Quality Changes in Fresh-Cut Lettuce When Subjected to Ultrasound Combined with Zinc Oxide Nanoparticle (ZnO NP) Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the ZnO NP Solution
2.3. Preparation of the Fresh-Cut Lettuce Samples
2.4. Firmness and Propectin
2.5. Colors and Browning Index
2.6. Chlorophyll and Cellulose
2.7. The Total Suspended Solids (TSSs)/the Titratable Acid (TA) Ratio and the Soluble Sugar Content (SSC)/TA Ratio
2.8. Total Phenolic Content
2.9. Antioxidant Enzymes
2.10. Antioxidant Capacity
2.11. Malondialdehyde and H2O2
2.12. GC-MS Analysis
2.13. The Microstructure
2.14. Data Analysis
3. Results and Analysis
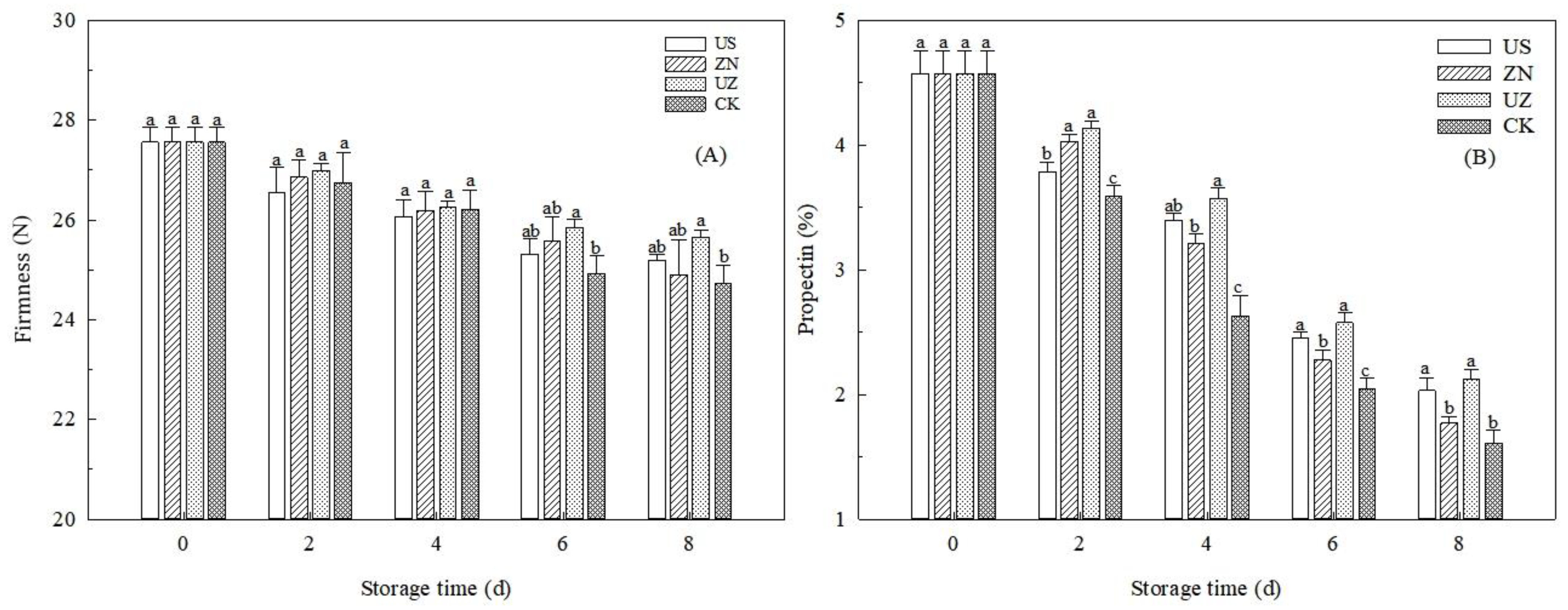
3.1. The Effects of Ultrasound Combined with ZnO NP Treatment on the Firmness and Propectin of Fresh-Cut Lettuce
3.2. Effects of Ultrasound Combined with ZnO NP Treatment on the Colors and BI of Fresh-Cut Lettuce
3.3. Effects of Ultrasound Combined with ZnO NP Treatment on the Chlorophyll and Cellulose of Fresh-Cut Lettuce
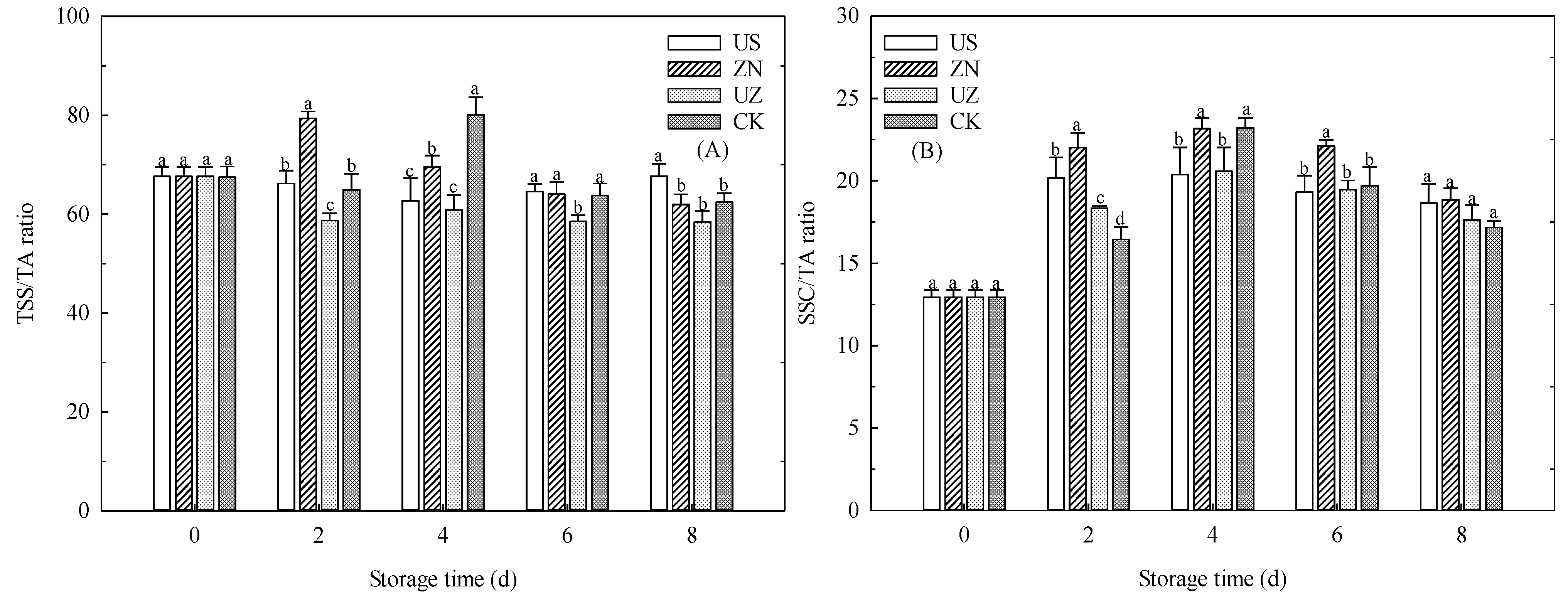
3.4. Effects of Ultrasound Combined with ZnO NP Treatment on the TSS/TA Ratio and SSC/TA Ratio of Fresh-Cut Lettuce
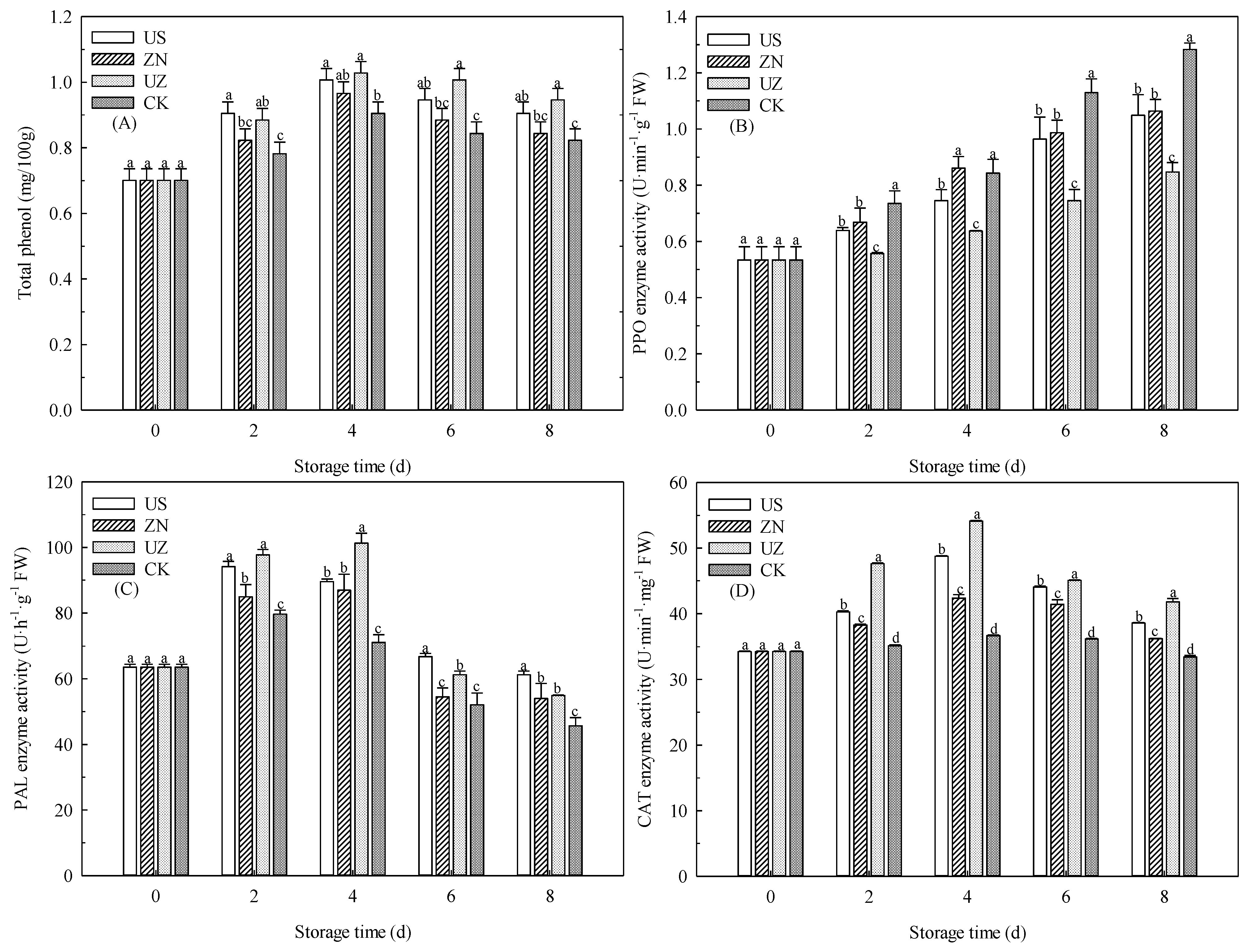
3.5. Effects of Ultrasound Combined with ZnO NP Treatment on the Total Phenolic Content and Activities of the Antioxidant Enzymes of Fresh-Cut Lettuce
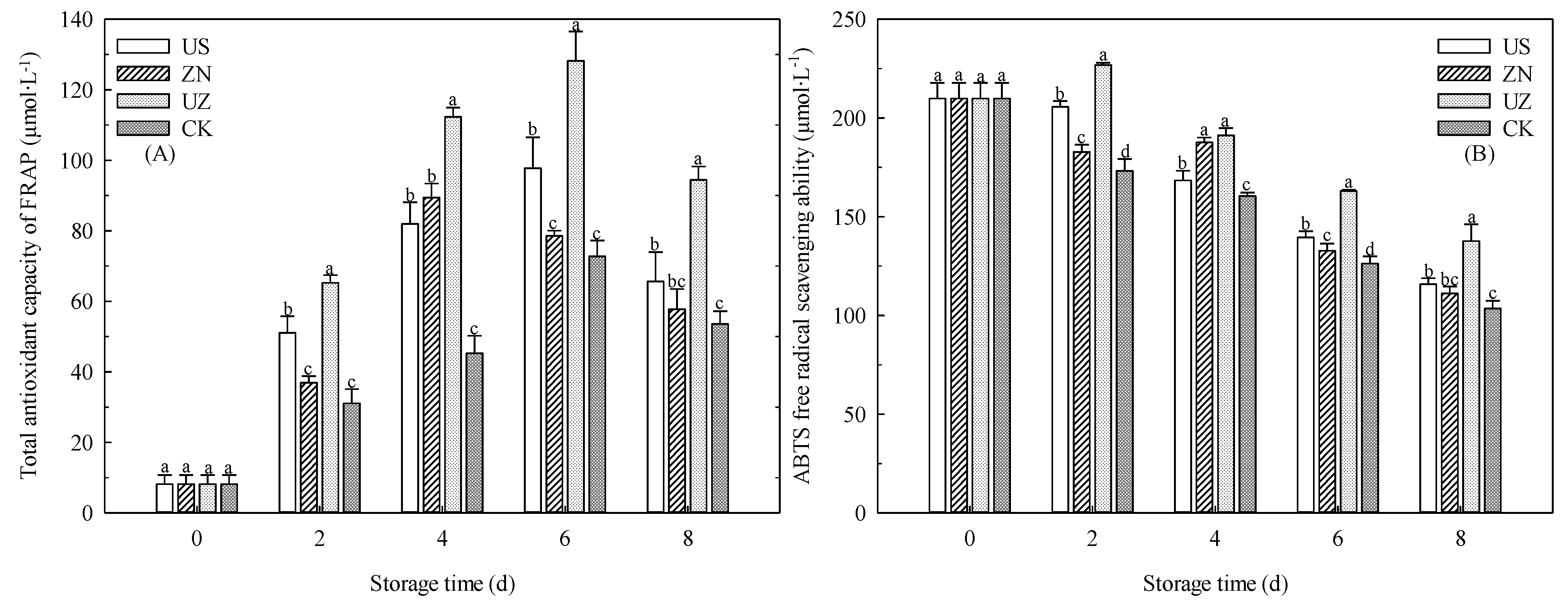
3.6. Effects of Ultrasound Combined with ZnO NP Treatment on the Antioxidant Capacity of Fresh-Cut Lettuce
3.7. Effects of Ultrasound Combined with ZnO NP Treatment on the Membrane Integrity of Fresh-Cut Lettuce
3.8. Effects of Ultrasound Combined with ZnO NP Treatment on the Volatile Organic Compounds of Fresh-Cut Lettuce
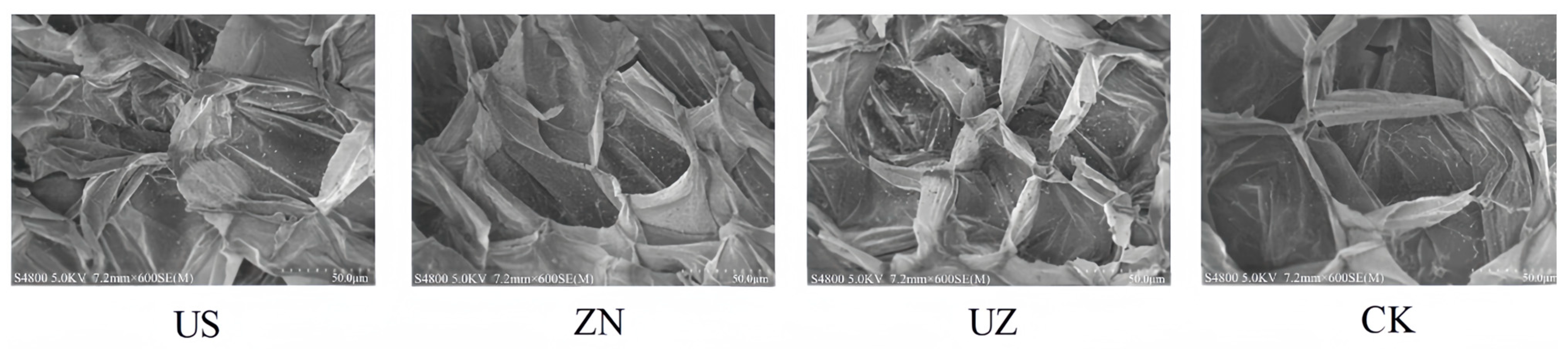
3.9. Effects of Ultrasound Combined with ZnO NP Treatment on the Microstructure of Fresh-Cut Lettuce
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banach, J.L.; van Bokhorst-van de Veen, H.; van Overbeek, L.S.; van der Zouwen, P.S.; Zwietering, M.H.; van der Fels-Klerx, H.J. Effectiveness of a peracetic acid solution on Escherichia coli reduction during fresh-cut lettuce processing at the laboratory and industrial scales. Int. J. Food Microbiol. 2020, 321, 108537. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhao, M.Y.; Phey, C.P.; Yang, H. Efficacy of low concentration acidic electrolysed water and levulinic acid combination on fresh organic lettuce (Lactuca sativa Var. Crispa L.) and its antimicrobial mechanism. Food Control 2019, 101, 241–250. [Google Scholar] [CrossRef]
