Optimization of the Hard Anodizing Process in Acidic Baths on AA6063 Aluminum Alloy Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hard Anodizing Process
2.3. Response Surface Methodology (RSM)
2.4. Characterization of Anodized Coatings
2.4.1. Measurements of Anodizing Thickness
2.4.2. Vickers Microhardness (HV)
2.4.3. Corrosion Measurements
3. Results and Discussion
3.1. Analysis of Response Surface Methodology
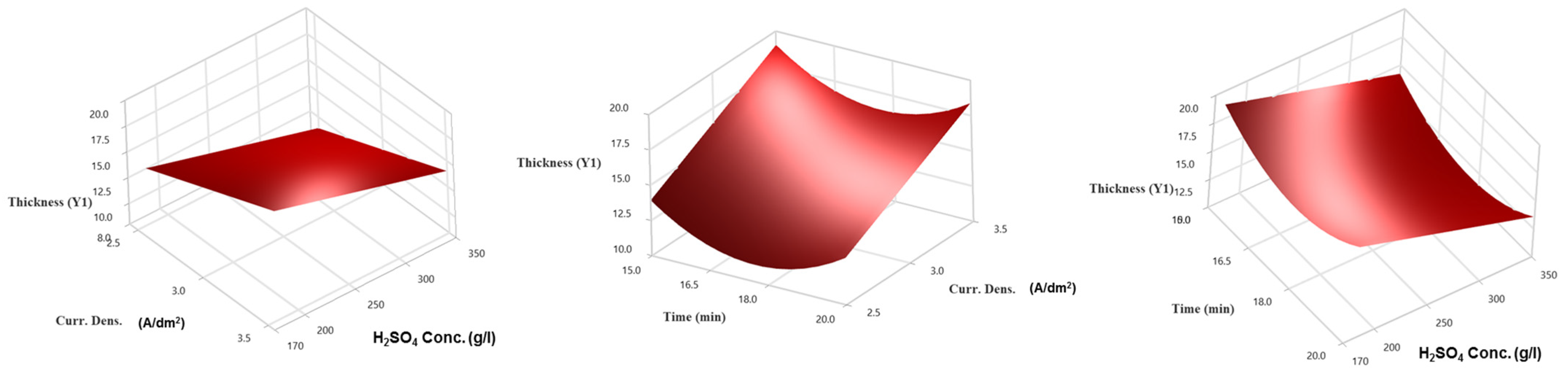
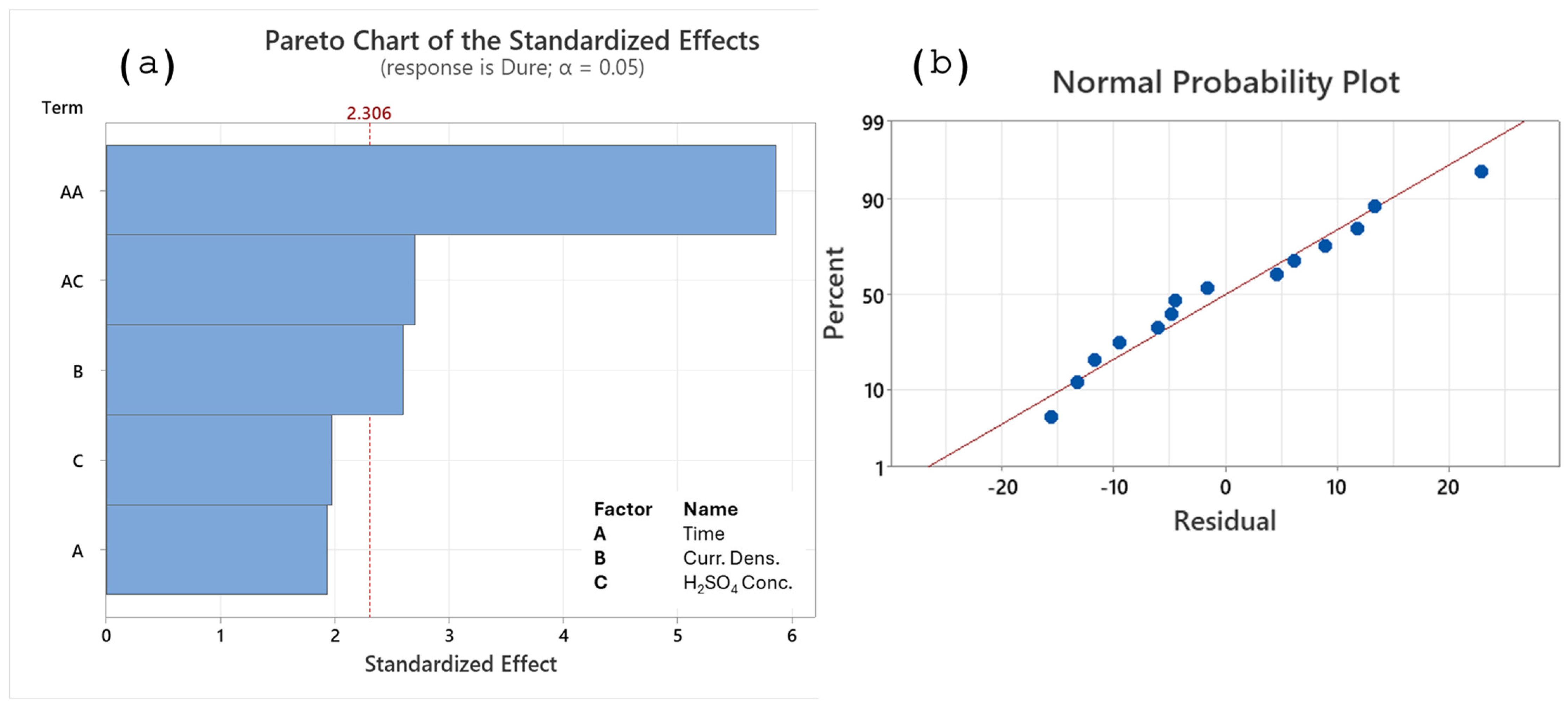
3.2. Analysis of Response Surface Regression for Thickness (Y1)
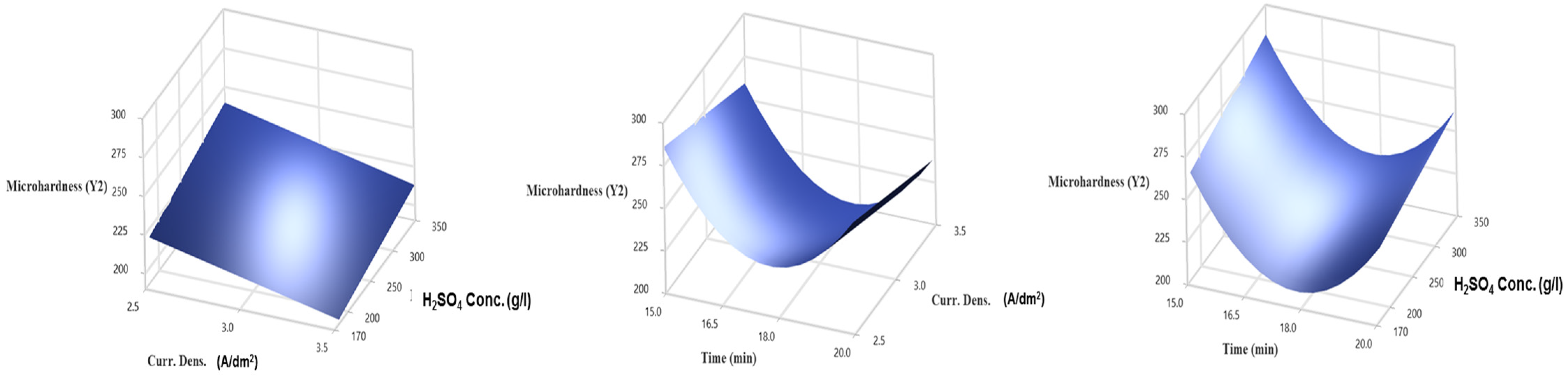
3.3. Response Surface Regression for Vickers Microhardness (Y2)
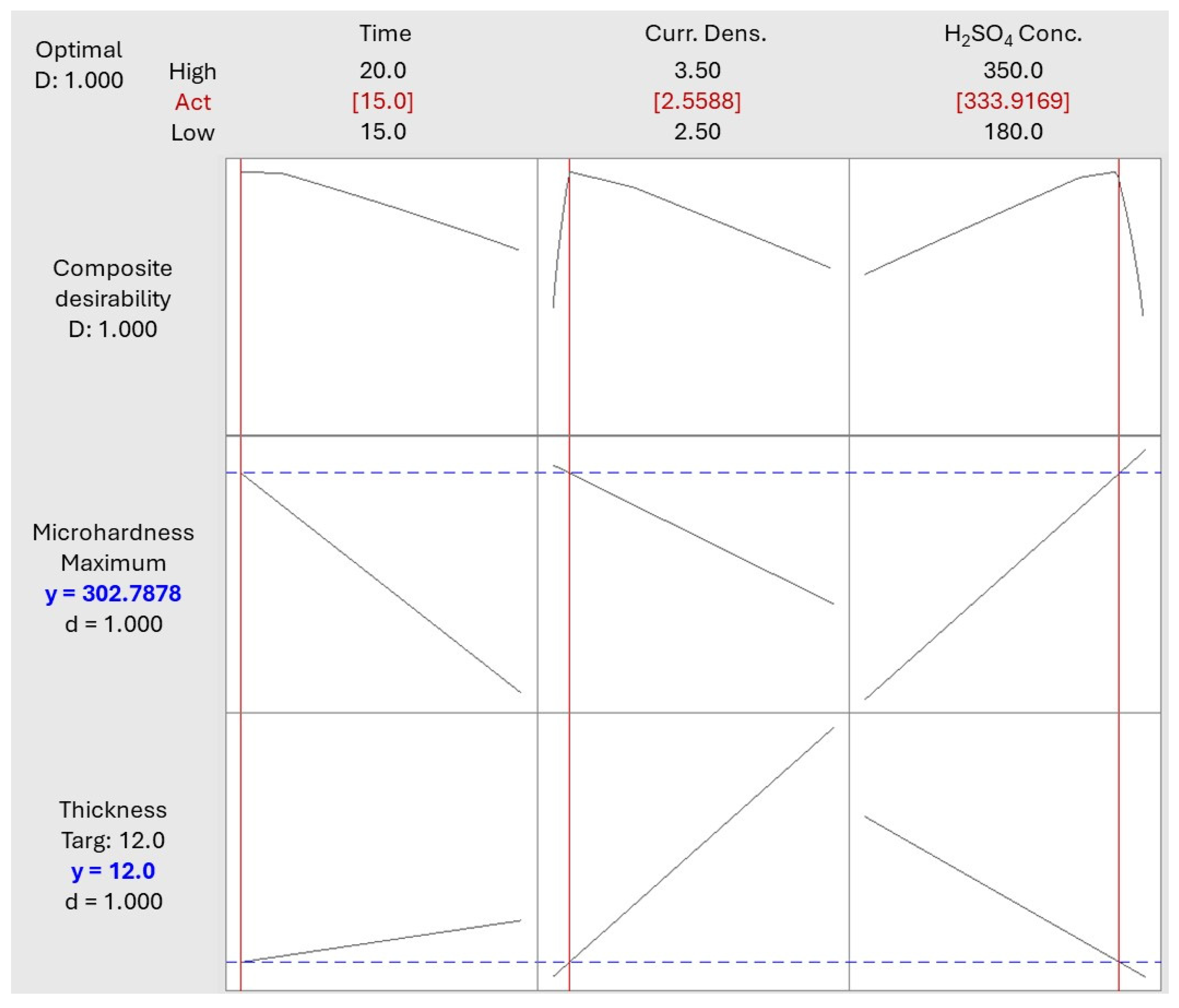
3.4. Optimization of the Anodization Process
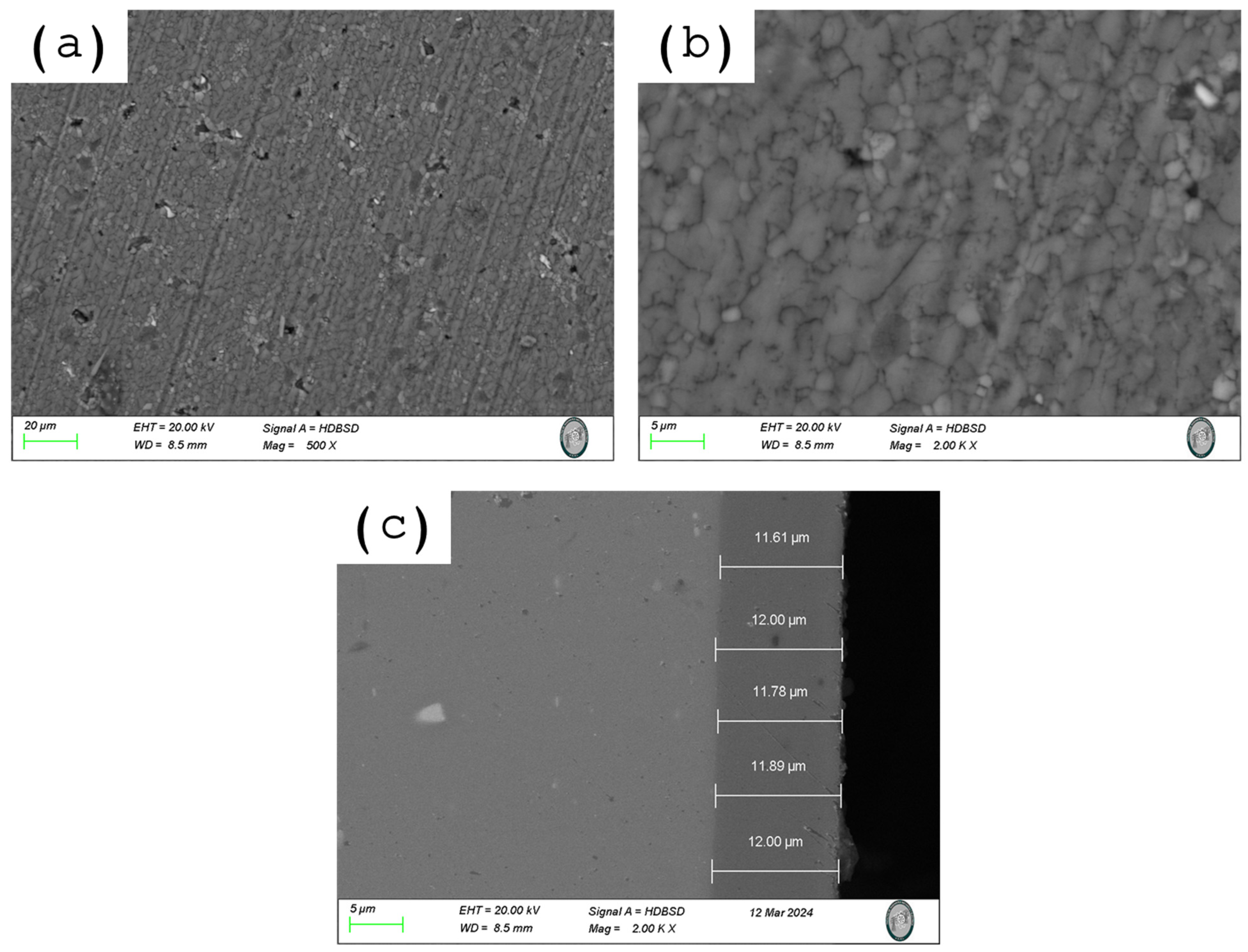
