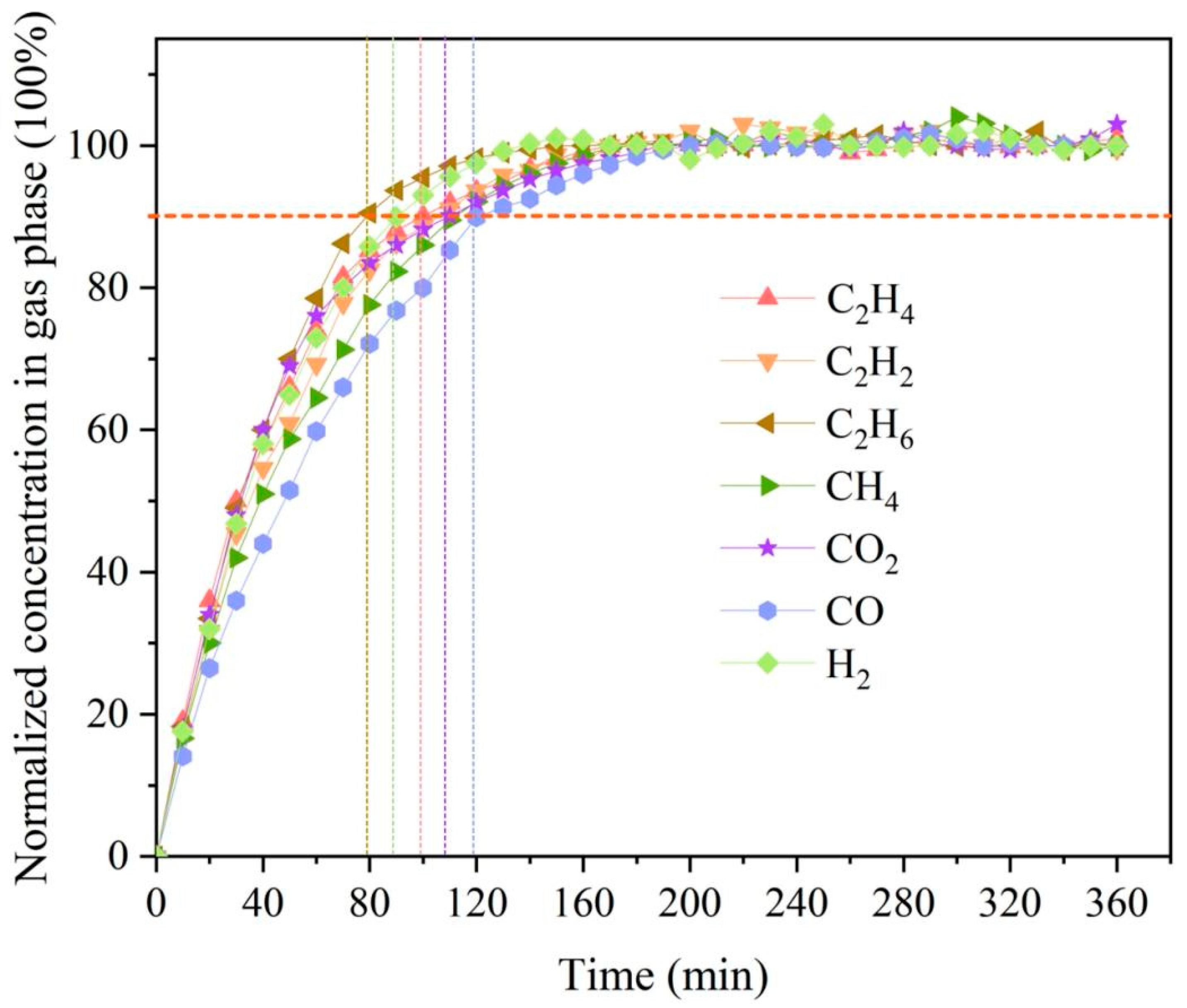
3.6. Membrane Separation Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, X.; Huang, S.; Zhang, X.; Zhu, Y.; An, G.; Du, Z. A transformer condition recognition method based on dissolved gas analysis features selection and multiple models fusion. Eng. Appl. Artif. Intell. 2023, 123, 106518–106526. [Google Scholar] [CrossRef]
- Chatterjee, S.; Haque, N.; Pradhan, A.K.; Dalai, S.; Chatter-jee, B. Estimation of conductivity at reduced time for sensing moisture content of oil-paper insulation. IEEE Sens. J. 2020, 21, 12999–13006. [Google Scholar] [CrossRef]
- Wang, H.; Qu, G.; Li, S. Role of metal passivator on production of hydrogen gas in insulating oil and oil-impregnated insulation paper. Energy Rep. 2024, 11, 1544–1550. [Google Scholar] [CrossRef]
- Li, Z.; Chen, W.; Yan, X.; Zhou, Q.; Wang, H. An outlier robust detection method for online monitoring data of dissolved gases in transformer oils. Flow. Meas. Instrum. 2025, 102, 102793–102800. [Google Scholar] [CrossRef]
- Soni, R.; Mehta, B. Diagnosis and prognosis of incipient faults and insulation status for asset management of power transformer using fuzzy logic controller & fuzzy clustering means. Elec. Power Syst. Res. 2023, 220, 109256.1–109256.18. [Google Scholar]
- Ahmed, R.; Liu, J.; Zhang, M.; Fan, X. Reliability and condition assessment techniques for oil-immersed power equipment under varying temperatures: A review. Energy Rep. 2025, 14, 1896–1916. [Google Scholar] [CrossRef]
- Amodu, I.O.; Raimi, M.; Ogbogu, M.N.; Benjamin, I.; Gulack, A.O.; Adeyinka, A.S.; Louis, H. Mn-doped covalent organic framework (COF), graphene, and their nanocomposite (Mn@GP/COF) as sensors for oil-dissolved gases in transformer: A computational study. Mater. Today Commun. 2024, 38, 108363–108379. [Google Scholar] [CrossRef]
- Kiran, S.R.; Mariprasath, T.; Basha, C.H.H.; Murali, M.; Reddy, M.B. Thermal degrade analysis of solid insulating materials immersed in natural ester oil and mineral oil by DGA. Mater. Today Proc. 2022, 52, 315–320. [Google Scholar] [CrossRef]
- Suwarno; Sutikno, H.; Prasojo, R.A.; Abu-Siada, A. Machine learning based multi-method interpretation to enhance dissolved gas analysis for power transformer fault diagnosis. Heliyon 2024, 10, 25975–25995. [Google Scholar] [CrossRef]
- Std C57.104-2019; Guide for the Interpretation of Gases Generated in Mineral Oil-Immersed Transformers. IEEE: New York City, NY, USA, 2019.
- Sun, C.; Ohodnicki, P.R.; Stewart, E.M. Chemical sensing strategies for real-time monitoring of transformer oil: A review. IEEE Sens. J. 2017, 18, 5786–5806. [Google Scholar] [CrossRef]
- Velasquez, R.M.A.; Velasquez, R.F.A. Enhanced fault detection in zig-zag transformers: Insights from dissolved gas analysis and transient current analysis. Results Eng. 2025, 25, 104166–104180. [Google Scholar] [CrossRef]