- Wang, J.; Fan, L. Effect of ultrasound treatment on microbial inhibition and quality maintenance of green asparagus during cold storage. Ultrason. Sonochem. 2019, 58, 104631. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Li, D.; Tang, D.; Huang, Z.; Kedbanglai, P.; Ge, Z.; Du, X.; Supapvanich, S. Effects of simultaneous ultrasonic and cysteine treatment on antibrowning and physicochemical quality of fresh-cut lotus roots during cold storage. Postharvest Biol. Technol. 2020, 168, 111294. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, X.; Yagoub, A.E.A.; Owusu-Ansah, P.; Wahia, H.; Ma, H.; Zhou, C. Effects of low frequency multi-mode ultrasound and it’s washing solution’s interface properties on freshly cut cauliflower. Food Chem. 2022, 366, 130683. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, J.; Teng, Z.; Luo, Y.; Yu, L.; Simko, I.; Chen, P. Identification of marker compounds for predicting browning of fresh-cut lettuce using untargeted UHPLC-HRMS metabolomics. Postharvest Biol. Technol. 2021, 180, 111626. [Google Scholar] [CrossRef]
- Zhu, Y.; Du, X.; Zheng, J.; Wang, T.; You, X.; Liu, H.; Liu, X. The effect of ultrasonic on reducing anti-browning minimum effective concentration of purslane extract on fresh-cut potato slices during storage. Food Chem. 2021, 343, 128401. [Google Scholar] [CrossRef]
- Fan, K.; Wu, J.; Chen, L. Ultrasound and its combined application in the improvement of microbial and physicochemical quality of fruits and vegetables: A review. Ultrason. Sonochem. 2021, 80, 105838. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Cui, Q.L.; Wang, Y.; Shi, F.; Liu, Y.P.; Liu, J.L.; Nie, G.W. Effect of carboxymethyl chitosan-gelatin-based edible coatings on the quality and antioxidant properties of sweet cherry during postharvest storage. Sci. Hortic. 2021, 289, 110462. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.Q.; Zeng, S.X.; Yuan, S.Z.; Yue, X.Z.; Tian, T.; Zhu, X.Q.; Zheng, S.F.; Xu, X.B.; Zuo, J.H.; et al. Browning mechanism in stems of fresh-cut lettuce. Food Chem. 2023, 405, 134575. [Google Scholar] [CrossRef]
- Liu, H.N.; Pei, M.S.; Wei, T.L.; Yu, Y.H.; Guo, D.L. Ultrasound treatment to modified atmospheric packaged fresh-cut cucumber: Influence on microbial inhibition and storage quality. Ultrason. Sonochem. 2019, 54, 162–170. [Google Scholar] [CrossRef]
