Abstract
Ophthalmic lens coatings are increasingly designed to combine optical, mechanical, and biological functions. This systematic review, registered in PROSPERO and conducted according to PRISMA 2020 guidelines, synthesized 54 experimental, preclinical, and clinical studies on coatings for spectacle lenses, contact lenses, and intraocular lenses. Spectacle lens studies consistently showed that anti-reflective and blue-light filtering coatings reduce glare perception, improve contrast sensitivity, and provide UV protection, while laboratory tests demonstrated significant reductions in impact resistance, with fracture energy of CR-39 lenses decreasing by up to 63% when coated. Contact lens research revealed that plasma and polymeric coatings reduce water contact angles from >100° to <20°, enhancing wettability, while antimicrobial strategies such as melamine binding or nanoparticle-based films achieved >80% reductions in bacterial adhesion. Drug-eluting approaches sustained antibiotic or antioxidant release for periods ranging from 24 h to 6 days, with improved ocular bioavailability compared with drops. Intraocular lens studies demonstrated that heparin surface modifications reduced postoperative flare and anterior chamber cells, and phosphorylcholine or alkylphosphocholine coatings suppressed lens epithelial cell proliferation. Drug-loaded coatings with methotrexate, gefitinib, or amikacin significantly inhibited posterior capsule opacification and infection in ex vivo and animal models. Collectively, coatings improve visual comfort, photoprotection, wettability, and biocompatibility, but clinical translation requires solutions to mechanical trade-offs, long-term stability, and regulatory challenges.
1. Introduction
The development of ophthalmic lenses, whether spectacle lenses, contact lenses, or intraocular lenses (IOLs), has traditionally focused on correcting refractive errors and restoring clear vision through improvements in optical materials, lens geometry, and surface coatings that reduce reflection, improve durability, and provide protection against ultraviolet (UV) radiation [1,2,3]. In the past few decades, the demands placed on these lenses have increased significantly with the proliferation of digital screens, a Light Emitting Diode (LED) lighting, and greater awareness of the role of light in circadian regulation and long-term retinal health [4]. As a result, not only are traditional optical coatings (anti-reflective, scratch-resistant, UV-blocking, photochromic, blue-light filtering) being refined, but functional coatings capable of more advanced manipulation of light, including luminescent or light-converting coatings, are gaining attention [5,6].
Luminescent thin film coatings have the ability to absorb certain wavelengths (e.g., UV or short-wavelength blue light) and re-emit them at longer, often visible, wavelengths. Such coatings hold promise for ophthalmic applications both for protection (e.g., by filtering potentially harmful high-energy light) and for enhancing visual performance (for example, by converting non-useful light into beneficial visible spectral components). Initial proof-of-concept studies show that glass or lens surfaces doped or coated with luminescent materials (such as rare earth ions or quantum dots) can increase visible light reaching photoreceptors under specific conditions, potentially benefiting situations with low light or for retinal degenerative conditions [7].
Parallel to these innovations, blue-light-filtering IOLs (often implemented via yellow chromophores or tinted materials) have been studied clinically for their effects on contrast sensitivity, color perception, and macular health [8,9,10]. For example, longitudinal randomized and comparative studies with blue-light-filtering IOLs versus non-tinted versions show reduced transmission of blue light and suggest potential protection of retinal cells, while generally showing minimal adverse effects on visual acuity and color discrimination over time [11]. Recent reviews summarize clinical outcomes and the ongoing debate about benefits versus perceptual trade-offs [12].
A new and rapidly developing direction is the incorporation of luminescent thin films, coatings that absorb incident light in one spectral band and re-emit it at longer wavelengths, onto optical substrates. Luminescent approaches can be broadly classified as down-conversion (absorbing higher-energy photons and re-emitting at longer wavelengths) and up-conversion (absorbing two or more lower-energy photons and emitting a single higher-energy photon). Materials that enable these processes include rare-earth dopants embedded in glass or polymer matrices, upconversion nanoparticles, and semiconductor quantum dots. In non-ophthalmic fields (e.g., smart windows, photovoltaics and bioimaging), luminescent conversion coatings have been shown to modify the spectral composition of transmitted light in useful ways, suggesting a route to novel ophthalmic functionalities such as enhanced visible output under scarce lighting, spectral reshaping to reduce retinal phototoxicity, or selective stimulation of specific photoreceptor subpopulations [13].
The translation of luminescent thin films into ophthalmic lenses is attractive but also technically demanding. Coatings must preserve high optical clarity and low scatter, maintain stable emission spectra and intensity over long wear periods, avoid introducing chromatic distortions or glare, and meet mechanical, chemical and biocompatibility requirements for eyewear and implantable devices. Manufacturing considerations, thin-film deposition on curved polymer or glass substrates, adhesion of luminescent dopants, durability of encapsulation, and regulatory safety for materials such as heavy-metal-containing quantum dots, add further complexity [14].
Despite these challenges, preliminary laboratory studies and cross-discipline advances suggest multiple potential clinical applications. Examples include (a) spectral protection against high-energy light while converting harmful UV or violet wavelengths into benign visible light to preserve scotopic sensitivity; (b) modulation of retinal irradiance in early or at-risk maculopathies; (c) augmentation of low-light visibility for people with rod- or cone-related dysfunction; and (d) integration with photochromic or anti-reflective stacks to yield multifunctional lens systems. Evidence from related domains (e.g., quantum-dot enhanced displays and luminescent polymer films) provides proof-of-principle for controlled spectral reshaping, though clinical evidence in humans remains scarce [15].
Given the multidisciplinary nature of the topic—bridging materials science, optics, vision science, and clinical ophthalmology—this review aims to provide a comprehensive synthesis of luminescent and advanced optical coatings applicable to ophthalmic lenses. It summarizes the main materials and deposition techniques, evaluates clinical and pre-clinical evidence on optical and functional performance, identifies key technical and regulatory challenges, and outlines future research directions toward safe and effective clinical translation.
In brief, the reviewed evidence indicates that luminescent and spectral-filtering coatings offer potential to enhance visual comfort and photoprotection while maintaining optical clarity, although their long-term stability and clinical validation remain open challenges.
2. Materials and Methods
2.1. Research Question and PICOS Framework
This systematic review was registered in PROSPERO (registration number: CRD420251155736) and conducted according to PRISMA 2020 guidelines [16] and AMSTAR-2 [17] methodological standards (Figure 1). A completed PRISMA checklist is provided as Supplementary Material (Supplementary File S1). The final literature search was completed on 18 September 2025. The research question was formulated using the PICOS framework to ensure methodological rigor and clinical relevance. Specifically, we aimed to evaluate whether ophthalmic lenses, including spectacle lenses, contact lenses, and intraocular lenses (Population), benefit from the application of coatings or surface modifications designed to enhance optical quality, mechanical durability, biocompatibility, or antimicrobial properties (Intervention), compared with uncoated or conventionally treated lenses (Comparator). Eligible outcomes included improvements in optical performance (e.g., glare reduction, contrast sensitivity, spectral filtering), mechanical resistance (e.g., abrasion, impact), surface wettability and antifouling properties, microbial adhesion, and biocompatibility markers such as postoperative inflammation or posterior capsule opacification. All study designs providing comparative experimental or clinical data were considered (Study design), encompassing in vitro, ex vivo, animal, and human trials across diverse geographic and clinical contexts. Subgroup analyses considered lens type (spectacles, contact lenses, IOLs), coating technology, and study setting as potential sources of heterogeneity. Through this comprehensive approach, our review sought to synthesize the available evidence on ophthalmic lens coatings and biomaterials and to provide insights into their translational potential for clinical practice and future innovation.

Figure 1.
PRISMA flow diagram of study selection.
2.2. Eligibility Criteria
Studies were excluded if they met any of the following conditions: (i) case reports or single-patient observations lacking generalizable outcomes; (ii) review articles (systematic or narrative), editorials, or conference abstracts without full data; (iii) duplicate publications derived from the same dataset or experimental series; or (iv) studies judged to present insufficient methodological detail or high risk of bias. Additional exclusions included reports lacking comparative data between coated and uncoated lenses or between different coating technologies; studies not addressing relevant outcomes related to optical performance, mechanical resistance, wettability, antimicrobial activity, or biocompatibility; and publications providing incomplete experimental, clinical, or preclinical data that precluded meaningful synthesis.
2.3. Information Sources
A comprehensive and systematic literature search was conducted using three major electronic databases: PubMed, Web of Science, and Scopus, without restrictions on publication date or language. To ensure complete coverage across the thematic domains of spectacle lenses, contact lenses, and intraocular lenses, as well as coating technologies and biomaterial modifications, the reference lists of all included articles were manually screened to identify additional relevant studies not captured by the initial search strategy.
