One-Step In Situ Electrochemical Synthesis of Polyaniline/CeO2 Composite Coating for Enhanced Corrosion Protection of Mild Steel
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Electropolymerization of Polyaniline and Polyaniline@CeO2 Coatings
2.3. Structure Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García-Cabezón, C.; Godinho, V.; Pérez-González, C.; Torres, Y.; Martín-Pedrosa, F. Electropolymerized polypyrrole silver nanocomposite coatings on porous Ti substrates with enhanced corrosion and antibacterial behavior for biomedical applications. Mater. Today Chem. 2023, 29, 101433. [Google Scholar] [CrossRef]
- Jadhav, R.S.; Hundiwale, D.G.; Mahulikar, P.P. Synthesis of nano polyaniline and poly-o-anisidine and applications in alkyd paint formulation to enhance the corrosion resistivity of mild steel. J. Coat. Technol. Res. 2009, 7, 449–454. [Google Scholar] [CrossRef]
- Yin, Y.; Prabhakar, M.; Ebbinghaus, P.; da Silva, C.C.; Rohwerder, M. Neutral inhibitor molecules entrapped into polypyrrole network for corrosion protection. Chem. Eng. J. 2022, 440, 135739. [Google Scholar] [CrossRef]
- Chen, Z.H.; Li, X.L.; Gong, B.; Scharnagl, N.; Zheludkevich, M.L.; Ying, H.J.; Yang, W.Z. Double Stimuli-Responsive Conducting Polypyrrole Nanocapsules for Corrosion-Resistant Epoxy Coatings. ACS Appl. Mater. Interfaces 2022, 15, 2067–2076. [Google Scholar] [CrossRef]
- Kumar, S.A.; Meenakshi, K.S.; Sankaranarayanan, T.S.N.; Srikanth, S. Corrosion resistant behaviour of PANI-metal bilayer coatings. Prog. Org. Coat. 2008, 62, 285–292. [Google Scholar] [CrossRef]
- Syugaev, A.V.; Maratkanova, A.N.; Smirnov, D.A. Polyaniline films electrodeposited on iron from oxalic acid solution: Linear dichroism of X-ray absorption and molecular arrangement. J. Solid State Electrochem. 2019, 23, 179–185. [Google Scholar] [CrossRef]
- Gupta, D.K.; Neupane, S.; Singh, S.; Karki, N.; Yadav, A.P. The effect of electrolytes on the coating of polyaniline on mild steel by electrochemical methods and its corrosion behavior. Prog. Org. Coat. 2021, 152, 106127. [Google Scholar] [CrossRef]