- Joshy, K.S.; Jose, J.; Li, T.; Thomas, M.; Shankregowda, A.M.; Sreekumaran, S.; Kalarikkal, N.; Thomas, S. Application of novel zinc oxide reinforced xanthan gum hybrid system for edible coatings. Int. J. Biol. Macromol. 2020, 151, 806–813. [Google Scholar] [CrossRef]
- Tornero, A.C.F.; Blasco, M.G.; Azqueta, M.C.; Acevedo, C.F.; Castro, C.S.; López, S.J.R. Antimicrobial ecological waterborne paint based on novel hybrid nanoparticles of zinc oxide partially coated with silver. Prog. Org. Coat. 2018, 121, 130–141. [Google Scholar] [CrossRef]
- Tantiwatcharothai, S.; Prachayawarakorn, J. Characterization of an antibacterial wound dressing from basil seed (Ocimum basilicum L.) mucilage-ZnO nanocomposite. Int. J. Biol. Macromol. 2019, 135, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Yuliani, S.; Ata, A.; Wardana, B.; Meindrawan, B.; Muchtadi, T.R. Nanocomposite edible coating from cassava starch, stearic acid and ZnO nanoparticles to maintain quality of fresh-cut mango cv. Arumanis. Ann. Univ. Dunarea Galati Fascicle VI-Food Technol. 2019, 42, 49–58. [Google Scholar]
- La, D.D.; Nguyen-Tri, P.; Le, K.H.; Nguyen, P.T.M.; Nguyen, M.D.-B.; Vo, A.T.K.; Nguyen, M.T.H.; Chang, S.W.; Tran, L.D.; Chung, W.J.; et al. Effects of antibacterial ZnO nanoparticles on the performance of a chitosan/gum arabic edible coating for post-harvest banana preservation. Prog. Org. Coat. 2021, 151, 106057. [Google Scholar] [CrossRef]
- Luo, L.; Wang, S.M.; Xu, W.W.; Pan, W.J. Study on the quality change of fresh-cut lotus root during storage with ultrasound-heat treatment. Food Ferment. Ind. 2022, 48, 203–210. [Google Scholar] [CrossRef]
- Xu, F.; Liu, S.; Xiao, Z.; Fu, L. Effect of ultrasonic treatment combined with 1-methylcyclopropene (1-MCP) on storage quality and ethylene receptors gene expression in harvested apple fruit. J. Food Biochem. 2019, 43, 12967. [Google Scholar] [CrossRef] [PubMed]
- Shahkoomahally, S.; Sarkhosh, A.; Richmond-Cosie, L.M.; Brecht, J.K. Physiological responses and quality attributes of muscadine grape (Vitis rotundifolia Michx) to CO2-enriched atmosphere storage. Postharvest Biol. Technol. 2021, 173, 111428. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Q.; Chen, W.; Guo, Q.; Xia, Y.; Wu, D.; Jing, D.; Liang, G. Melatonin treatment maintains quality and delays lignification in loquat fruit during cold storage. Sci. Hortic. 2021, 284, 233540344. [Google Scholar] [CrossRef]
