Activity in the Field of Blood Coagulation Processes of Poly(Lactide)-Zinc Fiber Composite Material Obtained by Magnetron Sputtering
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Polymers
2.1.2. Magnetron Usable Material
2.1.3. Microbiological Strains
- Staphylococcus aureus (ATCC 6538);
- Escherichia coli (ATCC 25922).
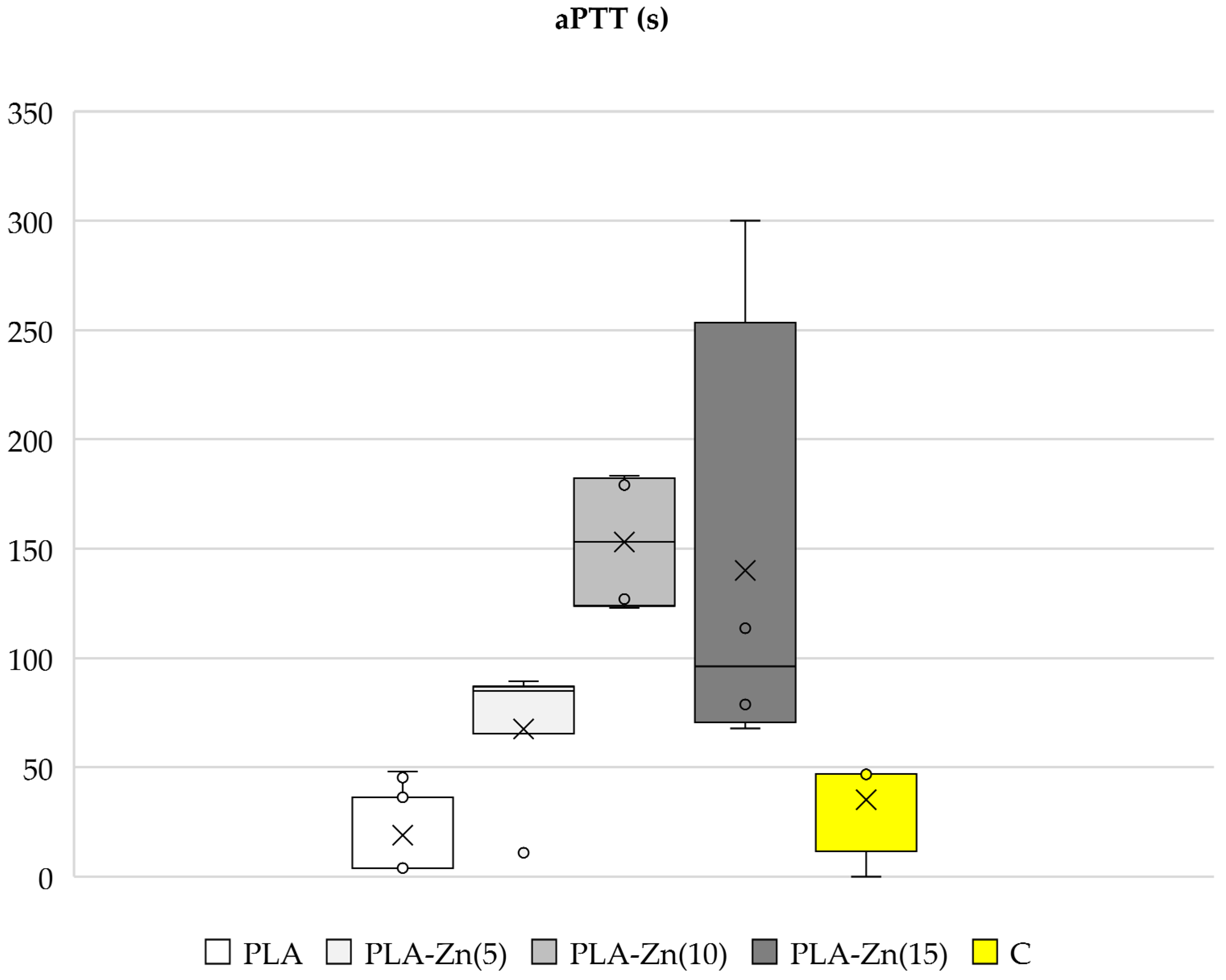
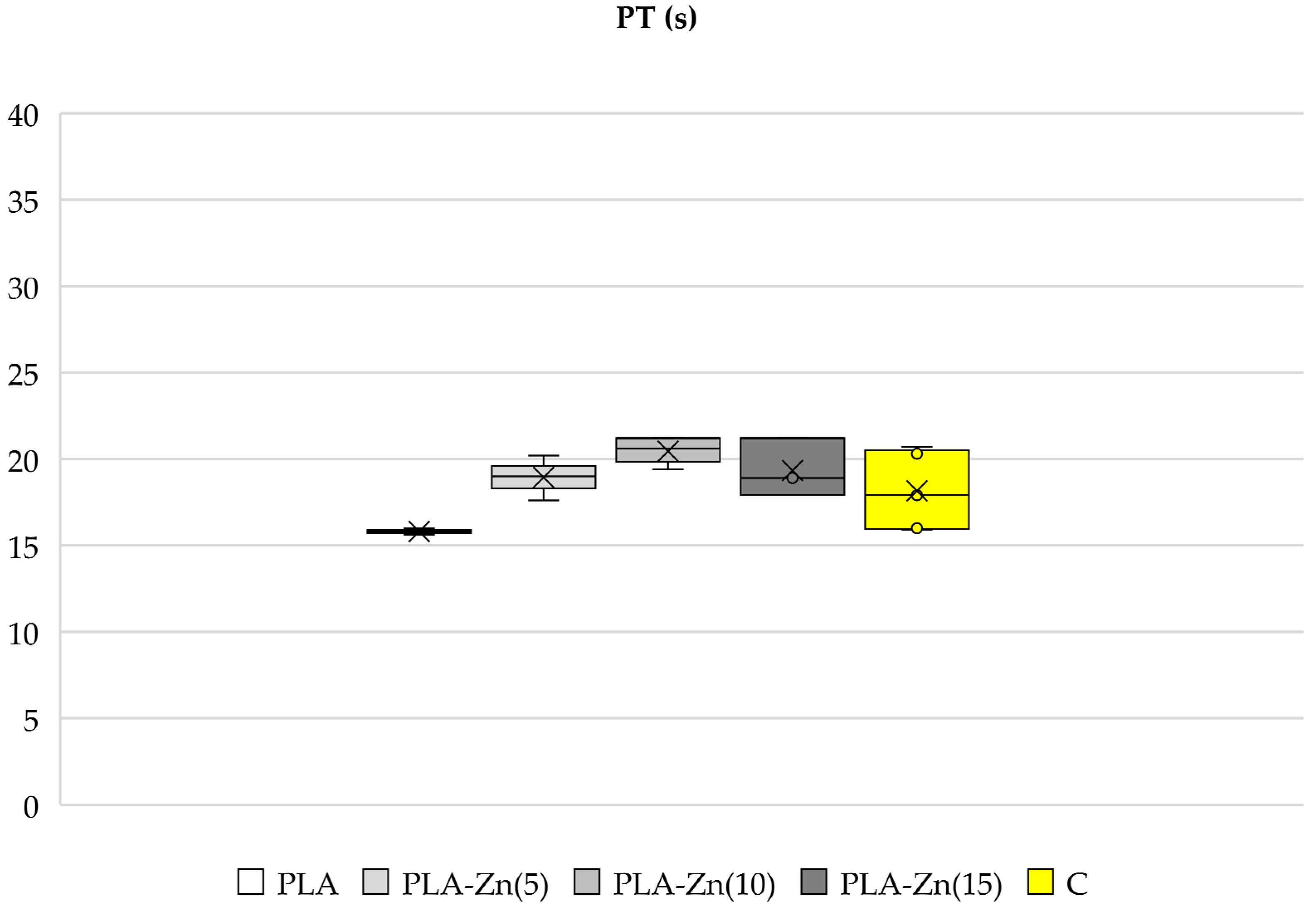
2.1.4. Activated Partial Thromboplastin Time (aPTT) and Prothrombin Time (PT)