- Bernard, M.C.; Joiret, S.; Goff, A.H.; Phong, P.V. Protection of iron against corrosion using a polyaniline layer: III. Spectroscopic analysis of the mechanisms accompanying the breakdown. J. Electrochem. Soc. 2001, 148, B304–B306. [Google Scholar] [CrossRef]
- Wang, M.X.; Yun, H.; Tan, K.Q.; Guo, A.Q.; Ling, J.W.; Jiang, F.F.; Shen, X.X.; Xu, Q.J. One-step electrochemical synthesis of poly(vinyl pyrrolidone) modified polyaniline coating on stainless steel for high corrosion protection performance. Prog. Org. Coat. 2020, 149, 105908. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, S.H.; Wang, P.; Lu, Z.X.; Chen, S.B.; Wang, L.S. Synthesis and corrosion protection of Nb doped TiO2 nanopowders modified polyaniline coating on 316 stainless steel bipolar plates for proton-exchange membrane fuel cells. Prog. Org. Coat. 2019, 137, 105327. [Google Scholar] [CrossRef]
- Jiang, L.; Syed, J.A.; Gao, Y.Z.; Lu, H.B.; Meng, X.K. Electrodeposition of Ni(OH)2 reinforced polyaniline coating for corrosion protection of 304 stainless steel. Appl. Surf. Sci. 2018, 440, 1011–1021. [Google Scholar] [CrossRef]
- Amegroud, H.; Boudalia, M.; Elhawary, M.; Garcia, A.J.; Bellaouchou, A.; Amin, H.M.A. Electropolymerized conducting polyaniline coating on nickel-aluminum bronze alloy for improved corrosion resistance in marine environment. Colloids Surf. A Physicochem. Eng. Asp. 2024, 691, 133909. [Google Scholar] [CrossRef]
- Pawar, P.; Gaikawad, A.B.; Patil, P.P. Electrochemical synthesis of corrosion protective polyaniline coatings on mild steel from aqueous salicylate medium. Sci. Technol. Adv. Mater. 2006, 7, 732–744. [Google Scholar] [CrossRef]
- Ganash, A.A.; Al-Nowaiser, F.M.; Al-Thabaiti, S.A.; Hermas, A.A. Comparison study for passivation of stainless steel by coating with polyaniline from two different acids. Prog. Org. Coat. 2011, 72, 480–485. [Google Scholar] [CrossRef]
