The Influence of Multi-Level Structure on the Bearing and Crack Propagation Mechanism of Tooth Enamel
Abstract
1. Introduction
2. Materials and Methods
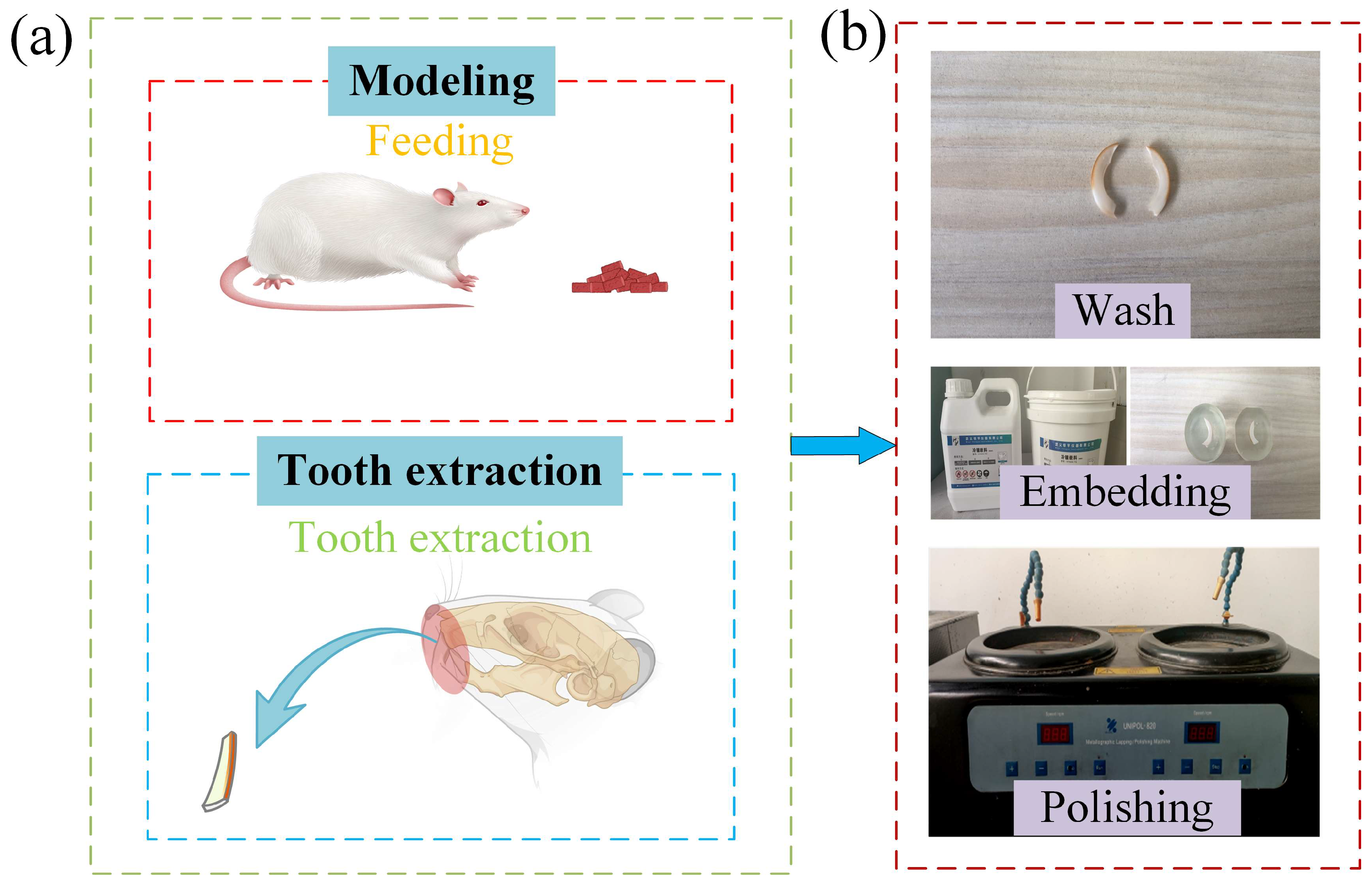
2.1. Rat Modeling
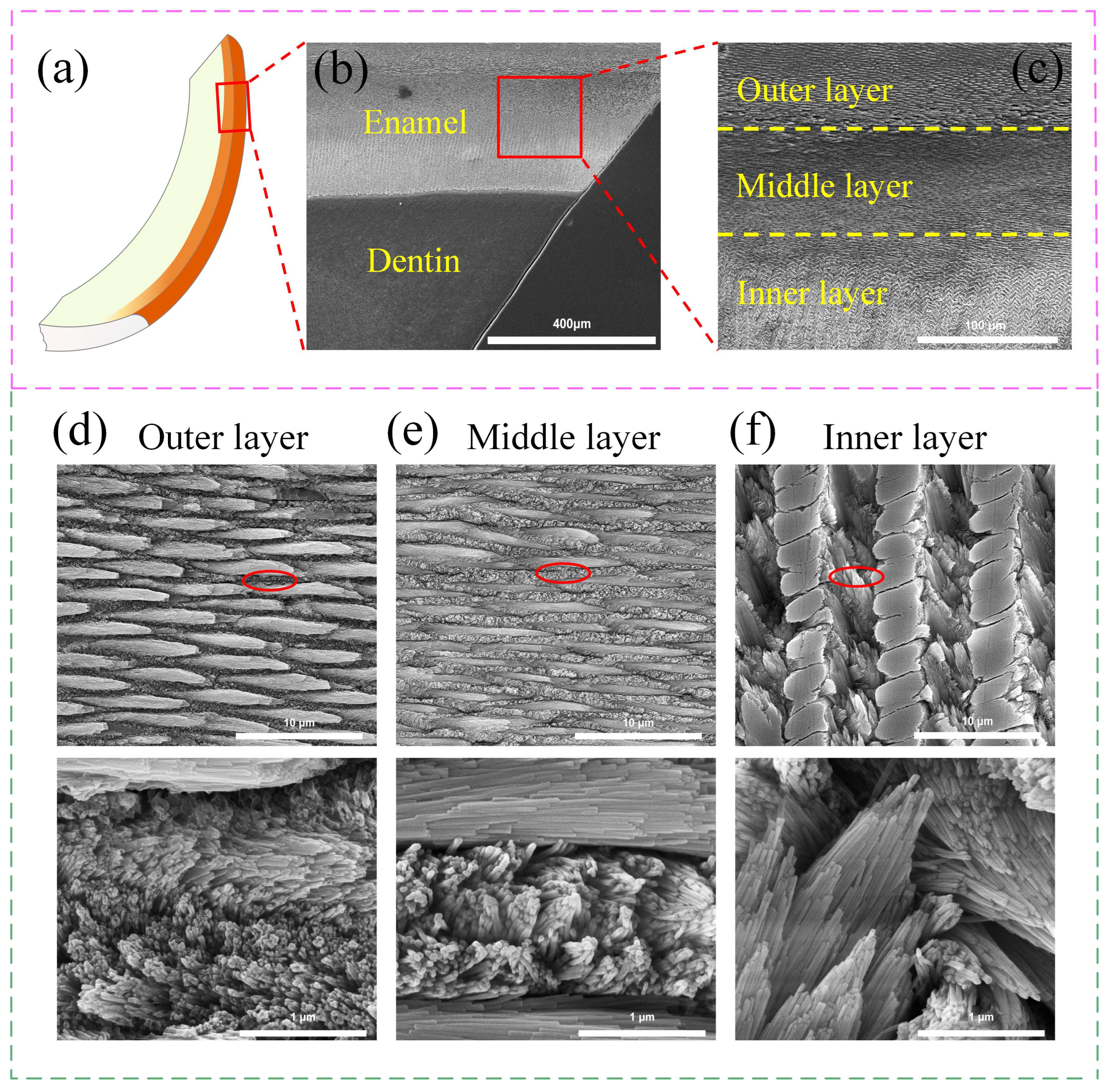
2.2. Methods for Preparing Tooth Samples
2.3. Microscopic Observation
2.4. Mechanical Performance Characterization
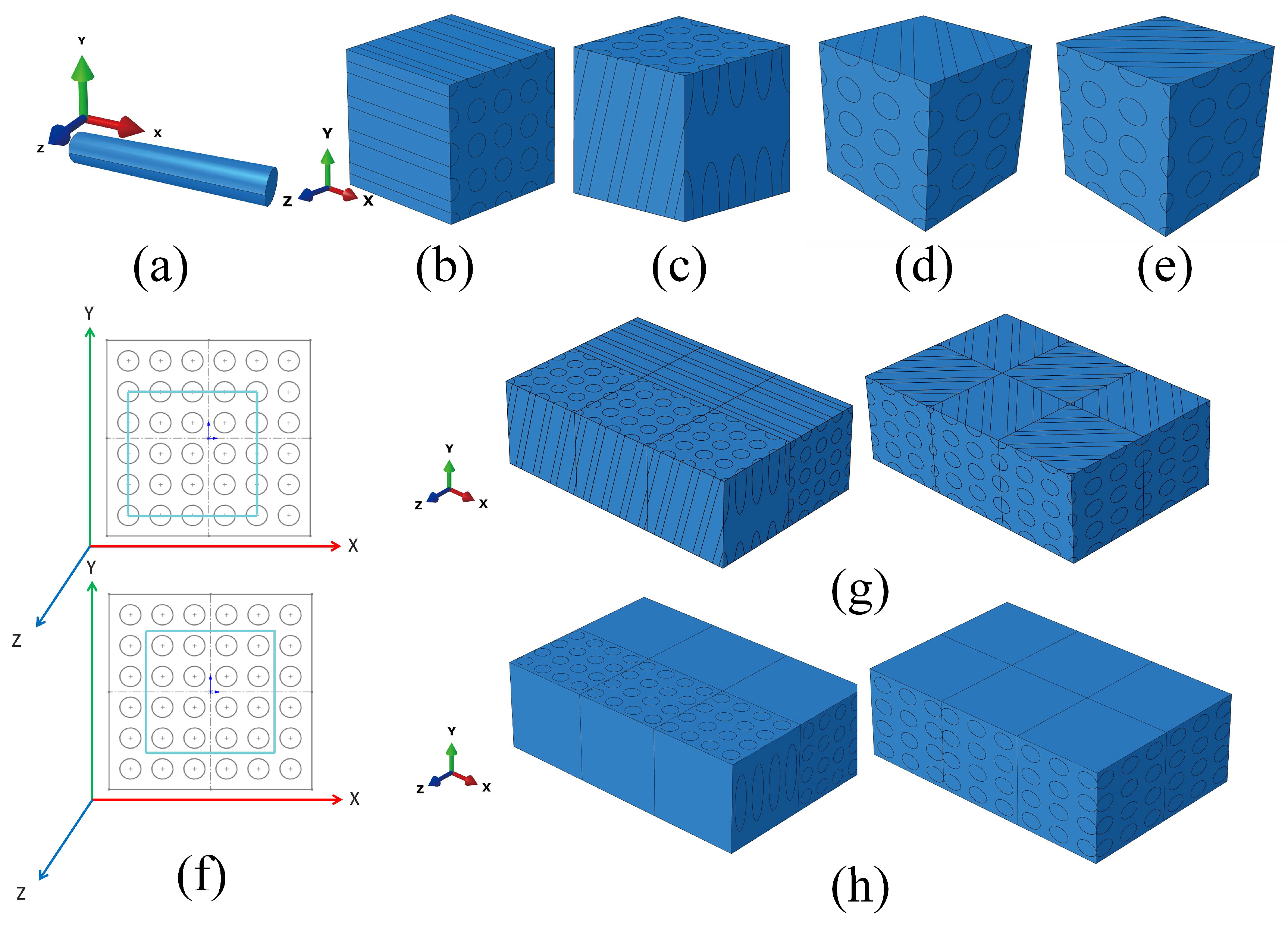
2.5. Modeling Method
2.6. Indentation Finite Element Model
2.7. Finite Element Model of Crack Analysis
3. Results and Discussion
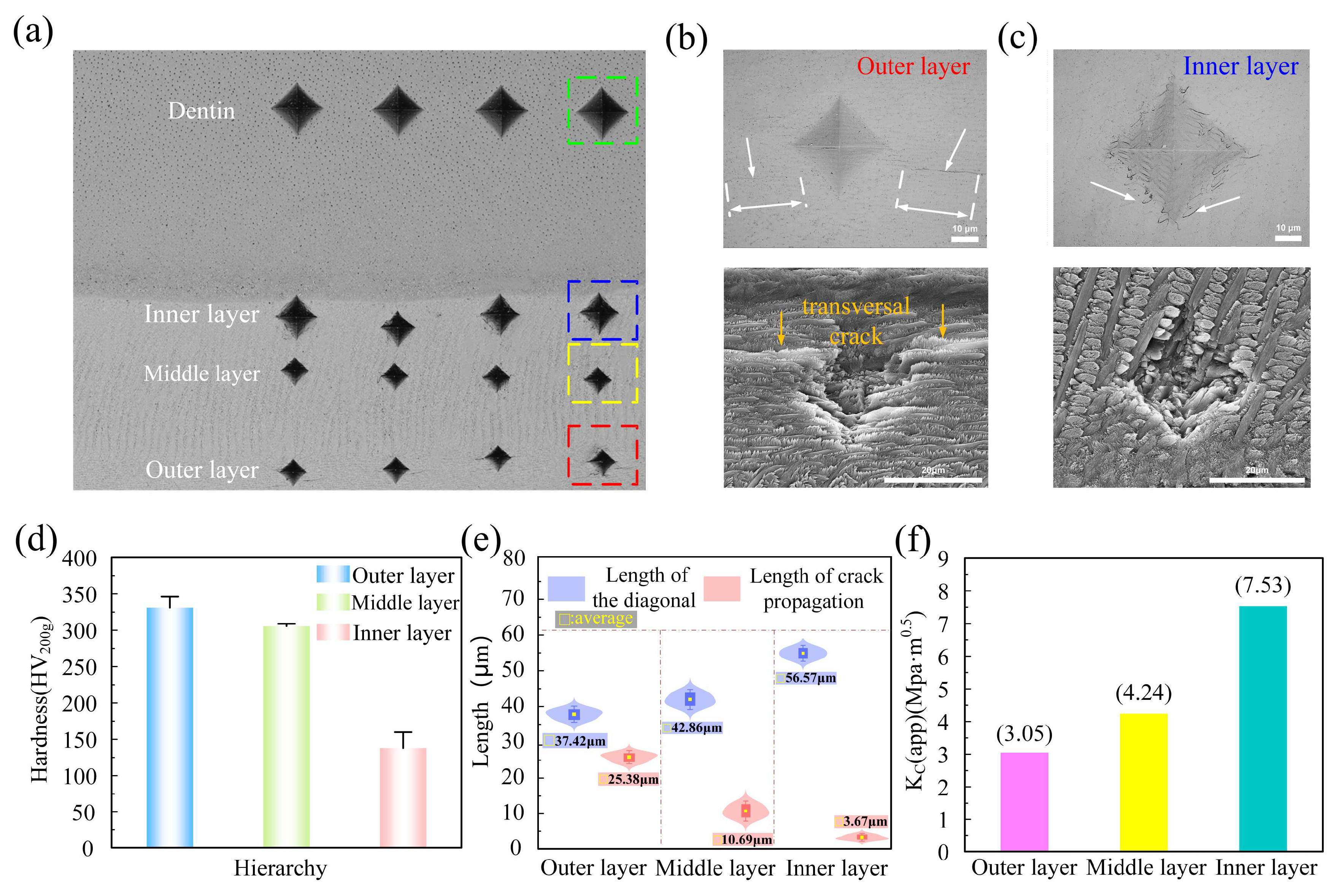
3.1. The Hardness and Fracture Toughness of Enamel from the Outer Layer to the Inner Layer
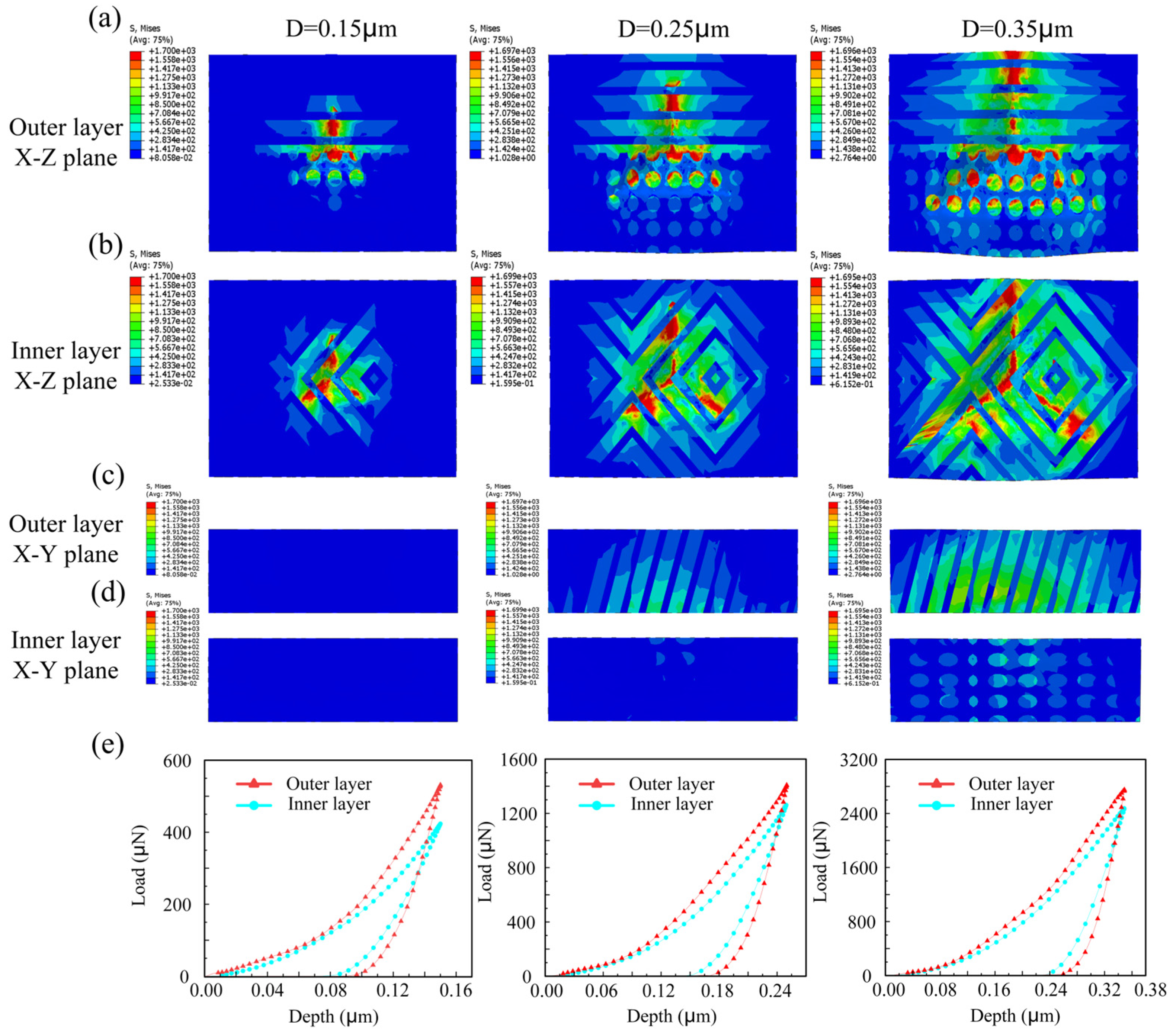
3.2. The Impact of Multi-Level Structure on Tooth Bearing Mechanism
3.2.1. Load–Indentation Depth Response Curve
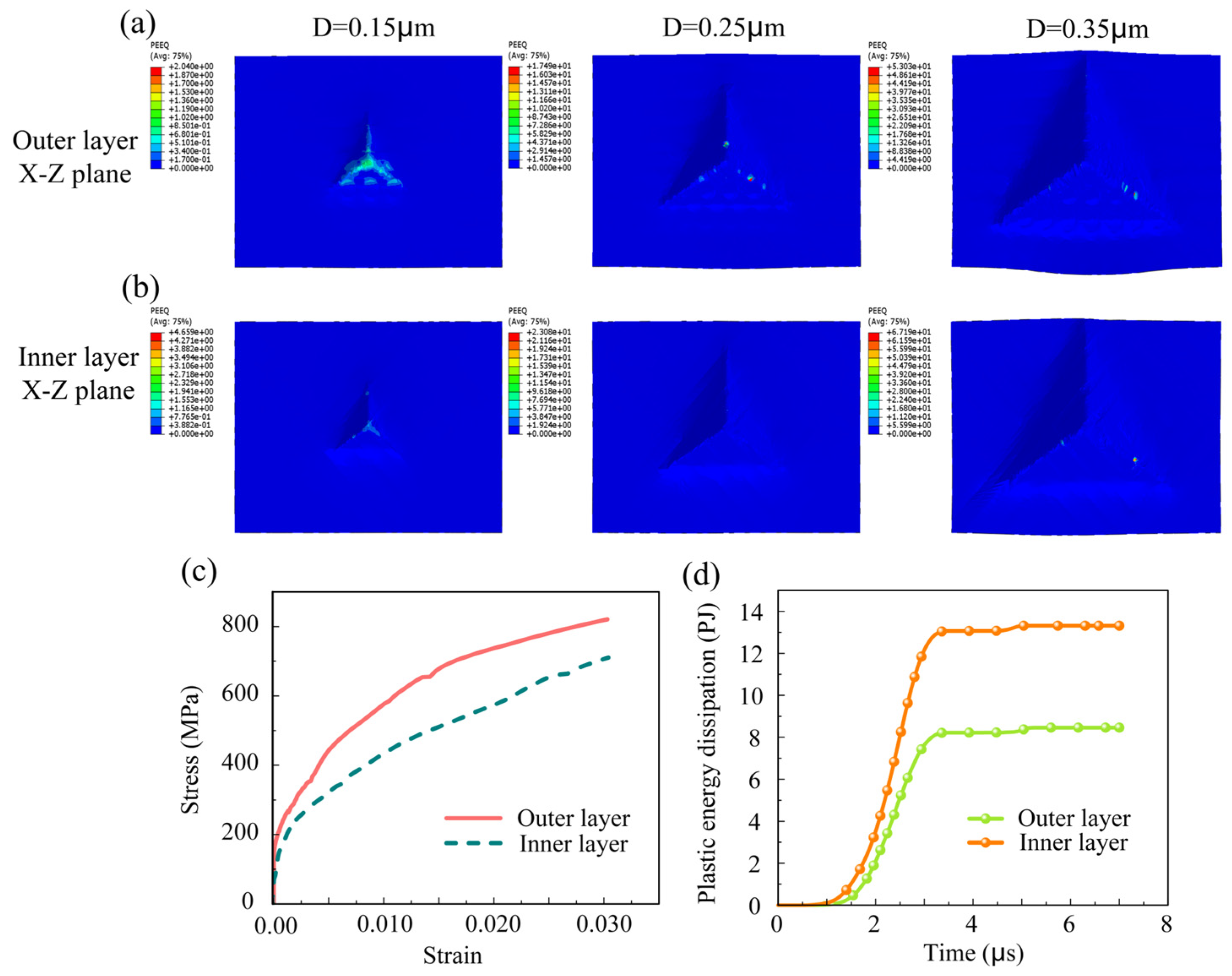
3.2.2. Stress–Strain Curve
3.3. The Influence of Multi-Level Structure on the Crack Propagation Mechanism of Teeth
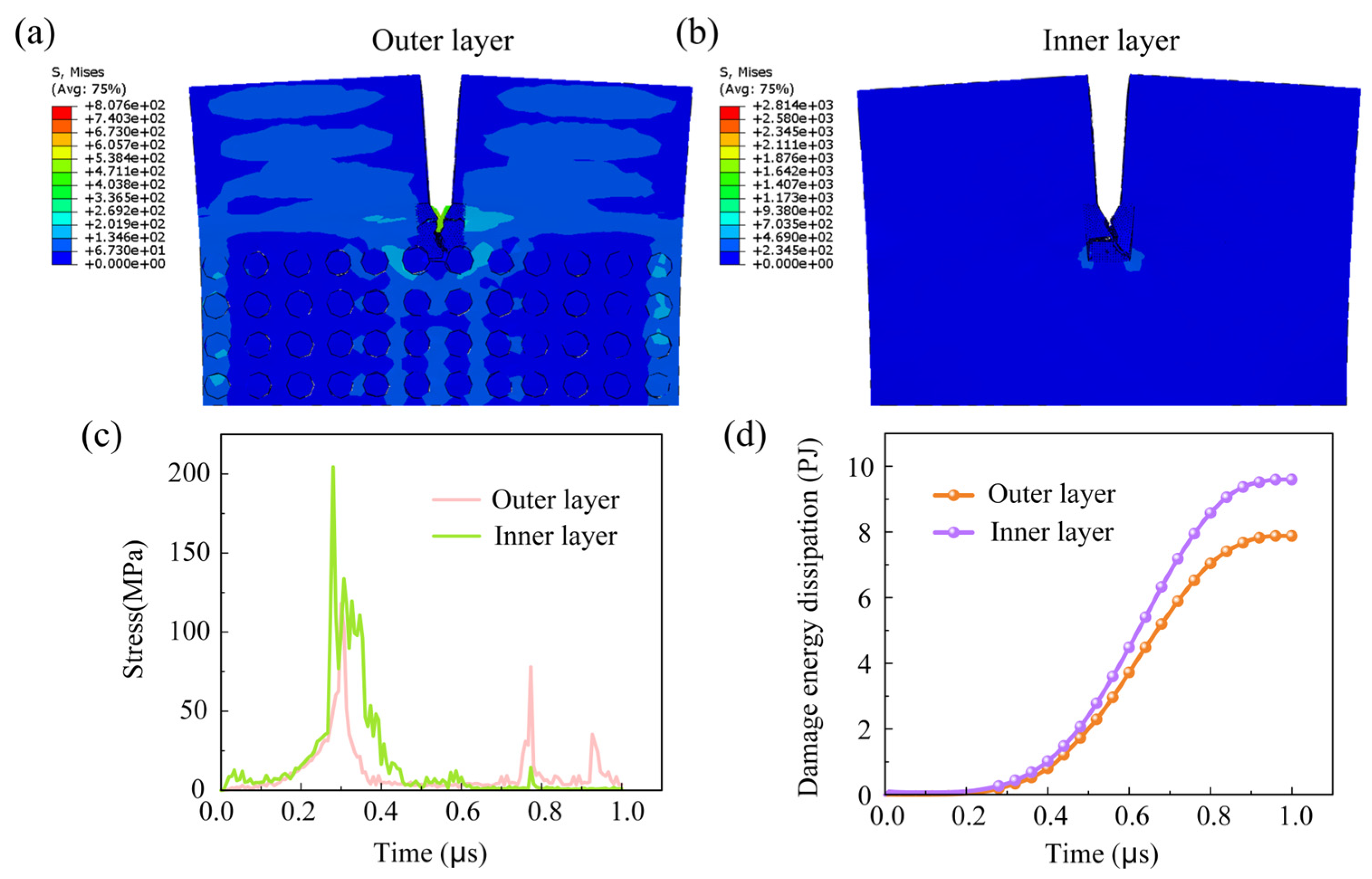
3.3.1. Observation of Crack Path
3.3.2. Time-Stress Curve
3.3.3. Crack Damage Energy Dissipation Curve
4. Conclusions
- (1)
- Research has indicated that the hardness of tooth enamel diminishes from its outermost layer to the inner layers, whereas its fracture toughness exhibits an incremental increase. Research indicates that the crack propagation patterns between the inner and outer layers can vary significantly, influenced by factors such as stress distribution and material properties.