2.2. Methods
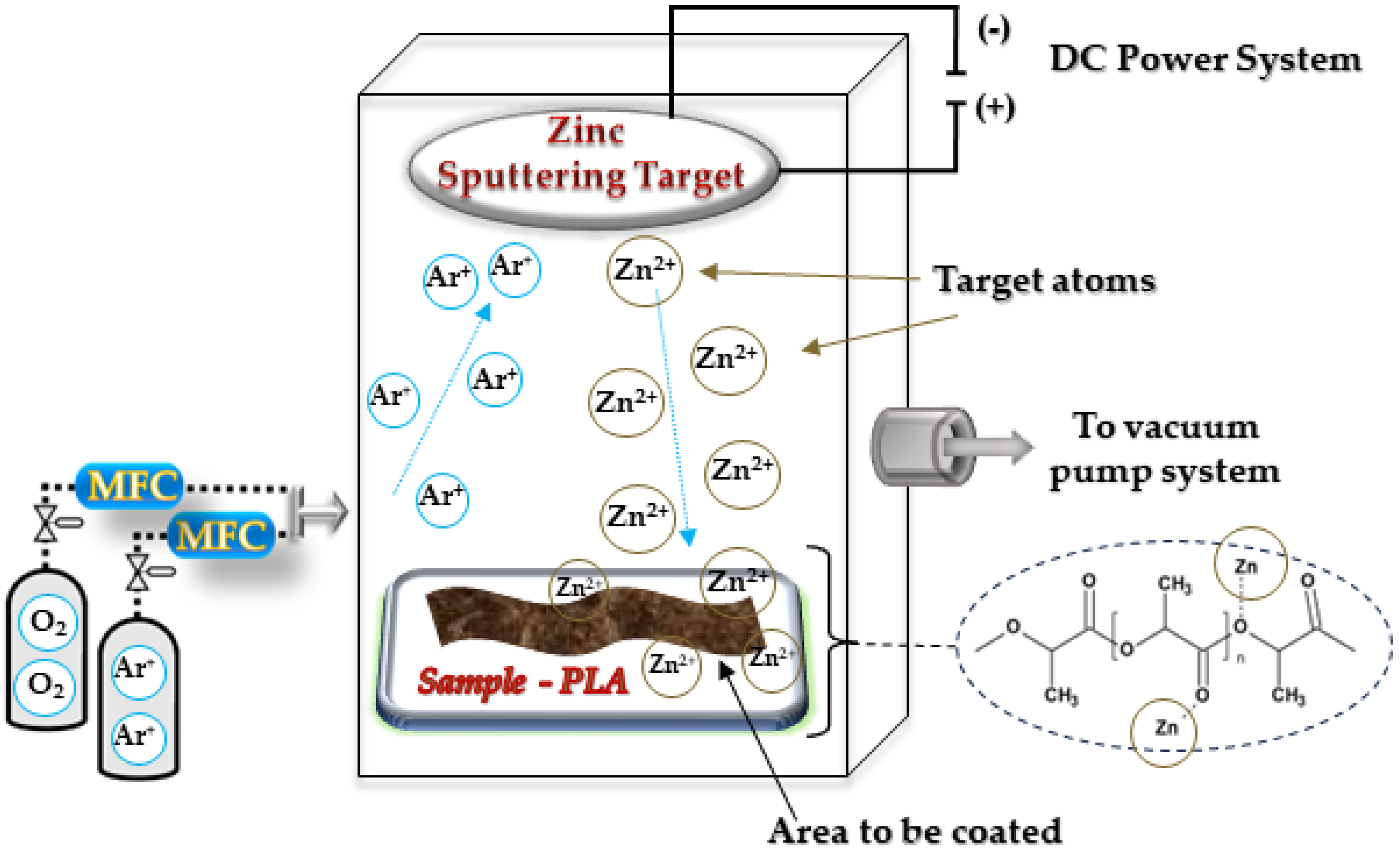
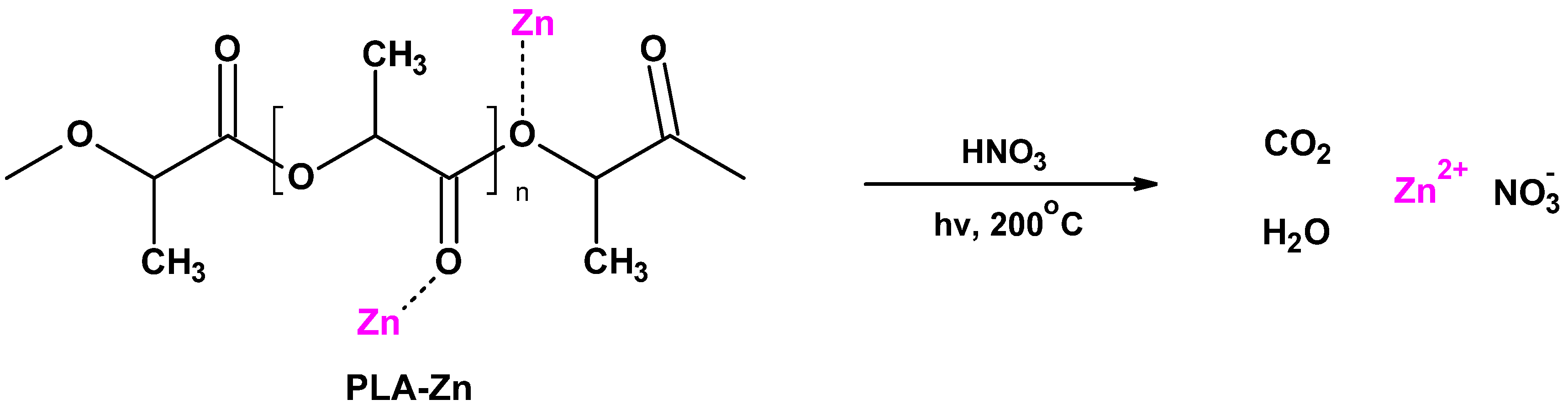
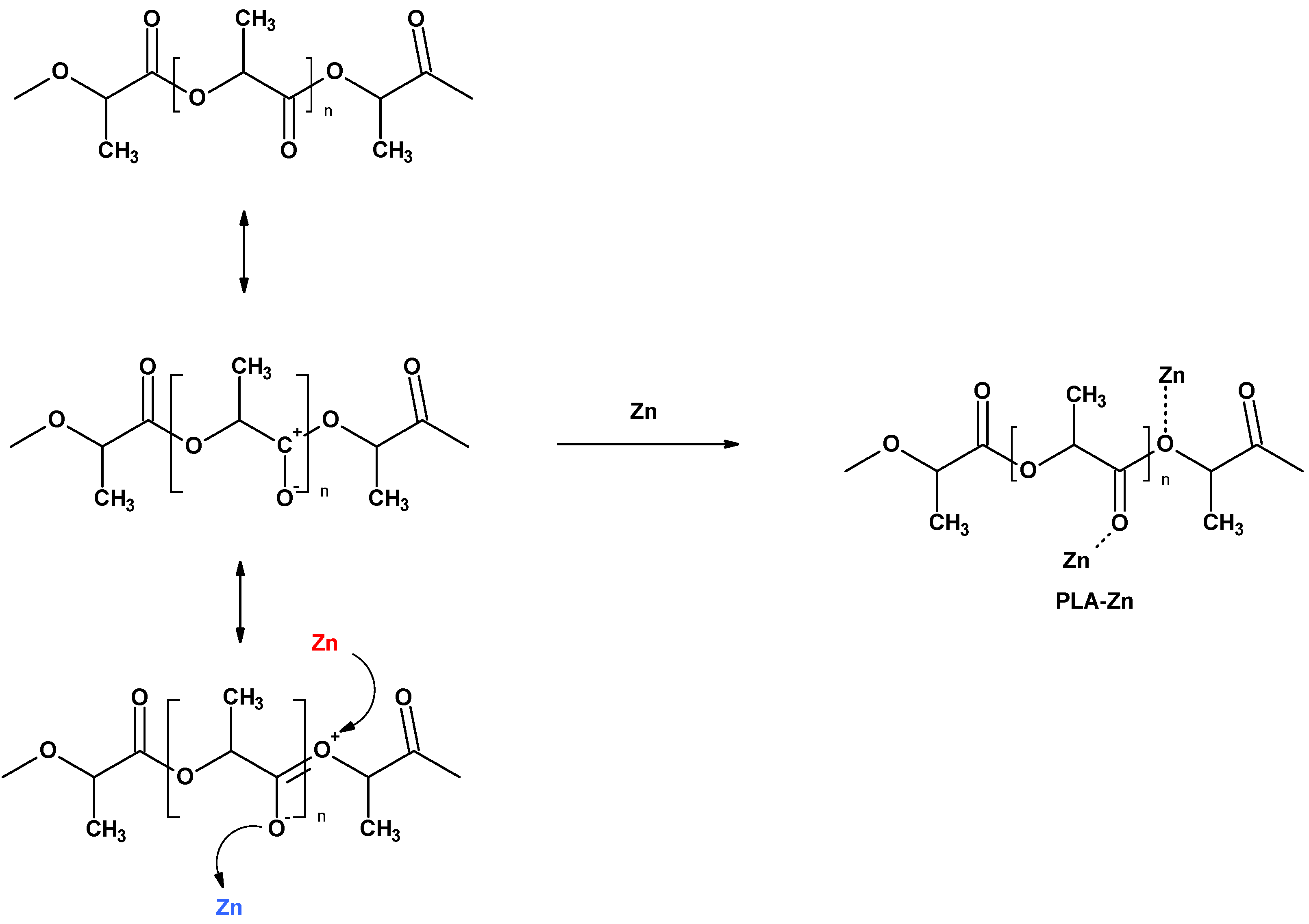
2.2.1. PLA–Zinc Composites (PLA-Zn) Synthesis
PLA Nonwoven Fabrics
PLA Nonwoven Zinc Coating (PLA-Zn Composite Synthesis)
- (1)
- The target–substrate distance—15 cm;
- (2)
- Deposition time: 5, 10, 15 min;
- (3)
- Working atmosphere—Ar;
- (4)
- Working pressure—1.8 × 10−3 mbar;
- (5)
- Power discharge—350–1000 W;
- (6)
- Power density—0.78 W/cm2.