- Kamaraj, K.; Sathiyanarayanan, S.; Venkatachari, G. Electropolymerised polyaniline films on AA 7075 alloy and its corrosion protection performance. Prog. Org. Coat. 2009, 64, 67–73. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, S.H.; Wang, P.; Chen, S.B.; Lu, Z.X.; Li, W.H. Electropolymerization and corrosion protection performance of the Nb: TiO2 nanofibers/polyaniline composite coating. J. Taiwan Inst. Chem. Eng. 2019, 103, 190–198. [Google Scholar] [CrossRef]
- Sasikumar, Y.; Kumar, A.M.; Gasem, Z.M.; Ebenso, E.E. Hybrid nanocomposite from aniline and CeO2 nanoparticles: Surface protective performance on mild steel in acidic environment. Appl. Surf. Sci. 2015, 330, 207–215. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Tian, S.W.; Lin, D.L.; Zhang, Z.H.; Li, G.C. Functional anti-corrosive and anti-bacterial surface coatings based on cuprous oxide/polyaniline microcomposites. Mater. Des. 2022, 216, 110589. [Google Scholar] [CrossRef]
- Jabri, H.A.; Devi, M.G.; Al-Shukaili, M.A. Development of polyaniline-TiO2 nano composite films and its application in corrosion inhibition of oil pipelines. J. Indian Chem. Soc. 2022, 100, 100826. [Google Scholar] [CrossRef]
- Sulistyaningsih, E.; Lestari, N. Coating of polyaniline-titanium dioxide (PANI-TiO2) composite for corrosion protection in low carbon steel. IOP Conf. Ser. Mater. Sci. Eng. 2020, 807, 012046. [Google Scholar] [CrossRef]
- Maruthi, N.; Faisal, M.; Raghavendra, N.; Prasanna, B.P.; Manohara, S.R.; Revanasiddappa, M. Anticorrosive polyaniline-coated copper oxide (PANI/CuO) nanocomposites with tunable electrical properties for broadband electromagnetic interference shielding. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621, 126611. [Google Scholar] [CrossRef]
- Maruthi, N.; Faisal, M.; Raghavendra, N.; Prasanna, B.P.; Manohara, S.R.; Revanasiddappa, M. Promising EMI shielding effectiveness and anticorrosive properties of PANI-Nb2O5 nanocomposites: Multifunctional approach. Synth. Met. 2021, 275, 116744. [Google Scholar] [CrossRef]
- Ashwini, I.S.; Pattar, J.; Anjaneyulu, P.; PrakashBabu, D.; Sreekanth, R.; Manohara, S.R.; Nagaraja, M. Synthesis and electrical properties of polyaniline–cerium oxide composites. Synth. Met. 2020, 270, 116588. [Google Scholar] [CrossRef]
