Research on the Mechanical Properties and Microstructure of Fly Ash, Slag, and Metakaolin Geopolymers
Abstract
1. Introduction
2. Test Materials
2.1. Material Introduction
2.2. Mix Proportion Design and Preparation
- (1)
- Modulus of alkali activator
- (2)
- Basic oxide equivalent
- (3)
- Water–solid ratio
2.3. Test Programs
2.3.1. Setting Time
- (1)
- Determination of initial setting time
- (2)
- Determination of final setting time
2.3.2. Compressive Strength and Flexural Strength
2.3.3. Microstructure Characterization
- (1)
- Phase analysis
- (2)
- Molecular structure analysis
- (3)
- Microanalysis
3. Results and Discussion
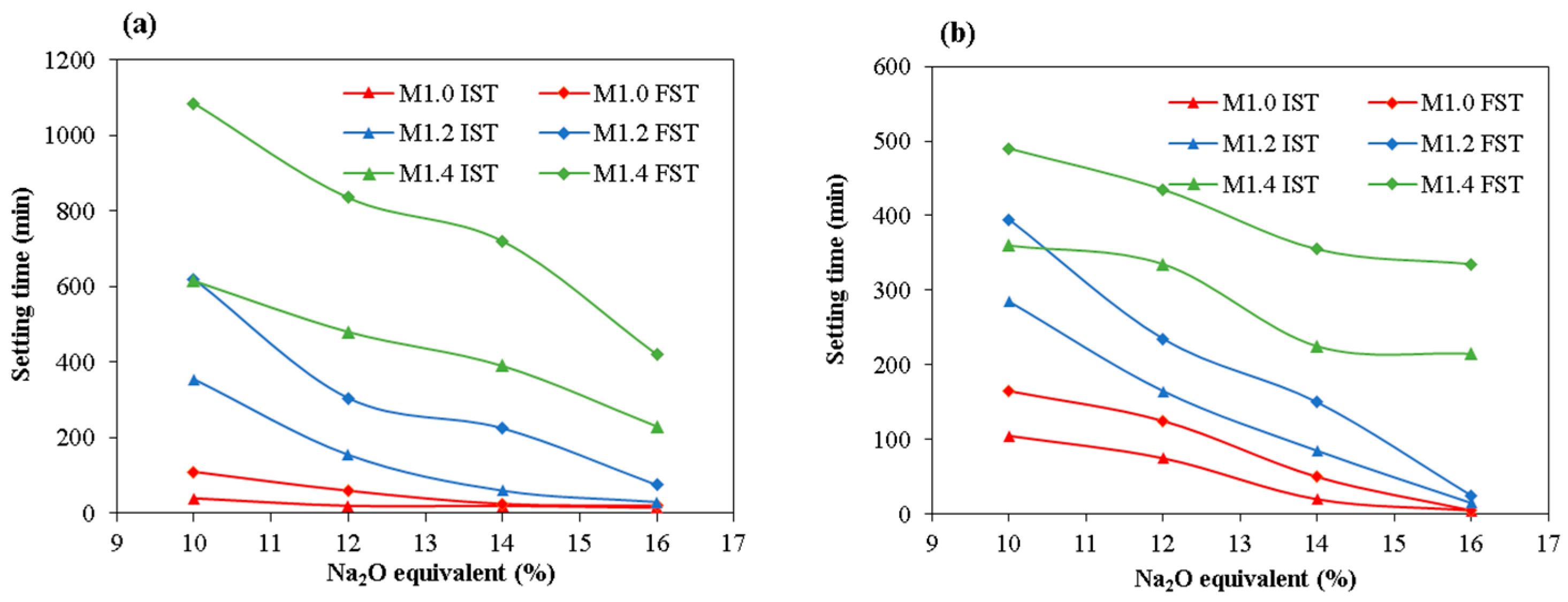
3.1. Setting Time
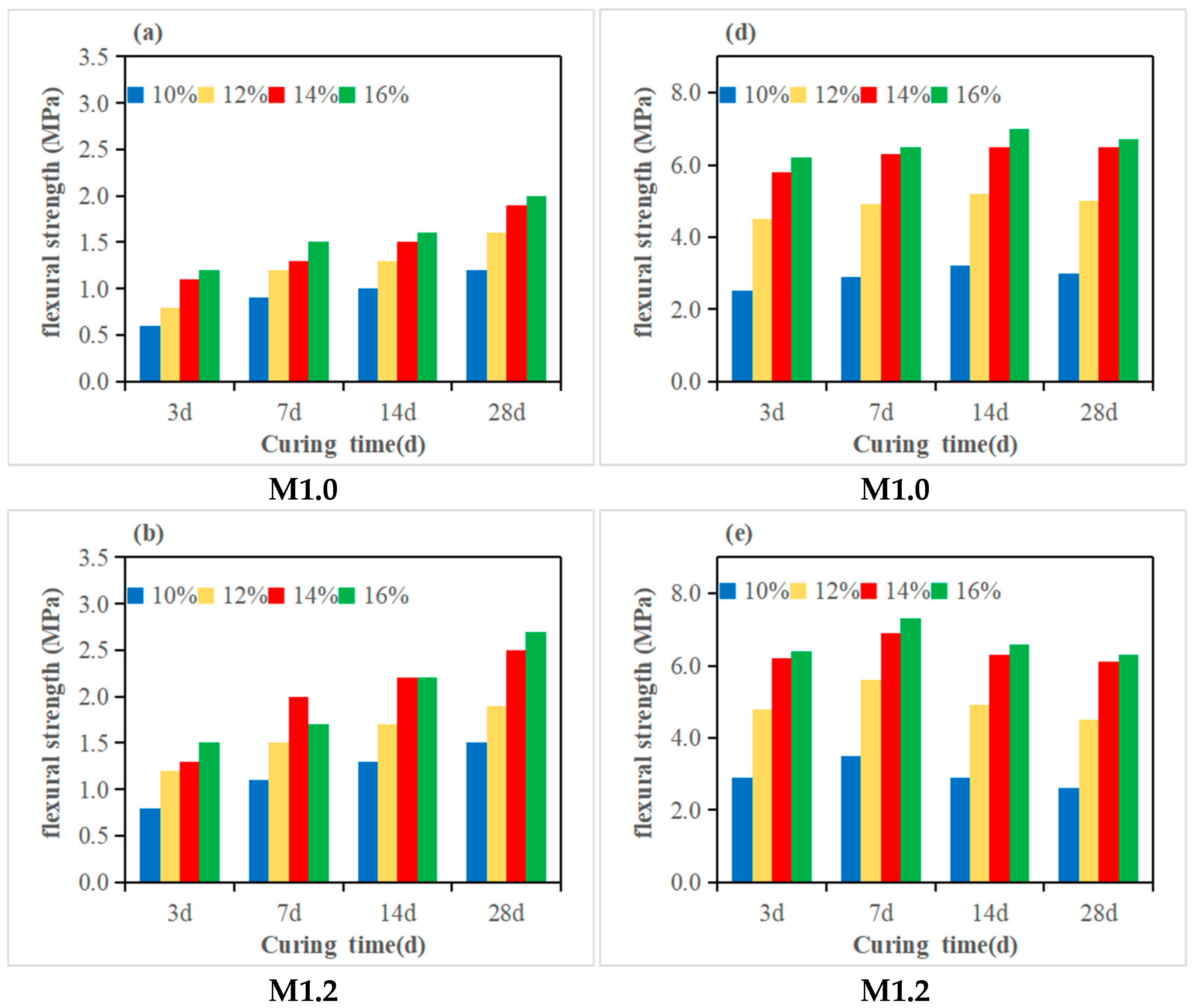
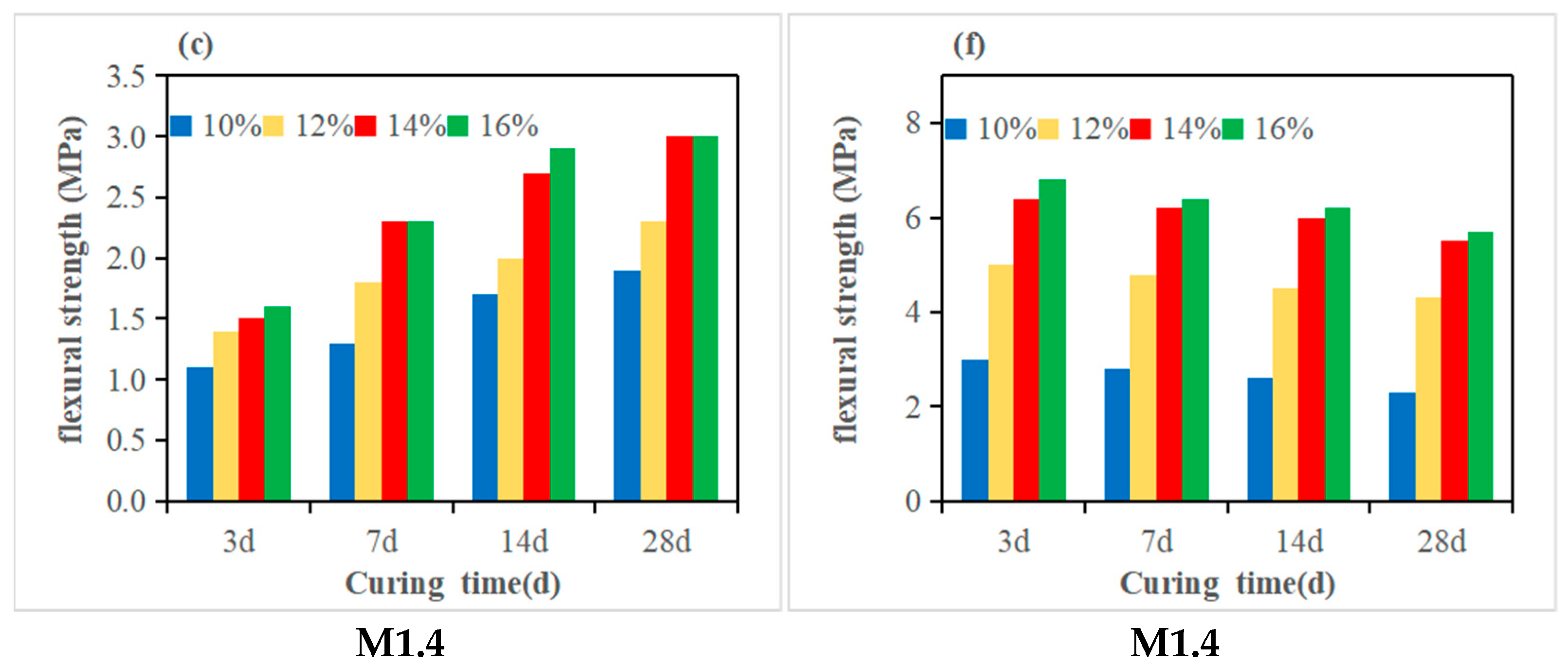
3.2. Compressive Strength and Flexural Strength
3.3. Characterization of Reaction Products
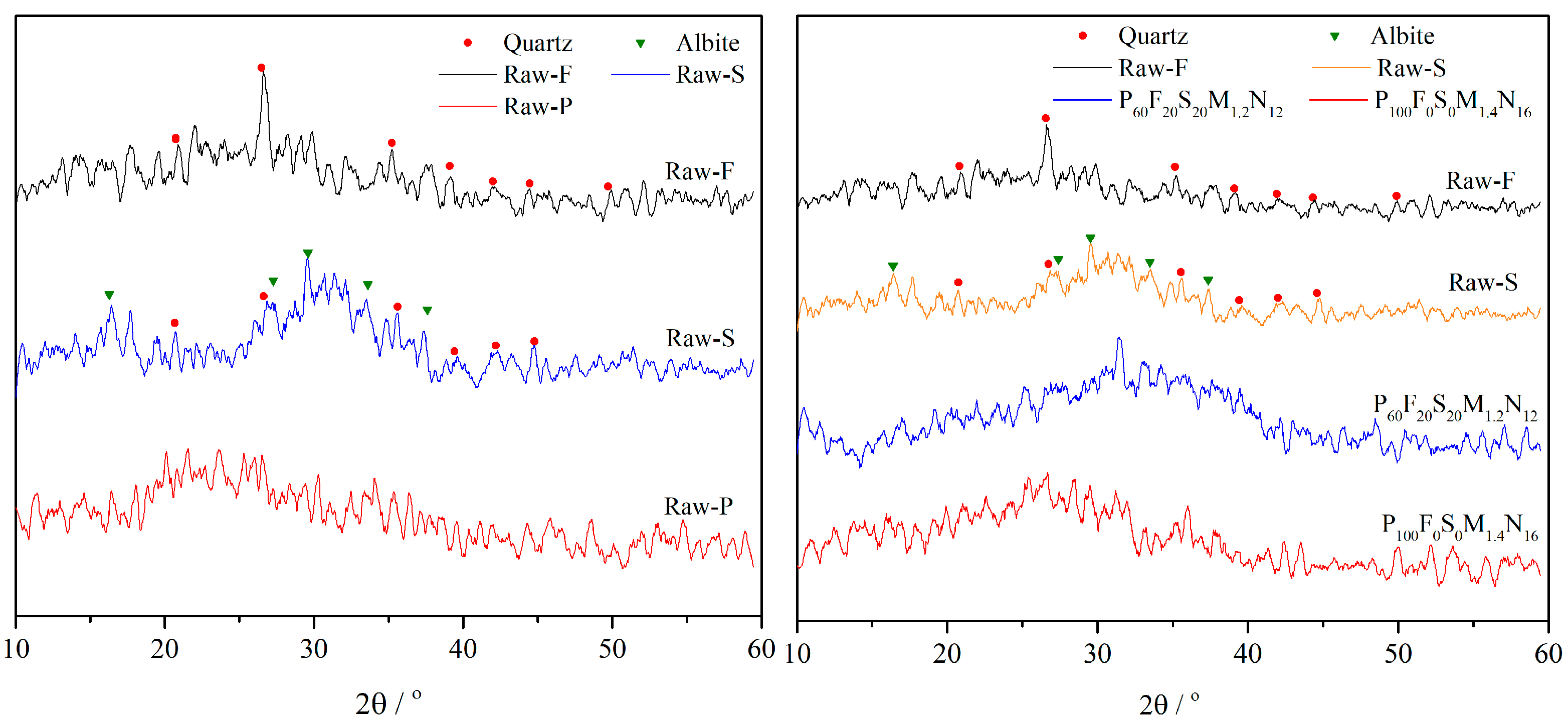