- (2)
- The outer layer of enamel predominantly consists of parallel or obliquely oriented crystals. In contrast to the cross-arranged crystal bundle configuration of the inner layer, the outer crystal bundles facilitate continuous stress distribution and demonstrate greater stiffness, rendering them more resistant to deformation.
- (3)
- Regarding crack propagation behavior, the uniform crystal orientation in the outer layer renders it more susceptible to crack initiation, with cracks typically propagating in a straight line. In contrast, the distinctively crossed crystal arrangement in the inner layer can absorb more energy during crack propagation by triggering mechanisms like crack deflection and branching, thereby effectively impeding crack growth. The plastic dissipation energy of the inner layer is significantly higher than that of the outer layer, resulting in greater toughness.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, B.H.; Gao, H.J. Mechanical properties of nanostructure of biological materials. J. Mech. Phys. Solids 2004, 52, 1963–1990. [Google Scholar] [CrossRef]
- Dunlop, J.W.C.; Fratzl, P. Multilevel architectures in natural materials. Scr. Mater. 2013, 68, 8–12. [Google Scholar] [CrossRef]
- Shaik, I.; Dasari, B.; Shaik, A.; Doos, M.; Kolli, H.; Rana, D.; Tiwari, R.V.C. Functional role of inorganic trace elements on enamel and dentin formation: A review. J. Pharm. Bioallied Sci. 2021, 13 (Suppl. 2), S952–S956. [Google Scholar] [CrossRef] [PubMed]
- Andrejovská, J.; Petruš, O.; Medveď, D.; Vojtko, M.; Riznič, M.; Kizek, P.; Dusza, J. Hardness and indentation modulus of human enamel and dentin. Surf. Interface Anal. 2023, 55, 270–278. [Google Scholar] [CrossRef]
- Chu, K.J.; Zhao, C.C.; Ren, F.Z. Measuring fracture toughness of human dental enamel at small scale using notched microcantilever beams. Biosurface Biotribology 2021, 7, 228–232. [Google Scholar] [CrossRef]
- Wilmers, J.; Wurmshuber, M.; Gescher, C.; Graupp, C.M.; Kiener, D.; Bargmann, S. Unraveling the orientation-dependent mechanics of dental enamel in the red-necked wallaby. Acta Biomater. 2024, 185, 254–265. [Google Scholar] [CrossRef]
- Hegedűs, M.; Kis, V.K.; Szabó, Á.; Kovács, I.; Rózsa, N.; Kovács, Z. Gradient structural anisotropy of dental enamel is optimized for enhanced mechanical behaviour. Mater. Des. 2023, 234, 112369. [Google Scholar] [CrossRef]
- Lei, L. The Wear-Resistant Mechanism of Human Tooth Enamel and the Research on Biomimetic Design of Dental Ceramics. Southwest Jiaotong University, Chengdu, China. 2022. Available online: https://kns.cnki.net/kcms2/article/abstract?v=MuKJieOJLT2Mbvb1Qe3EQSmzTVU6vVstID8OWwNmmHHY7Aq8TwU6AvxR3o9Q-vvVZ7Y5_qbAs5rg1mrJwdH8Q_5CRAa0Cuako3_HhJe9t0AbhWFDI2AAbE03KOdNoY7KCjAzoxw0gj3uRH-uqt5k3w==&uniplatform=NZKPT&language=CHS (accessed on 1 June 2022).