- Maruthi, N.; Faisal, M.; Raghavendra, N.; Prasanna, B.P.; Nandan, K.R.; Kumar, K.Y.; Prasad, S.B.B. Polyaniline/V2O5 composites for anticorrosion and electromagnetic interference shielding. Mater. Chem. Phys. 2020, 259, 124059. [Google Scholar] [CrossRef]
- Kumar, E.; Selvarajan, P.; Muthuraj, D. Preparation and characterization of polyaniline/cerium dioxide (CeO2) nanocomposite via in situ polymerization. J. Mater. Sci. 2012, 47, 7148–7156. [Google Scholar] [CrossRef]
- Li, Z.H.; Shen, Y.B.; Li, Y.B.; Zheng, F.; Liu, L.L. Doping effects of cerium ion on structure and electrochemical properties of polyaniline. Polym. Int. 2018, 67, 121–126. [Google Scholar] [CrossRef]
- Shetty, K.; Raj, K.; Mohan, N. Synthesis, characterization and corrosion studies of polyanailine (PANI)/ceriem dioxide (CeO2) nano composite. Mater. Today Proc. 2020, 27, 2158–2163. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.W.; Xie, Y.; Zhang, H.H.; Zhang, Z. Preparation and anti-corrosion performance of superhydrophobic silane/graphene oxide composite coating on copper. Surf. Coat. Technol. 2021, 423, 127622. [Google Scholar] [CrossRef]
- John, S.; Joseph, B.; Aravindakshan, K.K.; Joseph, A. Inhibition of mild steel corrosion in 1 M hydrochloric acid by 4-(N,N-dimethylaminobenzilidine)-3-mercapto-6-methyl-1,2,4-triazin (4H)-5-one (DAMMT). Mater. Chem. Phys. 2010, 122, 374–379. [Google Scholar] [CrossRef]
- Huang, X.Q.; Chen, Y.; Zhou, J.Q.; Zhang, Z.; Zhang, J.Q. Electrochemical nucleation and growth of Sn onto double reduction steel substrate from a stannous fluoborate acid bath. J. Electroanal. Chem. 2013, 709, 83–92. [Google Scholar] [CrossRef]
- Nautiyal, A.; Parida, S. Comparison of polyaniline electrodeposition on carbon steel from oxalic acid and salicylate medium. Prog. Org. Coat. 2016, 94, 28–33. [Google Scholar] [CrossRef]
- Mrad, M.; Amor, Y.B.; Dhouibi, L.; Montemor, F. Electrochemical study of polyaniline coating electropolymerized onto AA2024-T3 aluminium alloy: Physical properties and anticorrosion performance. Synth. Met. 2017, 234, 145–153. [Google Scholar] [CrossRef]
