Abstract
Robust engineering of two-dimensional (2D) materials via covalent grafting of organic molecules has been a great strategy for permanently tuningtheir physicochemical behaviors toward electrochemical energy applications. Herein, we demonstrated that a covalent functionalization approach of graphitic surfaces including graphene by a graftable porphyrin (g-Por) derivative, abbreviated as g-Por/HOPG or g-Por/G, is realizable. The efficiency of this approach is determined at both the molecular and global scales by using a state-of-the-art toolbox including cyclic voltammetry (CV), X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, atomic force microscopy (AFM), and scanning tunneling microscopy (STM). Consequently, g-Por molecules were proven to covalently graft on graphitic surfaces via C-C bonds, resulting in the formation of a robust novel hybrid 2D material visualized by AFM and STM imaging. Interestingly, the resulting robust molecular material was elucidated as a novel bifunctional catalyst for both the oxygen evolution (OER) and the hydrogen evolution reactions (HER) in acidic medium with highly catalytic stability and examined at the molecular level. These findings contribute to an in-depth understanding at the molecular level ofthe contribution of the synergetic effects of molecular structures toward the water-splitting process.
1. Introduction
Overuse of conventional fossil fuels such as coal, oil, and natural gas causes inescapably serious crises including energy depletion and global warming due to greenhouse gas emissions. Therefore, it is necessary to look for renewable and environmentally friendly solutions to replace these non-renewable resources to minimize the above-mentioned negative effects. Among others, hydrogen has been regarded as one of the most acceptable alternative energy carriers for the replacement of conventional fossil fuels due to its higher mass–energy density and regenerative, green, and sustainable nature [1,2,3]. Three main approaches have been employed for hydrogen production in industry, namely, steam–methane reforming, coal gasification, and water splitting. However, the first two methods still consume fossil fuels, thereby massively emitting CO2 gas into the environment [4]. In contrast, water splitting is regarded as a promising and green route for hydrogen production because its products are only hydrogen and oxygen, thereby reducing significantly secondary pollution to the environment [4]. Nevertheless, only 3%–5% of industrial hydrogen production hasbeen generated by watersplitting so far [5]. This unexpectedly low value is the result of the energy-consuming nature of the water-splitting process.In addition, this process is also kinetically controlled electrochemically due to sluggish interfacial charge transfer [6,7,8]. Theoretically, the water-splitting process involves two half-cell reactions, the O2 and H2 evolution reactions (OER and HER) [9]. Compared to the HER, which involvesonly two electron transfers, ensuring faster reaction kinetics [10], the OER is considered to be the bottleneck reaction from both the thermodynamic and kinetic points of view because it takes place through a multistep proton-coupled electron transfer, involving 4e− and 4H+ [11]. Therefore, seeking efficient and stable bifunctional electrocatalysts in order to increase the kinetics of the two half-cell reactions by lowering the energy barrier as well as to overcome their unfolding becomes crucial. To date, Ir/Ru-based metal oxides as well as the Pt-based precious metal alloys have been known as the best electrocatalysts for OER and HER in both basic and acidic media, respectively [12,13]. Nevertheless, despite significantly lowering the overpotentials as well as enhancing the conversion efficiency with respect to the water-splitting process, they are made from noble metals, thereby, limiting their utilization as practically promising electrocatalysts, particularly for large-scale applications. To deal with this challenge, numerous scientific studies focusing on non-precious metals as well as metal-free-based catalysts for enhancing the HER and OER processes have been conducted. For instance, metal and/or metal-oxide-based catalysts have received much attention due totheirlowcost, morphology and facets, as well as defect-tuned optoelectronic properties, which are favorable for overall water splitting [14,15,16,17,18,19]. Nonetheless, their inherent disadvantages such as low charge mobility and self-degradation have thus far resulted in low conversion efficiency and stability [20,21,22].
Other alternatives have been developed recently based on π-conjugated carbon materials due to their unique electrochemical properties that noticeablypromote the rate of the electrochemical reactions for HER and OER [23,24]. Among others, porphyrin-based materials are regarded as someof the most investigated materials in the field of molecular catalysts owing to their exceptional photochemical and photophysical properties [25]. Possessing four pyrrole units interconnected toeach other via methane bridges and a large π-conjugated electron system, all porphyrin derivatives can bind almost any known metal ions and be substituted by different functional moieties [26]. These intrinsic characteristics have provided great flexibility in engineering and designing surface-terminal functional groups, thereby enhancing the applicability of this class of molecule in electrocatalysis including HER and OER [27,28]. Thus far, metallated porphyrin-based catalysts with a metal center responsible for the catalytic activity have been exclusively investigated for water splitting [26,29,30,31]. Non-covalent functionalization of electrode surfaces has been employed for designing these types of heterogeneous catalysts [4,29,32,33,34]. Compared with other approaches, this approach dealing with two-dimensional (2D) materials including carbon-basedas well as transition metal dichalcogenides(TMDs)materials has been intensively focused on due to theintrinsic properties of the latter materials, including distinct thickness-dependent optoelectronic properties, large surface areas, and enormous experimental charge mobilities that facilitate the electrocatalytic activities [35,36,37,38,39]. In particular, a molecular approach to investigate the electrocatalytic HER process of a two-dimensional system consisting of catalytically active porphyrin molecules and atomically thin graphene electrodes was also conducted in order to verify the HER mechanism at the molecular scale [40].
Recently, it has been demonstrated that the catalytic activity of porphyrin-based complexes towards HER and OER can be activated by the N–H groups and N-lone pairs in the core of free-base porphyrins under acidic conditions [41,42,43]. The functional groups bearing either electron-donating or -withdrawing moieties substituted at the meso-position of the porphyrin ring can alter the basicity of porphyrin-based complexes that lead to distinct redox reactivity [44,45]. Motivated by this experimental proof-of-concept, several works investigating the catalytic activity of metal-free porphyrin-based complexes toward the bifunctional water-splitting process have been conducted so far [6,41]. Despite its non-destructive approach, thereby keeping the intrinsic characteristics of individuals intact, the stability of these electrocatalysts, particularly under such a strong acidic medium, which is employed frequently to operate proton-exchange-membrane-based water electrolysis [46], has become a bottleneck issue during electrolysis [8,47]. In order to deal with this drawback, covalent functionalization of porphyrin moieties to graphitic substrates has been suggested [48,49,50,51]. Advantageously, this approach enables the formation of heterogeneous materials even for such water-soluble porphyrin derivatives [52]. More importantly, upon covalent functionalization, controlled defects can be introduced that enable the bandgap tunability of semi-metal graphene [53]. Nevertheless, until now, such studies at the molecular scale on the enhancement of the electrocatalytic activity of heterogeneous materials have not been really reported, leading to a lack of insight into the location of the catalytic molecules attaching to the graphene surface, i.e., on defects, on functional groups, or the basal plane. Furthermore, bearing functional groups of different natures on its surface could also contribute to the overall catalytic effect of agraphitic-based catalyst [54]. Therefore, revealing the electrocatalytic roles of modified carbon materials including graphene at the molecular level is highly desirable.
In this study, we endeavored to employ a covalent molecular-anchoring strategy to functionalize graphitic surfaces including highly oriented pyrolytic graphite (HOPG), a model multilayered graphene, and CVD graphene on SiO2 (G-SiO2) by a graftable porphyrin derivative (g-Por). The efficiency of this approach was evaluated using cyclic voltammetry (CV), atomic force microscopy (AFM), scanning tunneling microscopy (STM), X-ray photoelectron spectroscopy (XPS), and Raman spectroscopy. Consequently, a full multilayer is obtained upon electrografting with 2 mM of g-Por-containing solution. Interestingly, the electrografted layer of g-Por is highly electrochemically stable within the investigated potential window and only removed by mechanics and/or thermal annealing, leaving behind pristine graphitic surfaces [55,56]. In particular, the g-Por-modified HOPG was revealed to be an effective bifunctional electrocatalyst for both the OER and HER processes in acidic medium. Our proposed approach provides a new way to covalently engineer graphenics and other 2D materials in a controlled manner applicable to water splitting as well as other energy conversion processes.