3.5. Characterization of AA6063 Anodized Coatings
3.5.1. Analysis of Coating Anodized Thickness
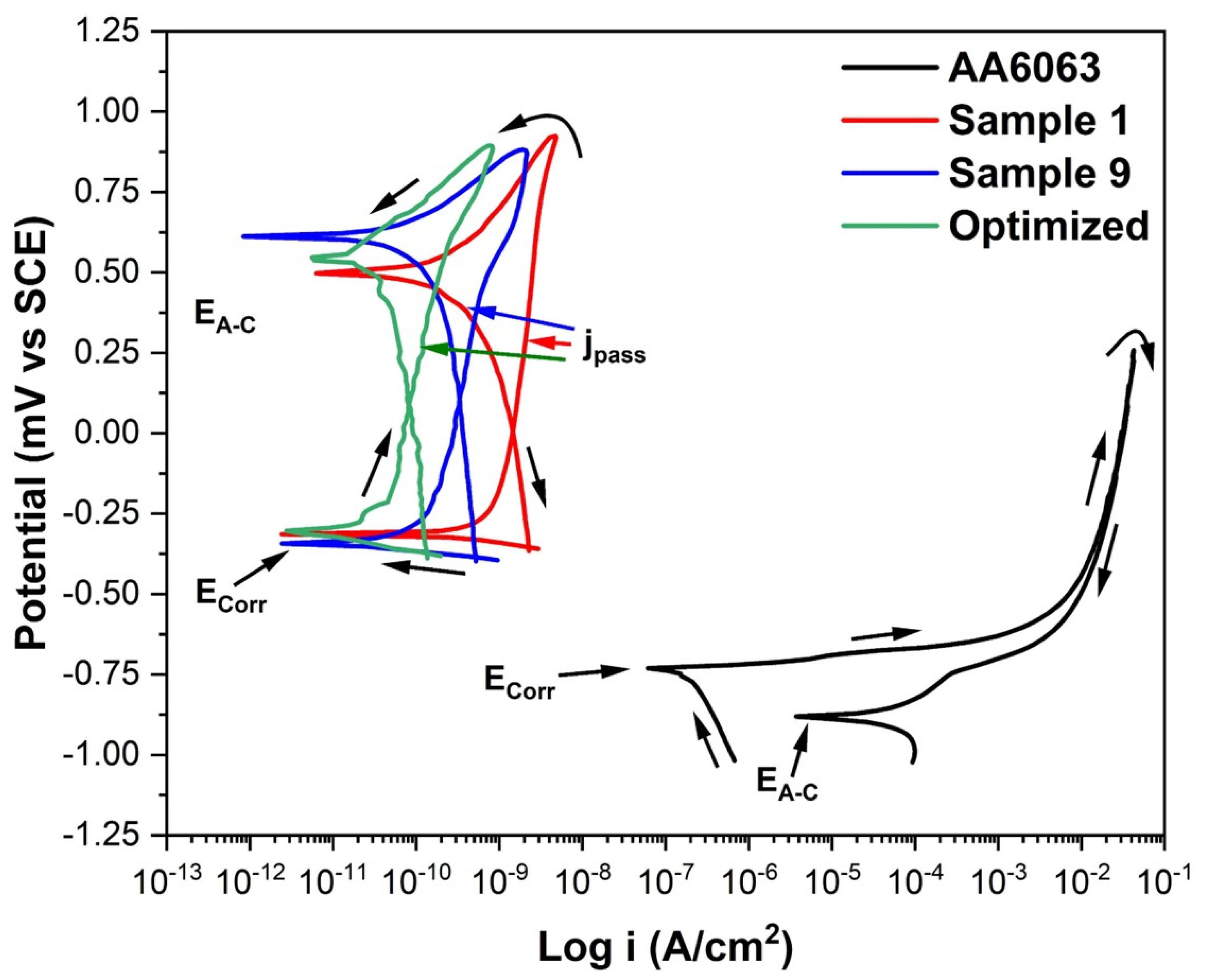
3.5.2. Corrosion Properties
4. Conclusions
- Using response surface methodology (RSM) and a central composite design (CCD), it was possible to create an experimental matrix with 20 combinations to obtain the response factors necessary to design response surface experiments.