- Nikzad, N.; Parastar, H. Evaluation of the effect of organic pollutants exposure on the antioxidant activity, total phenolic and total flavonoid content of lettuce (Lactuca sativa L.) using UV–Vis spectrophotometry and chemometrics. Microchem. J. 2021, 170, 106632. [Google Scholar] [CrossRef]
- Terefe, N.S.; Tepper, P.; Ullman, A.; Knoerzer, K.; Juliano, P. High pressure thermal processing of pears: Effect on endogenous enzyme activity and related quality attributes. Innov. Food Sci. Emerg. 2016, 33, 56–66. [Google Scholar] [CrossRef]
- Yeoh, W.K.; Ali, A. Ultrasound treatment on phenolic metabolism and antioxidant capacity of fresh-cut pineapple during cold storage. Food Chem. 2017, 216, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Gao, M.; Zheng, J.; Zhang, J.; Lu, L.; Liu, X. Novel browning alleviation technology for fresh-cut products: Preservation effect of the combination of Sonchus oleraceus L. extract and ultrasound in fresh-cut potatoes. Food Chem. 2021, 348, 129132. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, A.T.; Zhou, C.; Amanor-Atiemoh, R.; Ali, T.A.A.; Wahia, H.; Ma, H.; Sun, Y. Efficacy of dual-frequency ultrasound and sanitizers washing treatments on quality retention of cherry tomato. Innov. Food Sci. Emerg. 2020, 62, 102348. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, D.; Zhao, W.; Zheng, Y.; Wang, Y.; Wang, P.; Ma, Y.; Zhao, X. Low frequency ultrasound treatment enhances antibrowning effect of ascorbic acid in fresh-cut potato slices. Food Chem. 2022, 380, 132190. [Google Scholar] [CrossRef] [PubMed]
- Oyom, W.; Xu, H.; Liu, Z.; Long, H.; Li, Y.; Zhang, Z.; Bi, Y.; Tahergorabi, R.; Prusky, D. Effects of modified sweet potato starch edible coating incorporated with cumin essential oil on storage quality of ‘early crisp’. LWT 2022, 153, 112475. [Google Scholar] [CrossRef]
- Lu, C.; Ding, J.; Park, H.K.; Feng, H. High intensity ultrasound as a physical elicitor affects secondary metabolites and antioxidant capacity of tomato fruits. Food Control 2020, 113, 107176. [Google Scholar] [CrossRef]
- Khalid, S.; Malik, A.U.; Khan, A.S.; Khan, M.N.; Ullah, M.I.; Abbas, T.; Khalid, M.S. Tree age and fruit size in relation to postharvest respiration and quality changes in ‘Kinnow’ mandarin fruit under ambient storage. Sci. Hortic. 2017, 220, 183–192. [Google Scholar] [CrossRef]