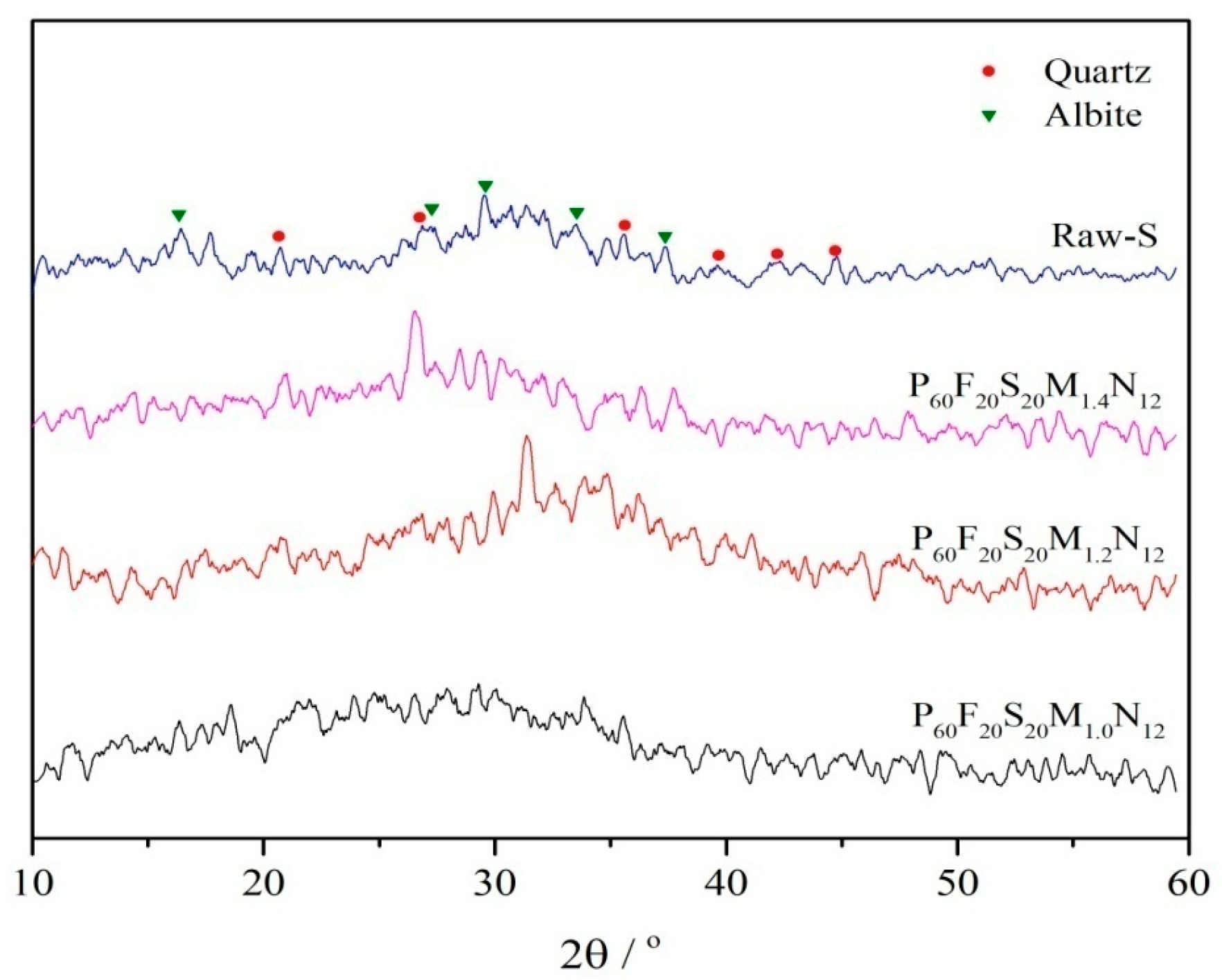
3.3.1. XRD Analysis
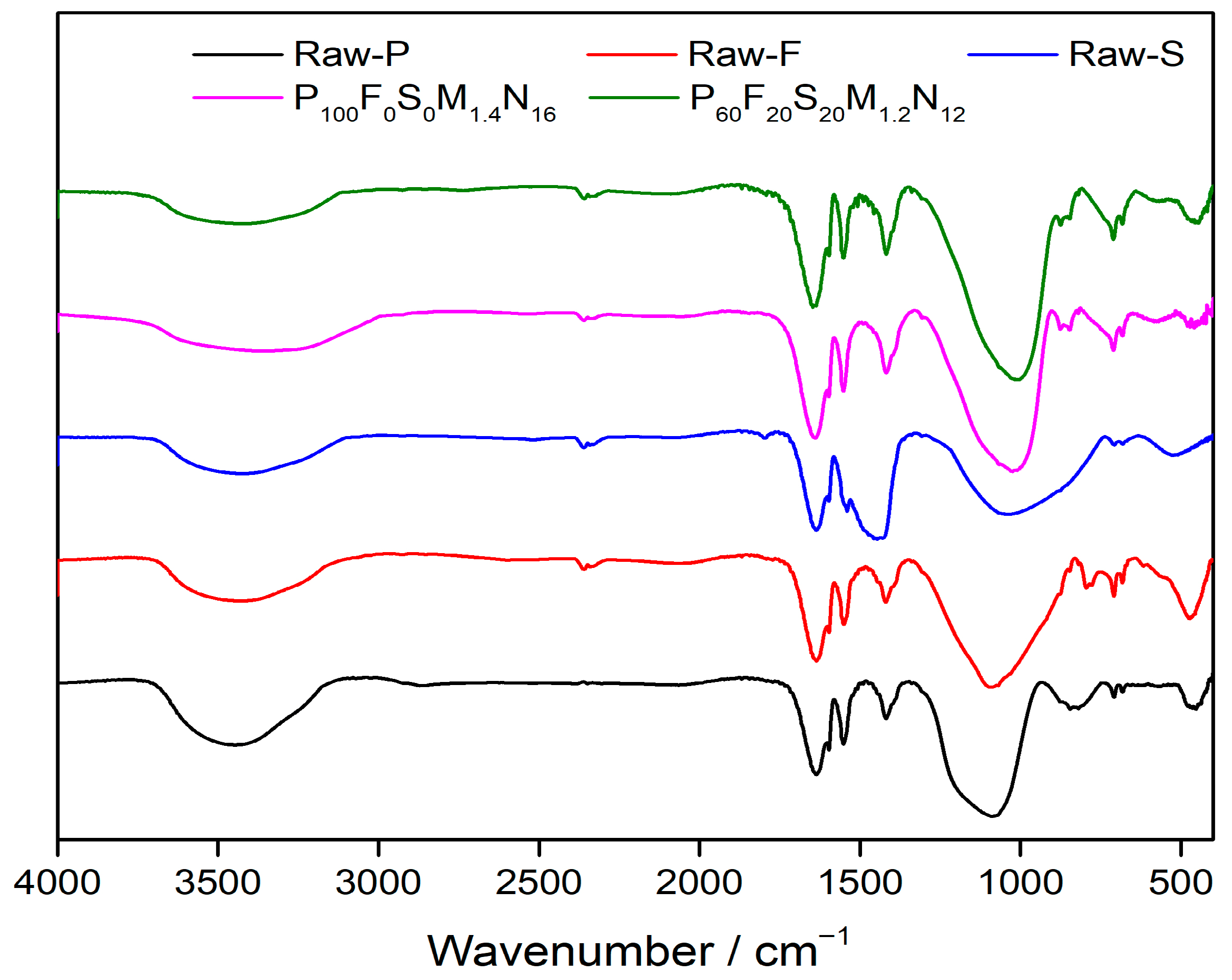
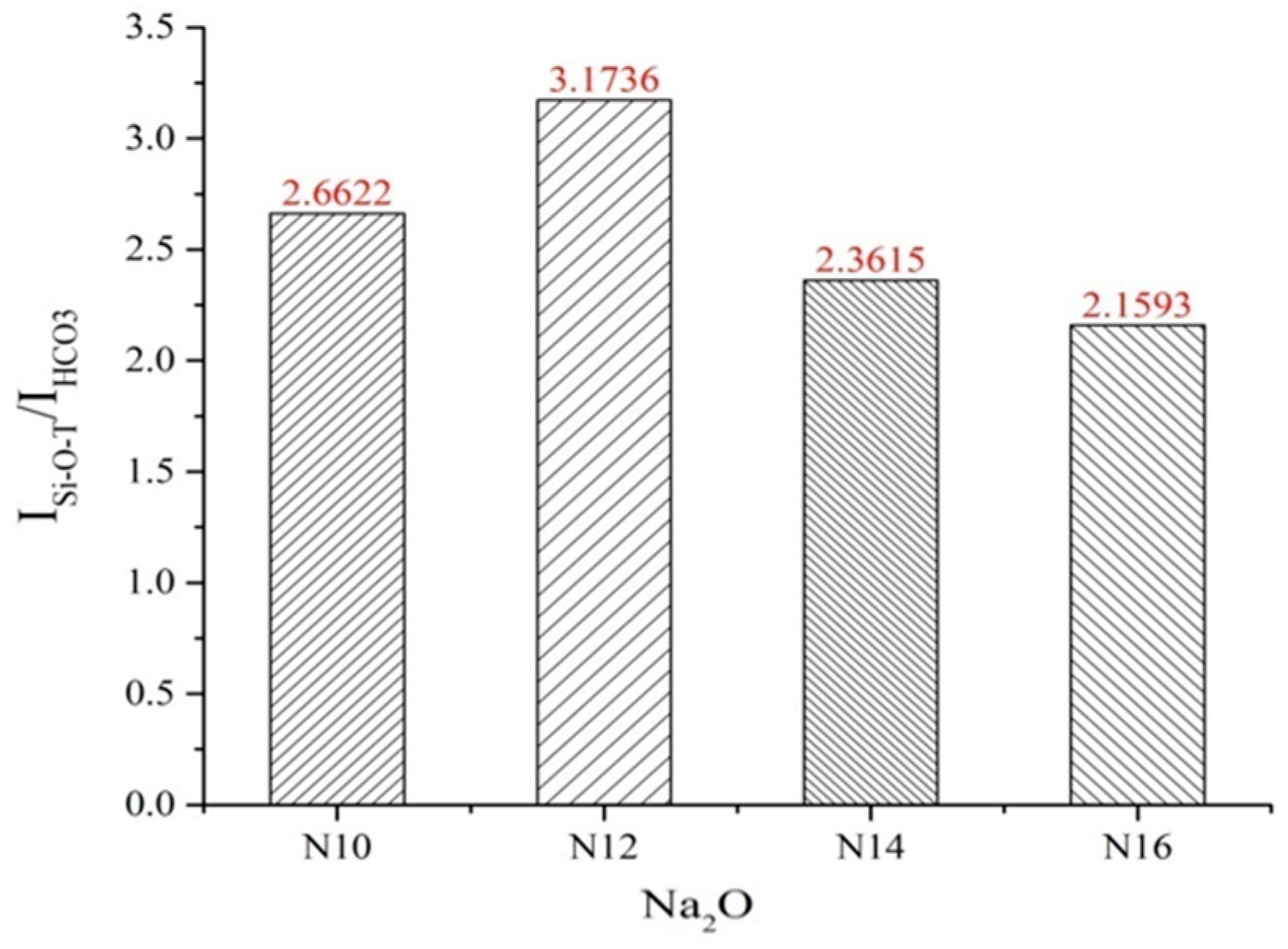
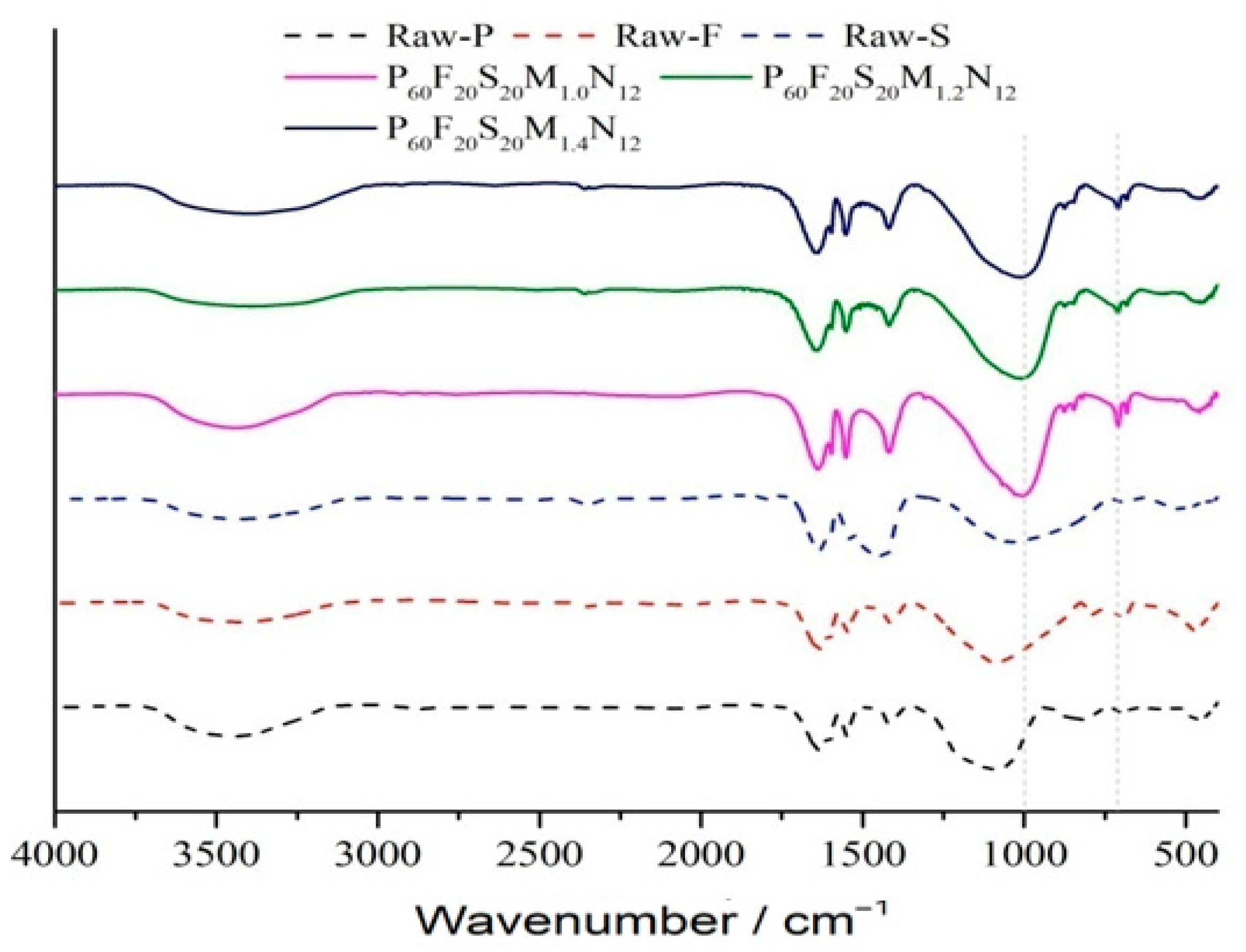
3.3.2. Infrared Spectroscopic Analysis
3.3.3. SEM Analysis
4. Conclusions
- (1)
- The setting time of the alkali activator increases with an increasing modulus within the range of 1.0 to 1.4. Under constant modulus conditions, the setting time decreases as the Na2O equivalent increases from 10% to 16%. The incorporation of fly ash and slag significantly reduces the setting time of the geopolymer system.