- Beniash, E.; Stifler, C.A.; Sun, C.Y.; Jung, G.S.; Qin, Z.; Buehler, M.J.; Gilbert, P.U.P.A. The hidden structure of human enamel. Nat. Commun. 2019, 10, 4383. [Google Scholar] [CrossRef]
- Wilmers, J.; Bargmann, S. Nature’s design solutions in dental enamel: Uniting high strength and extreme damage resistance. Acta Biomater. 2020, 107, 1–24. [Google Scholar] [CrossRef]
- Borrero-Lopez, O.; Constantino, P.J.; Bush, M.B.; Lawn, B.R. On the vital role of enamel prism interfaces and graded properties in human tooth survival. Biol. Lett. 2020, 16, 20200498. [Google Scholar] [CrossRef]
- Jia, Y.F. Mechanical Property Characterization and Behavior Study of Biological Hierarchical Structures at the Micro-Nano Scale. East China University of Science and Technology, Shanghai, China. 2014. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbname=CDFD2015&filename=1015553385.nh&dbcode=CDFD (accessed on 4 December 2017).
- Chai, H.; Lee, J.J.W.; Constantino, P.J.; Lawn, B.R. Remarkable resilience of teeth. Proc. Natl. Acad. Sci. USA 2009, 106, 7289–7293. [Google Scholar] [CrossRef]
- Meza-Lastra, H.; Muy-Rangel, M.D.; Valdez-Melchor, R.G. Recuperation levels of hemoglobin (Hb) in Wistar rats with fermented beet by Lactobacillus sporogenes. TIP Rev. Espec. En Cienc. Quím.-Biol. 2018, 21. [Google Scholar] [CrossRef]