- The analysis yielded models for both responses (thickness and microhardness) with an R-sq greater than 80%, sufficient to define the relationship between the variation in the model and the variation explained by the analysis.
- The most influential parameters in creating the alumina layer for thickness are current density, H2SO4 concentration, and the quadratic term for time.
- The results indicate that for Vickers microhardness, the most influential variables are the quadratic of time, the interaction between time and current density, and the concentration of H2SO4.
- With response optimization, a thickness of 11.85 µm and a Vickers microhardness of 297.7 were achieved, with an anodizing time of 15 min, a current density of 2.55 A/dm2, and an H2SO4 concentration of 333.91 g/L.
- The corrosion resistance of the anodized material also increases when anodized under the optimal conditions obtained, presenting low corrosion and passivation current densities and a more noble corrosion potential than the rest of the samples evaluated.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RSM | Response surface methodology |
| CCD | Composite central design |
| XRF | X-ray fluorescence |
| HV | Vickers microhardness |
| Curr. Dens. | Current density |
| H2SO4 Conc. | H2SO4 solution concentration |
| SCE | Saturated calomel electrode |
| CPPC | Cyclic potentiodynamic polarization curve |
| DOE | Design of experiments |
| ANOVA | Analysis of variance |
| Std Dev | Standard Deviation |
| Y1 | Thickness |
| Y2 | Vickers microhardness (HV) |
| ECorr | Corrosion potential |
| EA-C | Anodic-to-cathodic transition potential |
| jPass | Passivation current density |
| jCorr | Corrosion current density |
References
- Runge, J.M. A Brief History of Aluminum and Its Alloys. In The Metallurgy of Anodizing Aluminum; Springer: Cham, Switzerland, 2018; Volume XXII, pp. 1–63. [Google Scholar]
- Raj, V.; Rajaram, M.P.; Balasubramanian, G.; Vincent, S.; Kanagaraj, D. Pulse Anodizing—An Overview. Trans. IMF 2003, 81, 114–121. [Google Scholar] [CrossRef]
- Abdel-Salam, O.E.; Shoeib, M.A.; Elkilany, H.A. Characterization of the hard anodizing layers formed on 2014-T3 Al alloy, in sulphuric acid electrolyte containing sodium lignin sulphonate. Egypt. J. Petrol. 2017, 27, 497–504. [Google Scholar] [CrossRef]
- Roshani, M.; Rouhaghdam, A.S.; Aliofkhazraei, M.; Astaraee, A.H. Optimization of mechanical properties for pulsed anodizing of aluminum. Surf. Coat. Technol. 2017, 310, 17–24. [Google Scholar] [CrossRef]
- Cabral Miramontes, J.A.; Gaona Tiburcio, C.; Estupiñan López, F.; Lara-Banda, M.; Zambrano Robledo, P.; Nieves-Mendoza, D.; Maldonado Bandala, E.; Chacón Nava, J.; Almeraya Calderón, F. Corrosion Resistance of Hard Coat Anodized AA 6061 in Citric–Sulfuric Solutions. Coatings 2020, 10, 601. [Google Scholar] [CrossRef]
- Martínez Viademonte, M.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A Review on Anodizing of Aerospace Aluminum Alloys for Corrosion Protection. Coatings 2020, 10, 1106–1136. [Google Scholar] [CrossRef]
- Kwolek, P. Hard anodic coatings on aluminum alloys. Adv. Manuf. Sci. Technol. 2017, 41, 35–46. [Google Scholar]
- Gabe, D.R. Hard anodizing—What do we mean by hard? Met. Finish. 2002, 100, 52–58. [Google Scholar] [CrossRef]
- Vergara Guillén, L.E.; Nerey Carvajal, L.M.; Guedez Torcates, V.M. Modelo predictivo del espesor de la capa de óxido y microdureza en aluminio Al3003-B14 y Al6063-T6 anodizado usando análisis multifactorial. Ingeniare Rev. Chil. Ing. 2011, 19, 186–195. [Google Scholar] [CrossRef][Green Version]
- Ferreira, S.L.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Pouyafar, V.; Meshkabadi, R. Experimental investigation, modeling and optimization of parameters in hard anodizing of 6063 aluminum alloy using central composite design. Arab. J. Sci. Eng. 2024, 49, 11015–11029. [Google Scholar] [CrossRef]
- Nabavi, R.; Sarraf, S.; Soltanieh, M. Optimization of Hard Anodizing Process Parameters on 6061-T6 Aluminum Alloy Using Response Surface Methodology. J. Mater. Eng. Perform. 2024, 33, 10048–10061. [Google Scholar] [CrossRef]
- Samaniego-Gámez, P.O.; Almeraya-Calderon, F.; Maldonado-Bandala, E.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Olguin-Coca, J.; Lopez-Leon, L.D.; Silva Vidaurri, L.G.; Zambrano-Robledo, P.; Gaona-Tiburcio, C. Corrosion Behavior of AA2055 Aluminum-Lithium Alloys Anodized in the Presence of Sulfuric Acid Solution. Coatings 2021, 11, 1278. [Google Scholar] [CrossRef]