- (2)
- The molar ratio n(SiO2)/n(Al2O3) is a critical factor influencing geopolymer strength. Under fixed-modulus conditions, the effect of Na2O on strength becomes particularly pronounced. For high-metakaolin-based geopolymers, both flexural and compressive strengths increase continuously with n(SiO2)/n(Al2O3) ratios ranging from 2.47 to 2.97 and Na2O equivalents ranging from 10% to 16%, indicating that neither parameter has reached its critical threshold. When the n(SiO2)/n(Al2O3) ratio of fly ash- and slag-modified metakaolin reaches 3.28, the 28-day flexural and compressive strengths attain maximum values of 7.3 MPa and 63.5 MPa, respectively.
- (3)
- Based on XRD and FTIR spectral analyses, two optimal geopolymer formulations—P100F0S0M1.4N16 and P60F20S20M1.2N12—are identified under varying proportions of high-alkali metakaolin, fly ash, and slag. Shifts in the characteristic absorption peaks of metakaolin, fly ash, and slag confirm the occurrence of the geopolymerization reaction and the formation of geopolymer gels. SEM analysis further reveals that the P60F20S20M1.2N12 formulation exhibits the most compact microstructure and superior mechanical properties.
- (4)
- Using the fly ash and slag–metakaolin geopolymers with the best mechanical performance as a case study, XRD analysis demonstrates that the P60F20S20M1.2N12 geopolymer exhibits minimal characteristic peaks of quartz and albite, indicating a highly complete polymerization reaction. This leads to the formation of a substantial amount of amorphous silicate gel with excellent inter-gel bonding, resulting in optimal mechanical properties. FTIR analysis further confirms that this formulation exhibits the strongest relative Si-O-T absorption peak, the highest degree of geopolymerization, the most effective alkali activation, and, consequently, the greatest mechanical strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbhuiya, S.; Das, B.B. Life Cycle Assessment of construction materials: Methodologies, applications and future directions for sustainable decision-making. Case Stud. Constr. Mater. 2023, 1, e02326. [Google Scholar] [CrossRef]
- Bektaş, N.; Shmlls, M. Earthquake-Induced Waste Repurposing: A Sustainable Solution for Post-Earthquake Debris Management in Urban Construction. Buildings 2024, 14, 948. [Google Scholar] [CrossRef]
- Haigh, R.; Sandanayake, M.; Joseph, P.; Arun, M.; Yaghoubi, E.; Vrcelj, Z.; Sasi, S. The Thermo-Phase Change Reactivity of Textile and Cardboard Fibres in Varied Concrete Composites. Sustainability 2024, 16, 3221. [Google Scholar] [CrossRef]
- Woldesenbet, D.T.; Mohammed, J.J.; Negedu, S.D.; Henriques, J.; Bekele, E.A. Experimental investigation on partial cement replacement with binary blended bagasse ash and calcined dolomite for enhanced C-25 grade concrete performance. Sci. Rep. 2025, 15, 22844. [Google Scholar] [CrossRef]
- Johansson, L.; Bahrami, A.; Wallhagen, M.; Cehlin, M. A comprehensive review on properties of tailings-based low-carbon concrete: Mechanical, environmental, and toxicological performances. Dev. Built Environ. 2024, 18, 100428. [Google Scholar] [CrossRef]
- Klyuev, S.V.y.; Vatin, N.I.; Sabitov, L.S. Industrial and Civil Construction 2022: Selected Papers; Springer Nature: Cham, Switzerland, 2023; Volume 436. [Google Scholar]
- Le, V.S.; Sharko, A.; Sharko, O.; Stepanchikov, D.; Ercoli, R.; Nguyen, T.X.; Tran, D.H.; Buczkowska, K.E.; Dancova, P.; Łos, P.; et al. Multi-criteria optimization of geopolymer foam composition. J. Mater. Res. Technol. 2023, 26, 9049–9062. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Deng, Y.; Xu, F.; Hu, J.; Zhu, D.; Yu, Q.; Shi, C. Geopolymer composites for marine application: Structural properties and durability. Cem. Concr. Compos. 2024, 152, 105647. [Google Scholar] [CrossRef]
- Jamaludin, L.; Razak, R.A.; Abdullah, M.M.A.B.; Vizureanu, P.; Bras, A.; Imjai, T.; Sandu, A.V.; Abd Rahim, S.Z.; Yong, H.C. The Suitability of Photocatalyst Precursor Materials in Geopolymer Coating Applications: A Review. Coatings 2022, 12, 1348. [Google Scholar] [CrossRef]
- Yıldırım, A.C.; Toda, K.; Saito, T. Determination of the sorption mechanisms of sodium-alkalinized metakaolin-based geopolymers. Appl. Clay Sci. 2024, 251, 107303. [Google Scholar] [CrossRef]