- He, L.H.; Swain, M.V. Understanding the mechanical behaviour of human enamel from its structural and compositional characteristics. J. Mech. Behav. Biomed. Mater. 2008, 1, 18–29. [Google Scholar] [CrossRef]
- Borrero-Lopez, O.; Rodriguez-Rojas, F.; Constantino, P.J.; Lawn, B.R. Fundamental mechanics of tooth fracture and wear: Implications for humans and other primates. Interface Focus 2021, 11, 20200070. [Google Scholar] [CrossRef] [PubMed]
- Özcoban, H.; Yilmaz, E.D.; Schneider, G.A. Hierarchical microcrack model for materials exemplified at enamel. Dent. Mater. 2018, 34, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhou, L.; Li, M. Fracture parameters evaluation for the cracked nonhomogeneous enamel based on the finite element method and virtual crack closure technique. Facta Univ. Ser. Mech. Eng. 2023, 21, 323–338. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, D.; He, Q.C. Investigation of the effective wear of enamel accounting for microstructure and phase properties. Wear 2019, 432, 202941. [Google Scholar] [CrossRef]
- Pro, J.W.; Barthelat, F. Discrete element models of fracture in tooth enamel: Failure mode competition and statistical effects. J. Mech. Phys. Solids 2020, 137, 103868. [Google Scholar] [CrossRef]
- Ma, S.; Scheider, I.; Bargmann, S. Continuum damage modeling and simulation of hierarchical dental enamel. Model. Simul. Mater. Sci. Eng. 2016, 24, 045014. [Google Scholar] [CrossRef]