2. Materials and Methods
All chemicals including 5,10,15,20-tetrakis(4-aminophenyl)-21H,23H-porphine (abbreviated as a-Por), hydrochloric acid (37%), sulfuric acid (98%), and sodium nitrite (>97%) were purchased from Sigma-Aldrich and used without further purification. Graftable porphyrin, abbreviated as g-Por, was insitu generated from its corresponding aniline, i.e., a-Por. In detail, an excess amount of 0.1 M NaNO2 was added to a-Por-containing electrolyte right before being injected into the electrochemical cell, followed by stirring for 2 min. It is worth noting that concentrated HCl (1M) solution was employed to completely dissolve the a-Por compound followed by diluting the solution to the expected concentration, i.e., 50 mM. High-purity water (Milli-Q purification system, Burlington, MA, USA, <3 ppm, >18 MΩ cm) was used for all prepared electrolytes, including 5 mM H2SO4 and 1M HCl + X mM a-Por (X = 0.2 and 2 mM). All electrolytes were degassed by Ar (grade 5.0, Praxair, Danbury, CT, USA) for several hours before use.
HOPG (ZYB grade) was ordered from Momentive Performance Materials and CVD graphene on SiO2 (G-SiO2) was purchased from Graphenea. Prior to every experiment, G-SiO2 was carefully washed dropwise with toluene and dried in a stream of N2, whereas HOPG was freshly cleaved using scotch tape.
All voltammetry (CV) measurements were performed using both a Biologic potentiostat (VSP; Bio-Logic Science Instruments, Singapore) and an Autolab PGSTAT101 potentiostat (Metrohm_AutolabBV, Utrecht, The Netherlands) integrated with a lab-built single-compartment three-electrode cell holding a working electrode area of 38.5 mm2. Pt wire and Ag/AgCl (saturated KCl) served as counter and reference electrodes, respectively. The g-Por grafted samples were rinsed with hot toluene (90°) followed by Milli-Q water and finally dried using a N2 stream to remove any non-covalently functionalized species. The working potential was measured with respect to Ag/AgCl and then converted for the reversible hydrogen electrode (RHE) according to the following Equation (1):
ERHE = EAg/AgCl/KCl + 0.197 V + (0.0591 pH)
Raman measurements were performed with a 632.8 (nm) He–Ne laser using an Omega Scope 1000 (AIST-NT, Stamford, CT, USA). Raman scattering was collected and directed to a Raman spectrograph (HoribaJY, Kyoto, Japan, iHR-320) equipped with a cooled charge-coupled device (CCD) camera operated at −100 °C (Andor Technology, Belfast, UK, DU920P) through a dichroic mirror, a pinhole, and a long-pass filter (Chroma Technology Corporation, Bellows Falls, VT, USA, HQ645LP). All of the measurements were performed under ambient conditions.
XPS measurements were conducted on a K-alpha X-ray photoelectron spectrometer (Thermo VG, Oxford, UK) employing a monochromated Al Kα (hν = 1486.6 (eV), 3 (mA) emission) source. The analyzer was operated in fixed analyzer transmission (FAT) mode with survey scans taken with a pass energy of 200 (eV) and high-resolution scans with a pass energy of 40 eV. The binding energy was referenced to the Ag 3d5/2 peak at 368.21 (eV) measured under the same operating conditions.
AFM measurements were acquired in tapping mode under ambient air conditions with silicon cantilevers (spring constant of 21–60 (N/m) and a resonance frequency of ca. 300 kHz, Olympus, Tokyo, Japan) using a Multimode SPM (DI) with a Nanoscope IV controller.
STM experiments were performed in constant-current mode using a Pico STM system (Agilent, Santa Clara, CA, USA). STM tips were prepared by mechanical cutting Pt/Ir wire (80%/20%, diameter 0.25 (mm)). STM and AFM data analysis was performed using WSxM 5.0 [57].
3. Results
3.1. Electrochemical and Structural Properties of Pristine HOPG Surface
In order to guarantee the electro-inactiveness and freshness of the HOPG surface, a model multilayered graphene surface, within the measured potential regime, initial CV and STM measurements were performed in asupporting electrolyte of 5 mM H2SO4.Figure 1a shows the cyclic voltammogram (CV) describing the electrochemical behavior of a HOPG electrode contacted to the supporting electrolyte of 5 mM H2SO4 and measured within the potential range of +2.2 (V) to −1.0 (V) vs. RHE. As can be seen, the obtained CV is featureless within a broad potential window that is limited by two surface reaction processes, namely, the hydrogen evolution reaction (HER) at the cathodic, and the copper dissolution reaction (OER) at the anodic potential limits, demonstrating that the HOPG surface is not affected by the presence of the sulfate (SO42−) anions. This means that the HOPG surface remains intact within the measured potential regime. This was then demonstrated at the molecular scaleemploying STM imaging as shown in Figure 1b,c.The results show that the pristine HOPG surface is atomically flat, featuring a characteristic hexagonal carbon lattice without any adsorbates or defects. This feature is beneficial for the investigation of covalent functionalization at the molecular scale.
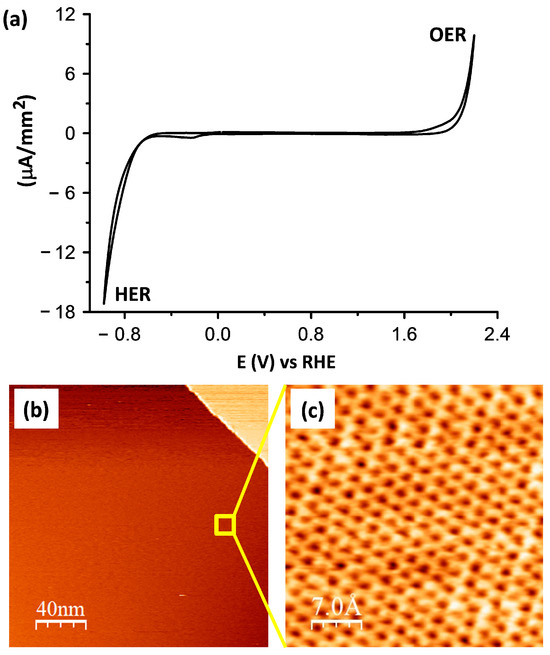
Figure 1.
(a) Voltammetric curve of a HOPG electrode in pure 5 mM H2SO4 electrolyte showing a featureless curve except for characteristic anodic OER and cathodic HER, scan rate dE/dt = 50 (mV/s); (b) STM images illustrating (b) the surface morphology and (c) atomic lattice of the top-most carbon layer with the corresponding measuring parameters: It = 0.1 (nA), Ub = −0.6 (V); and It = 0.4 (nA), Ub = −0.6 (V).