- Amran, Y.H.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean production and properties of geopolymer concrete; A review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Mi, B.; Zhao, H.; Lu, M.; Zhou, Y.; Xue, Y. Synthesis of Electrolytic Manganese Slag–Solid Waste-Based Geopolymers: Compressive Strength and Mn Immobilization. Materials 2024, 17, 1431. [Google Scholar] [CrossRef]
- Janga, S.; Raut, A.N.; Murmu, A.L. Assessment of Thermal and Mechanical Properties of Fly Ash Geopolymer Blocks with a Sustainability Perspective using Multi-Criteria Decision-Making Approach. J. Build. Eng. 2024, 88, 109261. [Google Scholar] [CrossRef]
- Chen, K.; Liu, Q.; Chen, B.; Zhang, S.; Ferrara, L.; Li, W. Effect of raw materials on the performance of 3D printing geopolymer: A review. J. Build. Eng. 2024, 84, 108501. [Google Scholar] [CrossRef]
- Nenadović, S.; Gulicovski, J.; Mirković, M.; Kljajević, L.; Bošković, I.; Vukčević, M.; Nenadović, M. Structural, Mechanical and Chemical Properties of Low Content Carbon Geopolymer. Sustainability 2022, 14, 4885. [Google Scholar] [CrossRef]
- Abbas, R.; Abdelzaher, M.A.; Shehata, N.; Tantawy, M.A. Production, characterization and performance of green geopolymer modified with industrial by-products. Sci. Rep. 2024, 14, 5104. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, H.; Li, Y.; Liu, F.; Li, Q.; Mao, Y.; Yang, A. Geopolymers prepared from industrial solid waste: Comprehensive properties and application prospects. Environ. Res. 2025, 278, 121518. [Google Scholar] [CrossRef]
- Odimegwu, T.C.; Kaish, A.B.M.A.; Jamil, M.; Zain, M.F.M.; Turlanbekov, A.; Al Zand, A.W. Mechanical Characterization of Geopolymer Paste and Mortar Fabricated from Alum Sludge and Fly Ash. Buildings 2023, 13, 2118. [Google Scholar] [CrossRef]
- Sbahieh, S.; McKay, G.; Al-Ghamdi, S.G. Comprehensive Analysis of Geopolymer Materials: Properties, Environmental Impacts, and Applications. Materials 2023, 16, 7363. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Guo, T.; Han, P.; Wang, X.; Ma, F.; He, B. Durability study and mechanism analysis of red mud-coal metakaolin geopolymer concrete under a sulfate environment. Constr. Build. Mater. 2023, 409, 133990. [Google Scholar] [CrossRef]
- Wang, J.; Guo, R.; Wu, A.; Xiao, B.; Ruan, Z. Strength and Microstructural Characteristics of Activated Fly Ash–Cement Paste. Processes 2024, 12, 2356. [Google Scholar] [CrossRef]
- Lăzărescu, A.V.; Szilagyi, H.; Baeră, C.; Ioani, A. The Effect of Alkaline Activator Ratio on the Compressive Strength of Fly Ash-Based Geopolymer Paste. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209, 012064. [Google Scholar] [CrossRef]
- Lăzărescu, A.; Szilagyi, H.; Ioani, A.; Baeră, C. Parameters Affecting the Mechanical Properties of Fly Ash-Based Geopolymer Binders—Experimental Results. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012015. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Luhar, I.; Luhar, S. A Comprehensive Review on Fly Ash-Based Geopolymer. J. Compos. Sci. 2022, 6, 219. [Google Scholar] [CrossRef]
- Kanagaraj, B.; Lubloy, E.; Anand, N.; Hlavicka, V.; Kiran, T. Investigation of physical, chemical, mechanical, and microstructural properties of cement-less concrete—State-of-the-art review. Constr. Build. Mater. 2023, 365, 130020. [Google Scholar] [CrossRef]
- Mishra, J.; Nanda, B.; Patro, S.K.; Krishna, R.S. Sustainable Fly Ash Based Geopolymer Binders: A Review on Compressive Strength and Microstructure Properties. Sustainability 2022, 14, 15062. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, K.; Li, W.; Shi, G.; Lu, P. Effect of slag on the mechanical properties and bond strength of fly ash-based engineered geopolymer composites. Compos. Part B Eng. 2019, 164, 747–757. [Google Scholar] [CrossRef]
- Böke, N.; Birch, G.D.; Nyale, S.M.; Petrik, L.F. New synthesis method for the production of coal fly ash-based foamed geopolymers. Constr. Build. Mater. 2015, 75, 189–199. [Google Scholar] [CrossRef]
- Oikonomopoulos, I.K.; Perraki, M.; Tougiannidis, N.; Perraki, T.; Kasper, H.U.; Gurk, M. Clays from Neogene Achlada lignite deposits in Florina basin (Western Macedonia, N. Greece): A prospective resource for the ceramics industry. Appl. Clay Sci. 2015, 103, 1–9. [Google Scholar] [CrossRef]