- Kamada, K.; Higashikawa, K.; Inada, M.; Enomoto, N.; Hojo, J. Photoassisted Anodic Electrodeposition of Ceria Thin Films. J. Phys. Chem. C 2007, 111, 14508–14513. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.M.; Du, X.Q.; Chen, Y.; Zhang, Z.; Zhang, J.Q. Influences of the main anodic electroplating parameters on cerium oxide films. Appl. Surf. Sci. 2014, 305, 330–336. [Google Scholar] [CrossRef]
- Lyutov, V.; Kabanova, V.; Gribkova, O.; Nekrasov, A.; Tsakova, V. Electrochemically-obtained polysulfonic-acids doped polyaniline films—A comparative study by electrochemical, microgravimetric and XPS methods. Polymers 2020, 12, 1050. [Google Scholar] [CrossRef]
- Wang, N.; Feng, J.T.; Chen, J.; Wang, J.N.; Yan, W. Adsorption mechanism of phosphate by polyaniline/TiO2 composite from wastewater. Chem. Eng. J. 2017, 316, 33–40. [Google Scholar] [CrossRef]
- Zhang, H.H.; Zhang, X.; Bian, H.; Zhang, L.; Chen, Y.; Yang, Y.; Zhang, Z. Benzotriazole loaded CeO2 nano-containers towards superior anti-corrosive silane coating for protection of copper. Colloids Surf. A Physicochem. Eng. Asp. 2023, 682, 132844. [Google Scholar] [CrossRef]
- Joseph, A.; Mathew, K.P.J.; Vandana, S. Zirconium-Doped Ceria Nanoparticles as Anticorrosion Pigments in Waterborne Epoxy–Polymer Coatings. ACS Appl. Nano Mater. 2020, 4, 834–849. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.C.; He, H.Q.; Ouyang, L.; Yuan, S.J. A stearic Acid/CeO2 bilayer coating on AZ31B magnesium alloy with superhydrophobic and self-cleaning properties for corrosion inhibition. J. Alloys Compd. 2020, 834, 155210. [Google Scholar] [CrossRef]
- Lyu, L.; Xie, Q.; Yang, Y.Y.; Wang, R.R.; Cen, W.F.; Luo, S.Y.; Yang, W.S.; Gao, Y.; Xiao, Q.Q.; Zou, P.; et al. A novel CeO2 Hollow-Shell sensor constructed for high sensitivity of acetone gas detection. Appl. Surf. Sci. 2022, 571, 151337. [Google Scholar] [CrossRef]