- Ali, M.S.; Bakar, A.H.A.; Omar, A.; Jaafar, A.S.A.; Mohamed, S.H. Conventional methods of dissolved gas analysis using oil-immersed power transformer for fault diagnosis: A review. Electr. Power Syst. Res. 2023, 216, 109064–109079. [Google Scholar] [CrossRef]
- Uddin, A.I.; Yaqoob, U.; Chung, G.S. Dissolved hydrogen gas analysis in transformer oil using Pd catalyst decorated on ZnO nanorod array. Sens. Actuators B Chem. 2016, 226, 90–95. [Google Scholar] [CrossRef]
- Fei, S.; Wang, M.; Miao, Y.; Tu, J.; Liu, C. Particle swarm optimization based support vector machine for forecasting dissolved gases content in power transformer oil. Energy Convers. Manag. 2009, 50, 1604–1609. [Google Scholar] [CrossRef]
- Park, M.S.; Lee, J.H.; Park, Y.; Yoo, R.; Park, S.; Jung, H.; Kim, W.; Lee, H.S.; Lee, W. Doping effects of ZnO quantum dots on the sensitive and selective detection of acetylene for dissolved-gas analysis applications of transformer oil. Sens. Actuators B Chem. 2019, 299, 126992–127001. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, W.; Xia, J.; Gu, W.; Zhou, Q. First-principles investigation of Pd modified HfTe2 monolayer membranes: Detection of dissolved gas in transformer oil. Colloids Surf. A Physicochem. Eng. Asp. 2025, 726, 137782–137793. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, L.; Yang, Z.; Cui, Y.; Wang, L.; Jiang, J.; Guo, L. A new testing method for the dielectric response of oil-immersed transformer. IEEE Trans. Ind. Electron. 2020, 67, 10833–10843. [Google Scholar] [CrossRef]
- de Faria, H., Jr.; Costa, J.G.S.; Olivas, J.L.M. A review of monitoring methods for predictive maintenance of electric power transformers based on dissolved gas analysis. Renew. Sustain. Energy Rev. 2015, 46, 201–209. [Google Scholar] [CrossRef]
- Sun, H.; Huang, Y.; Huang, C. A Review of Dissolved Gas Analysis in Power Transformers. Energy Procedia 2012, 14, 1220–1225. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Q.; Wang, Z.; Dai, J. A highly sensitive low-pressure TDLAS sensor for detecting dissolved CO and CO2 in transformer insulating oil. Opt. Laser Technol. 2024, 174, 110622–110630. [Google Scholar] [CrossRef]
- Chen, T.; Ma, F.; Zhao, Y.; Zhao, Y.; Wan, L.; Li, K.; Zhang, G. Portable ppb-level acetylene photoacoustic sensor for transformer on-field measurement. Optik 2021, 243, 167440–167448. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Li, T.; Zhang, C.; Yu, L.; Luo, Z.; Luo, B.; Tian, B.; Wang, H. Complementary MEMS gas sensor array and lightweight deep learning for DGA in transformer fault diagnosis. Sens. Actuators B Chem. 2025, 444, 138482–138494. [Google Scholar] [CrossRef]
- Li, C.; Chen, K.; Zhao, J.; Qi, H.; Zhao, X.; Ma, F.; Han, X.; Guo, M.; An, R. High-sensitivity dynamic analysis of dissolved gas in oil based on differential photoacoustic cell. Opt. Lasers Eng. 2023, 161, 107394–107401. [Google Scholar] [CrossRef]