- Alishavandi, M.; Ebadi, M.; Kokabi, A.H. Optimization of parameters for the friction stir processing and welding of aa1050 aluminum alloy. Iran. J. Mater. Sci. Eng. 2021, 18, 1–11. [Google Scholar]
- Pulido, H.G.; De la Vara Salazar, R.; Carrasco, A.C.; Sánchez, M.O. Análisis y Diseño de Experimentos, 2nd ed.; McGraw-Hill/Interamericana Editores: Ciudad de México, Mexico, 2008; pp. 413–420. [Google Scholar]
- Khuri, A.; Mukhopadhyay, S. Response Surface Methodology. Wiley Interdiscipl. Rev. Comput. Stat. 2010, 2, 128–149. [Google Scholar] [CrossRef]
- Samaniego-Gámez, P.; Almeraya-Calderón, F.; Martin, U.; Ress, J.; Gaona-Tiburcio, C.; Silva-Vidaurri, L.; Cabral-Miramontes, J.; Bastidas, J.M.; Chacón-Nava, J.G.; Bastidas, D.M. Efecto del tratamiento de sellado en el comportamiento frente a corrosión de la aleación anodizada de aluminio-litio AA2099. Rev. Met. 2020, 56, e180. [Google Scholar] [CrossRef]
- Chen, W.-H.; Uribe, M.C.; Kwon, E.E.; Lin, K.-Y.A.; Park, Y.-K.; Ding, L.; Saw, L.H. A comprehensive review of thermoelectric generation optimization by statistical approach: Taguchi method, analysis of variance (ANOVA), and response surface methodology (RSM). Renew. Sustain. Energy Rev. 2022, 169, 112917. [Google Scholar] [CrossRef]
- Antony, J. Design of Experiments for Engineers and Scientists, 2nd ed.; Elsevier: Waltham, MA, USA, 2023; pp. 73–76. [Google Scholar]
- Rashid, K.H.; Khadom, A.A.; Mahood, H.B. Aluminum ASA 6061 anodizing process by chromic acid using Box-Wilson central composite design: Optimization and corrosion tendency. Met. Mater. Int. 2021, 27, 4059–4073. [Google Scholar] [CrossRef]
- Moradi, M.; Hashemi, R.; Kasaeian-Naeini, M. Experimental investigation of parameters in fused filament fabrication 3D printing process of ABS plus using response surface methodology. Int. J. Adv. Manuf. Technol. 2023, 1–18. [Google Scholar] [CrossRef]
- Elhami, S.; Razfar, M.R.; Farahnakian, M.; Rasti, A. Application of GONNS to Predict Constrained Optimum Surface Roughness in Face Milling of High-Silicon Austenitic stainless Steel. Int. J. Adv. Manufact. Technol. 2012, 66, 975–986. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.; Almeraya-Calderón, F.; López, F.E.; Lara Banda, M.; Olguín-Coca, J.; López-León, L.D.; Castañeda-Robles, I.; Alcalá, M.Á.E.; Zambrano-Robledo, P.; Gaona-Tiburcio, C. Citric Acid as an Alternative to Sulfuric Acid for the Hard-Anodizing of AA6061. Metals 2021, 11, 1838. [Google Scholar] [CrossRef]
- Georgantzia, E.; Gkantou, M.; Kamaris, G.S. Aluminium alloys as structural material: A review of research. Eng. Struct. 2021, 227, 111372. [Google Scholar] [CrossRef]
- Alwitt, R.S.; McClung, R.C.; Jacobs, S. Anodized Aluminum Coatings for Thermal Control, Part I: Coating Process and Stresses. In Proceedings of the AIAA Materials Specialist Conference, Dallas, TX, USA, 16–17 April 1992; pp. 39–45, AIAA-922158-CP, AAIA Technical Papers (A92-31285 12-23). [Google Scholar]
- Martinez-Ramos, C.; Gaona-Tiburcio, C.; Estupiñan-López, F.; Cabral-Miramontes, J.; Maldonado-Bandala, E.; Nieves-Mendoza, D.; Baltazar-Zamora, M.A.; Landa-Ruiz, L.; Galvan-Martinez, R.; Almeraya-Calderón, F. Smart Corrosion Monitoring in AA2055 Using Hidden Markov Models and Electrochemical Noise Signal Processing. Materials 2025, 18, 2865. [Google Scholar] [CrossRef]
- Belwalkar, A.; Grasing, E.; Van Geertruyden, W.; Huang, Z.; Misiolek, W. Effect of Processing Parameters on Pore Structure and Thickness of anodic Aluminum Oxide (AAO) Tubular Membranes. J. Membr. Sci. 2008, 319, 192–198. [Google Scholar] [CrossRef]
- Curioni, M.; Gionfini, T.; Vicenzo, A.; Skeldon, P.; Thompson, G.E. Optimization of anodizing cycles for enhanced performance. Surf. Interface Anal. 2013, 45, 1485–1489. [Google Scholar] [CrossRef]
- Almeraya-Calderón, F.; Montoya-Rangel, M.; Nieves-Mendoza, D.; Jáquez-Muñoz, J.M.; Diaz-Olivares, A.; Lara-Banda, M.; Maldonado-Bandala, E.; Estupinan-Lopez, F.; Cabral-Miramontes, J.; Olguin-Coca, J.; et al. Corrosion Behavior of Advanced High-Strength Steels (AHSS) in Chloride Solutions for Automotive Applications. Metals 2025, 15, 1116. [Google Scholar] [CrossRef]
- Bensalah, W.; Elleuch, K.; Feki, M.; Wery, M.; Ayedi, H. Optimization of anodic layer properties on aluminium in mixed oxalic/sulphuric acid bath using statistical experimental methods. Surf. Coat. Technol. 2007, 201, 7855–7864. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Biles, W.E.; Swain, J.J. Optimization and Industrial Experimentation, 1st ed.; Wiley: New York, NY, USA, 1980. [Google Scholar]
- ASTM E3-95; Standard Practice for Preparation of Metallographic Specimens. ASTM International: WEST Conshohocken, PA, USA, 1995.