3.2. Electrochemical Functionalization of HOPG by g-Por
The pristine HOPG surface characterized above was employed for the covalent functionalization of g-Por using an electrografting process [58]. Figure 2a illustrates CV curves of the HOPG surface brought into contact with a working electrolyte of 2 mM g-Por + 50 mM HCl + access 0.1 M NaNO2 and conducted within the potential range of +0.5 (V) to −1.0 (V) vs. RHE. As can be seen, within the investigated potential regime, an identically irreversible reduction peak located at E1 = +0.21 (V) (RD) accompanying two others at E2 = −0.18 (V) (RP1) and E3 = −0.44 (V) (RP2) vs. RHE, respectively, are observed in the first scan (black curve). The first reductive peak (RD) is absent in subsequent cycles and the others become insignificant (red curve). This indicates that surface modification is blocked under this experimental condition [58]. In order to define the derivation of these peaks, we carried out electrochemical measurements of a-Por solution with and without adding NaNO2 (Figure S1). Based on this experiment, it is reasonable to assign the RD peak in the first scan to the reduction of the diazonium ions (N2+), which were insitu generated upon adding the NaNO2, in order to form the corresponding aryl radicals that immediately covalently attach to graphitic surfaces via C-C bonds. The suppression of this peak in subsequent scans results from the covalent g-Por layer formed on the HOPG surface during the first cycle [56,59]. Meanwhile, the two further reductive peaks (i.e., RP1 and RP2), which were also observed upon measuring with respect to the NaNO2 free solution (Figure S1), are probably caused by the reduction of the porphyrin ring [60,61].
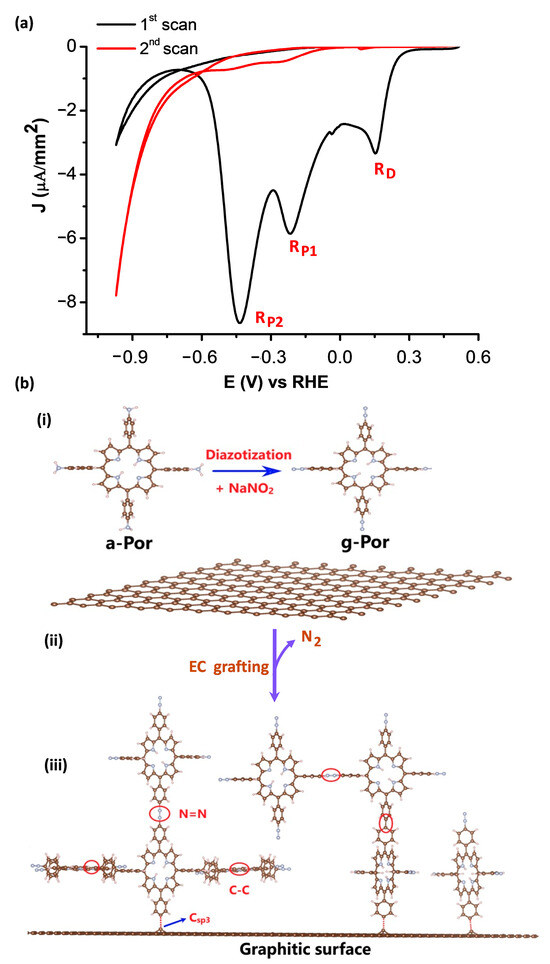
Figure 2.
(a) Cyclic voltammograms of HOPG in 2 mM g-Por + 50 mM HCl + access 0.1 M NaNO2. Scan rate dE/dt = 50 (mV/s). (b) Schemes showing (i) the formation of g-Por diazonium cation from its corresponding aniline, (ii) electrochemical reduction of g-Por cations to the corresponding radicals and (iii) covalent grafting of g-Por radicals on the graphitic surface.
It can be realized that, this overall covalent grafting process involves three steps as proposed in Figure 2b. Step one relates to theinsitu generation of g-Por cations by reaction of the corresponding amine with NaNO2 in the acidic medium as illustrated by Scheme (i). Step 2 involves the electrochemical reduction of g-Por cations accompaniedby the release of N2 molecules to form the aryl radicals (Scheme (ii)). Step 3 is the covalent attachment of the highly reactive g-Por radicals to either the carbon lattice or the pre-grafted g-Por molecules via C-C and/or azo (-N=N-) bondings, thereby further developing the grafted film thickness, as described by Scheme (iii) in Figure 2b.
3.3. Covalent Nature and Structural Properties of g-Por/HOPG
In order to verify the efficiency of the applied functionalization protocol, an integrated toolbox including spectroscopic and microscopic techniques was used.
The covalent nature of the bond formed between the g-Por moieties and the HOPG surface was demonstrated by Raman spectroscopy, which provides the degree of carbon lattice defects via the D-band intensity measurement. Therefore, this is assigned as an experimentally reasonable approach to characterize the degree of covalent functionalization [62,63]. A direct comparison between the Raman spectra of pristine HOPG surface (black curve) and g-Por/HOPG is illustrated in Figure 3a. All displayed Raman spectra were averaged over five sample spots. As a result, the typical peak at 1579 (cm−1), the so-called G-band of the carbon lattice, is observed in both spectra [58]. In particular, the spectrum of the pristine HOPG does not show any D-band peak evolution, meaning that the employed HOPG surface is defect-free [64]. Contrarily, the Raman spectrum of g-Por/HOPG shows an identical D-band peak located at 1350 ± 10 (cm−1) that is activated exclusively upon introducing Csp3-based defects into the Csp2 lattice (marked by the blue arrow in Figure 3a and the corresponding inserted figure) [64]. The observed result provides a firm basis to demonstrate C-C covalent bonding formation upon electrografting of g-Por molecules to the HOPG surface.
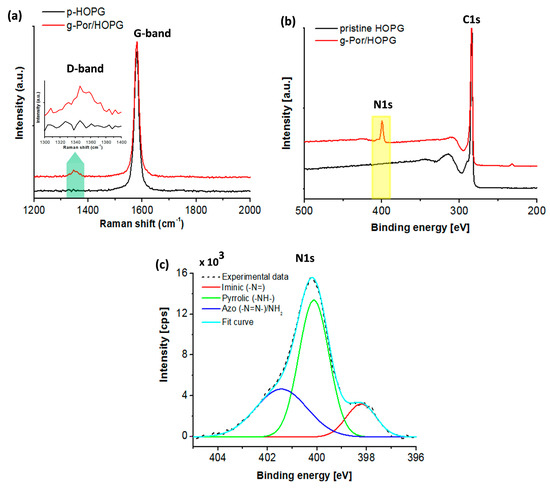
Figure 3.
(a) Raman spectra of HOPG before (black curve) and after grafting of g-Por moieties (red curve) showing an evolution of the D-band. The spectra were averaged over 5 sample spots; Integration time: 100 (s); (b) XPS survey spectra of pristine HOPG before (black curve) and after being functionalized by g-Por molecules (red curve); (c) XPS high-resolution spectrum of core-level nitrogen element showing three distinct components.
To obtain adetailed insight into the chemical properties of the g-Por functionalized HOPG surface, XPS characterization was used as shown in Figure 3b,c. Compared to the survey spectrum of pristine HOPG as illustrated by the black curve in Figure 3b, the g-Por/HOPG spectrum presents an additional signal at ~400 (eV) coupled with the typically characterized C1s (284.4 (eV)) seen in the spectrum of the pristine material [65,66]. This is assigned to the nitrogen (N1s) core-level signature generated from the g-Por/HOPG (red curve). Deconvoluting the XPS spectrum of the N 1s peak shows that it is formed of three peaks centered at 398.03 (eV), 400.21 (eV), and 400.85 (eV) (Figure 3c). The first two peaks can be assigned to the iminic (-N=) and pyrrolic (-NH-) groups within the porphyrin ring [67,68,69]. Meanwhile, the last peak at 400.85 eV peak can be assigned to the azo (-N=N-) and/or amine groups [70,71,72,73]. Combining these results with those of the Raman measurement, it becomes evident that the observed N1s signal on the g-Por/HOPG sample reveals a successful grafting of the g-Por moieties to the HOPG surface.