- Hong, T.; Kang, J.; Wang, S.; Li, Y.; Jing, X. Transformer oil-soaking stability and gas-permeation performance of Teflon AF2400 membranes. Mater. Lett. 2025, 401, 139235–139238. [Google Scholar] [CrossRef]
- Qu, L.; Papaioannou, E.I. Development of mixed ionic and electronic conducting materials for gas separation membranes: A critical overview. Chem. Eng. J. 2024, 496, 153791–153803. [Google Scholar] [CrossRef]
- Liu, S.; Ma, L.; Chen, C.; Chen, C.; Lin, Y.S. Highly gas permeable, ultrathin Teflon AF2400/γ-alumina composite hollow fiber membranes for dissolved gas analysis. J. Membr. Sci. 2017, 540, 243–250. [Google Scholar] [CrossRef]
- Song, H.; Wang, H.; Wang, H.; Liu, Y.; Liu, S.; Chen, C. Rapid evaluation method for oil-gas separation membrane utilizing mass spectrometry. Chin. J. Anal. Chem. 2025, 53, 100515–100523. [Google Scholar] [CrossRef]
- Criscuoli, A.; Macedonio, F.; Brunetti, A.; Tocci, E.; Drioli, E. Impact of Membrane Engineering on the Process Engineering Progresses: Towards a Sustainable Development. Chem. Eng. Process. Process Intensif. 2023, 189, 109385. [Google Scholar] [CrossRef]
- Buggenhout, S.V.; Verbeke, R.; Davenport, D.M.; Vankelecom, I.F. Drying polymer membranes for preservation: A review. J. Membr. Sci. 2025, 731, 124190–124207. [Google Scholar] [CrossRef]
- Bakar, N.; Abu-Siada, A.; Islam, S. A review of dissolved gas analysis measurement and interpretation techniques. IEEE Electr. Insul. Mag. 2014, 30, 39–49. [Google Scholar] [CrossRef]
- Chen, T.; Li, K.; Liao, Z.; Xie, X.; Zhang, G. Influence of oil status on membrane-based gas-oil separation in DGA. Sensors 2022, 22, 3629. [Google Scholar] [CrossRef]
- Elmanovich, I.V.; Kondratenko, M.S.; Kolomytkin, D.O.; Gallyamov, M.O.; Khokhlov, A.R. Active layer materials coated with Teflon AF nano-films deposited from solutions in supercritical CO2 for fuel cell applications. Int. J. Hydrog. Energy 2013, 38, 10592–10601. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Weber, S.G. Morphology and free volume of nanocomposite Teflon AF 2400 films and their relationship to transport behavior. J. Membr. Sci. 2013, 443, 115–123. [Google Scholar] [CrossRef]
- Li, C.; Ma, F.; Sun, C.; Qi, H.; Han, X.; Guo, M.; Chen, K. In-situ detection of dissolved C2H2/CH4 with frequency-division-multiplexed fiber-optic photoacoustic sensor. Sens. Actuators B Chem. 2025, 435, 137651–137659. [Google Scholar] [CrossRef]
- Han, Y.; Ding, F.; Hao, C.; Lv, S.; Wang, X. The oil-gas separation characteristics of ceramic/Teflon AF2400 composite membrane. Sep. Purif. Technol. 2012, 88, 19–23. [Google Scholar] [CrossRef]
- State Grid Corporation of China. Technical Specification for Online Monitoring Devices of Dissolved Gases in Transformer Oil: Q/GDW 10536-2021; State Grid Corporation of China: Bejing, China, 2021; pp. 5–6. [Google Scholar]
- IEC 60567; Oil-Filled Electrical Equipment—Sampling of Gases and Analysis of Free and Dissolved Gases—Guidance. IEC: Geneva, Switzerland, 2011.