- Zhang, S.; Fang, X.; Wu, W.; Tong, C.; Chen, H.; Yang, H.; Gao, H. Effects of negative air ions treatment on the quality of fresh shiitake mushroom (Lentinus edodes) during storage. Food Chem. 2022, 371, 131200. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Fan, L. Quality changes in fresh-cut asparagus with ultrasonic-assisted washing combined with cinnamon essential oil fumigation. Postharvest Biol. Technol. 2022, 187, 111873. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.; Hou, Y.; Pan, Y.; Shi, L.; Li, X. Effects of harvest maturity stage on postharvest quality of winter jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit during cold storage. Sci. Hortic. 2021, 277, 109778. [Google Scholar] [CrossRef]
- Fialho, L.; Ramôa, S.; Parenzan, S.; Guerreiro, I.; Catronga, H.; Soldado, D.; Guerreiro, O.; García, V.G.; e Silva, P.O.; Jerónimo, E. Effect of regulated deficit irrigation on pomegranate fruit quality at harvest and during cold storage. Agric. Water Manag. 2021, 251, 106869. [Google Scholar] [CrossRef]
- Ho, P.L.; Tran, D.T.; Hertog, M.L.A.T.M.; Nicolaï, B.M. Effect of controlled atmosphere storage on the quality attributes and volatile organic compounds profile of dragon fruit (Hylocereus undatus). Postharvest Biol. Technol. 2021, 173, 111406. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Yu, Y.; Wu, Z.; Wang, H. Combination of ozone and ultrasonic-assisted aerosolization sanitizer as a sanitizing process to disinfect fresh-cut lettuce. Ultrason Sonochem 2021, 76, 105622. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Ma, Q.; Zhang, J.; Jiang, K.; Cai, S.; Wang, W.; Wang, J.; Sun, J. Cactus polysaccharides enhance preservative effects of ultrasound treatment on fresh-cut potatoes. Ultrason. Sonochem. 2022, 90, 106205. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, J.L.G.; Justus, A.; de Oliveira, L.F.; Alves, G.E. Osmotic Dehydration of Tomato Assisted by Ultrasound: Evaluation of the Liquid Media on Mass Transfer and Product Quality. Int. J. Food Eng. 2015, 11, 505–516. [Google Scholar] [CrossRef]