- Tan, B.C.; Xiang, B.; Zhang, S.T.; Qiang, Y.J.; Xu, L.H.; Chen, S.J.; He, J.H. Papaya leaves extract as a novel eco-friendly corrosion inhibitor for Cu in H2SO4 medium. J. Colloid Interface Sci. 2020, 582, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wu, M.P.; Miao, X.J.; Zhao, Z.S.; Gong, Y.L. Microstructure and corrosion behavior of CeO2/FeCoNiCrMo high-entropy alloy coating prepared by laser cladding. J. Alloys Compd. 2022, 890, 161826. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, L.L.; Li, D.R.; Chen, J.X.; Zhao, Y.C.; Liu, L.; Shuai, C.J.; Yang, K.; Atrens, A.; Zhao, M.C. Effect of grain refinement and crystallographic texture produced by friction stir processing on the biodegradation behavior of a Mg-Nd-Zn alloy. J. Mater. Sci. Technol. 2018, 35, 777–783. [Google Scholar] [CrossRef]
- Wu, Y.M.; Jiang, F.W.; Qiang, Y.J.; Zhao, W.J. Synthesizing a novel fluorinated reduced graphene oxide-CeO2 hybrid nanofiller to achieve highly corrosion protection for waterborne epoxy coatings. Carbon 2021, 176, 39–51. [Google Scholar] [CrossRef]
- Qian, Y.; Zheng, W.; Chen, W.G.; Feng, T.; Liu, T.X.; Fu, Y.Q. Enhanced functional properties of CeO2 modified graphene/epoxy nanocomposite coating through interface engineering. Surf. Coat. Technol. 2021, 409, 126819. [Google Scholar] [CrossRef]
- Ge, C.Y.; Yang, X.G.; Hou, B.R. Synthesis of polyaniline nanofiber and anticorrosion property of polyaniline–epoxy composite coating for Q235 steel. J. Coat. Technol. Res. 2011, 9, 59–69. [Google Scholar] [CrossRef]
- Zhang, X.L. Electrochemical Evidence of Corrosion Resistance of Polyaniline Film on the Copper Surface. Int. J. Electrochem. Sci. 2020, 15, 4470–4480. [Google Scholar] [CrossRef]
- Ren, Y.J.; Zeng, C.L. Effect of conducting composite polypyrrole/polyaniline coatings on the corrosion resistance of type 304 stainless steel for bipolar plates of proton-exchange membrane fuel cells. J. Power Sources 2008, 182, 524–530. [Google Scholar] [CrossRef]