- IEC 60599; Mineral Oil-Filled Electrical Equipment in Service—Guidance on the Interpretation of Dissolved and Free Gases Analysis. IEC: Geneva, Switzerland, 2015.
- Mousavi, S.M.; Raveshiyan, S.; Amini, Y.; Zadhoush, A. A critical review with emphasis on the rheological behavior and properties of polymer solutions and their role in membrane formation, morphology, and performance. Adv. Colloid. Interface Sci. 2023, 319, 102986–103018. [Google Scholar] [CrossRef]
- Lin, D.; Chang, C.; Chen, T.; Cheng, L. Microporous PVDF membrane formation by immersion precipitation from water/TEP/PVDF system. Desalination 2002, 145, 25–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.; Yi, S.; Setiawana, L.; Hu, X.; Fane, A.G. Novel chemical surface modification to enhance hydrophobicity of polyamide-imide (PAI) hollow fiber membranes. J. Membr. Sci. 2011, 380, 241–250. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Z.; Huang, Y.; Liu, A.; Wang, H.; Zhou, R.; Kang, S.; Li, J.; Li, N. Thermally stable polyamide-imide (PAI) hierarchical porous separator for lithium metal batteries. Adv. Membr. 2025, 5, 100168–100177. [Google Scholar] [CrossRef]
- Helali, N.; Rastgar, M.; Ismail, M.F.; Sadrzadeh, M. Development of underwater superoleophobic polyamide-imide (PAI) microfiltration membranes for oil/water emulsion separation. Sep. Purif. Technol. 2020, 238, 116451–116464. [Google Scholar] [CrossRef]
- Li, X.; Shan, H.; Cao, M.; Li, B. Mussel-inspired modification of PTFE membranes in a miscible THF-Tris buffer mixture for oil-in-water emulsions separation. J. Membr. Sci. 2018, 555, 237–249. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, Y.; Liu, J.; Li, X.; Li, B.; Wang, S. Preparation of re-entrant and anti-fouling PVDF composite membrane with omniphobicity for membrane distillation. J. Membr. Sci. 2020, 595, 117563–117576. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, B.; Wang, Z.; Li, B. Fabrication of omniphobic PVDF composite membrane with dual-scale hierarchical structure via chemical bonding for robust membrane distillation. J. Membr. Sci. 2021, 622, 119038–119052. [Google Scholar] [CrossRef]


| Spinning Parameters | Value |
|---|---|
| PAI concentration (wt%) | 24, 27, 30 |
| NMP concentration in core liquid (wt%) | 65 |
| The casting solution flow rate (mL/min) | 2.0 |
| The core liquid flow rate (mL/min) | 1.0 |
| Air gap (cm) | 40 |
| Coagulation bath composition (anhydrous ethanol:H2O) (volume ratio) | 80:20 |
| The casting solution temperature (°C) | 25 |
| The coagulation bath temperature (°C) | 25 |
| Spinneret jet (mm) | 1.0 (inner diameter), 1.8 (outer diameter) |
| Immersion Solution Compositions and Dip-Coating Conditions | Value |
|---|---|
| Teflon AF2400 concentration (wt%) | 0.5, 1.0, 1.5, 2.0 |
| Operating pressure (MPa) | −0.05 |
| The immersion solution temperature (°C) | 25 |
| The dip-coating time (s) | 30 |
| Membrane | C1s | N1s | O1s | F1s |
|---|---|---|---|---|