2.4. Search Methods for Identification of Studies
The search strategy combined controlled vocabulary and free-text terms related to ophthalmic lenses, surface coatings, and biomaterial modifications. Keywords encompassed general descriptors of lens types (e.g., spectacles, contact lenses, intraocular lenses), coating processes and technologies (e.g., thin films, nanocoatings, sol–gel, hybrid surface treatments), and functional outcomes (e.g., antireflective, scratch-resistant, hydrophobic, UV- or blue-light filtering, luminescent and light-management properties). Complete search strategies tailored to each database are provided in Supplementary File S2.
Two reviewers independently assessed study eligibility at both the title/abstract screening and full-text review stages. Discrepancies were resolved through discussion and consensus. No language restrictions were applied, and studies published in languages other than English or Portuguese were translated and included when relevant data were available.
2.5. Data Extraction and Data Items
Two authors (A.P.O. and C.M.P.) independently extracted data from all eligible studies. For each included article, key characteristics were collected, including first author’s name, year of publication, country or institutional setting, study design (in vitro, in vivo, ex vivo, or clinical), lens type (spectacle, contact lens, intraocular lens), coating or surface modification investigated, coating technique, comparator, and primary outcomes. Discrepancies in data extraction or study inclusion were resolved through discussion and consensus, without the need for a third reviewer. Record management, including duplicate removal and tracking of study eligibility, was conducted using Microsoft Excel.
The primary variables extracted included coating technology (e.g., antireflective, scratch-resistant, hydrophobic/oleophobic, UV- or blue-light filtering, luminescent, antimicrobial, or drug-eluting), lens material (e.g., CR-39, polycarbonate, silicone hydrogel, rigid gas permeable, acrylic, or polymethylmethacrylate (PMMA)), and methodological approach (e.g., laboratory testing, tribological assays, surface chemistry, animal models, clinical trials). Additional variables, such as sample size (when applicable), outcome domains (optical performance, mechanical resistance, wettability, antimicrobial activity, biocompatibility), and reported limitations or conflicts of interest (e.g., patents, industrial funding), were also recorded to support synthesis and identify methodological heterogeneity across study types.
3. Results
3.1. Study Selection
A total of 440 records were initially retrieved from PubMed (n = 117), Web of Science (n = 123), and Scopus (n = 200) (Figure 1). After removal of duplicates and screening of titles and abstracts, 351 records were excluded for not being related to ophthalmic lenses, coatings, biomaterials, lacking assessment of relevant optical, surface, or biocompatibility parameters, lacking comparative analysis, or being case reports or review articles. Subsequently, 89 full-text articles were assessed for eligibility. Of these, 40 were excluded due to non-comparative data, dissimilar demographics, incomplete data, high risk of bias, or unavailability of shared data. Additionally, six relevant studies were identified through manual review of reference lists. In total, 54 studies met the inclusion criteria and were included in the qualitative synthesis [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71].
3.2. Study Characteristics
Supplementary File S3 summarizes the key characteristics of the 54 studies included in this synthesis, which investigated ophthalmic lenses, surface coatings, and biomaterials across diverse contexts, including spectacles, soft and rigid contact lenses, and intraocular lenses. The studies encompassed both laboratory-based experimental work (e.g., materials science, in vitro assays, tribology, and optical characterization) and clinical or preclinical research, ranging from crossover trials and randomized controlled studies in patients to in vivo animal models and ex vivo human capsular bag experiments.
Research objectives varied widely, including the development and evaluation of anti-reflective (AR), scratch-resistant (SR), superhydrophobic, and antifog coatings for spectacles; the modification of contact lens surfaces with antimicrobial peptides, polyphenolic layers, phosphorylcholine derivatives, or nanocomposites to improve wettability, reduce microbial adhesion, and deliver drugs; and the engineering of IOLs surfaces with heparin, alkylphosphocholines, methotrexate- or gefitinib-loaded slow-release matrices, and poly (2-methacryloyloxyethyl phosphorylcholine) (MPC) polymers to reduce postoperative inflammation, prevent posterior capsule opacification (PCO), and enhance biocompatibility.
Methodological approaches included advanced coating techniques such as plasma polymerization, vapor deposition, sol–gel processing, plasma-enhanced chemical vapor deposition (PECVD), laser ablation, nanoelectrospray, and mussel-inspired chemistries, as well as functional assays like protein adsorption analysis, microbial adhesion tests, fibroblast proliferation assays, tribological friction studies, UV–Vis spectroscopy, X-ray Photoelectron Spectroscopy (XPS), Atomic Force Microscopy (AFM), and Transmission Electron Microscopy (TEM). Clinical and preclinical designs evaluated patient-reported outcomes (e.g., glare reduction, comfort), contrast sensitivity, aqueous flare after cataract surgery, and efficacy in animal keratitis or capsular bag models.
Lens types investigated spanned CR-39, polycarbonate, Phoenix/Trivex, mid- and high-index polymers, silicone hydrogel and hydrogel soft lenses, rigid gas permeable (RGP) materials, and hydrophobic/hydrophilic acrylic or PMMA IOLs. Outcomes included enhanced visual comfort and contrast, reduced glare, improved wettability and antifouling, antimicrobial activity against bacteria, fungi, and Acanthamoeba, sustained drug release, inhibition of lens epithelial cells (LECs) adhesion and proliferation, and suppression of PCO.
Proposed solutions and implications covered the use of durable AR and scratch-resistant coatings for spectacles, multifunctional nanostructured films for optical devices, antimicrobial and antifouling surface treatments for contact lenses to prevent keratitis, and bioactive or drug-eluting coatings for IOLs to reduce inflammatory complications and posterior capsule opacification. Several studies also emphasized trade-offs, such as decreased impact resistance with some AR/SR coatings or color shifts with certain nanocoatings, highlighting the need for balanced optimization of optical, mechanical, and biological performance in future lens innovations.
3.3. Outcomes
3.3.1. Spectacle Lens Coatings
Spectacle lens coatings have been investigated extensively across clinical, laboratory, and materials science contexts, revealing a complex interplay between improved wearer experience, optical performance, and mechanical durability. Early clinical work demonstrated that coatings such as AR layers could meaningfully alter subjective visual experiences. In a crossover study, Bachman and Weaver [19] found that progressive addition lens wearers consistently preferred AR-coated CR-39 lenses compared with uncoated controls, reporting less glare and reflections with no measurable changes in visual acuity. Similarly, Coupland and Kirkham [28] tested a small cohort using magnesium fluoride AR coatings and showed that contrast sensitivity improved both with and without glare, indicating that even relatively simple AR designs can provide perceptual advantages. Ross and Bradley [55] confirmed these results, documenting reductions in glare perception and improved contrast in challenging light conditions, with patients expressing a clear preference for AR lenses despite no measurable acuity gains. Expanding on this, Leung, Li, and Kee [44] conducted a randomized comparison of blue-light filtering strategies and demonstrated that both AR-based filters and brown-tinted lenses reduced phototoxicity risk by 10–23% without altering contrast, color vision, or glare sensitivity, although subtle differences emerged in age-dependent preferences. Liou et al. [46] further advanced the protective rationale, showing in a UVB mouse model that both UV400 and photochromic coatings on CR-39 lenses prevented keratitis, visual loss, and inflammatory damage, offering preclinical validation that spectral filtering coatings can protect not only subjectively but also biologically.
While these clinical studies highlight wearer-centered benefits, a series of laboratory investigations has consistently raised concerns about the mechanical trade-offs introduced by coatings. Chou and Hovis [25,26] conducted ballistic and abrasion experiments on CR-39 and Phoenix lenses, finding that uncoated lenses, although more vulnerable to scratches, maintained superior impact resistance, whereas SR + AR coatings improved abrasion resistance but sharply reduced ballistic thresholds. Corzine et al. [29] provided quantitative support, demonstrating that AR coatings reduced fracture energy of CR-39 lenses by up to 63% and SR coatings by 57%, while AR-prep layers had no effect, indicating that coating chemistry rather than preparation per se drives mechanical weakening. Chou, Gupta, and Hovis [24] extended these findings to polycarbonate lenses, showing that multilayer anti-reflective (MAR) coatings combined with reduced thickness (2 mm) produced the lowest resistance in sharp missile tests, leading them to recommend against such configurations in industrial protection settings. Further work by Chou, Yuen, and Dain [27] tested mid-index organics (CR-39, Phoenix, Trilogy, Nikon, EYAS) and again observed that coatings sometimes reduced impact resistance, with AR effects highly dependent on base-coat interactions, raising compliance issues with safety standards. Chou, Dain, and Cheng [23] also studied the impact of UV exposure, finding that prolonged radiation reduced impact resistance across several coated materials, most notably in Trilogy AR lenses, whereas polycarbonate retained the highest resilience. Stroud [61] evaluated localized surface defects across glass, hard resin, and polycarbonate, showing that scratches, pits, and power aberrations were more frequent in polycarbonate lenses, largely due to thickness variations in scratch-resistant coatings, underscoring the importance of quality control in manufacturing. Together, these studies provide a consistent message: while coatings confer optical and comfort benefits, they may compromise the structural integrity of spectacle lenses, particularly under occupational or high-impact conditions.