- ASTM B487-85; Standard Test Method for Measurement of Metal and Oxide Coating Thickness by Microscopical Examination of Cross Section. ASTM International: WEST Conshohocken, PA, USA, 2020.
- ASTM E92-17; Standard Test Method for Vickers Hardness of Metallic Materials. ASTM International: WEST Conshohocken, PA, USA, 2017.
- Franco, M.; Krishna, T.H.; Pillai, A.M.; Rajendra, A.; Sharma, A.K. A comparative study on the corrosion behavior of hard anodic coatings on AA6061 obtained using dc and pulsed dc power sources. Acta Metall. Sin. Engl. Lett. 2013, 26, 647–656. [Google Scholar] [CrossRef]
- Gaona-Tiburcio, C.; Almeraya-Calderón, F.; Chacon-Nava, J.G.; Matutes-Aquino, J.A.; Martinez-Villafañe, A. Electrochemical response of permanente magnets in different solutions. J. Alloys Compd. 2004, 369, 78–80. [Google Scholar] [CrossRef]
- Loto, R.T.; Adeleke, A.J. Corrosion of aluminum alloy metal matrix composites in neutral chloride solutions. Fail. Anal. Prev. 2016, 16, 874–885. [Google Scholar] [CrossRef]
- ASTM G61-86; Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron-, Nickel-, or Cobalt-Based Alloy. ASTM International: West Conshohocken, PA, USA, 2018.
- Li, W.; Hu, S.J.; Cheng, S.W. Robust design and analysis for manufacturing processes with parameter interdependency. J. Manuf. Syst. 2002, 21, 93–100. [Google Scholar] [CrossRef]
- Chen, D.C.; Chen, C.F. Use of Taguchi method to study a robust design for the sectioned beams curvature during rolling. J. Mater. Process. Technol. 2007, 190, 130–137. [Google Scholar] [CrossRef]
- Brahmi, M.; Ba, M.; Hidri, Y.; Hassen, A. factorial experimental design intended for the optimization of the alumina purification conditions. J. Mol. Struct. 2018, 1157, 567–578. [Google Scholar] [CrossRef]
- Martínez-Ramos, C.; Olguin-Coca, J.; Lopez-Leon, L.D.; Gaona-Tiburcio, C.; Lara-Banda, M.; Maldonado-Bandala, E.; Castañeda-Robles, I.; Jaquez-Muñoz, J.M.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; et al. Electrochemical Noise Analysis Using Experimental Chaos Theory, Power Spectral Density and Hilbert–Huang Transform in Anodized Aluminum Alloys in Tartaric–Phosphoric–Sulfuric Acid Solutions. Metals 2023, 13, 1850. [Google Scholar] [CrossRef]
- Marwan, N.; Webber, C.L.; Macau, E.E.N.; Viana, R.L. Introduction to Focus Issue: Recurrence Quantification Analysis for Understanding Complex Systems. Chaos 2018, 28, 85601. [Google Scholar] [CrossRef]
- Figueiredo, J.; Vila, M.; Fiúza, A.; Góis, J.; Futuro, A.; De Dinis, M.L.; Martins, D. A Holistic Approach in Re-Mining Old Tailings Deposits for the Supply of Critical-Metals: A Portuguese Case Study. Minerals 2019, 9, 638. [Google Scholar] [CrossRef]
- Man, L.; Kumar, P.; Teng, T.T.; Wasewar, K. Design of Experiments for Malachite Green Dye Removal from Wastewater Using Thermolysis–Coagulation–Flocculation. Desalin. Water Treat. 2012, 40, 260–271. [Google Scholar] [CrossRef]
- Rajendra, A.; Parmar, B.J.; Sharma, A.K.; Bhojraj, H.; Nayak, M.M.; Rajanna, K. Hard anodisation of aluminium and its application to sensorics. Surf. Eng. 2005, 21, 193–197. [Google Scholar] [CrossRef]
- Mohammadi, I.; Afshar, A.; Ahmadi Seyedkhani, S. Nanoporous anodized aluminum thickness optimization through pulse current mode. J. Adv. Mater. Process. 2015, 3, 11–24. [Google Scholar]
- Garcia-Vergara, S.J.; Skeldon, P.; Thompson, G.E.; Hashimoto, T.; Habazaki, H. Compositional evidence for flow in anodic films on aluminum under high electric fields. J. Electrochem. Soc. 2007, 154, C540. [Google Scholar] [CrossRef]
- De Graeve, I.; Terryn, H.; Thompson, G.E. Influence of local heat development on film thickness for anodizing aluminumin sulfuric acid. J. Electrochem. Soc. 2003, 150, B158–B165. [Google Scholar] [CrossRef]
- Almeraya-Calderon, F.; Villegas-Tovar, M.; Maldonado-Bandala, E.; Nieves-Mendoza, D.; Méndez-Ramírez, C.T.; Baltazar-Zamora, M.A.; Olguín-Coca, J.; Lopez-Leon, L.D.; Santiago-Hurtado, G.; Almaguer-Cantu, V.; et al. Electrochemical Noise Analysis in Passivated Martensitic Precipitation-Hardening Stainless Steels in H2SO4 and NaCl Solutions. Metals 2025, 15, 837. [Google Scholar] [CrossRef]
- Baiwei, Z.; Seifeddine, S.; Persson, O.Å.; Jarfors Anders, E.W.; Leisner, P.; Zanella, C. A study of formation and growth of the anodized surface layer on cast Al-Si alloys based on different analytical techniques. Mater. Des. 2016, 101, 254–262. [Google Scholar]