- Santos, J.R.; Mattoso, L.H.; Motheo, A.J. Investigation of corrosion protection of steel by polyaniline films. Electrochim. Acta 1998, 43, 309–313. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Zhang, Z.; Shi, L.Q.; Zhang, F.; Li, S.Q.; Zeng, R.C. Corrosion resistance of a self-healing multilayer film based on SiO2 and CeO2 nanoparticles layer-by-layer assembly on Mg alloys. Mater. Lett. 2019, 237, 14–18. [Google Scholar] [CrossRef]
- Özyılmaz, A.T.; Erbil, M.; Yazıcı, B. The electrochemical synthesis of polyaniline on stainless steel and its corrosion performance. Curr. Appl. Phys. 2006, 6, 1–9. [Google Scholar] [CrossRef]
- Jafari, Y.; Ghoreishi, S.M.; Shabani-Nooshabadi, M. Electrochemical deposition and characterization of polyaniline-graphene nanocomposite films and its corrosion protection properties. J. Polym. Res. 2016, 23, 91. [Google Scholar] [CrossRef]
- Fatahiamirdehi, M.; Mahani, M.; Mirseyed, S.F.; Rostamian, A.; Ostadhassan, M. Enhancing corrosion resistance of 316L stainless steel through electrochemical deposition of polyaniline coatings in acidic environments. J. Mater. Sci. 2024, 59, 14716–14727. [Google Scholar] [CrossRef]
- Qiu, C.; Liu, D.; Jin, K.; Fang, L.; Xie, G.; Robertson, J. Electrochemical functionalization of 316 stainless steel with polyaniline-graphene oxide: Corrosion resistance study. Mater. Chem. Phys. 2017, 198, 90–98. [Google Scholar] [CrossRef]
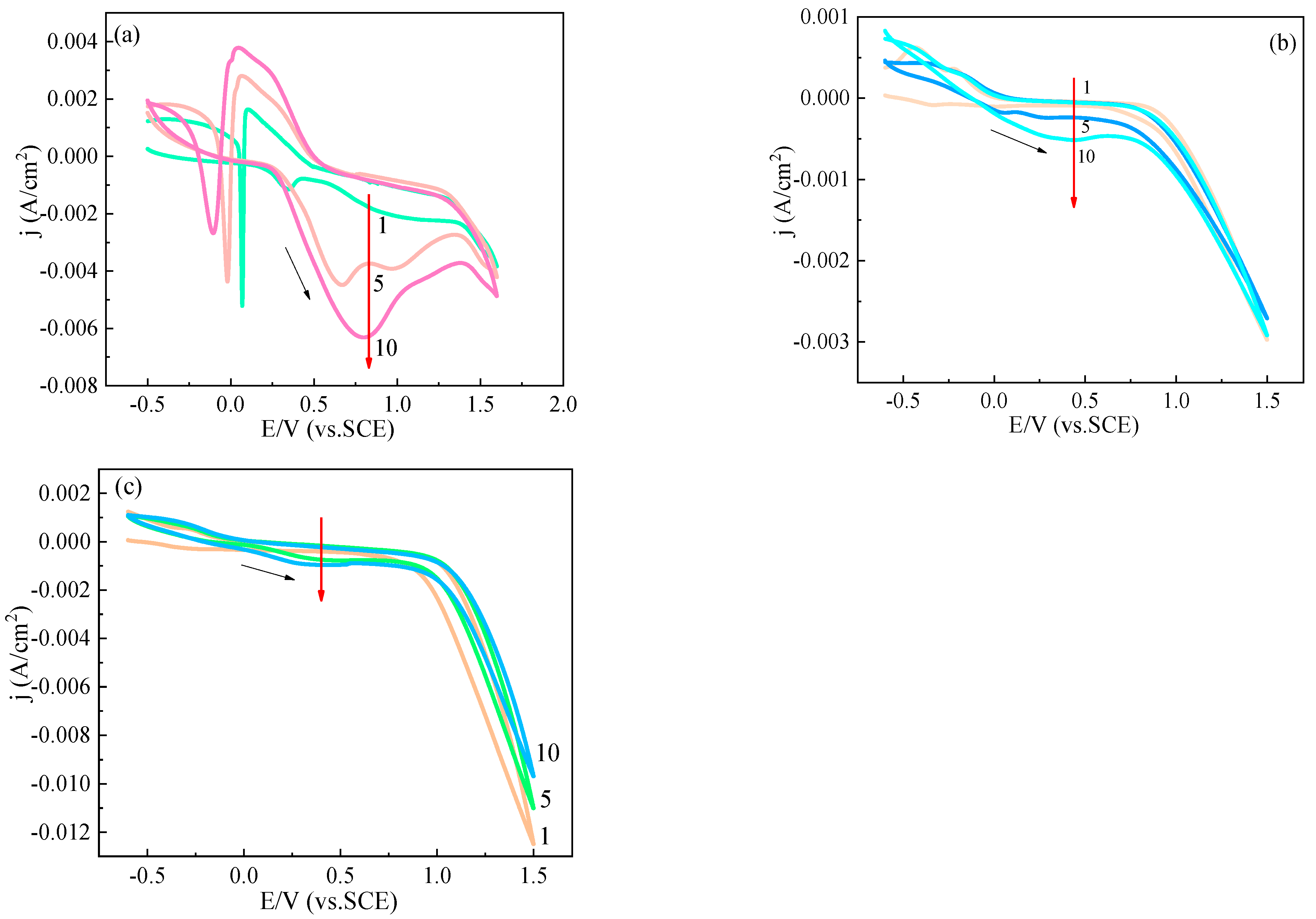
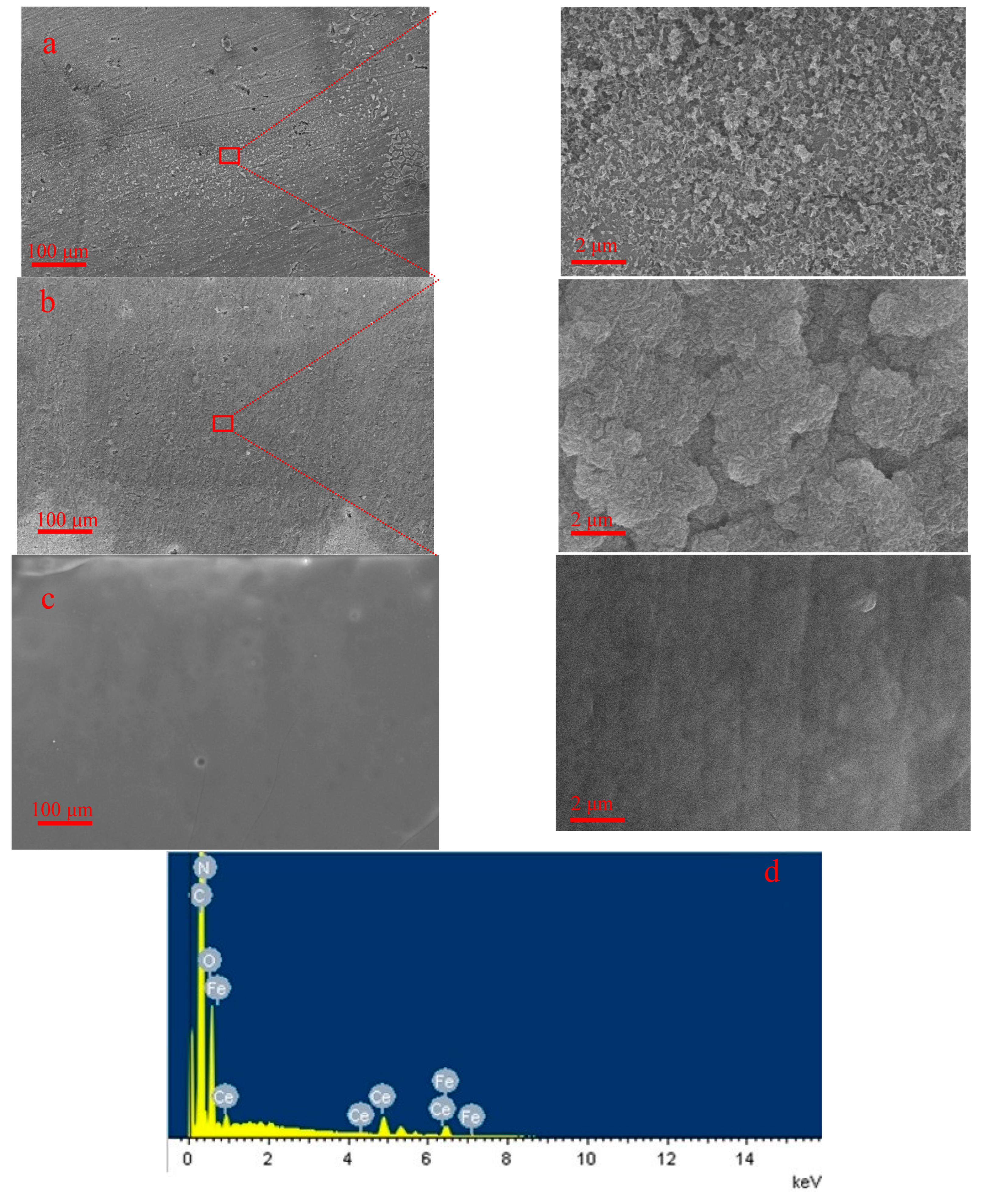
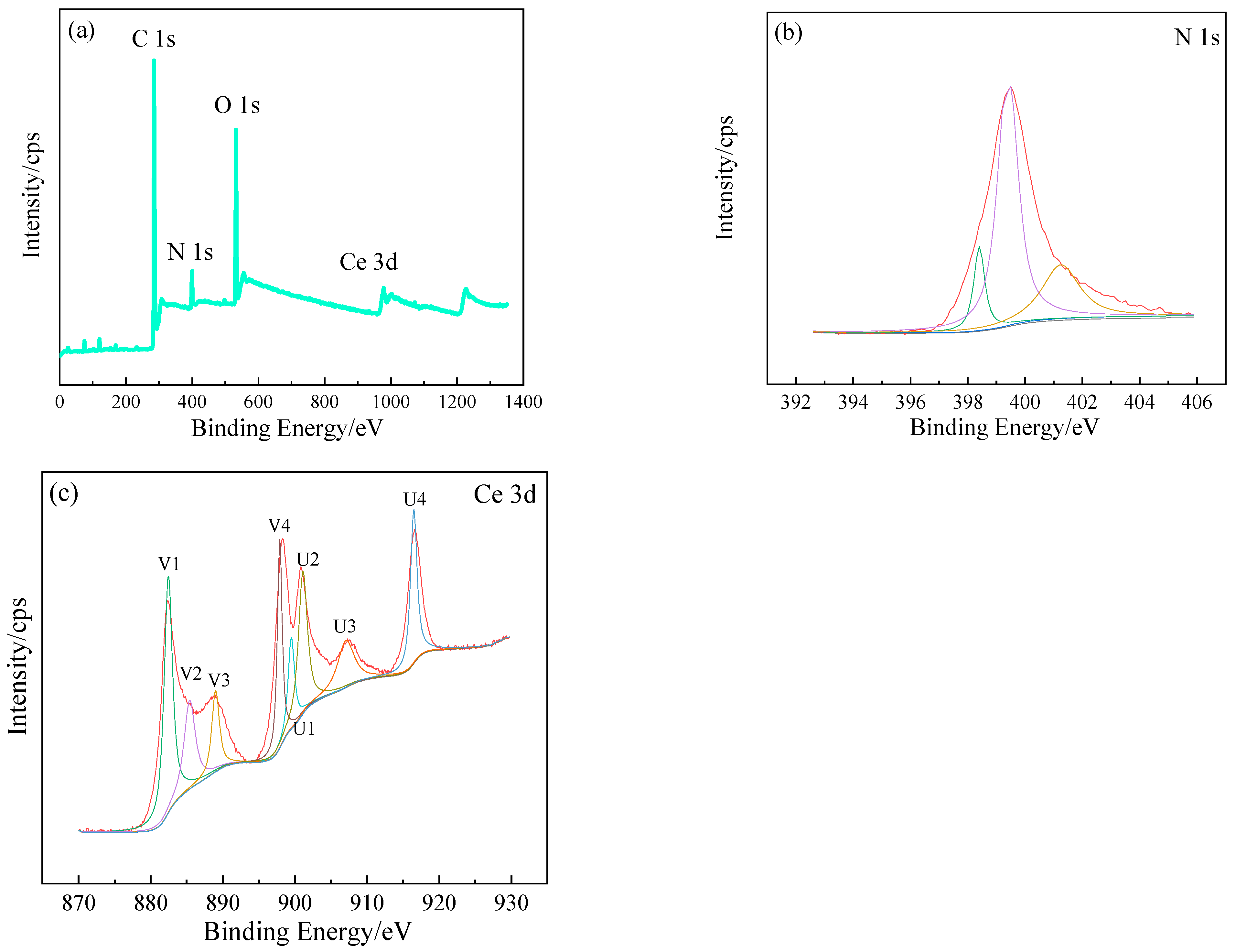
| Element | C | Si | Mn | Cr | Ni | Cu | Fe |
|---|---|---|---|---|---|---|---|
| % | 0.17 | 0.15 | 0.39 | 0.11 | 0.13 | 0.32 | balance |
| Sample | Aniline (mol/L) | Oxalic Acid (mol/L) | Benzoic Acid (mol/L) | Cerium Nitrate (mol/L) | Ammonium Acetate (mol/L) |
|---|---|---|---|---|---|
| 1 | 0.1 | 0.3 | - | - | - |
| 2 | 0.1 | - | 0.04 | - | - |
| 3 | 0.1 | - | 0.04 | 0.1 | 0.1 |
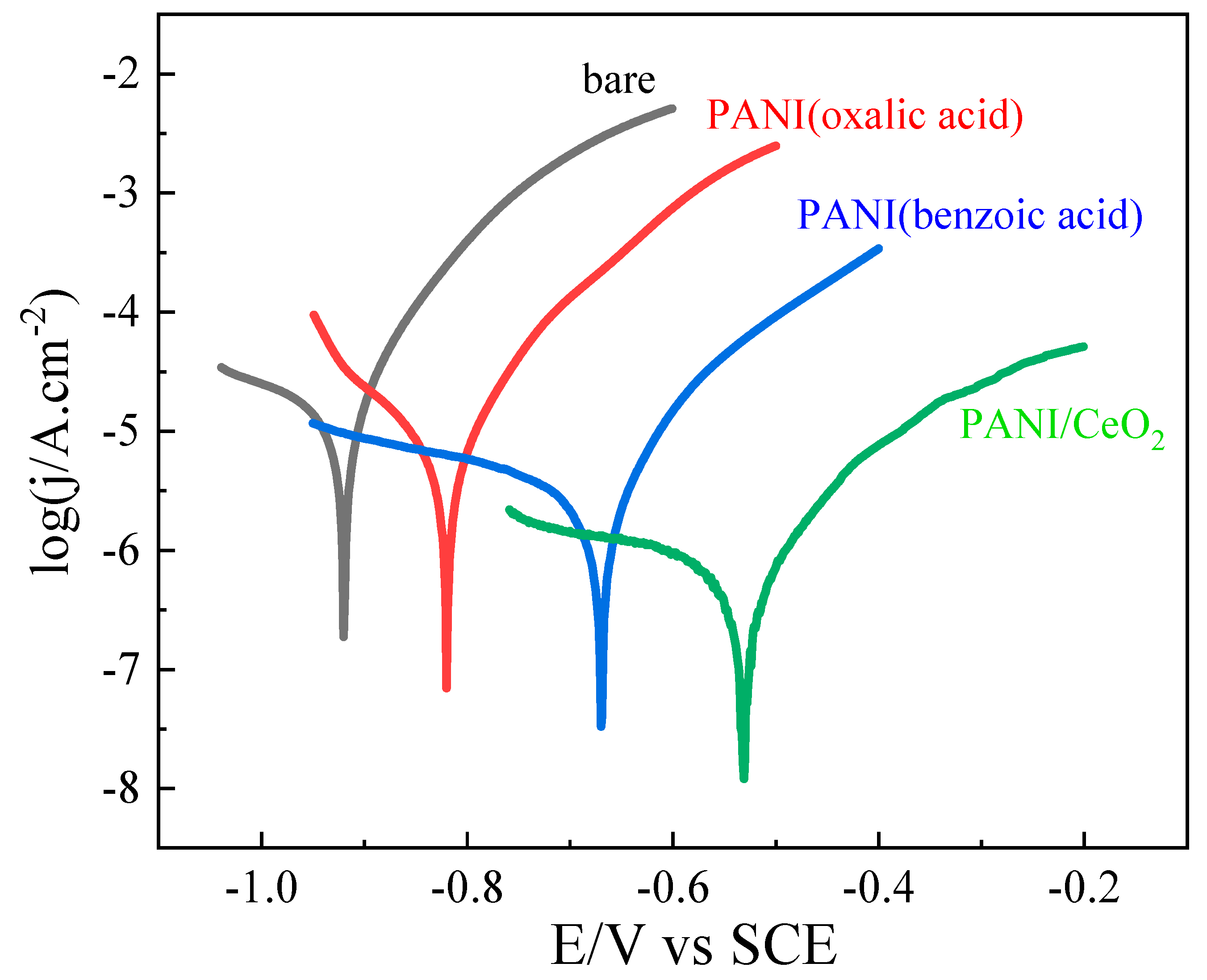
| Condition | Ecorr. (V) | jcorr. (μA.cm−2) | IE% |
|---|---|---|---|
| bare | −0.923 | 34.62 | - |
| polyaniline (oxalic acid) | −0.836 | 8.185 | 76.4 |
| polyaniline (benzoic acid) | −0.675 | 1.096 | 96.8 |
| polyaniline/CeO2 | −0.543 | 0.632 | 98.2 |