At the same time, a dynamic body of materials science research has sought to engineer coatings with multifunctional properties that transcend the limitations of conventional AR and SR layers. Bozukova et al. [20] created hydrogel lenses incorporating zinc sulfide nanocomposites, which exhibited partial blue-light filtering, stable mechanical performance, and biocompatibility with lens epithelial cells, offering a conceptual foundation for biologically integrated ophthalmic materials. Gu, Ma, and He [35] developed carbon dot–polyvinyl alcohol hybrids fabricated at room temperature, which combined ultraviolet shielding, antifogging, light conversion, and notable durability, though without yet reaching clinical translation. Kats et al. [39] demonstrated that ultrathin germanium dielectric films (5–25 nm) deposited by e-beam evaporation on metallic substrates could produce broadband absorption resonances and vivid angle-insensitive colors, suggesting potential future ophthalmic applications in tunable filters. Kim [42] investigated graded-index AR stacks using silicon dioxide (SiO2), magnesium fluoride (MgF2), and indium tin oxide, achieving lower reflectance and enhanced violet-light blocking compared with commercial lenses, albeit with a slight violet tint. Huang et al. [36] contributed further by developing hydrophobic silica-based AR coatings via a template-free sol–gel route, achieving water contact angles of 126° and transmittance values up to 99.3% on PMMA substrates, thus combining optical enhancement with antifouling properties relevant to ophthalmic plastics. Paulson et al. [49] demonstrated the industrial feasibility of advanced coatings through reactive sputtering, achieving reflectance below 0.7% and hardness up to 18 GPa, surpassing commercial strengthened glass and already applied across millions of consumer optics, thereby illustrating scalability. Quiroga et al. [51] introduced rewritable gradient-index liquid crystal prototypes that maintained stability under UV photoalignment and thermal erasure cycles, opening the door to dynamic spectacle lenses for presbyopia correction. Schottner, Rose, and Posset [57] provided evidence on ORMOCER® sol–gel hybrids that combined abrasion resistance with UV-curability and compatibility with AR and photochromic layers, marking an early but influential example of hybrid multifunctional coatings. Xu et al. [68] contributed further with hierarchical nanostructures mimicking lotus leaves, achieving >95% transmittance across 530–1340 nm, superhydrophobicity (162° contact angle), and robust thermal and chemical resistance, presenting a proof-of-concept for highly durable and multifunctional AR coatings for ophthalmic lenses.
Taken together, these 20 studies highlight the tension and convergence in spectacle coating research. Clinical and preclinical work consistently demonstrates that AR, UV400, photochromic, and blue-light filtering coatings improve visual comfort, reduce glare, and provide meaningful protection against phototoxicity. In contrast, laboratory evaluations reveal that many conventional coatings reduce mechanical resilience, raising significant safety concerns for occupational and industrial applications, especially in thinner lenses. Meanwhile, materials science innovations are rapidly expanding the possibilities of what coatings can achieve, from nanocomposite filtering to dynamic gradient-index modulation, with industrial-scale methods such as reactive sputtering already demonstrating practical viability. The trajectory of spectacle coating development thus reflects an ongoing effort to integrate optical performance, wearer comfort, biological protection, and mechanical safety into unified solutions. Achieving this integration will require bridging the translational gap between experimental materials and clinically validated, durable lens products. As these advances converge, the next generation of spectacle lenses may overcome the current trade-offs, offering wearers both enhanced visual experiences and uncompromised protection.
3.3.2. Contact Lens Coatings
Contact lens coatings have been extensively investigated across clinical, preclinical, and laboratory domains, reflecting an ongoing attempt to address the long-standing challenges of comfort, fouling, infection, and drug delivery associated with both soft and RGP lenses. Early work demonstrated that surface modifications using plasma polymerization could meaningfully alter the wettability of silicone-based materials. Ho and Yasuda [38] pioneered the deposition of ultrathin methane-derived plasma films on silicone lenses, producing a reduction in hydrophobicity and tackiness without severely compromising oxygen permeability, and thereby laying the foundation for subsequent hydrophilic coatings. Decades later, Yin et al. [70] extended this approach to fluorosilicone acrylate RGP lenses, where argon plasma treatment introduced oxygen-containing moieties, reduced water contact angles from 107° to approximately 20°, and increased hydrophilicity, though high plasma power induced etching and surface degradation. Similarly, Shin, Jang, Kwon, and Mah [60] applied low-temperature air plasma to fluorosilicone RGPs, showing a marked reduction in contact angle and chemical transformation to silica-like layers, with wettability improvements largely attributable to surface chemistry rather than topography. Wang et al. [65] advanced plasma and deposition methods further by employing PECVD to coat orthokeratology lenses with N-vinylpyrrolidone and poly (ethyleneglycol) methacrylate (PEGMA), which yielded hydrophilic, protein-resistant surfaces stable for 90 days, representing a durable modification compatible with clinical replacement cycles.
In parallel with these physical and chemical strategies, bioinspired coatings have emerged as promising antifouling and lubricating approaches. Chang et al. [21] reported a mucin-inspired surface grafting technique that covalently immobilized mucin layers on silicone hydrogel lenses, transforming hydrophobic substrates into highly hydrophilic, low-friction surfaces that resisted lipid adsorption and significantly reduced tribological wear under simulated eyelid motion. Rosenhek-Goldian et al. [54] corroborated these findings by showing that mucin-functionalized hydrophobic substrates supported hydration layers capable of producing ultralow coefficients of friction, thereby mimicking natural tear-film lubrication. Extending bioinspired chemistries, Demian et al. [32] created a mussel-inspired polydopamine/tannin coating reinforced with chitosan, generating superhydrophilic silicone hydrogel surfaces within 15 min of immersion, with marked lipid repellence and partial Gram-positive antimicrobial activity. Yu et al. [71] developed a distinct hydrophilic polymerization method by coating commercial silicone hydrogels (ACUVUE® Advance) with a thin poly (dimethylacrylamide) film, which reduced contact angle from 106° to 45° and lowered the corneal friction coefficient by more than sixfold, highlighting the correlation between lubricity and wearer comfort. Vivero-López et al. [64] pursued another biomimetic avenue in RGPs, constructing dual-layer phenolic and peptoid coatings that improved antifouling performance, reduced protein adsorption, and conferred stable wettability, demonstrating the feasibility of multifunctional layering on rigid materials.
The antimicrobial potential of surface-functionalized lenses has been intensively explored, given the risks of keratitis and microbial keratopathy. Dutta et al. [33] engineered melamine-coated lenses, showing broad-spectrum activity against bacteria, fungi, and Acanthamoeba, with sustained efficacy even after autoclaving, while retaining biocompatibility with mammalian cells. Cheng et al. [22] complemented this with catechol–polyethyleneglycol–urea (catechol–PEG–urea) functionalized coatings, deposited via simple aqueous immersion, that conferred antifouling and antibacterial properties persisting over seven days, including resilience to steam sterilization. Dai et al. [30] advanced the concept further by developing glycocalyx-mimetic coatings on therapeutic bandage lenses, incorporating polydopamine with silver/copper nanoparticles and thiolated heparin, achieving >80% bacterial reduction, decreased protein adsorption, and therapeutic benefit in rabbit keratitis models. Santos et al. [56] introduced additional multifunctional designs using polyphenolic grafting on RGPs, demonstrating decreased microbial adhesion alongside enhanced surface wettability, emphasizing the adaptability of phenolic polymers to rigid platforms.
Controlled drug delivery via contact lens coatings has emerged as another frontier. Danion, Arsenault, and Vermette [31] immobilized levofloxacin-loaded liposomes onto lens surfaces, achieving sustained antibiotic release over six days with complete inhibition of S. aureus at lower inocula, although higher bacterial burdens required an initial burst. Shi et al. [58] employed nano-electrospray deposition of poly (lactic-co-glycolic acid) (PLGA) films encapsulating antiallergic and antiglaucoma drugs (ketotifen, latanoprost, bimatoprost), demonstrating sustained delivery modulated by drug hydrophobicity, though sterilization via autoclave degraded polymer matrices, underscoring industrialization challenges. Singh et al. [59] developed phosphorylcholine (MPC)-based hydrogel platforms capable of loading >10 mg/g of resveratrol and releasing it over 24 h, yielding superior ocular bioavailability in rabbits compared with eye drops, while retaining antifouling properties and antioxidant activity. Tam et al. [62] explored hydrophilic monomer-based surface coatings engineered to facilitate drug uptake and prolonged release profiles, further bridging the gap between comfort and therapeutic efficacy.