- Chen, K.S.; Chen, H.T.; Chang, T.C. The construction and application of Six Sigma quality indices. Int. J. Prod. Res. 2017, 55, 2365–2384. [Google Scholar] [CrossRef]
- Schneider, M.; Liebmann, T.; Langklotz, U.; Michaelis, A. Microelectrochemical investigation of anodic oxide formation on the aluminum alloy AA2024. Electrochim. Acta 2017, 249, 198–205. [Google Scholar] [CrossRef]
- O’sullivan, J.P.; Wood, G.C. The morphology and mechanism of formation of porous anodic films on aluminium. Proc. R. Soc. Lond. A 1970, 317, 511–543. [Google Scholar]
- Kikuchi, T.; Takenaga, A.; Natsui, S.; Suzuki, R. Advanced hard anodic alumina coatings via etidronic acid anodizing. Surf. Coat. Technol. 2017, 326, 72–78. [Google Scholar] [CrossRef]
- Totaro, P.; Khusid, B. Multistep Anodization of 7075–T6 Aluminum Alloy. Surf. Coat. Technol. 2021, 421, 127407. [Google Scholar] [CrossRef]
- Stępniowski, W.; Moneta, M.; Karczewski, K.; Michalska-Domańska, M.; Czujko, T.; Mol, J.M.C.; Buijnsters, J. Fabrication of copper nanowires via electrodeposition in anodic aluminum oxide templates formed by combined hard anodizing and electrochemical barrier layer thinning. J. Electroanal. Chem. 2017, 809, 59–66. [Google Scholar] [CrossRef]
- Han, X.Y. Improved two-step anodization technique for ordered porous anodic aluminum membranes. J. Electroanal. Chem. 2011, 655, 56–64. [Google Scholar] [CrossRef]
- Mehdizade, M.; Soltanieh, M.; Eivani, A.R. Investigation of anodizing time and pulse voltage modes on the corrosion behavior of nanostructured anodic layer in commercial pure aluminum. Surf. Coat. Technol. 2018, 358, 741–752. [Google Scholar] [CrossRef]
- Lerner, L.M. Hard anodizing of aerospace aluminium alloys. Trans. Inst. Met. Finish. 2010, 88, 21–24. [Google Scholar] [CrossRef]
- Khamaj, J.A. Cyclic polarization analysis of corrosion behavior of ceramic coating on 6061 Al/SiCp composite for marine applications. Prot. Met. Phys. Chem. Surf. 2016, 52, 886–893. [Google Scholar] [CrossRef]
- Silverman, D.C. Practical Corrosion Prediction Using Electrochemical Techniques, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 1129–1166. [Google Scholar]
- Mansouri, K.; Ibrik, K.; Bensalah, N.; Abdel-Wahab, A. Anodic dissolution of pure aluminum during electrocoagulation process: Influence of supporting electrolyte, initial pH and current density. Ind. Eng. Chem. Res. 2011, 50, 13362–13372. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, G.; Meng, X.; Zhang, A. High Performance Alumina Films Prepared by Direct Current Plus Pulse Anodisation. Surf. Eng. 2014, 30, 455–459. [Google Scholar] [CrossRef]
- Ali, H. Review of Porous Anodic Aluminium Oxide (AAO) Applications for Sensors MEMS Biomedical devices. Trans. IMF 2017, 95, 290–296. [Google Scholar] [CrossRef]

| Material | Elements | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Al | Si | Fe | Cu | Mn | Mg | Cr | Ti | Zn | |
| AA6063 | Bal | 0.55 | 0.06 | 0.004 | 0.006 | 0.83 | 0.002 | 0.002 | 0.007 |
| Factor | Variable | Name | Minimum | Maximum |
|---|---|---|---|---|
| A | Time | Time (min) | 15 | 20 |
| B | Current density | Curr. Dens. (A/dm2) | 2.5 | 3.5 |
| C | H2SO4 solution concentration | H2SO4 Conc. (g/L) | 180 | 350 |
| Sample | Run Order | Design Variables | |||||
|---|---|---|---|---|---|---|---|
| Time (min) | Current Density (A/dm2) | H2SO4 Conc. (g/L) | |||||
| Actual Value | A | Actual Value | B | Actual Value | C | ||
| 1 | 1 | 15 | −1 | 2.5 | −1 | 180 | −1 |
| 2 | 6 | 20 | +1 | 2.5 | −1 | 180 | −1 |
| 3 | 7 | 15 | −1 | 3.5 | +1 | 180 | −1 |
| 4 | 17 | 20 | +1 | 3.5 | +1 | 180 | −1 |
| 5 | 13 | 15 | −1 | 2.5 | −1 | 350 | +1 |
| 6 | 8 | 20 | +1 | 2.5 | −1 | 350 | +1 |
| 7 | 16 | 15 | −1 | 3.5 | +1 | 350 | +1 |
| 8 | 11 | 20 | +1 | 3.5 | +1 | 350 | +1 |
| 9 | 5 | 17.5 | 0 | 3 | 0 | 265 | 0 |
| 10 | 9 | 17.5 | 0 | 3 | 0 | 265 | 0 |
| 11 | 12 | 17.5 | 0 | 3 | 0 | 265 | 0 |
| 12 | 20 | 17.5 | 0 | 3 | 0 | 265 | 0 |
| 13 | 15 | 17.5 | 0 | 3 | 0 | 265 | 0 |
| 14 | 10 | 17.5 | 0 | 3 | 0 | 265 | 0 |
| Samples | Time (min) | Current Density (A/dm2) | H2SO4 Conc. (g/L) | Coating Thickness (Y1) (µm) | Std Dev Thickness (Y1) (µm) | Microhardness (Y2) (HV) | Std Dev Microhardness (Y2) (HV) |
|---|---|---|---|---|---|---|---|
| 1 | 15 | 2.5 | 180 | 14.67 | 0.128 | 273.5 | 14.448 |