- Ritchie, R.O. The conflicts between strength and toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef]
- Rivera, C.; Arola, D.; Ossa, A. Indentation damage and crack repair in human enamel. J. Mech. Behav. Biomed. Mater. 2013, 21, 178–184. [Google Scholar] [CrossRef]
- Bargmann, S.; Scheider, I.; Xiao, T.; Yilmaz, E.; Schneider, G.A.; Huber, N. Towards bio-inspired engineering materials: Modeling and simulation of the mechanical behavior of hierarchical bovine dental structure. Comput. Mater. Sci. 2013, 79, 390–401. [Google Scholar] [CrossRef]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Wan, B.Y.; Man, Z.Y.; Li, K.C.; Swain, M.V.; Li, Q. On elastoplastic behavior of porous enamel–An indentation and numerical study. Acta Biomater. 2024, 183, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Juha, S.; Ortiz, C.; Boyce, M.C. Anisotropic design of a multilayered biological exoskeleton. J. Mater. Res. 2009, 24, 3477–3494. [Google Scholar] [CrossRef]
- Spears, I.R. A three-dimensional finite element model of prismatic enamel: A re-appraisal of the data on the Young’s modulus of enamel. J. Dent. Res. 1997, 76, 1690–1697. [Google Scholar] [CrossRef]
- He, L.H.; Fujisawa, N.; Swain, M.V. Elastic modulus and stress–strain response of human enamel by nano-indentation. Biomaterials 2006, 27, 4388–4398. [Google Scholar] [CrossRef]