Alongside antimicrobial and drug-eluting strategies, multiple groups have sought to understand and mitigate lens fouling. Peng et al. [50] established a model-blink cell that replicates tear-film rupture and reformation, showing that silicone hydrogels develop discrete protein–lipid deposits consistent with in vivo spoilation, and validating the system as a robust screening platform for antifouling coatings and care regimens. Rickert et al. [53] evaluated protein and lipid fouling across commercial silicone hydrogel and hydrogel lenses, demonstrating that material composition and surface treatment substantially influence microbial colonization, with Balafilcon A exhibiting particularly high susceptibility, likely due to hydrophobicity and acid–base surface properties. Willis et al. [67] earlier examined surfactant and protein-based surface layers, reporting short-term improvements in wettability but highlighting their transient durability. Refaei et al. [52] and Tsai et al. [63] further studied surface energy and deposition patterns across lenses, showing that surface modifications alter lysozyme accumulation and microbial adhesion in clinically relevant ways.
Rigid lens coatings also represent a critical area of innovation, particularly for scleral and corneal gas permeable (GP) wearers where wettability and protein deposition strongly influence comfort. Yamasaki et al. [69] investigated the role of care solutions containing hyaluronic acid derivatives (HAD) on poly (ethyleneglycol)-coated RGPs (Hydra-PEG®). They found that HAD enhanced wettability in both PEG-coated and uncoated lenses, reduced lysozyme deposition, and protected PEG layer integrity under repeated cycling, whereas solutions lacking HAD led to deteriorated wettability and coating loss. This demonstrates that coating performance is not solely material dependent but strongly modulated by care regimens.
Taken together, these 24 studies illustrate the multifaceted progression of contact lens coating technologies, from early plasma-based approaches that provided proof-of-concept for modifying hydrophilicity without compromising oxygen permeability, to contemporary bioinspired and polymeric strategies that integrate antifouling, lubrication, antimicrobial, and drug delivery functions. Clinical and preclinical investigations confirm that covalently bound antimicrobial peptides, liposome- and nanoparticle-based drug reservoirs, and glycocalyx-mimetic nanocoatings can reduce microbial adhesion and keratitis risk, while hydrophilic polymer films, mucin analogues, and zwitterionic platforms consistently improve wettability and tribological performance. Importantly, evidence from rigid gas permeable and scleral lenses demonstrates that the durability of coatings is strongly influenced by care regimens, with hyaluronic acid derivative–containing solutions prolonging PEG-coating efficacy and minimizing protein fouling. Collectively, these findings reveal a convergence toward multifunctional, durable, and biocompatible coatings capable of enhancing comfort, safety, and therapeutic utility, but they also highlight divergent technological routes, from biomimetic lubricating layers to nanoparticle antimicrobials and polymeric drug-eluting depots, that must be carefully balanced for clinical translation. Achieving this integration will require coating stability, scalable manufacturing, and regulatory acceptance, while prioritizing wearer-centered outcomes of comfort, infection prevention, and visual quality.
3.3.3. Intraocular Lens Coatings
IOL surface modifications have been investigated over several decades in an effort to improve postoperative outcomes, minimize inflammatory complications, and prevent PCO. Early research centered on heparin surface modification (HSM) of PMMA IOLs, which was hypothesized to reduce postoperative uveal inflammation by conferring hydrophilicity and anticoagulant properties. Arthur et al. [18] demonstrated in a clinical evaluation that patients implanted with heparin-modified PMMA lenses exhibited reduced flare and fewer anterior chamber cells in the early postoperative period compared with standard PMMA IOLs, suggesting improved uveal biocompatibility. Lundberg et al. [47] provided complementary laboratory data, showing that heparin-coated IOLs significantly reduced the adhesion of Staphylococcus epidermidis under dynamic flow conditions, supporting a dual rationale for HSM lenses: decreased inflammation and reduced bacterial colonization. Werner et al. [66] corroborated these findings through explant analyses, showing fewer giant cell deposits and less surface fibrosis on heparin-coated lenses compared with non-coated controls, further validating the concept that heparinization improved ocular tolerance.
Beyond heparin, other biocompatible coatings have been explored to reduce LEC proliferation and migration, which are central to PCO. Okajima et al. [48] coated hydrophobic acrylic lenses with MPC, a zwitterionic polymer known to mimic cell membrane phospholipids. Their in vitro experiments demonstrated that MPC-coated lenses suppressed LEC adhesion, proliferation, and migration beneath the IOL optic, particularly in collagen IV matrices that simulate capsular bag substrates. These findings suggested that phosphorylcholine-based surface modification could enhance capsular biocompatibility and reduce the fibrotic component of PCO. Similarly, Eibl et al. [34] investigated alkylphosphocholine (APC)-coated IOLs in preclinical models, showing that such lenses inhibited LEC proliferation while being well tolerated in corneal systems, reinforcing the role of phosphocholine derivatives as promising antifibrotic coatings.
Clinical studies have also supported these laboratory findings. Krall et al. [43] conducted a randomized trial of heparin-coated hydrophobic acrylic lenses, reporting a significant reduction in aqueous flare and cells during the early postoperative weeks compared with unmodified acrylic lenses. These improvements were attributed to reduced protein adsorption and diminished inflammatory cell activation, suggesting that surface chemistry remained influential even in modern foldable acrylic designs. Hussain et al. [37] expanded the paradigm by incorporating pharmacologic strategies directly into IOL coatings, experimenting with antiproliferative drug loading to inhibit LEC activity. Although their studies were preliminary, they highlighted the translational potential of combining mechanical and chemical strategies to suppress secondary cataract formation.
Building upon these concepts, Kassumeh et al. [40,41] introduced drug-eluting IOL platforms aimed at targeted PCO prevention. In 2018, they reported a PLGA-based coating incorporating methotrexate (MTX), an antiproliferative agent, onto acrylic IOL surfaces. Their ex vivo human capsular bag model demonstrated marked inhibition of LEC proliferation and PCO development, with sustained drug release over several days [40]. Extending this strategy, Kassumeh et al. [41] evaluated gefitinib, an epidermal growth factor receptor (EGFR) inhibitor, embedded in similar polymeric coatings. Gefitinib-coated lenses effectively suppressed LEC growth and migration in vitro and in ex vivo models, suggesting the feasibility of tailoring coatings to target specific molecular pathways involved in capsular fibrosis. Together, these studies established the proof-of-principle that IOLs could act as local drug delivery depots, addressing PCO at its source.
More recently, Li et al. [45] developed multifunctional coatings that integrated both antifouling and antimicrobial capabilities. By combining zwitterionic phosphorylcholine layers with covalently bound amikacin, they created IOLs capable of loading approximately 35.5 µg of antibiotic and releasing it in a sustained manner for up to 30 days. In rabbit implantation studies, these coatings prevented bacterial colonization, reduced biofilm formation, and maintained good biocompatibility, while simultaneously decreasing lens epithelial adhesion. Such multifunctional coatings exemplify the current trajectory of innovation: lenses that combine passive antifouling with active pharmacological defense, bridging infection control and PCO prevention.
While most investigations focus on preventive strategies, it is also important to recognize the limitations and complications associated with IOL coatings.
Taken together, these 10 studies highlight the evolution of IOL surface modification strategies from early heparinization of rigid PMMA lenses to contemporary multifunctional, drug-eluting hydrophobic acrylic platforms. Early clinical and laboratory evidence consistently demonstrated that heparin surface modification improved uveal biocompatibility and reduced inflammatory sequelae, findings subsequently expanded to phosphocholine and alkylphosphocholine coatings that specifically targeted LEC behavior and PCO. Clinical trials have supported reduced flare and early inflammation with coated lenses, while ex vivo and in vivo preclinical work has provided strong evidence that local drug delivery via methotrexate-, gefitinib-, or amikacin-eluting coatings can significantly suppress LEC proliferation, fibrosis, and infection. At the same time, review of explanted lenses reminds us that surface modifications may introduce long-term stability issues, underscoring the need for rigorous testing. Overall, the trajectory of IOL coating research reflects a convergence toward multifunctional and bioactive designs capable of improving surgical outcomes, reducing complications, and potentially transforming the IOL from a passive refractive element into an active therapeutic device. Future clinical translation will depend on balancing coating stability, sterilization compatibility, and manufacturing scalability with the promise of reducing PCO, enhancing ocular biocompatibility, and delivering sustained pharmacological protection.
A conceptual overview of coating strategies across all ophthalmic lens types (including spectacle, contact, and intraocular lenses) is summarized in Figure 2, which integrates the main materials, functional effects, advantages, and biomedical implications described in Section 3.3.1, Section 3.3.2 and Section 3.3.3.