- Liu, K.; Yuan, C.; Chen, Y.; Li, H.; Liu, J. Combined effects of ascorbic acid and chitosan on the quality maintenance and shelf life of plums. Sci. Hortic. 2014, 176, 45–53. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Jiang, Y.; Zhang, S.; Lin, Y.; Wang, Z. Phomopsis longanae Chi-induced pericarp browning and disease development of harvested longan fruit in association with energy status. Postharvest Biol. Technol. 2014, 93, 24–28. [Google Scholar] [CrossRef]
- Wang, T.; Yun, J.; Zhang, Y.; Bi, Y.; Zhao, F.; Niu, Y. Effects of ozone fumigation combined with nano-film packaging on the postharvest storage quality and antioxidant capacity of button mushrooms (Agaricus bisporus). Postharvest Biol. Technol. 2021, 176, 111501. [Google Scholar] [CrossRef]
- Han, Y.-F.; Cao, G.-X.; Gao, X.-J.; Xia, M. Isolation and characterisation of the sesquiterpene lactones from Lactuca sativa L var. anagustata. Food Chem. 2010, 120, 1083–1088. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wang, Y.; Wang, J.; Xu, Y.; Yi, S.; Zhu, W.; Mi, H.; Li, T.; Li, J. Combined ultrasound and heat pretreatment improve the enzymatic hydrolysis of clam (Aloididae aloidi) and the flavor of hydrolysates. Innov. Food Sci. Emerg. 2021, 67, 102596. [Google Scholar] [CrossRef]
- García, C.J.; Gil, M.I.; Tomas-Barberan, F.A. LC–MS untargeted metabolomics reveals early biomarkers to predict browning of fresh-cut lettuce. Postharvest Biol. Technol. 2018, 146, 9–17. [Google Scholar] [CrossRef]
- Nagdalian, A.; Blinov, A.; Gvozdenko, A.; Golik, A.; Rekhman, Z.; Rzhepakovsky, I.; Kolesnikov, R.; Avanesyan, S.; Blinova, A.; Pirogov, M.; et al. Effect of MnO2 Nanoparticles Stabilized with Cocamidopropyl Betaine on Germination and Development of Pea (Pisum sativum L.) Seedlings. Nanomaterials 2024, 14, 959. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, A.T.; Zhou, C. Novel assisted/unassisted ultrasound treatment: Effect on respiration rate, ethylene production, enzymes activity, volatile composition, and odor of cherry tomato. LWT 2021, 149, 111779. [Google Scholar] [CrossRef]
- Wang, J.; Huang, K.; Wu, Z.; Yu, Y. Effects of ultrasound-assisted low-concentration chlorine washing on ready-to-eat winter jujube (Zizyphus jujuba Mill. cv. Dongzao): Cross-contamination prevention, decontamination efficacy, and fruit quality. Ultrason. Sonochem. 2022, 82, 105905. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, J.; Liu, E.; Yang, M.; Chen, S.; Hu, F.; Ma, H.; Liu, Z.; Yu, X. Enhancing the taste of raw soy sauce using low intensity ultrasound treatment during moromi fermentation. Food Chem. 2019, 298, 124928. [Google Scholar] [CrossRef]