- Alkan, M.; Hopa, Ç.; Yilmaz, Z.; Güler, H. The effect of alkali concentration and solid/liquid ratio on the hydrothermal synthesis of zeolite NaA from natural kaolinite. Microporous Mesoporous Mater. 2005, 86, 176–184. [Google Scholar] [CrossRef]
- Alujas, A.; Fernández, R.; Quintana, R.; Scrivener, K.L.; Martirena, F. Pozzolanic reactivity of low grade kaolinitic clays: Influence of calcination temperature and impact of calcination products on OPC hydration. Appl. Clay Sci. 2015, 108, 94–101. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Rose, V.; de Gutierrez, R.M. Evolution of binder structure in sodium silicate-activated slag-metakaolin blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Mozgawa, W. The relation between structure and vibrational spectra of natural zeolites. J. Mol. Struct. 2001, 596, 129–137. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]









| Chemical Composition | SiO2 | Al2O3 | Fe2O3 | K2O | TiO2 | CaO | Na2O | MgO | Loss |
|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 50.14 | 41.11 | 0.76 | 0.55 | 0.24 | 0.17 | 0.06 | 0.06 | 6.91 |
| Chemical Composition | CaO | SiO2 | Al2O3 | MgO | SO3 | TiO2 | MnO | Fe2O3 | K2O | Na2O | SrO | Loss |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 35.9 | 34.1 | 15.36 | 6.58 | 2.5 | 2.41 | 1.07 | 0.83 | 0.06 | 0.4 | 0.13 | 0.66 |
| Chemical Composition | SiO2 | Al2O3 | Fe2O3 | CaO | TiO2 | MgO | SO3 | Na2O | P2O5 | SrO | Loss |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 61.88 | 25.3 | 4.28 | 3.96 | 1.33 | 1.27 | 0.73 | 0.46 | 0.31 | 0.12 | 0.36 |
| Technical Parameters | Na2O Content (%) | SiO2 Content (%) | Modulus | Density (kg/m3) | Baume Degrees |
|---|---|---|---|---|---|
| Measured value | 8.5 | 26.5 | 3.1 | 1480 | 40 |
| Table | n(SiO2)/n(Al2O3) | n(Na2O)/n(Al2O3) | n(H2O)/n(Al2O3) |
|---|---|---|---|
| P100F0S0M1.0N10 | 2.47 | 0.4 | 6.75 |
| P100F0S0M1.0N12 | 2.55 | 0.48 | 6.72 |
| P100F0S0M1.0N14 | 2.63 | 0.56 | 6.69 |
| P100F0S0M1.0N16 | 2.71 | 0.64 | 6.66 |
| P100F0S0M1.2N10 | 2.55 | 0.4 | 6.61 |
| P100F0S0M1.2N12 | 2.65 | 0.48 | 6.55 |
| P100F0S0M1.2N14 | 2.74 | 0.56 | 6.49 |
| P100F0S0M1.2N16 | 2.84 | 0.64 | 6.43 |
| P100F0S0M1.4N10 | 2.63 | 0.4 | 6.46 |
| P100F0S0M1.4N12 | 2.75 | 0.48 | 6.38 |
| P100F0S0M1.4N14 | 2.86 | 0.56 | 6.29 |
| P100F0S0M1.4N16 | 2.97 | 0.64 | 6.21 |
| P60F20S20M1.0N10 | 3.06 | 0.5 | 8.46 |
| P60F20S20M1.0N12 | 3.16 | 0.6 | 8.42 |
| P60F20S20M1.0N14 | 3.26 | 0.7 | 8.39 |
| P60F20S20M1.0N16 | 3.36 | 0.8 | 8.35 |
| P60F20S20M1.2N10 | 3.15 | 0.5 | 8.28 |
| P60F20S20M1.2N12 | 3.28 | 0.6 | 8.21 |
| P60F20S20M1.2N14 | 3.4 | 0.7 | 8.14 |
| P60F20S20M1.2N16 | 3.52 | 0.8 | 8.07 |
| P60F20S20M1.4N10 | 3.26 | 0.5 | 8.1 |
| P60F20S20M1.4N12 | 3.4 | 0.6 | 8.0 |
| P60F20S20M1.4N14 | 3.54 | 0.7 | 7.89 |
| P60F20S20M1.4N16 | 3.68 | 0.8 | 7.78 |
| Wave Number/cm−1 | Key Type | References |
|---|---|---|
| 796, 778, 692, 688 | Quartz | [29] |
| 1091, 1078 | Vibration Si-O-Si | [30] |
| 1640, 1632 | Bending vibration H-O-H | [31] |
| 1453, 1385 | Asymmetric stretching O-C-O | [32,33] |
| 560 | Stretching vibration Al-O | [34] |
| 580 | Asymmetric stretching vibration T-O | [34] |
| 1024 | Asymmetric stretching vibration Si-O-Ta | [35] |
| 460 | Bending vibration T-O | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Z.; Li, Z.; Wang, P.; Song, Z.; Wu, L. Research on the Mechanical Properties and Microstructure of Fly Ash, Slag, and Metakaolin Geopolymers. Coatings 2025, 15, 1258. https://doi.org/10.3390/coatings15111258
Xing Z, Li Z, Wang P, Song Z, Wu L. Research on the Mechanical Properties and Microstructure of Fly Ash, Slag, and Metakaolin Geopolymers. Coatings. 2025; 15(11):1258. https://doi.org/10.3390/coatings15111258
Chicago/Turabian StyleXing, Zhiqiang, Zekang Li, Peng Wang, Zeming Song, and Li Wu. 2025. "Research on the Mechanical Properties and Microstructure of Fly Ash, Slag, and Metakaolin Geopolymers" Coatings 15, no. 11: 1258. https://doi.org/10.3390/coatings15111258
APA StyleXing, Z., Li, Z., Wang, P., Song, Z., & Wu, L. (2025). Research on the Mechanical Properties and Microstructure of Fly Ash, Slag, and Metakaolin Geopolymers. Coatings, 15(11), 1258. https://doi.org/10.3390/coatings15111258