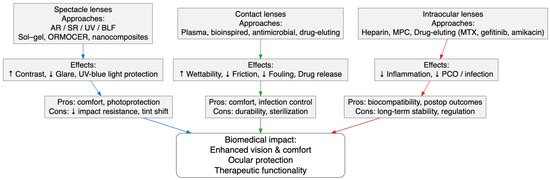
Figure 2.
Conceptual diagram summarizing coating materials and functional approaches across spectacle, contact, and intraocular lenses, showing their main effects, advantages, limitations, and biomedical relevance.
4. Discussion
The synthesis of 54 studies in this review demonstrates that ophthalmic lens coatings have progressed from traditional anti-reflective and scratch-resistant layers to multifunctional systems that integrate optical, mechanical, and biological performance. Our findings confirm that coatings improve subjective visual comfort and provide meaningful protection against phototoxicity, but they also reveal mechanical trade-offs and translational challenges. This duality closely mirrors the conclusions of Samson’s classical review of ophthalmic lens coatings, which already emphasized the importance of anti-reflective treatments for wearer satisfaction while warning of their potential influence on durability and safety [72]. More recently, Martínez-Pérez et al. [73] provided meta-analytic evidence that spectral filters, including blue-light and UV-blocking coatings, reduce glare and enhance contrast sensitivity, consistent with the clinical and preclinical studies identified in our review. Both bodies of work confirm that wearer-centered optical benefits are robust, although mechanical vulnerabilities remain underexplored.
The laboratory evidence synthesized here, showing that AR and scratch-resistant coatings reduce impact resistance in CR-39 and polycarbonate lenses, also resonates with the concerns raised historically by Samson [72] and is consistent with the trade-offs reported by Martínez-Pérez et al. [73]. Our findings extend these observations by highlighting new materials science approaches, such as sol–gel silica and ORMOCER® hybrids, that aim to preserve mechanical stability while maintaining optical performance. While these innovations are promising, no large-scale clinical validation is yet available, which marks a clear divergence between experimental development and real-world application.
The results concerning contact lens coatings illustrate a broader transition from surface wettability modifications toward multifunctional bioinspired, antimicrobial, and drug-eluting platforms. This trajectory aligns closely with recent reviews such as Franco et al. [74], Nguyen et al. [75], and Zhao et al. [76], which all conclude that while coatings can improve lens comfort and drug bioavailability, scalability and sterilization remain critical obstacles. Our findings reinforce these limitations, since many of the strategies identified, such as plasma treatments and peptide coatings, were tested only in vitro or in short-term preclinical models. The overlap is also evident when comparing our review with the systematic mapping of therapeutic lenses provided by Rykowska et al. [77], who noted the potential of biodegradable and drug-loaded platforms for long-term disease management. Unlike our included studies, however, Rykowska et al. emphasize biodegradable scaffolds and controlled degradation as strategies for drug release, which were not widely represented in the literature we analyzed.
Drug-eluting contact lenses appear as one of the most innovative directions. Our synthesis confirms that liposome-based reservoirs, PLGA coatings, and phosphorylcholine hydrogels achieve sustained delivery, though industrial translation is limited by degradation during sterilization and variable release kinetics. These results are highly consistent with Ciolino et al. [78], who pioneered the concept using PLGA and pHEMA films for antibiotic delivery. Later reviews, such as Zhao et al. [76] and Gao et al. [79], have echoed the same message: therapeutic contact lenses can achieve clinically meaningful drug levels, but the challenge is producing stable, reproducible, and regulatory-acceptable products. Compared with our review, which synthesizes mainly experimental reports, these external reviews emphasize the regulatory and translational bottlenecks that must be addressed.
An additional area where our results converge with the literature is in the exploration of nanomaterials. We identified multiple studies that used nanoparticles for antimicrobial or light-converting purposes, showing potential for multifunctionality but also raising safety questions. Similar concerns are reflected in Baghban et al. [80], who discussed nanomaterial-laden contact lenses for glaucoma diagnosis and therapy. Both our review and theirs underline the potential of nanotechnology for enhanced functionality, but also the lack of long-term biocompatibility and toxicity data, particularly for quantum dots or metallic nanoparticles. The main difference lies in scope: our synthesis focuses on coatings across spectacles, contact lenses, and IOLs, whereas Baghban et al. provide a disease-specific perspective, centered on glaucoma management.
For intraocular lenses, our findings highlight the evolution from heparin surface modifications to multifunctional and drug-eluting coatings, including methotrexate- and gefitinib-loaded polymers. These results confirm the broader trajectory of ocular implant coatings described in parallel reviews, although most of those focus on contact lenses rather than IOLs. The parallels with drug-eluting contact lens research are clear: in both cases, polymer-based coatings offer controlled drug release and enhanced biocompatibility, yet both remain at proof-of-concept stages with limited clinical evidence. The added complexity for IOLs is the requirement of sterilization, long-term stability inside the eye, and strict regulatory scrutiny, challenges less prominent for removable devices such as spectacles or contact lenses.
The comparison of our synthesis with external reviews shows a strong convergence in recognizing that the optical benefits of coatings are well established and that multifunctionality is becoming the dominant trend. Our review adds value by demonstrating that these themes—optical enhancement, antimicrobial protection, drug delivery, and durability trade-offs—recur across spectacles, contact lenses, and intraocular lenses, rather than being confined to a single platform. This wider perspective contrasts with most existing reviews, which typically focus on one device type, such as drug-eluting contact lenses [74,76,78]. At the same time, important divergences emerge: works such as Rykowska et al. [77] and Baghban et al. [79] emphasize biodegradable scaffolds or disease-specific nanomaterial strategies, whereas our findings underline mechanical safety and optical performance trade-offs that remain particularly relevant for spectacles and intraocular lenses. Future research will therefore need to bridge these perspectives, integrating clinically meaningful optical and mechanical endpoints with advanced drug delivery and nanotechnology approaches, to enable multifunctional coatings to progress from laboratory development to safe and widely adopted ophthalmic devices.
Several strengths emerge from this synthesis. It integrates evidence across spectacles, contact lenses, and intraocular lenses, thereby providing a panoramic view that is rarely captured in previous reviews restricted to a single lens category. By combining clinical trials, preclinical models, and laboratory investigations, it enables comparison across optical, mechanical, biological, and therapeutic outcomes, offering a balanced assessment of both efficacy and safety. Another strength is the inclusion of emerging functional strategies such as bioinspired antifouling layers, nanoparticle-based antimicrobial systems, and drug-eluting coatings, which together illustrate the rapid diversification of approaches to lens surface engineering. Nonetheless, important limitations must be acknowledged. Laboratory and animal results may not accurately predict long-term clinical performance in humans, and the heterogeneity of study designs, coating methods, and outcome measures makes direct quantitative comparison difficult. Many of the most innovative technologies remain at the proof-of-concept stage, with only limited progression toward regulatory approval or large-scale commercialization. In addition, follow-up periods in clinical studies were generally short, raising concerns about durability, stability under sterilization, and safety over the extended lifetimes expected for ophthalmic devices. These methodological and translational gaps constrain the generalizability of current evidence and highlight the need for more rigorous validation.
From a clinical and translational perspective, the findings of this review underscore that coatings already deliver tangible benefits in terms of glare reduction, visual comfort, and protection against harmful wavelengths, but that further progress is required before next-generation multifunctional systems become standard of care. For spectacles, the main challenge is balancing perceptual benefits with adequate impact resistance in occupational and pediatric settings. For contact lenses, durability, resistance to fouling, and compatibility with sterilization and care regimens will dictate which antimicrobial or drug-eluting coatings achieve clinical viability. For intraocular lenses, early clinical trials confirm the potential of surface modification to reduce postoperative inflammation and posterior capsule opacification, while drug-eluting platforms open the door to lenses functioning as therapeutic depots. Future research should therefore prioritize standardized evaluation methods across optical, mechanical, and biological endpoints, long-term biocompatibility and safety testing, and the development of industrial processes that allow stable, reproducible coatings on complex curved substrates. Equally important will be early engagement with regulatory bodies to ensure that safety, toxicity, and durability standards are met, particularly when materials involve nanoparticles or bioactive agents. Interdisciplinary collaboration between materials science, clinical ophthalmology, and regulatory science will be essential to bridge the translational gap and accelerate the responsible integration of multifunctional coatings into everyday ophthalmic practice. These relationships between coating functionality, material innovations, and translational challenges are summarized in Figure 3, which provides a conceptual mind map of the review’s main findings.

Figure 3.
Conceptual mind map summarizing the main findings of the review.