| Classification | The Aroma Components | Area/% | |||
|---|---|---|---|---|---|
| US | ZnO NPs | US + ZnO NPs | CK | ||
| Benzenes | Toluene | 1.82 ± 0.11 a | 1.19 ± 0.09 b | 1.11 ± 0.08 b | 0.58 ± 0.03 c |
| p-Xylene | 2.00 ± 0.15 a | ||||
| o-Xylene | 1.01 ± 0.08 a | ||||
| 1,4-Bis(trimethylsilyl)benzene | 16.29 ± 1.04 a | ||||
| Benzene, 1,2,4,5-tetramethy | 0.59 ± 0.02 a | ||||
| Benzene, 1,2,3,5-tetramethyl | 0.56 ± 0.03 a | ||||
| Ethylbenzene | 0.21 ± 0.02 a | ||||
| Benzene, 1,3-dimethyl | 0.05 ± 0.00 a | ||||
| Benzene, 1-ethyl-2,4-dimethyl | 1.79 ± 0.09 a | ||||
| Benzene, 1-methyl-3-(1-methylethyl) | 0.24 ± 0.00 a | ||||
| p-Cymene | 0.06 ± 0.00 a | ||||
| Benzene, 2-ethyl-1,3-dimethyl | 0.24 ± 0.02 a | ||||
| Benzene, 1,2,3,4-tetramethyl | 0.36 ± 0.02 b | 1.59 ± 0.11 a | |||
| Alkanes | Ethylene oxide | 10.08 ± 0.64 a | |||
| Heptane, 2,2,4,6,6-pentamethyl | 1.62 ± 0.10 a | 1.34 ± 0.07 b | 1.41 ± 0.07 ab | 1.6 ± 0.09 a | |
| Silane, [[4-[1,2-bis[(trimethylsilyl)oxy]ethyl]-1,2-phenylene]bis(oxy)]bis[trimethyl | 0.71 ± 0.06 a | ||||
| Silane, trimethyl[5-methyl-2-(1-methylethyl)phenoxy] | 13.71 ± 0.86 a | 13.16 ± 0.84 a | |||
| 3-Amino-2-phenazinol ditms | 0.39 ± 0.01 a | ||||
| Amines | Formamide, N-ethyl-N-phenyl | 24.16 ± 1.53 a | |||
| Chlorodifluoroacetamide | 11.15 ± 0.68 a | ||||
| 4-Amino-5-imidazole carboxamide,N,N,O- tris(trimethylsilyl) | 0.54 ± 0.03 a | ||||
| 1H-Indole-3-ethanamine, 6-fluoro-.beta.-methyl | 21.83 ± 1.39 b | 26.24 ± 1.64 ab | 27.28 ± 1.68 a | ||
| 2-Methylamino-N-phenyl-acetamide | 15.39 ± 0.93 a | ||||
| N-Benzyl-N-ethyl-p-isopropylbenzamide | 0.14 ± 0.00 a | ||||
| Acetamide, N-[(4.alpha.,5.alpha.)-cholestan-4-yl] | 0.21 ± 0.01 a | ||||
| Aldehydes | 1-Cyclohexene-1-carboxaldehyde, 4-(1-methylethenyl)-, (S) | 0.3 ± 0.02 a | |||
| Ethers | 2′,6′-Dihydroxyacetophenone, bis(trimethylsilyl) ether | 11.52 ± 0.70 a | 0.33 ± 0.01 b | ||
| Phenolics | 1,2-Benzenediol, 3,5-bis(1,1-dimethylethyl) | 12.91 ± 0.82 a | |||
| Alkenes | 2-Propen-1-amine, 2-bromo-N-methyl | 5.29 ± 0.32 b | 20.44 ± 1.27 a | ||
| Nitriles | 2-Amino-4-dimethylaminomethylenepentanedinitrile | 3.34 ± 0.17 c | 13.01 ± 0.85 b | 20.07 ± 1.21 a | |
| Ureas | 1-(6-Methyl-benzothiazole-2-yl)-3-(4-methyl-benzoyl)-thiourea | 1.06 ± 0.04 a | 0.32 ± 0.01 c | 0.38 ± 0.02 c | 0.64 ± 0.02 b |