- Tvergaard, V.; Hutchinson, J.W. The relation between crack growth resistance and fracture process parameters in elastic-plastic solids. J. Mech. Phys. Solids 1992, 40, 1377–1397. [Google Scholar] [CrossRef]
- Al-Mukhtar, A.; Könke, C. Crack simulation in human teeth. Mater. Des. Process. Commun. 2021, 3, e200. [Google Scholar] [CrossRef]
- Shen, J.F.; Zhu, S.Q.; Kong, Y.Y.; Li, X.P.; Chen, M.M.; Zhou, Q.X.; Fan, Y.J.; Xia, J. Effect of iron deficiency and supplementation on tooth structure and properties. Wear 2025, 205817. [Google Scholar] [CrossRef]
- Anderson, R.A.; McBrayer, L.D.; Herrel, A. Bite force in vertebrates: Opportunities and caveats for use of a nonpareil whole-animal performance measure. Biol. J. Linn. Soc. 2008, 93, 709–720. [Google Scholar] [CrossRef]
- Fong, H.; Sarikaya, M.; White, S.N.; Snead, M.L. Nano-mechanical properties profiles across dentin–enamel junction of human incisor teeth. Mater. Sci. Eng. C 1999, 7, 119–128. [Google Scholar] [CrossRef]
- Mao, F.Y.; Li, C.K.; Meng, J.; Li, Q.; Bai, B.; Wang, Y.Q.; Zhang, Z.Q.; Zhao, L.X.; Wang, B.Y. Introduction to terminologies of tooth enamel microstructures and aproposal for their standard Chinese translations. Vertebr. Palasiat. 2017, 55, 347–366. [Google Scholar] [CrossRef]
- Liu, S.Y.; Xu, Y.Z.; Liao, R.C.; He, G.; Ding, L.; An, B.B.; Zhang, D.S. On fracture behavior of inner enamel: A numerical study. Appl. Math. Mech. 2023, 44, 931–940. [Google Scholar] [CrossRef]