5. Conclusions
This comprehensive synthesis of 54 studies highlights the significant advances made in the development of ophthalmic lens coatings and biomaterials across spectacles, contact lenses, and intraocular lenses. Collectively, the evidence demonstrates that surface modifications can meaningfully improve visual performance, comfort, and ocular health. Clinical investigations consistently show that anti-reflective, spectral-filtering, photochromic, and luminescent coatings enhance wearer experience and provide photoprotective benefits, although laboratory findings caution against mechanical compromises that must be addressed in safety-critical applications.
For contact lenses, innovations in plasma-based, biomimetic, and polymeric coatings have expanded their functionality beyond vision correction, enabling improved wettability, reduced fouling, antimicrobial activity, and sustained drug delivery. These approaches are converging toward multifunctional platforms that prioritize both patient comfort and ocular health, though challenges remain in achieving long-term durability, sterilization compatibility, and scalable manufacturing.
In intraocular lenses, the trajectory of research has progressed from heparin surface modifications to sophisticated drug-eluting and luminescent coatings capable of reducing postoperative inflammation, preventing posterior capsule opacification, and mitigating infectious risks. These advances point to a future where IOLs evolve from passive implants to active therapeutic and light-modulating devices.
Despite these promising developments, translation to routine clinical use will require harmonization of optical, mechanical, and biological performance, alongside rigorous evaluation of long-term stability and regulatory validation. The next generation of ophthalmic lenses will likely be defined by multifunctionality, integrating comfort, protection, light modulation, and therapy into durable and scalable designs. Achieving this vision will depend on multidisciplinary collaboration bridging materials science, clinical ophthalmology, and regulatory science, with the ultimate goal of improving visual outcomes and quality of life for patients worldwide.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coatings15111246/s1, Supplementary File S1: PRISMA 2020 checklist; Supplementary File S2: Full electronic database search strategies (PubMed, Web of Science, Scopus); Supplementary File S3: Baseline characteristics of the 54 included studies.
Author Contributions
Conceptualization, C.M.-P. and A.P.O.; methodology, C.M.-P. and A.P.O.; software, C.M.-P. and A.P.O.; validation, C.M.-P. and A.P.O.; formal analysis, C.M.-P. and A.P.O.; investigation, C.M.-P. and A.P.O.; resources, C.M.-P. and A.P.O.; data curation, C.M.-P. and A.P.O.; writing—original draft preparation, C.M.-P. and A.P.O.; writing—review and editing, C.M.-P. and A.P.O.; visualization, C.M.-P. and A.P.O.; supervision, C.M.-P. and A.P.O.; project administration, C.M.-P. and A.P.O.; funding acquisition, C.M.-P. and A.P.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wu, K.Y.; Khammar, R.; Sheikh, H.; Marchand, M. Innovative Polymeric Biomaterials for Intraocular Lenses in Catarac Surgery. J. Funct. Biomater. 2024, 15, 391. [Google Scholar] [CrossRef]
- Abdulamier, A.A.; Shaker, L.M.; Al-Amiery, A.A. Advancements in the Chemistry of Contact Lenses: Innovations and Applications. Results Chem. 2024, 12, 101872. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Fan, W.; Zhong, Q.; Xue, R.; Li, S.; Song, Z.; Tao, Y. Eye of the Future: Unlocking the Potential Utilization of Hydrogels in Intraocular Lenses. Bioeng. Transl. Med. 2024, 9, e10664. [Google Scholar] [CrossRef]
- Wong, N.A.; Bahmani, H. A Review of the Current State of Research on Artificial Blue Light Safety as It Applies to Digital Devices. Heliyon 2022, 8, e10282. [Google Scholar] [CrossRef]
- Hee Han, D. Development of UV Blocking Lens with Photochromic Function with Refractive Index of 1.67. Int. J. Appl. Eng. Technol. 2021, 3. Available online: https://romanpub.com/resources/ijaet%20v3-2-2021%2001.pdf (accessed on 23 September 2025).
- Citek, K. Anti-Reflective Coatings Reflect Ultraviolet Radiation. Optometry 2008, 79, 143–148. [Google Scholar] [CrossRef]
- Li, L.; Sahi, S.K.; Peng, M.; Lee, E.B.; Ma, L.; Wojtowicz, J.L.; Malin, J.H.; Chen, W. Luminescence- and Nanoparticle-Mediated Increase of Light Absorption by Photoreceptor Cells: Converting UV Light to Visible Light. Sci. Rep. 2016, 6, 20821. [Google Scholar] [CrossRef]
- Popov, I.; Jurenova, D.; Valaskova, J.; Sanchez-Chicharro, D.; Stefanickova, J.; Waczulikova, I.; Krasnik, V. Effect of Blue Light Filtering Intraocular Lenses on Visual Perception. Medicina 2021, 57, 559. [Google Scholar] [CrossRef]
- Downie, L.E.; Keller, P.R. Blue-Light Filtering Intraocular Lenses (IOLs) for Protecting Macular Health. Cochrane Database Syst. Rev. 2018, 5, CD011977. [Google Scholar] [CrossRef]
- Kara-Junior, N.; Espindola, R.F.; Gomes, B.A.F.; Ventura, B.; Smadja, D.; Santhiago, M.R. Effects of Blue Light–Filtering Intraocular Lenses on the Macula, Contrast Sensitivity, and Color Vision after a Long-Term Follow-Up. J. Cataract Refract. Surg. 2011, 37, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Kontadakis, G.A.; Plainis, S.; Moschandreas, J.; Tsika, C.; Pallikaris, I.G.; Tsilimbaris, M.K. In Vivo Evaluation of Blue-Light Attenuation with Tinted and Untinted Intraocular Lenses. J. Cataract Refract. Surg. 2011, 37, 1031–1037. [Google Scholar] [CrossRef]
- Kohnen, T.; Hammond, B.R. Blue Light Filtration in Intraocular Lenses: Effects on Visual Function and Systemic Health. Clin. Ophthalmol. 2024, 18, 1575–1586. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Du, Z.; Pan, J.; Huang, Y. Multifunctional Upconversion Nanoparticles Transforming Photoacoustic Imaging: A Review. Nanomaterials 2025, 15, 1074. [Google Scholar] [CrossRef]
- Gallardo-Rivera, O.; Rivera, A.; Meza Espinoza, L.O.; Lazcano Ortiz, Z. Upconversion-Enhanced Luminescence in PMMA Doped with Rare Earth Ions by Plasmonic Resonance with Metallic Nanoparticles. ACS Omega 2025, 10, 11806–11816. [Google Scholar] [CrossRef]
- Marchini, F.; Chiatti, C.; Fabiani, C.; Pisello, A.L. Development of an Innovative Translucent–Photoluminescent Coating for Smart Windows Applications: An Experimental and Numerical Investigation. Renew. Sustain. Energy Rev. 2023, 184, 113530. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Arthur, S.N.; Peng, Q.; Apple, D.J.; Escobar-Gomez, M.; Bianchi, R.; Pandey, S.K.; Werner, L. Effect of Heparin Surface Modification in Reducing Silicone Oil Adherence to Various Intraocular Lenses. J. Cataract Refract. Surg. 2001, 27, 1662–1669. [Google Scholar] [CrossRef]
- Bachman, W.; Weaver, J. Comparison Between Anti-Reflection Coated and Uncoated Spectacle Lenses for Presbyopic Highway Patrol Troopers. J. Am. Optom. Assoc. 1997, 70, 103–109. [Google Scholar]