The morphology of the g-Por/HOPG material was investigated usingthe AFM method (Figure 4a,b). The results show that the HOPG surface is fully covered by g-Por molecules, forming a homogeneously compact film with ameasured root-mean-square (RMS) roughness of 0.92. AFM-tip-assisted degrafting was conducted on the g-Por/HOPG sample to quantitatively measure the thickness of the functionalized layers (Figure S2). This showed that the thickness value found with this experimental condition validated with the green line in Figure S2a is about 4.7 ± 0.2 (nm) (Figure S2b), which is approximately equal to atrilayer of g-Por assuming that the grafted g-Por molecules in the first layer align perpendicularly with respect to the HOPG surface.
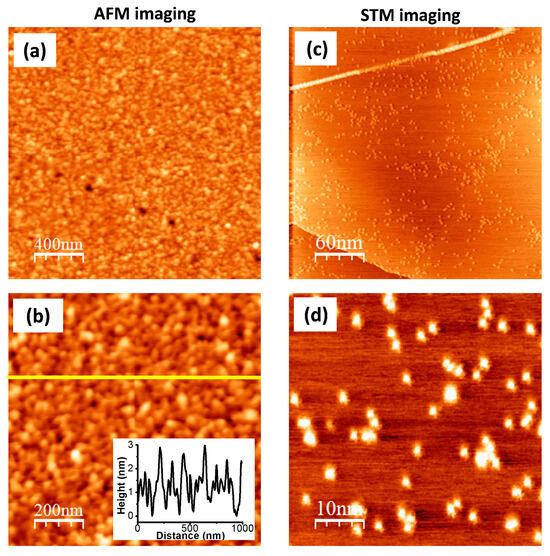
Figure 4.
(a,b) AFM images of g-Por/HOPG showing a multilayered formation of the grafted g-Por moieties on HOPG surface; (c,d) High-resolution STM images of g-Por/HOPG with different scales. Tunneling parameters: It = 0.1 (nA), Ub = −0.6 (V).
The STM technique was employed to identify the grafted sites on the surface owing to its high sensitivity to the local density of states (LDOS) at the surface, which is probably affected by the on-site covalent modification [58,74]. Figure 4c,d illustrate both large-scale and high-resolution STM images of the g-Por/HOPG surface. These show randomly bright clusters with variable dimensions reflecting the different grafted configurations as proposed in Figure 2b. The different visualizations by AFM and STMcan be interpreted via the unmatched scanning mechanisms of these techniques. The AFM method is used to visualize the morphology of the topmost grafted layer, whereas the STM enables visualization of the molecules directly anchored to the HOPG surface.
Encouraged by the successful grafting on HOPG, the used protocol for covalent functionalization was also extended to CVD graphene on SiO2 (G-SiO2). The output of this approach was examined by Raman spectroscopy and AFM imaging. Figure 5a shows a comparison ofthe Raman spectra of the pristine G-SiO2 and g-Por/G-SiO2 surfaces. The results show that the pristine G-SiO2displaysatypical peak at 1597 (cm−1), which is characteristic of the G-band of the carbon lattice (the black spectrum in Figure 5a). In addition, a very tiny peak at 1352 (cm−1) (assigned as the D-band) is recorded in the pristine G-SiO2spectrum demonstrating the presence of some negligibly random defects throughout the employed graphene surface. By contrast, the D-band peak is of significant size in the g-Por/G-SiO2spectrum, implying that the defect density of the graphene surface increased owing to the formation of C-C covalent bonds between the graphene lattice and g-Por moieties upon electrochemical grafting [74,75]. The morphological derivation of G-SiO2 after being grafted by g-Por was also verified by AFM imaging as shown in Figure 5b,c and Figure S3. This showed a homogeneously flat surface with a measured root mean square (RMS) roughness of about 0.77, including wrinkles that originated from synthesis processes visualized for the pristine G-SiO2 (Figure S3a–c) [76,77,78]. In contrast, upon being functionalized by g-Por molecules, the G-SiO2 morphology was obviously altered by the formation of a homogeneous grafted layer coupled with bright clusters generated from physisorbed side products, which resulted in a significant increase in its measured RMS roughnessto 2.02 as illustrated in Figure 5b,c and Figure S3d–f. The developments in D-band intensity and RMS roughness are both evidence for a successful covalent functionalization of g-Por onto the G-SiO2 surface.
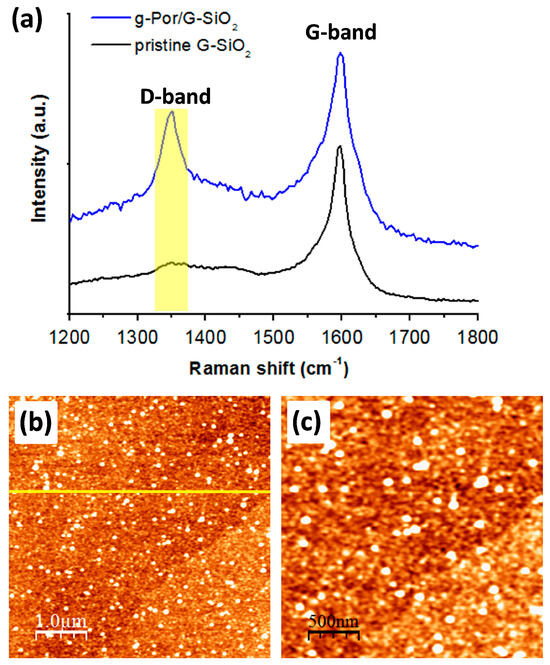
Figure 5.
Raman spectra (a) and AFM images (b,c) of g-Por/G-SiO2 showing significant developments in the D-band intensity and the RMS surface roughness upon the grafting of g-Por molecules as demonstrated by the line profile shown in the insert Figure 5b, which was measured along with the yellow line, respectively.
3.4. Electrocatalytic Activities of g-Por/HOPG
Motivated by the positive results of the electrochemical and structural characterizations, it is reasonable to investigate the electrocatalytic performance of g-Por/HOPG with respect to both the HER and OER processes in an aqueous acidic solution containing 0.1 M H2SO4 using linear sweep voltammetry (LSV). The results are shown in Figure 6. The potential range for HER is from 0 (V) to −0.78 (V) vs. RHE, whereas that for OER is from 0.0 (V) to +2.0 (V) vs. RHE. At asteady state, the g-Por/HOPG electrode exhibits much better catalytic activity in the HER compared to pristine HOPG (black and red curves in Figure 6a) with an initial onset overpotential (η) of ca. 0.25 (V) vs.RHE. The calculated overpotentials to sustain a catalytic current of 3.0 mA.cm−2 for the pristine HOPG and g-Por/HOPG electrodes are 0.75 (V) and 0.39 (V) vs.RHE, respectively. More importantly, the steady-stateg-Por/HOPG electrode reached the benchmarking catalytic current density of 10 (mA.cm−2) at the overpotential of 0.53 (V) vs.RHE. This improvement can be assigned to the g-Por grafted layer that was constructed from the vertical covalent anchoring of either single g-Por molecule or g-Por clusters as proposed in Figure 2b. As a result, the interspaces between them facilitate the imine groups within the porphyrin ring accessibly catalyzing the HER process [42,79].
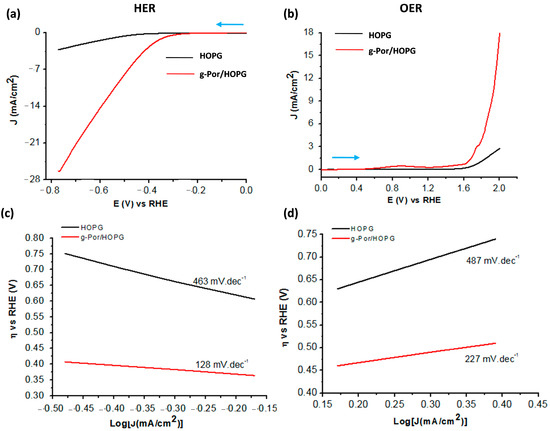
Figure 6.