| Ketones | 7-Methoxy-2,3-diphenyl-4H-chrome-4-one | 0.31 ± 0.01 a | |||
| Hydrazines | N-Phenyl-N’-(4-N,N-diethylaminobenzylidene) hydrazine | 0.12 ± 0.00 a | |||
| Acids | Arsenous acid, tris(trimethylsilyl) ester | 3.33 ± 0.16 a | |||
| Azoles | 5H-Naphtho[2,3-c]carbazole, 5-methyl | 0.47 ± 0.03 a | |||
| 1,2,4-Oxadiazole, 5-(4-nitrophenyl)-3-phenyl | 0.29 ± 0.01 a | ||||
| Quinolines | Benzo[h]quinoline, 2,4-dimethyl | 1.46 ± 0.08 b | 2.07 ± 0.13 a | ||
| 6-Chloro-3-ethyl-2-methyl-4-phenylquinoline | 0.27 ± 0.01 a | ||||
| Thiadiazines | 5-Methyl-2-phenylindolizine | 0.76 ± 0.03 a | |||
| Silicon | Silanediamine, 1-chloro-N,N,N’,N’,1-pentamethyl | 24.9 ± 1.47 a | |||
| Others | Carbon dioxide | 11.33 ± 0.69 b | 13.09 ± 0.86 b | 51.3 ± 3.11 a | |
| Benzenes | 22.27 | 3.54 | 1.71 | 2.17 | |
| Alkanes | 11.7 | 2.05 | 15.51 | 14.76 | |
| Amines | 35.85 | 37.22 | 26.38 | 27.49 | |
| Aldehydes | 0.3 | ||||
| Ethers | 11.52 | 0.33 | |||
| Phenolics | 12.91 | ||||
| Alkenes | 5.29 | 20.44 | |||
| Nitriles | 3.34 | 13.01 | 20.07 | ||
| Ureas | 1.06 | 0.32 | 0.38 | 0.64 | |
| Ketones | 0.31 | ||||
| Hydrazines | 0.12 | ||||
| Acids | 3.33 | ||||
| Azoles | 0.76 | ||||
| Quinolines | 1.73 | 2.07 | |||
| Thiadiazines | 0.76 | ||||
| Silicon | 24.9 | ||||
| Carbon dioxide | 11.33 | 13.09 | 51.3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Dong, Y.; Xu, W.; Wang, S.; Zhu, J.; Xu, Y.; Xu, M. Quality Changes in Fresh-Cut Lettuce When Subjected to Ultrasound Combined with Zinc Oxide Nanoparticle (ZnO NP) Treatment. Coatings 2024, 14, 943. https://doi.org/10.3390/coatings14080943
Xu X, Dong Y, Xu W, Wang S, Zhu J, Xu Y, Xu M. Quality Changes in Fresh-Cut Lettuce When Subjected to Ultrasound Combined with Zinc Oxide Nanoparticle (ZnO NP) Treatment. Coatings. 2024; 14(8):943. https://doi.org/10.3390/coatings14080943
Chicago/Turabian StyleXu, Xianmeng, Yulu Dong, Weiwen Xu, Shunmin Wang, Jiahui Zhu, Yudie Xu, and Min Xu. 2024. "Quality Changes in Fresh-Cut Lettuce When Subjected to Ultrasound Combined with Zinc Oxide Nanoparticle (ZnO NP) Treatment" Coatings 14, no. 8: 943. https://doi.org/10.3390/coatings14080943
APA StyleXu, X., Dong, Y., Xu, W., Wang, S., Zhu, J., Xu, Y., & Xu, M. (2024). Quality Changes in Fresh-Cut Lettuce When Subjected to Ultrasound Combined with Zinc Oxide Nanoparticle (ZnO NP) Treatment. Coatings, 14(8), 943. https://doi.org/10.3390/coatings14080943