- Liu, S.Y.; Xu, Y.Z.; An, B.B.; Zhang, D.S. Interaction of rod decussation and crack growth in enamel. Comput. Methods Biomech. Biomed. Eng. 2022, 26, 700–709. [Google Scholar] [CrossRef]
- Bajaj, D.; Arola, D. Role of prism decussation on fatigue crack growth and fracture of human enamel. Acta Biomater. 2009, 5, 3045–3056. [Google Scholar] [CrossRef]
- Bajaj, D.; Arola, D.D. On the R-curve behavior of human tooth enamel. Biomaterials 2009, 30, 4037–4046. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Y.; Xin, H.; Zhu, S.; Chen, M.; Fan, Y.; Xia, J. The Influence of Multi-Level Structure on the Bearing and Crack Propagation Mechanism of Tooth Enamel. Coatings 2025, 15, 1255. https://doi.org/10.3390/coatings15111255
Kong Y, Xin H, Zhu S, Chen M, Fan Y, Xia J. The Influence of Multi-Level Structure on the Bearing and Crack Propagation Mechanism of Tooth Enamel. Coatings. 2025; 15(11):1255. https://doi.org/10.3390/coatings15111255
Chicago/Turabian StyleKong, Yiyun, Haiyan Xin, Siqi Zhu, Mengmeng Chen, Yujie Fan, and Jing Xia. 2025. "The Influence of Multi-Level Structure on the Bearing and Crack Propagation Mechanism of Tooth Enamel" Coatings 15, no. 11: 1255. https://doi.org/10.3390/coatings15111255
APA StyleKong, Y., Xin, H., Zhu, S., Chen, M., Fan, Y., & Xia, J. (2025). The Influence of Multi-Level Structure on the Bearing and Crack Propagation Mechanism of Tooth Enamel. Coatings, 15(11), 1255. https://doi.org/10.3390/coatings15111255