Comparative electrocatalytic activities of pristine HOPG and g-Por/HOPG for (a) HER and (b) OER in 0.1 M H2SO4 solution. The scan directions are highlighted by blue arrows; (c,d) Tafel plots showing significant enhancements with respect to the OER and particularly the HER catalyzed by g-Por grafted layer.
Likewise, Figure 6b shows the steady OER electrocatalytic activities of pristine HOPG and g-Por/HOPG in 01 M H2SO4aqueous solution. The g-Por/HOPG showsbetter catalytic activity in the OER compared to pristine HOPG. The G-Por/HOPG has an initial onset overpotential of ca. 0.32 (V) vs.RHE and reaches a current density of 3.0 (mA.cm−2) at the overpotential of 0.52 (V) vs.RHE, while the pristine HOPG plateaus at 0.7 (mA.cm−2). To reach the benchmarking catalytic current density of 10 (mA.cm−2), the overpotential measuredfor the steady-state g-Por/HOPG electrode wasfound to be ca. 0.7 (V) vs.RHE. Based on the obtained results, it becomes evident that the g-Por/HOPG material favorably catalyzes the HER over the OER. A comparison of the overall water-splitting activities of the graphene-based electrocatalysts employed in this work is summarized in Table S1.
In order to further understand the kinetics of HER and OER during the electrolysis process, Tafel plots were drawn based on the LSV curves using the Tafel Equation (2) as follows:
in which η is the overpotential, J is the current density, and b is the Tafel slope (Figure 6c,d). The Tafel slopes calculated for g-Por/HOPG with respect to HER as well as OER are 128 (mV.dec−1) (Figure 6c) and 231 (mV.dec−1) (Figure 6d), respectively. These calculated values indicate that the g-Por/HOPG is preferably efficient for the HER rather than OER under an acidic environment. Based on the obtained Tafel slope for the HER, enables the conclusion that this process is operated following the Volmer–Heyrovsky mechanism, namely [41,80]:
η = a + b.log(J)
H2O + e− = Hads + OH− and H2O + e− + Hads = H2 + OH−
The stability of this catalyst was also elucidated by repeatedly cycling in the same potential range followed by AFM imaging (Figure S4). This shows that the catalytic activity of the g-Por/HOPG was insignificantly reduced for up to 50 scans continuously. This was further confirmed by AFM results captured right after the samples were scanned for 50 cycles, which showed that the g-Por/HOPG surface was nearly intact following the electrocatalytic process.
4. Conclusions
In this contribution, we experimentally demonstrated the feasibility of covalently functionalizing graphitic surfaces including graphene by electrografting g-Por moieties onto them employing a combinative state-of-the-art toolbox comprising CV, Raman spectroscopy, AFM, and STM techniques. The results show that the g-Por molecules covalently attach to bothgraphitic surfaces, i.e., HOPG and G-SiO2, and/or the as-grafted g-Por moieties to form a robust multilayered thinfilm. The fabricated g-Por/HOPG enhances both the OER and particularly, the HER in acidic medium, through a process that follows the Volmer–Heyrovsky mechanism. The stability of the grafted g-Por layer under catalytic conditions at the molecular level was experimentally examined. This showed that the catalytic activity of the g-Por/HOPG was insignificantly reduced for up to 50 scans continuously. These findings also contribute to an in-depth understanding at the molecular level of the synergetic effects of molecular structures toward HER and OER. Our findings also open a new pathway to robustly functionalizeother 2D materials applicable to the field of renewable energy conversion and storage.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coatings14060745/s1, Figure S1: The comparative CVs of HOPG in g-Por solution with (black curve) and without (red curve) NaNO2 showing similar two reductive peaks (noted as RP1 and RP2) that are assigned to the reductions of the porphyrin ring [https://doi.org/10.1016/j.susc.2012.08.013]. Contrarily, the first peak (RD) merely observed with the solution containing NaNO2 is attributed to the reduction of the diazonium ions, which were in-situ generated upon adding the NaNO2, in order to form the corresponding aryl radicals that covalently attach to graphitic surfaces via C-C bonds; Figure S2: Nanoshaving using AFM tip was employed to effectively remove the g-Por grafted molecules from the HOPG substrate, thereby regenerating pristine graphite underneath (a). Based on the topography profile measured along with the green line in (b), it enables estimation of the thickness of the g-Por grafted to be about 4.7 ± 0.2 nm; Figure S3: AFM images showing the G-SiO2 surface before and after being electrografted by g-Por moieties in which the wrinkles characterized for pristine G-SiO2 surface is covered by the g-Por grafted layer (a,b,d,e) resulting in an obvious increase of the surface roughness (c,f); Figure S4: Stability test by LSV (a) and AFM imaging (b,c) for the g-Por/HOPG electrode shows an insignificant change in its catalytic activity as well as surface morphology upon 50 scans; Table S1: A comparison of the overall water splitting activities of graphene based electrocatalysts with our current work. The higher overpotentials counted for our designed material are probably assigned to the dilute acidic electrolyte, i.e., 0.1 M, employed in our experiments. References [81,82,83,84,85,86,87,88] are cited in Supplementary Materials.