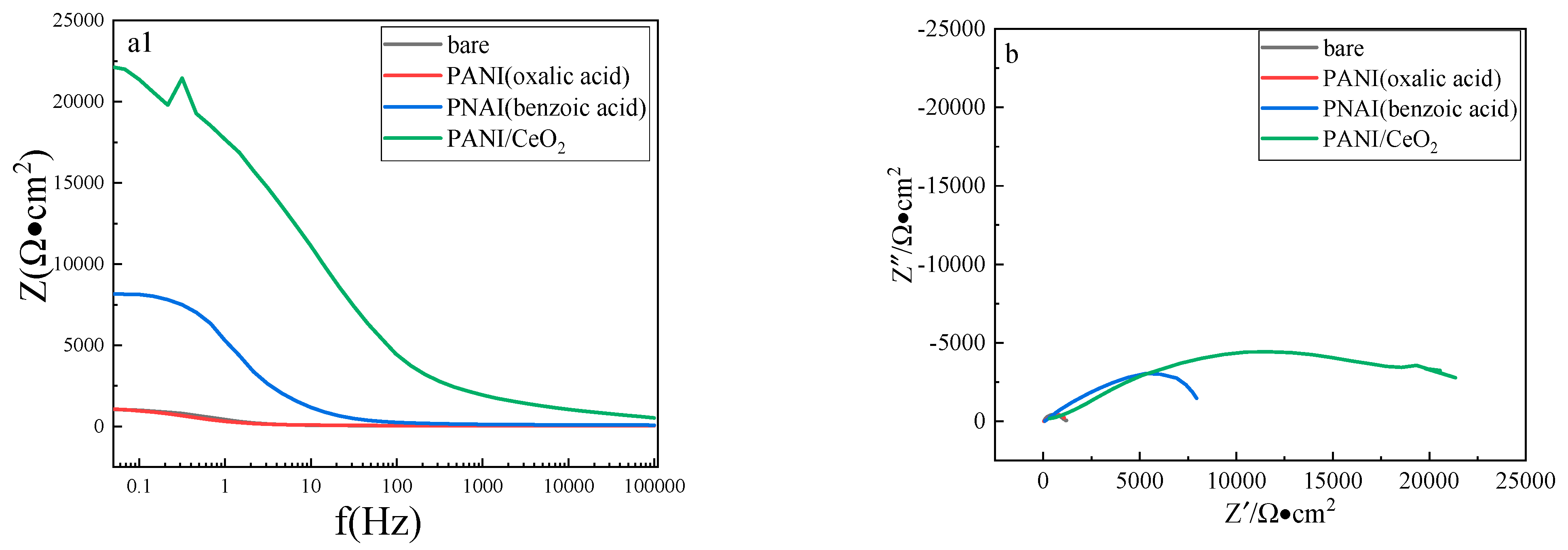
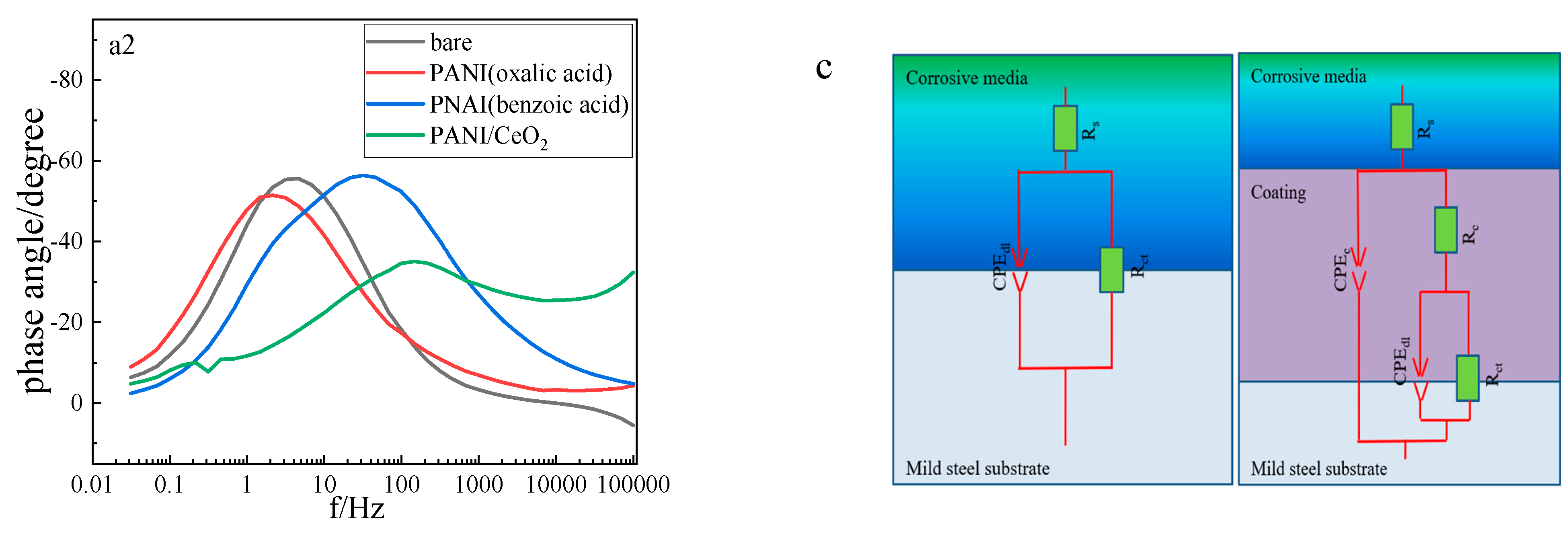
| Condition | Rct kΩ.cm2 | CPEdl Ω−1·cm−2·sn | nc | Rc Ω.cm2 | CPEc Ω−1·cm−2·sn | ndl | Rp kΩ.cm2 | PE% |
|---|---|---|---|---|---|---|---|---|
| bare | 1.06 | 3.81 × 10−5 | 0.83 | - | - | - | 1.06 | - |
| polyaniline (oxalic acid) | 1.14 | 3.17 × 10−5 | 0.83 | 27.7 | 9.57 × 10−5 | 0.73 | 1.17 | 9.4 |
| polyaniline (benzoic acid) | 11.3 | 6.59 × 10−6 | 0.84 | 990 | 5.38 × 10−5 | 0.77 | 12.3 | 91.4 |
| polyaniline/ CeO2 | 23.6 | 3.26 × 10−6 | 0.86 | 4754 | 2.93 × 10−5 | 0.81 | 28.4 | 96.3 |
| Sample | Electrolyte | IE% | Ref. |
|---|---|---|---|
| polyaniline | benzoic acid | 96.8 | this work |
| polyaniline/CeO2 | benzoic acid + cerium nitrate | 98.2 | this work |
| polyaniline | oxalic acid | 81.4 | [51] |
| polyaniline | sodium potassium tartrate | 82.5 | [7] |
| polyaniline/graphene | sulfuric acid + grahpene | 97.0 | [52] |
| polyaniline | sulfuric acid | 94.4 | [53] |
| polyaniline-GO | sodium dodecyl sulfate+GO | 98.4 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Du, X.; Yang, Z.; Chen, Y. One-Step In Situ Electrochemical Synthesis of Polyaniline/CeO2 Composite Coating for Enhanced Corrosion Protection of Mild Steel. Coatings 2025, 15, 74. https://doi.org/10.3390/coatings15010074
Zhou H, Du X, Yang Z, Chen Y. One-Step In Situ Electrochemical Synthesis of Polyaniline/CeO2 Composite Coating for Enhanced Corrosion Protection of Mild Steel. Coatings. 2025; 15(1):74. https://doi.org/10.3390/coatings15010074
Chicago/Turabian StyleZhou, Haoyao, Xiaoqing Du, Zhongnian Yang, and Yu Chen. 2025. "One-Step In Situ Electrochemical Synthesis of Polyaniline/CeO2 Composite Coating for Enhanced Corrosion Protection of Mild Steel" Coatings 15, no. 1: 74. https://doi.org/10.3390/coatings15010074
APA StyleZhou, H., Du, X., Yang, Z., & Chen, Y. (2025). One-Step In Situ Electrochemical Synthesis of Polyaniline/CeO2 Composite Coating for Enhanced Corrosion Protection of Mild Steel. Coatings, 15(1), 74. https://doi.org/10.3390/coatings15010074