- Bozukova, D.; Pagnoulle, C.; Pauw-Gillet, M.-C.; Vertruyen, B.; Jérôme, R.; Jérôme, C. Hydrogel Nanocomposites: A Potential UV/Blue Light Filtering Material for Ophthalmic Lenses. J. Biomater. Sci. Polym. Ed. 2010, 22, 1947–1961. [Google Scholar] [CrossRef]
- Chang, Y.M.; Wang, Y.S.; Chen, H.Y. Controlling Superhydrophobicity on Complex Substrates Based on a Vapor-Phase Sublimation and Deposition Polymerization. ACS Appl. Mater. Interfaces 2023, 15, 48754–48763. [Google Scholar] [CrossRef]
- Cheng, W.; Yang, C.; Ding, X.; Engler, A.C.; Hedrick, J.L.; Yang, Y.Y. Broad-Spectrum Antimicrobial/Antifouling Soft Material Coatings Using Poly(Ethylenimine) as a Tailorable Scaffold. Biomacromolecules 2015, 16, 1967–1977. [Google Scholar] [CrossRef]
- Chou, B.R.; Dain, S.J.; Cheng, B.B. Effect of Ultraviolet Exposure on Impact Resistance of Ophthalmic Lenses. Optom. Vis. Sci. 2015, 92, 1154–1160. [Google Scholar] [CrossRef]
- Chou, B.R.; Gupta, A.; Hovis, J. The Effect of Multiple Antireflective Coatings and Center Thickness on Resistance of Polycarbonate Spectacle Lenses to Penetration by Pointed Missiles. Optom. Vis. Sci. 2005, 82, 964–969. [Google Scholar] [CrossRef]
- Chou, B.R.; Hovis, J.K. Durability of Coated CR-39 Industrial Lenses. Optom. Vis. Sci. 2003, 80, 703–707. [Google Scholar] [CrossRef]
- Chou, B.R.; Hovis, J.K. Effect of Multiple Antireflection Coatings on Impact Resistance of Hoya Phoenix Spectacle Lenses. Clin. Exp. Optom. 2006, 89, 86–89. [Google Scholar] [CrossRef]
- Chou, B.R.; Yuen, G.S.C.; Dain, S.J. Ballistic Impact Resistance of Selected Organic Ophthalmic Lenses. Clin. Exp. Optom. 2011, 94, 568–574. [Google Scholar] [CrossRef]
- Coupland, S.G.; Kirkham, T.H. Improved Contrast Sensitivity with Antireflective Coated Lenses in the Presence of Glare. Can. J. Ophthalmol. 1981, 16, 136–140. [Google Scholar]
- Corzine, J.C.; Greer, R.B.; Bruess, R.D.; Lee, G.K.; Scaief, A.L.E.E. Effects of Coatings on the Fracture Resistance of Ophthalmic Lenses. Optom. Vis. Sci. 1996, 73, 8–15. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, J.; Zhang, S.; Li, L.; Qu, C.; Chen, J.; Lu, L. Ag/Cu Nanoparticles-Loaded Glycocalyx Biomimetic Corneal Bandage Lenses for Combatting Bacterial Keratitis. J. Control. Release 2024, 376, 382–394. [Google Scholar] [CrossRef]
- Danion, A.; Arsenault, I.; Vermette, P. Antibacterial Activity of Contact Lenses Bearing Surface-Immobilized Layers of Intact Liposomes Loaded with Levofloxacin. J. Pharm. Sci. 2007, 96, 2350–2363. [Google Scholar] [CrossRef]
- Demian, P.; Nagaya, D.; Refaei, R.; Iwai, K.; Hasegawa, D.; Baba, M.; Messersmith, P.B.; Lamrani, M. Enhancing Performance of Silicone Hydrogel Contact Lenses with Hydrophilic Polyphenolic Coatings. J. Funct. Biomater. 2024, 15, 321. [Google Scholar] [CrossRef]
- Dutta, D.; Cole, N.; Kumar, N.; Willcox, M.D.P. Broad Spectrum Antimicrobial Activity of Melimine Covalently Bound to Contact Lenses. Invest. Ophthalmol. Vis. Sci. 2013, 54, 175–182. [Google Scholar] [CrossRef]
- Eibl, K.H.; Wertheimer, C.; Kernt, M.; Wolf, A.; Kook, D.; Haritoglou, C.; Kampik, A. Alkylphosphocholines for Intraocular Lens Coating. J. Cataract Refract. Surg. 2013, 39, 438–445. [Google Scholar] [CrossRef]
- Gu, X.; Ma, J.; He, J. Fabrication of Robust Carbon Dots Containing Coatings with UV-Shielding, Light Conversion, and Antifogging Multiple Functions. Langmuir 2024, 40, 1461–1469. [Google Scholar] [CrossRef]
- Huang, X.; Yuan, Y.; Liu, S.; Zhang, L.; Hong, R. Preparation of Hydrophobic Broadband Antireflective SiO2 Coating on Flexible Poly(Methyl Methacrylate) Substrates. Colloids Surf. A 2018, 538, 519–525. [Google Scholar] [CrossRef]
- Hussain, S.; Donempudi, S.; Tammishetti, S.; Garikapati, K.R.; Bhadra, M.P. Cell Adhesion Resistant, UV Curable, Polymer Zinc Oxide Nanocomposite Materials for Intraocular Lens Application. Polym. Adv. Technol. 2018, 29, 1234–1241. [Google Scholar] [CrossRef]
- Ho, C.-P.; Yasuda, H. Ultrathin Coating of Plasma Polymer of Methane Applied on the Surface of Silicone Contact Lenses. J. Biomed. Mater. Res. 1988, 22, 919–937. [Google Scholar] [CrossRef]
- Kats, M.A.; Blanchard, R.; Genevet, P.; Capasso, F. Nanometre Optical Coatings Based on Strong Interference Effects in Highly Absorbing Media. Nat. Mater. 2013, 12, 20–24. [Google Scholar] [CrossRef]
- Kassumeh, S.A.; Wertheimer, C.M.; von Studnitz, A.; Hillenmayer, A.; Priglinger, C.; Wolf, A.; Mayer, W.J.; Teupser, D.; Holdt, L.M.; Priglinger, S.G.; et al. Poly(Lactic-Co-Glycolic) Acid as a Slow-Release Drug-Carrying Matrix for Methotrexate Coated onto Intraocular Lenses to Conquer Posterior Capsule Opacification. Curr. Eye Res. 2018, 43, 702–708. [Google Scholar] [CrossRef]
- Kassumeh, S.; Kueres, A.; Hillenmayer, A.; von Studnitz, A.; Elhardt, C.; Ohlmann, A.; Priglinger, S.G.; Wertheimer, C.M. Development of a Drug-Eluting Intraocular Lens to Deliver Epidermal Growth Factor Receptor Inhibitor Gefitinib for Posterior Capsule Opacification Prophylaxis. Eur. J. Ophthalmol. 2021, 31, 436–444. [Google Scholar] [CrossRef]
- Kim, K.C. Effective Graded Refractive-Index Anti-Reflection Coating for High Refractive-Index Polymer Ophthalmic Lenses. Mater. Lett. 2015, 160, 158–161. [Google Scholar] [CrossRef]
- Krall, E.M.; Arlt, E.M.; Jell, G.; Strohmaier, C.; Bachernegg, A.; Emesz, M.; Grabner, G.; Dexl, A.K. Intraindividual Aqueous Flare Comparison after Implantation of Hydrophobic Intraocular Lenses with or without a Heparin-Coated Surface. J. Cataract Refract. Surg. 2014, 40, 1363–1370. [Google Scholar] [CrossRef]
- Leung, T.W.; Li, R.W.H.; Kee, C.S. Blue-Light Filtering Spectacle Lenses: Optical and Clinical Performances. PLoS ONE 2017, 12, e0169114. [Google Scholar] [CrossRef]
- Li, K.; Yu, L.; Ma, L.; Xia, J.; Peng, J.; Hu, P.; Liu, G.; Ye, J. Surface Modification of Commercial Intraocular Lens by Zwitterionic and Antibiotic-Loaded Coating for Preventing Postoperative Endophthalmitis. Colloids Surf. B Biointerfaces 2023, 222, 113093. [Google Scholar] [CrossRef]
- Liou, J.-C.; Teng, M.-C.; Tsai, Y.-S.; Lin, E.-C.; Chen, B.-Y. UV-Blocking Spectacle Lens Protects against UV-Induced Decline of Visual Performance. Mol. Vis. 2015, 21, 846–856. [Google Scholar]
- Lundberg, F.; Gouda, I.; Larm, O.; Galin, M.A.; Sa Ljungh, A. A New Model to Assess Staphylococcal Adhesion to Intraocular Lenses under in Vitro Flow Conditions. Biomaterials 1998, 19, 1727–1733. [Google Scholar] [CrossRef]
- Okajima, Y.; Saika, S.; Sawa, M. Effect of Surface Coating an Acrylic Intraocular Lens with Poly(2-Methacryloyloxyethyl Phosphorylcholine) Polymer on Lens Epithelial Cell Line Behavior. J. Cataract Refract. Surg. 2006, 32, 666–671. [Google Scholar] [CrossRef]
- Paulson, C.A.; Price, J.J.; Koch, K.W.; Kim, C.-G.; Oh, J.-H.; Lin, L.; Subramanian, A.N.; Zhang, B.; Amin, J.; Mayolet, A.; et al. Industrial-Grade Anti-Reflection Coatings with Extreme Scratch Resistance. Opt. Lett. 2019, 44, 5977–5980. [Google Scholar] [CrossRef]