Author Contributions
Conceptualization, T.M.T.H.; methodology, T.M.T.H.; validation, T.H.P. and T.M.T.H.; investigation, T.H.P. and T.M.T.H.; data curation, T.H.P.; writing—original draft preparation, T.H.P. and T.M.T.H.; writing—review and editing, T.M.T.H.; visualization, T.M.T.H.; project administration, T.M.T.H.; funding acquisition, T.M.T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Ministry of Education and Training of Vietnam under grant number B2022-DQN-07.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors thank Steven De Feyterand co-workers (KU Leuven, Belgium) for access to the scanning probe microscopies and Raman spectroscopy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pu, Z.; Zhang, G.; Hassanpour, A.; Zheng, D.; Wang, S.; Liao, S.; Chen, Z.; Sun, S. Regenerative fuel cells: Recent progress, challenges, perspectives and their applications for space energy system. Appl. Energy 2021, 283, 116376. [Google Scholar] [CrossRef]
- Le, T.T.; Sharma, P.; Bora, B.J.; Tran, V.D.; Truong, T.H.; Le, H.C.; Nguyen, P.Q.P. Fueling the future: A comprehensive review of hydrogen energy systems and their challenges. Int. J. Hydrogen Energy 2024, 54, 791–816. [Google Scholar] [CrossRef]
- Ali, A.; Shen, P.K. Nonprecious metal’s graphene-supported electrocatalysts for hydrogen evolution reaction: Fundamentals to applications. Carbon Energy 2020, 2, 99–121. [Google Scholar] [CrossRef]
- Bai, F.; Bao, Y.; Li, Q. Porphyrin and macrocycle derivatives for electrochemical water splitting. MRS Bull. 2020, 45, 569–573. [Google Scholar] [CrossRef]
- Yin, H.-J.; Wang, Z.; Zhao, Z.-Y.; Jiang, X.-Y.; Yu, J.-Y.; Yang, L.-M.; Zhang, Y.-M.; Liu, W.; Ni, C.-L. Synthesis, crystal structure and properties of electro-catalysis for hydrogen production of a molecular nickel catalyst based on bis(1,2,5-thiadiazole-3,4-dithiolate) ligand. J. Mol. Struct. 2023, 1274, 134501. [Google Scholar] [CrossRef]
- Taranu, B.-O.; Fagadar-Cosma, E. Catalytic Properties of Free-Base Porphyrin Modified Graphite Electrodes for Electrochemical Water Splitting in Alkaline Medium. Processes 2022, 10, 611. [Google Scholar] [CrossRef]
- Sanati, S.; Abazari, R.; Morsali, A. Enhanced electrochemical oxygen and hydrogen evolution reactions using an NU-1000@NiMn-LDHS composite electrode in alkaline electrolyte. Chem. Commun. 2020, 56, 6652–6655. [Google Scholar] [CrossRef] [PubMed]
- Hötger, D.; Etzkorn, M.; Morchutt, C.; Wurster, B.; Dreiser, J.; Stepanow, S.; Grumelli, D.; Gutzler, R.; Kern, K. Stability of metallo-porphyrin networks under oxygen reduction and evolution conditions in alkaline media. Phys. Chem. Chem. Phys. 2019, 21, 2587–2594. [Google Scholar] [CrossRef]
- Peng, J.; Dong, W.; Wang, Z.; Meng, Y.; Liu, W.; Song, P.; Liu, Z. Recent advances in 2D transition metal compounds for electrocatalytic full water splitting in neutral media. Mater. Today Adv. 2020, 8, 100081. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Dai, H. Strongly Coupled Inorganic/Nanocarbon Hybrid Materials for Advanced Electrocatalysis. J. Am. Chem. Soc. 2013, 135, 2013–2036. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, T.; Cao, H.; Cui, S.; Du, P. Self-supported Ni2P nanosheets on low-cost three-dimensional Fe foam as a novel electrocatalyst for efficient water oxidation. J. Energy Chem. 2020, 42, 71–76. [Google Scholar] [CrossRef]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.-K.; Liu, L.-M.; et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Sun, Z.; Chen, X.; Zhu, G.; Sun, B.; Yamauchi, Y.; Liu, S. Recent advances in Ru/Ir-based electrocatalysts for acidic oxygen evolution reaction. Appl. Catal. B Environ. 2024, 343, 123584. [Google Scholar] [CrossRef]
- Majhi, K.C.; Yadav, M. Transition Metal-Based Chalcogenides as Electrocatalysts for Overall Water Splitting. ACS Eng. Au 2023, 3, 278–284. [Google Scholar] [CrossRef]
- Li, S.; Li, E.; An, X.; Hao, X.; Jiang, Z.; Guan, G. Transition metal-based catalysts for electrochemical water splitting at high current density: Current status and perspectives. Nanoscale 2021, 13, 12788–12817. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qin, P.; Luo, Y.; Ruan, Q.; Liu, L.; Wu, Y.; Li, Q.; Xu, Y.; Liu, R.; Chu, P.K. Recent progress and perspective of cobalt-based catalysts for water splitting: Design and nanoarchitectonics. Mater. Today Energy 2022, 23, 100911. [Google Scholar] [CrossRef]
- Kazemi, A.; Manteghi, F.; Tehrani, Z. Metal Electrocatalysts for Hydrogen Production in Water Splitting. ACS Omega 2024, 9, 7310–7335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cui, J.; Eslava, S. Oxygen Evolution Catalysts at Transition Metal Oxide Photoanodes: Their Differing Roles for Solar Water Splitting. Adv. Energy Mater. 2021, 11, 2003111. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Balogun, M.S.; Tong, Y.; Huang, Y. Oxygen vacancy–based metal oxides photoanodes in photoelectrochemical water splitting. Mater. Today Sustain. 2022, 18, 100118. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Sun, F.; Qin, J.; Wang, Z.; Yu, M.; Wu, X.; Sun, X.; Qiu, J. Energy-saving hydrogen production by chlorine-free hybrid seawater splitting coupling hydrazine degradation. Nat. Commun. 2021, 12, 4182. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Moreno, P.; Serrato, J.C.; Willison, J.C.; Magnin, J.-P. Photohydrogen production from lactose and lactate by recombinant strains of Rhodobacter capsulatus: Modeling and optimization. Int. J. Hydrogen Energy 2018, 43, 21231–21245. [Google Scholar] [CrossRef]
- Wang, J.; Kong, H.; Zhang, J.; Hao, Y.; Shao, Z.; Ciucci, F. Carbon-based electrocatalysts for sustainable energy applications. Prog. Mater. Sci. 2021, 116, 100717. [Google Scholar] [CrossRef]
- Zhai, Q.; Pan, Y.; Dai, L. Carbon-Based Metal-Free Electrocatalysts: Past, Present, and Future. Acc. Mater. Res. 2021, 2, 1239–1250. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, A.; Joshi, C.; Singh, R.; Saran, S.; Jain, S.L. Heterostructured nanocomposite tin phthalocyanine@mesoporous ceria (SnPc@CeO2) for photoreduction of CO2 in visible light. RSC Adv. 2015, 5, 42414–42421. [Google Scholar] [CrossRef]
- Zhang, W.; Lai, W.; Cao, R. Energy-Related Small Molecule Activation Reactions: Oxygen Reduction and Hydrogen and Oxygen Evolution Reactions Catalyzed by Porphyrin- and Corrole-Based Systems. Chem. Rev. 2017, 117, 3717–3797. [Google Scholar] [CrossRef] [PubMed]
- Castro-Cruz, H.M.; Macías-Ruvalcaba, N.A. Porphyrin-catalyzed electrochemical hydrogen evolution reaction. Metal-centered and ligand-centered mechanisms. Coord. Chem. Rev. 2022, 458, 214430. [Google Scholar] [CrossRef]