| PAI | 90.76% | 5.95% | 3.29% | --- |
| Teflon/PAI | 82.8% | 4.43% | 3.7% | 9.06% |
| Membrane | Tensile Stress at Break/MPa | Elongation at Break/% | Young’s Modulus/MPa |
|---|---|---|---|
| PAI | 34.8 ± 0.1 | 65.8 ± 0.2 | 563.9 ± 0.3 |
| Teflon/PAI | 42.8 ± 0.3 | 72.1 ± 0.1 | 656.5 ± 0.2 |
| Membrane Substance | Modification Method | Treated Membrane | Omniphobicity | Feed Composition | Temperature Difference (°C) | Oil–Gas Separation Equilibrium Time (h) | Ref. |
|---|---|---|---|---|---|---|---|
| Ceramic membrane tube (7.5 mm inner diameter, 12.5 mm outer diameter, 100 mm length, and 50 nm pore size) | Coating by 1.8 wt% Teflon AF2400 | Teflon AF2400 (an 8 μm separation thickness) | --- | Transformer oilcontained H2, CO2, CO, CH4, C2H4, C2H6 and C2H2 | 20 | 5 | [36] |
| FEP membrane (a thickness of 100 μm) | --- | FEP with a thickness of 0.3 mm | --- | Insulating oil solution contained 58.4 ppm H2, 479.1 ppm CO2, 164.9 ppm CO, 17.5 ppm CH4, 16.6 ppm, C2H4, 16.8 ppm C2H6, 15.2 ppm C2H2 | 25 | --- | [28] |
| Macroporous α-Al2O3 hollow fiber membrane | Coating with a 0.5 wt% Teflon AF2400 solution | Teflon AF2400/γ-Al2O3/α-Al2O3 (the thickness of the Teflon AF2400 layer is about 270 nm) | --- | --- | --- | --- | [27] |
| PAI hollow fiber membrane | Vacuum-assisted dip-coating by 1.0 wt% Teflon AF2400 solution | Teflon/PAI (a 1.11 μm separation thickness) | 156.3 ± 1.8° and 123.0 ± 2.5° toward DI water and mineral insulating oil, respectively | Insulating oil solution contained 16.84 μL·L−1 C2H4, 3.64 μL·L−1 C2H2, 24.01 μL·L−1 C2H6, 12.46 μL·L−1 CH4, 1475.29 μL·L−1 CO2, 457.24 μL·L−1 CO and 65.94 μL·L−1 H2 | 50 ± 1 | 2 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Yang, Q.; Jin, Y.; Meng, Y.; Shen, L.; Zhu, X.; Gao, H.; Chen, C. Novel Omniphobic Teflon/PAI Composite Membrane Prepared by Vacuum-Assisted Dip-Coating Strategy for Dissolved Gases Separation from Transformer Oil. Coatings 2025, 15, 1319. https://doi.org/10.3390/coatings15111319
Zhang W, Yang Q, Jin Y, Meng Y, Shen L, Zhu X, Gao H, Chen C. Novel Omniphobic Teflon/PAI Composite Membrane Prepared by Vacuum-Assisted Dip-Coating Strategy for Dissolved Gases Separation from Transformer Oil. Coatings. 2025; 15(11):1319. https://doi.org/10.3390/coatings15111319
Chicago/Turabian StyleZhang, Wei, Qiwei Yang, Yuanyuan Jin, Yanzong Meng, Leyu Shen, Xuran Zhu, Haifeng Gao, and Chuan Chen. 2025. "Novel Omniphobic Teflon/PAI Composite Membrane Prepared by Vacuum-Assisted Dip-Coating Strategy for Dissolved Gases Separation from Transformer Oil" Coatings 15, no. 11: 1319. https://doi.org/10.3390/coatings15111319
APA StyleZhang, W., Yang, Q., Jin, Y., Meng, Y., Shen, L., Zhu, X., Gao, H., & Chen, C. (2025). Novel Omniphobic Teflon/PAI Composite Membrane Prepared by Vacuum-Assisted Dip-Coating Strategy for Dissolved Gases Separation from Transformer Oil. Coatings, 15(11), 1319. https://doi.org/10.3390/coatings15111319