| 2 | 20 | 2.5 | 180 | 16.02 | 0.027 | 265.1 | 15.266 |
| 3 | 15 | 3.5 | 180 | 23.01 | 0.203 | 223.2 | 12.531 |
| 4 | 20 | 3.5 | 180 | 20.10 | 0.149 | 247.4 | 17.941 |
| 5 | 15 | 2.5 | 350 | 11.56 | 0.556 | 296.7 | 17.023 |
| 6 | 20 | 2.5 | 350 | 12.25 | 0.161 | 268.2 | 14.512 |
| 7 | 15 | 3.5 | 350 | 16.65 | 0.154 | 296.5 | 14.828 |
| 8 | 20 | 3.5 | 350 | 15.11 | 0.123 | 229.3 | 8.416 |
| 9 | 17.5 | 3.0 | 265 | 14.89 | 0.425 | 214.7 | 10.737 |
| 10 | 17.5 | 3.0 | 265 | 14.84 | 0.214 | 225.2 | 11.261 |
| 11 | 17.5 | 3.0 | 265 | 12.96 | 0.751 | 206.8 | 10.340 |
| 12 | 17.5 | 3.0 | 265 | 10.44 | 0.746 | 211.4 | 10.573 |
| 13 | 17.5 | 3.0 | 265 | 14.49 | 0.305 | 200.8 | 10.048 |
| 14 | 17.5 | 3.0 | 265 | 14.91 | 0.223 | 239.2 | 11.965 |
| Source | GL | Ajust. SS | Ajust. MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 4 | 114.256 | 28.5639 | 10.56 | 0.002 |
| Lineal | 3 | 94.167 | 31.3890 | 11.60 | 0.002 |
| Time | 1 | 0.727 | 0.7270 | 0.27 | 0.617 |
| Curr. Dens. | 1 | 51.922 | 51.9221 | 19.19 | 0.002 |
| H2SO4 Conc. | 1 | 41.518 | 41.5179 | 15.34 | 0.004 |
| Cuadratic | 1 | 20.088 | 20.0885 | 7.42 | 0.023 |
| Time × Time | 1 | 20.088 | 20.0885 | 7.42 | 0.023 |
| Error | 9 | 24.353 | 2.7059 | ||
| Lack-of-Fit | 4 | 8.383 | 2.0957 | 0.66 | 0.648 |
| Pure error | 5 | 15.970 | 3.1941 | ||
| Total | 13 | 138.609 | |||
| S = 1.64496 | R-sq(adj) = 74.62% | ||||
| R-sq = 82.43% | R-sq(pre) = 59.22% | ||||
| Source | GL | Ajust. SS | Ajust. MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 5 | 11,927.1 | 2385.4 | 11.19 | 0.002 |
| Lineal | 3 | 3065.3 | 1021.8 | 4.79 | 0.034 |
| Time | 1 | 797.6 | 797.6 | 3.74 | 0.089 |
| Curr. Dens. | 1 | 1435.4 | 1435.4 | 6.73 | 0.032 |
| H2SO4 Conc. | 1 | 832.3 | 832.3 | 3.90 | 0.084 |
| Cuadratic | 1 | 7308.8 | 7308.8 | 34.29 | 0.000 |
| Time × Time | 1 | 7308.8 | 7308.8 | 34.29 | 0.000 |
| Interaction of 2 factors | 1 | 1552.9 | 1552.9 | 7.28 | 0.027 |
| Time × H2SO4 Conc. | 1 | 1552.9 | 1552.9 | 7.28 | 0.027 |
| Error | 8 | 1705.4 | 213.2 | ||
| Lack-of-Fit | 3 | 745.2 | 248.4 | 1.29 | 0.373 |
| Pure error | 5 | 960.2 | 192.0 | ||
| Total | 13 | 13,632.5 | |||
| S = 14.6004 | R-sq(adj) = 79.67% | ||||
| R-sq = 87.49% | R-sq(pre) = 50.99% | ||||
| Sample | Ecorr (V vs. SCE) | EA–C (V vs. SCE) | jpass (A/cm2) | jCorr (A/cm2) | Hysteresis |
|---|---|---|---|---|---|
| AA6063 | −0.751 | −0.880 | --- | 1.91 × 10−7 | Positive |
| No. 1 | −0.314 | 0.499 | 2.01 × 10−9 | 4.65 × 10−10 | Negative |
| No. 9 | −0.344 | 0.611 | 4.13 × 10−9 | 5.57 × 10−11 | Negative |
| Optimized | −0.238 | 0.540 | 1.21 × 10−10 | 6.69 × 10−13 | Negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral-Miramontes, J.; Gaona-Tiburcio, C.; Maldonado-Bandala, E.; Juarez-Alejandro, L.; Baltazar-Zamora, M.A.; Estupiñan-Lopez, F.; Landa-Ruiz, L.; Olguin-Coca, J.; Lopez-Leon, L.D.; Nieves-Mendoza, D.; et al. Optimization of the Hard Anodizing Process in Acidic Baths on AA6063 Aluminum Alloy Using Response Surface Methodology. Coatings 2025, 15, 1306. https://doi.org/10.3390/coatings15111306
Cabral-Miramontes J, Gaona-Tiburcio C, Maldonado-Bandala E, Juarez-Alejandro L, Baltazar-Zamora MA, Estupiñan-Lopez F, Landa-Ruiz L, Olguin-Coca J, Lopez-Leon LD, Nieves-Mendoza D, et al. Optimization of the Hard Anodizing Process in Acidic Baths on AA6063 Aluminum Alloy Using Response Surface Methodology. Coatings. 2025; 15(11):1306. https://doi.org/10.3390/coatings15111306
Chicago/Turabian StyleCabral-Miramontes, José, Citlalli Gaona-Tiburcio, Erick Maldonado-Bandala, Lino Juarez-Alejandro, Miguel Angel Baltazar-Zamora, Francisco Estupiñan-Lopez, Laura Landa-Ruiz, Javier Olguin-Coca, Luis Daimir Lopez-Leon, Demetrio Nieves-Mendoza, and et al. 2025. "Optimization of the Hard Anodizing Process in Acidic Baths on AA6063 Aluminum Alloy Using Response Surface Methodology" Coatings 15, no. 11: 1306. https://doi.org/10.3390/coatings15111306
APA StyleCabral-Miramontes, J., Gaona-Tiburcio, C., Maldonado-Bandala, E., Juarez-Alejandro, L., Baltazar-Zamora, M. A., Estupiñan-Lopez, F., Landa-Ruiz, L., Olguin-Coca, J., Lopez-Leon, L. D., Nieves-Mendoza, D., Jaquez-Muñoz, J. M., & Almeraya-Calderon, F. (2025). Optimization of the Hard Anodizing Process in Acidic Baths on AA6063 Aluminum Alloy Using Response Surface Methodology. Coatings, 15(11), 1306. https://doi.org/10.3390/coatings15111306