- Heppe, N.; Gallenkamp, C.; Paul, S.; Segura-Salas, N.; von Rhein, N.; Kaiser, B.; Jaegermann, W.; Jafari, A.; Sergueev, I.; Krewald, V.; et al. Substituent Effects in Iron Porphyrin Catalysts for the Hydrogen Evolution Reaction. Chem. Eur. J. 2023, 29, e202202465. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, X.-P.; Zhao, B.; Li, P.; Qi, J.; Guo, X.; Wang, B.; Lei, H.; Zhang, W.; Apfel, U.-P.; et al. Enzyme-Inspired Iron Porphyrins for Improved Electrocatalytic Oxygen Reduction and Evolution Reactions. Angew. Chem. Int. Ed. 2021, 60, 7576–7581. [Google Scholar] [CrossRef]
- Udry, G.A.O.; Tiessler-Sala, L.; Pugliese, E.; Urvoas, A.; Halime, Z.; Maréchal, J.-D.; Mahy, J.-P.; Ricoux, R. Photocatalytic Hydrogen Production and Carbon Dioxide Reduction Catalyzed by an Artificial Cobalt Hemoprotein. Int. J. Mol. Sci. 2022, 23, 14640. [Google Scholar] [CrossRef]
- Hayat, A.; Sohail, M.; Ali, H.; Taha, T.A.; Qazi, H.I.A.; Ur Rahman, N.; Ajmal, Z.; Kalam, A.; Al-Sehemi, A.G.; Wageh, S.; et al. Recent Advances and Future Perspectives of Metal-Based Electrocatalysts for Overall Electrochemical Water Splitting. Chem. Rec. 2023, 23, e202200149. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z.; Medforth, C.J.; Shelnutt, J.A. Self-Assembly and Self-Metallization of Porphyrin Nanosheets. J. Am. Chem. Soc. 2007, 129, 2440–2441. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yin, H.; Liu, P.; Wang, Y.; Yao, X.; Tang, Z.; Zhao, H. Molecular engineering of Ni–/Co–porphyrin multilayers on reduced graphene oxide sheets as bifunctional catalysts for oxygen evolution and oxygen reduction reactions. Chem. Sci. 2016, 7, 5640–5646. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liu, S.; Wang, J.; Zhang, W.; Tian, T.; Sun, J.; Bai, F. Self-assembled supramolecular nanostructure photosensitizers for photocatalytic hydrogen evolution. APL Mater. 2020, 8, 120706. [Google Scholar] [CrossRef]
- Fareza, A.R.; Nugroho, F.A.A.; Abdi, F.F.; Fauzia, V. Nanoscale metal oxides–2D materials heterostructures for photoelectrochemical water splitting—A review. J. Mater. Chem. A 2022, 10, 8656–8686. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Neves, M.G.P.M.S.; Trindade, T. Functionalization of Graphene Oxide with Porphyrins: Synthetic Routes and Biological Applications. ChemPlusChem 2020, 85, 1857–1880. [Google Scholar] [CrossRef]
- Huynh, T.M.T.; Phan, T.H.; Phillipson, R.; Volodine, A.; De Feyter, S. Doping of graphene via adlayer formation of electrochemically reduced dibenzyl viologen. J. Mater. Chem. C 2022, 10, 2696–2702. [Google Scholar] [CrossRef]
- Seo, S.; Lee, K.; Min, M.; Cho, Y.; Kim, M.; Lee, H. A molecular approach to an electrocatalytic hydrogen evolution reaction on single-layer graphene. Nanoscale 2017, 9, 3969–3979. [Google Scholar] [CrossRef]
- Ge, Y.; Lyu, Z.; Marcos-Hernández, M.; Villagrán, D. Free-base porphyrin polymer for bifunctional electrochemical water splitting. Chem. Sci. 2022, 13, 8597–8604. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Rodríguez-López, N.; Villagrán, D. Hydrogen gas generation using a metal-free fluorinated porphyrin. Chem. Sci. 2018, 9, 4689–4695. [Google Scholar] [CrossRef] [PubMed]
- Patra, B.C.; Khilari, S.; Manna, R.N.; Mondal, S.; Pradhan, D.; Pradhan, A.; Bhaumik, A. A Metal-Free Covalent Organic Polymer for Electrocatalytic Hydrogen Evolution. ACS Catal. 2017, 7, 6120–6127. [Google Scholar] [CrossRef]
- Ito, A.; Konishi, K.; Aida, T. Free bases of chiral N-substituted porphyrins as catalysts for asymmetric reaction. Tetrahedron Lett. 1996, 37, 2585–2588. [Google Scholar] [CrossRef]
- Medforth, C.J.; Senge, M.O.; Smith, K.M.; Sparks, L.D.; Shelnutt, J.A. Nonplanar distortion modes for highly substituted porphyrins. J. Am. Chem. Soc. 1992, 114, 9859–9869. [Google Scholar] [CrossRef]
- Lin, Y.; Dong, Y.; Wang, X.; Chen, L. Electrocatalysts for the Oxygen Evolution Reaction in Acidic Media. Adv. Mater. 2023, 35, 2210565. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.-M.; Zhu, Y.-P.; Chen, P.; Ma, T.-Y. Recent Advances in Transition-Metal-Mediated Electrocatalytic CO2 Reduction: From Homogeneous to Heterogeneous Systems. Catalysts 2017, 7, 373. [Google Scholar] [CrossRef]
- Ahmed, A.; Devi, G.; Kapahi, A.; Kundan, S.; Katoch, S.; Bajju, G.D. Covalently linked porphyrin-graphene oxide nanocomposite: Synthesis, characterization and catalytic activity. J. Mater. Sci. Mater. Electron. 2019, 30, 19738–19751. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, R.K.; Ram, K.; Aguiar, A.; Koh, J.; Sobral, A.J. Graphene oxide modified cobalt metallated porphyrin photocatalyst for conversion of formic acid from carbon dioxide. J. CO2 Util. 2018, 27, 107–114. [Google Scholar] [CrossRef]
- Santos, C.I.; Goncalves, G.; Cicuendez, M.; Mariz, I.; Silva, V.S.; Oliveira, H.; Campos, F.; Vieira, S.I.; Marques, P.A.; Maçôas, E.M. Biocompatible hybrids based on nanographene oxide covalently linked to glycolporphyrins: Synthesis, characterization and biological evaluation. Carbon 2018, 135, 202–214. [Google Scholar] [CrossRef]
- Berijani, K.; Farokhi, A.; Hosseini-Monfared, H.; Janiak, C. Enhanced enantioselective oxidation of olefins catalyzed by Mn-porphyrin immobilized on graphene oxide. Tetrahedron 2018, 74, 2202–2210. [Google Scholar] [CrossRef]
- Wei, P.J.; Yu, G.Q.; Naruta, Y.; Liu, J.G. Covalent grafting of carbon nanotubes with a biomimetic heme model compound to enhance oxygen reduction reactions. Angew. Chem. Int. Ed. 2014, 53, 6659–6663. [Google Scholar] [CrossRef] [PubMed]
- Wetzl, C.; Silvestri, A.; Garrido, M.; Hou, H.L.; Criado, A.; Prato, M. The Covalent Functionalization of Surface-Supported Graphene: An Update. Angew. Chem. Int. Ed. 2023, 62, e202212857. [Google Scholar] [CrossRef] [PubMed]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Antonietti, M.; García, H. Active sites on graphene-based materials as metal-free catalysts. Chem. Soc. Rev. 2017, 46, 4501–4529. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.M.T.; Phan, T.H.; Ivasenko, O.; Mertens, S.F.L.; De Feyter, S. Nanoconfined self-assembly on a grafted graphitic surface under electrochemical control. Nanoscale 2017, 9, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.M.T.; Nguyen, D.D.; Hoang, N.H.; Phan, T.H. Reversible Tuning of Surface Properties of Graphene-like Material via Covalently Functionalized Hydrophobic Layer. Crystals 2023, 13, 635. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gomez-Rodriguez, J.; Colchero, J.; Gómez-Herrero, J.; Baro, A. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.; Phan, T.H.; Fujita, Y.; Li, Z.; Ivasenko, O.; Vanderlinden, W.; Van Gorp, H.; Frederickx, W.; Lu, G.; Tahara, K.; et al. Covalent Modification of Graphene and Graphite Using Diazonium Chemistry: Tunable Grafting and Nanomanipulation. ACS Nano 2015, 9, 5520–5535. [Google Scholar] [CrossRef]
- Tahara, K.; Kubo, Y.; Lindner, B.; Hashimoto, S.; Hirose, S.; Brown, A.; Hirsch, B.; Daukiya, L.; De Feyter, S.; Tobe, Y. Steric and Electronic Effects of Electrochemically Generated Aryl Radicals on Grafting of the Graphite Surface. Langmuir 2019, 35, 2089–2098. [Google Scholar] [CrossRef]