- Peng, C.C.; Fajardo, N.P.; Razunguzwa, T.; Radke, C.J. In Vitro Spoilation of Silicone-Hydrogel Soft Contact Lenses in a Model-Blink Cell. Optom. Vis. Sci. 2015, 92, 768–780. [Google Scholar] [CrossRef][Green Version]
- Quiroga, J.A.; Canga, I.; Alonso, J.; Crespo, D. Reversible Photoalignment of Liquid Crystals: A Path toward the Creation of Rewritable Lenses. Sci. Rep. 2020, 10, 5739. [Google Scholar] [CrossRef]
- Refaei, R.; Lee, K.; Lee, G.A.; Demian, P.; El Mansouri, F.; Messersmith, P.B.; Lamrani, M.; Khaddor, M.; Allali, N. Functionalized Surface Coatings for Rigid Contact Lenses. J. Funct. Biomater. 2024, 15, 154. [Google Scholar] [CrossRef]
- Rickert, C.A.; Wittmann, B.; Fromme, R.; Lieleg, O. Highly Transparent Covalent Mucin Coatings Improve the Wettability and Tribology of Hydrophobic Contact Lenses. ACS Appl. Mater. Interfaces 2020, 12, 28024–28033. [Google Scholar] [CrossRef]
- Rosenhek-Goldian, I.; Kampf, N.; Klein, J. Trapped Aqueous Films Lubricate Highly Hydrophobic Surfaces. ACS Nano 2018, 12, 10075–10083. [Google Scholar] [CrossRef]
- Ross, J.; Bradley, A. Visual Performance and Patient Preference: A Comparison of Anti-Reflection Coated and Uncoated Spectacle Lenses. J. Am. Optom. Assoc. 1997, 68, 361–366. [Google Scholar] [PubMed]
- Santos, L.; Rodrigues, D.; Lira, M.; Oliveira, R.; Yebra-Pimentel Vilar, E. The Influence of Surface Treatment on Hydrophobicity, Protein Adsorption and Microbial Colonisation of Silicone Hydrogel Contact Lenses. Contact Lens Anterior Eye 2007, 30, 183–188. [Google Scholar] [CrossRef]
- Schottner, G.; Rose, K.; Posset, U. Scratch and Abrasion Resistant Coatings on Plastic Lenses—State of the Art, Current Developments and Perspectives. J. Sol-Gel Sci. Technol. 2003, 27, 71–79. [Google Scholar] [CrossRef]
- Shi, J.; Xu, L.; Qiu, D. Effective Antifogging Coating from Hydrophilic/Hydrophobic Polymer Heteronetwork. Adv. Sci. 2022, 9, 2200072. [Google Scholar] [CrossRef]
- Singh, A.; Li, P.; Beachley, V.; McDonnell, P.; Elisseeff, J.H. A Hyaluronic Acid-Binding Contact Lens with Enhanced Water Retention. Cont. Lens Anterior Eye 2015, 38, 79–84. [Google Scholar] [CrossRef]
- Shin, H.S.; Jang, J.K.; Kwon, Y.S.; Mah, K.C. Surface Modification of Rigid Gas Permeable Contact Lens Treated by Using a Low-Temperature Plasma in Air. J. Korean Phys. Soc. 2009, 55, 2436–2440. [Google Scholar] [CrossRef]
- Stroud, J.S. Localized Defects in Ophthalmic Lenses. Optom. Vis. Sci. 1989, 66, 141–145. [Google Scholar] [CrossRef]
- Tam, C.H.; Alexander, M.S.; Sanderson, J.; Qi, S. Selectively Coated Contact Lenses by Nanoelectrospray (NES) to Fabricate Drug-Eluting Contact Lenses for Treating Ocular Diseases. Med. Eng. Phys. 2024, 124, 104110. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Hsieh, Y.C.; Lin, Y.H.; Chang, H.C.; Tang, Y.H.; Huang, K.C. Fabrication of Hydrophilic Surface on Rigid Gas Permeable Contact Lenses to Enhance the Wettability Using Ultraviolet Laser System. Micromachines 2019, 10, 394. [Google Scholar] [CrossRef]
- Vivero-Lopez, M.; Pereira-Da-Mota, A.F.; Carracedo, G.; Huete-Toral, F.; Parga, A.; Otero, A.; Concheiro, A.; Alvarez-Lorenzo, C. Phosphorylcholine-Based Contact Lenses for Sustained Release of Resveratrol: Design, Antioxidant and Antimicrobial Performances, and in Vivo Behavior. ACS Appl. Mater. Interfaces 2022, 14, 55431–55446. [Google Scholar] [CrossRef]
- Wang, T.J.; Lin, W.P.; Guo, S.P. Increased Hydrophilicity and Anti-Fouling Effect of Orthokeratology Lenses Coated with NVP and PEGMA by Plasma-Enhanced Chemical Vapor Deposition. J. Coat. Technol. Res. 2024, 21, 737–745. [Google Scholar] [CrossRef]
- Werner, L.; Legeais, J.M.; Nagel, M.D.; Renard, G. Evaluation of Teflon-Coated Intraocular Lenses in an Organ Culture Method. J. Biomed. Mater. Res. 1999, 46, 347–354. [Google Scholar] [CrossRef]
- Willis, S.L.; Court, J.L.; Redman, R.P.; Wang, J.-H.; Leppard, S.W.; O’Byrne, V.J.; Small, S.A.; Lewis, A.L.; Jones, S.A.; Stratford, P.W. A Novel Phosphorylcholine-Coated Contact Lens for Extended Wear Use. Biomaterials 2001, 22, 3261–3272. [Google Scholar] [CrossRef]
- Xu, L.; Geng, Z.; He, J.; Zhou, G. Mechanically Robust, Thermally Stable, Broadband Antireflective, and Superhydrophobic Thin Films on Glass Substrates. ACS Appl. Mater. Interfaces 2014, 6, 9029–9035. [Google Scholar] [CrossRef]
- Yamasaki, K.; Nakagawa, H.; Motohiro, C.; Jones, L.; Hui, A. The Impact of a Hyaluronic Acid Derivative-Containing Care System on the Wettability of PEG-Coated Rigid Lenses. Cont. Lens Anterior Eye 2025, 102490. [Google Scholar] [CrossRef]
- Yin, S.; Wang, Y.; Ren, L.; Zhao, L.; Kuang, T.; Chen, H.; Qu, J. Surface Modification of Fluorosilicone Acrylate RGP Contact Lens via Low-Temperature Argon Plasma. Appl. Surf. Sci. 2008, 255, 483–485. [Google Scholar] [CrossRef]
- Yu, Y.; Macoon, R.; Chauhan, A. Improving Wettability and Lubricity of Commercial Contact Lenses by Polymerizing a Thin Film of Dimethylacryamide. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123974. [Google Scholar] [CrossRef]
- Samson, F. Ophthalmic Lens Coatings. Surf. Coat. Technol. 1996, 81, 79–86. [Google Scholar] [CrossRef]
- Martinez-Perez, C.; Oliveira, A.P. Meta-Analysis of Materials and Treatments Used in Ophthalmic Lenses: Implications for Lens Characteristics. Materials 2024, 17, 5949. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Contact Lenses as Ophthalmic Drug Delivery Systems: A Review. Polymers 2021, 13, 1102. [Google Scholar] [CrossRef]
- Nguyen, D.C.T.; Dowling, J.; Ryan, R.; McLoughlin, P.; Fitzhenry, L. Pharmaceutical-Loaded Contact Lenses as an Ocular Drug Delivery System: A Review of Critical Lens Characterization Methodologies with Reference to ISO Standards. Cont. Lens Anterior Eye 2021, 44, 101487. [Google Scholar] [CrossRef]
- Zhao, L.; Song, J.; Du, Y.; Ren, C.; Guo, B.; Bi, H. Therapeutic Applications of Contact Lens-Based Drug Delivery Systems in Ophthalmic Diseases. Drug Deliv. 2023, 30, 2219419. [Google Scholar] [CrossRef]
- Rykowska, I.; Nowak, I.; Nowak, R.; Michałkiewicz, O. Biodegradable Contact Lenses for Targeted Ocular Drug Delivery: Recent Advances, Clinical Applications, and Translational Perspectives. Molecules 2025, 30, 2542. [Google Scholar] [CrossRef]
- Ciolino, J.B.; Hoare, T.R.; Iwata, N.G.; Behlau, I.; Dohlman, C.H.; Langer, R.; Kohane, D.S. A Drug-Eluting Contact Lens. Invest. Ophthalmol. Vis. Sci. 2009, 50, 3346–3352. [Google Scholar] [CrossRef]
- Gao, D.; Yan, C.; Wang, Y.; Yang, H.; Liu, M.; Wang, Y.; Li, C.; Li, C.; Cheng, G.; Zhang, L. Drug-Eluting Contact Lenses: Progress, Challenges, and Prospects. Biointerphases 2024, 19, 040801. [Google Scholar] [CrossRef]
- Baghban, R.; Talebnejad, M.R.; Meshksar, A.; Heydari, M.; Khalili, M.R. Recent Advancements in Nanomaterial-Laden Contact Lenses for Diagnosis and Treatment of Glaucoma, Review and Update. J. Nanobiotechnol. 2023, 21, 402. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).