- Phan, T.H.; Wandelt, K. Self-assembly of metal free porphyrin layers at copper-electrolyte interfaces: Dependence on substrate symmetry. Surf. Sci. 2013, 607, 82–91. [Google Scholar] [CrossRef]
- Wilson, G.S.; Neri, B.P. Cyclic voltammetry of porphyrins and metalloporphyrins. Ann. N. Y. Acad. Sci. 1973, 206, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Jin, Z.; Kim, K.K.; Hilmer, A.J.; Paulus, G.L.C.; Shih, C.-J.; Ham, M.-H.; Sanchez-Yamagishi, J.D.; Watanabe, K.; Taniguchi, T.; et al. Understanding and controlling the substrate effect on graphene electron-transfer chemistry via reactivity imprint lithography. Nat. Chem. 2012, 4, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, S.; Bekyarova, E.; Itkis, M.E.; Zhang, H.; Shepperd, K.; Hicks, J.; Sprinkle, M.; Berger, C.; Lau, C.N.; deHeer, W.A.; et al. Spectroscopy of Covalently Functionalized Graphene. Nano Lett. 2010, 10, 4061–4066. [Google Scholar] [CrossRef] [PubMed]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Chen, I.W.P.; Huang, C.-Y.; Jhou, S.-H.S.; Zhang, Y.-W. Exfoliation and Performance Properties of Non-Oxidized Graphene in Water. Sci. Rep. 2014, 4, 3928. [Google Scholar] [CrossRef] [PubMed]
- Aarva, A.; Sainio, S.; Deringer, V.L.; Caro, M.A.; Laurila, T. X-ray Spectroscopy Fingerprints of Pristine and Functionalized Graphene. J. Phys. Chem. C 2021, 125, 18234–18246. [Google Scholar] [CrossRef] [PubMed]
- Akaike, K.; Aoyama, K.; Dekubo, S.; Onishi, A.; Kanai, K. Characterizing electronic structure near the energy gap of graphitic carbon nitride based on rational interpretation of chemical analysis. Chem. Mater. 2018, 30, 2341–2352. [Google Scholar] [CrossRef]
- Tourabi, M.; Nohair, K.; Traisnel, M.; Jama, C.; Bentiss, F. Electrochemical and XPS studies of the corrosion inhibition of carbon steel in hydrochloric acid pickling solutions by 3, 5-bis (2-thienylmethyl)-4-amino-1, 2, 4-triazole. Corros. Sci. 2013, 75, 123–133. [Google Scholar] [CrossRef]
- Orqusha, N. Grafting of the gold surface by heterocyclic moieties derived through electrochemical oxidation of amino triazole–an experimental and “ab initio” study. RSC Adv. 2022, 12, 23017–23025. [Google Scholar] [CrossRef]
- Mesnage, A.; Lefèvre, X.; Jégou, P.; Deniau, G.; Palacin, S. Spontaneous Grafting of Diazonium Salts: Chemical Mechanism on Metallic Surfaces. Langmuir 2012, 28, 11767–11778. [Google Scholar] [CrossRef]
- Chaussé, A.; Chehimi, M.M.; Karsi, N.; Pinson, J.; Podvorica, F.; Vautrin-Ul, C. The Electrochemical Reduction of Diazonium Salts on Iron Electrodes. The Formation of Covalently Bonded Organic Layers and Their Effect on Corrosion. Chem. Mater. 2002, 14, 392–400. [Google Scholar] [CrossRef]
- Islam, M.; Achour, A.; Saeed, K.; Boujtita, M.; Javed, S.; Djouadi, M.A. Metal/Carbon Hybrid Nanostructures Produced from Plasma-Enhanced Chemical Vapor Deposition over Nafion-Supported Electrochemically Deposited Cobalt Nanoparticles. Materials 2018, 11, 687. [Google Scholar] [CrossRef] [PubMed]
- Hunger, R.; Jaegermann, W.; Merson, A.; Shapira, Y.; Pettenkofer, C.; Rappich, J. Electronic Structure of Methoxy-, Bromo-, and Nitrobenzene Grafted onto Si(111). J. Phys. Chem. B 2006, 110, 15432–15441. [Google Scholar] [CrossRef] [PubMed]
- Trung Huynh, T.M.; Nguyen, T.L.; Phan, T.H. Engineering the Surface Chemistry of Graphite and Graphene by Covalently Anchored Triazole Derivative. J. Electrochem. Soc. 2023, 170, 106510. [Google Scholar] [CrossRef]
- Phan, T.H.; Van Gorp, H.; Li, Z.; Trung Huynh, T.M.; Fujita, Y.; Verstraete, L.; Eyley, S.; Thielemans, W.; Uji, I.H.; Hirsch, B.E.; et al. Graphite and Graphene Fairy Circles: A Bottom-Up Approach for the Formation of Nanocorrals. ACS Nano 2019, 13, 5559–5571. [Google Scholar] [CrossRef]
- Pereira, V.M.; Neto, A.C.; Liang, H.; Mahadevan, L. Geometry, mechanics, and electronics of singular structures and wrinkles in graphene. Phys. Rev. Lett. 2010, 105, 156603. [Google Scholar] [CrossRef]
- Kumar, K.; Kim, Y.-S.; Yang, E.-H. The influence of thermal annealing to remove polymeric residue on the electronic doping and morphological characteristics of graphene. Carbon 2013, 65, 35–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Zhou, C. Review of chemical vapor deposition of graphene and related applications. Acc. Chem. Res. 2013, 46, 2329–2339. [Google Scholar] [CrossRef]
- Elghamry, I.; Alablan, A.S.; Abdelsalam, M.E. Hydrogen Gas Generation Using Self-Assembled Monolayers (SAMs) of 5,10,15,20-Tetrakis (p-Thiophenol) Porphyrin on a Gold Electrode. Catalysts 2023, 13, 1355. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Li, S.; Yu, Z.; Yang, Y.; Liu, Y.; Zou, H.; Yang, H.; Jin, J.; Ma, J. Nitrogen-doped truncated carbon nanotubes inserted into nitrogen-doped graphene nanosheets with a sandwich structure: A highly efficient metal-free catalyst for the HER. J. Mater. Chem. A 2017, 5, 6405. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, J.; Yang, M.; Fang, Z.; Jian, J.; Yu, D.; Chen, X.; Dai, L. Ultrathin Black Phosphorus-on-Nitrogen Doped Graphene for Efficient Overall Water Splitting: Dual Modulation Roles of Directional Interfacial Charge Transfer. J. Am. Chem. Soc. 2019, 141, 4972. [Google Scholar] [CrossRef]
- Tian, Y.; Ye, Y.; Wang, X.; Peng, S.; Wei, Z.; Zhang, X.; Liu, W. Three-dimensional N-doped, plasma-etched graphene: Highly active metal-free catalyst for hydrogen evolution reaction. Appl. Catal. A Gen. 2017, 529, 127. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Liu, S.; Shao, G.; Zhao, X. A covalent organic framework/graphene aerogel electrocatalyst for enhanced overall water splitting. Nanoscale 2022, 14, 16944. [Google Scholar] [CrossRef]
- Truong, L.; Jerng, S.-K.; Roy, S.B.; Jeon, J.H.; Kim, K.; Akbar, K.; Yi, Y.; Chun, S.-H. Chrysanthemum-Like CoP Nanostructures on Vertical Graphene Nanohills as Versatile Electrocatalysts for Water Splitting. ACS Sustain. Chem. Eng. 2019, 7, 4625. [Google Scholar] [CrossRef]
- Wang, J.; Yang, W.; Liu, J. CoP2 nanoparticles on reduced graphene oxide sheets as a super-efficient bifunctional electrocatalyst for full water splitting. J. Mater. Chem. A 2016, 4, 4686. [Google Scholar] [CrossRef]
- Jiao, L.; Zhou, Y.-X.; Jiang, H.-L. Metal–organic framework-based CoP/reduced graphene oxide: High-performance bifunctional electrocatalyst for overall water splitting. Chem. Sci. 2016, 7, 1690. [Google Scholar] [CrossRef]
- Zhao, Z.; Schipper, D.E.; Leitner, A.P.; Thirumalai, H.; Chen, J.-H.; Xie, L.; Qin, F.; Alam, M.K.; Grabow, L.C.; Chen, S.; et al. Bifunctional metal phosphide FeMnP films from single source metal organic chemical vapor deposition for efficient overall water splitting. Nano Energy 2017, 39, 444. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).