2.2.2. PLA–Zn Composite Physico-Chemical Characterization
PLA–Zn Composite Characterization
- Zinc concentration determination
PLA–Zn Composite Morphology
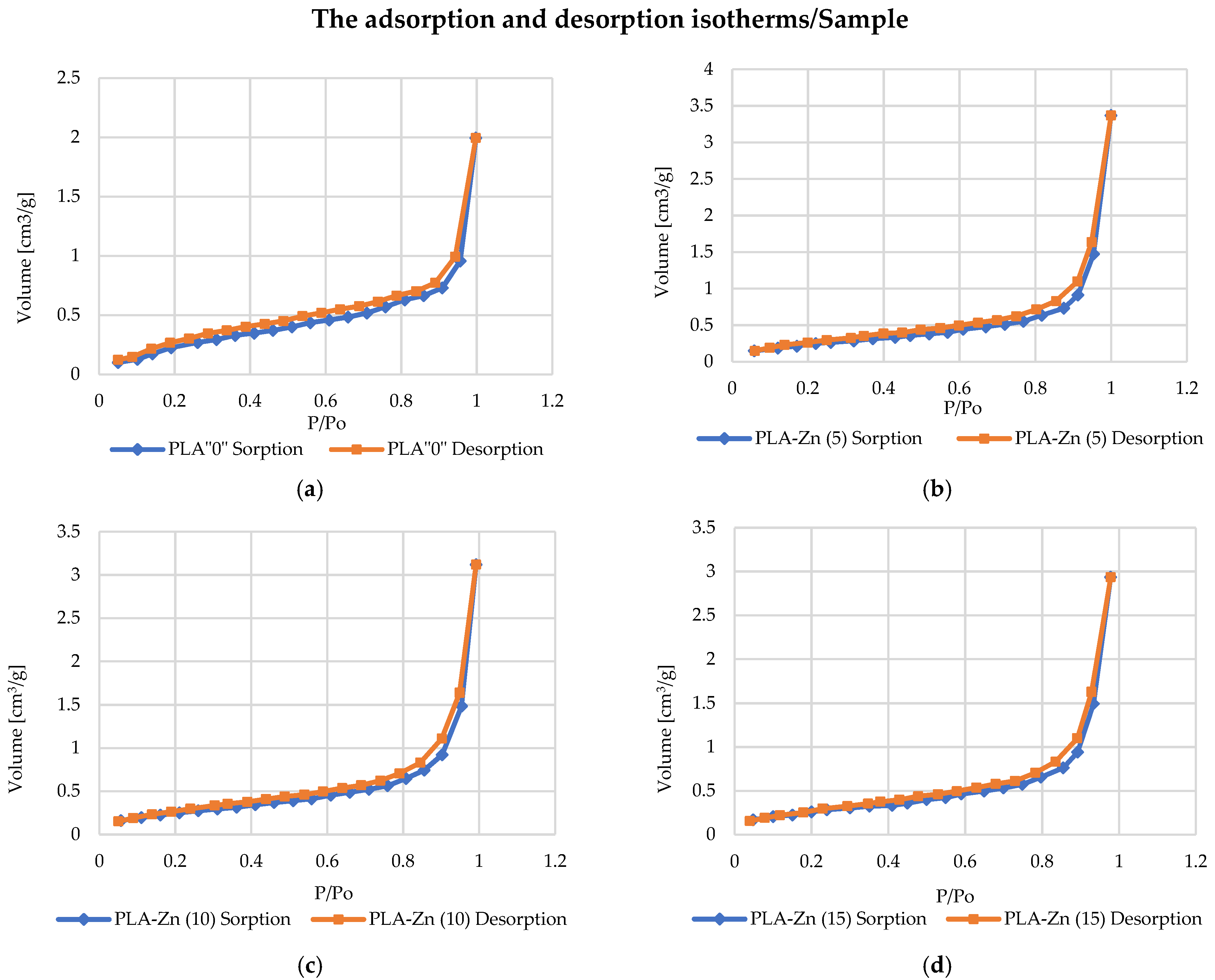
PLA–Zn Composite Specific Surface Area and Total Pore Volume
2.2.3. PLA–Zn Composite Biochemical Characterization
Activated Partial Thromboplastin Time (aPTT) and Prothrombin Time (PT)
PLA–Zn Composite Antimicrobial Properties
3. Results and Discussion
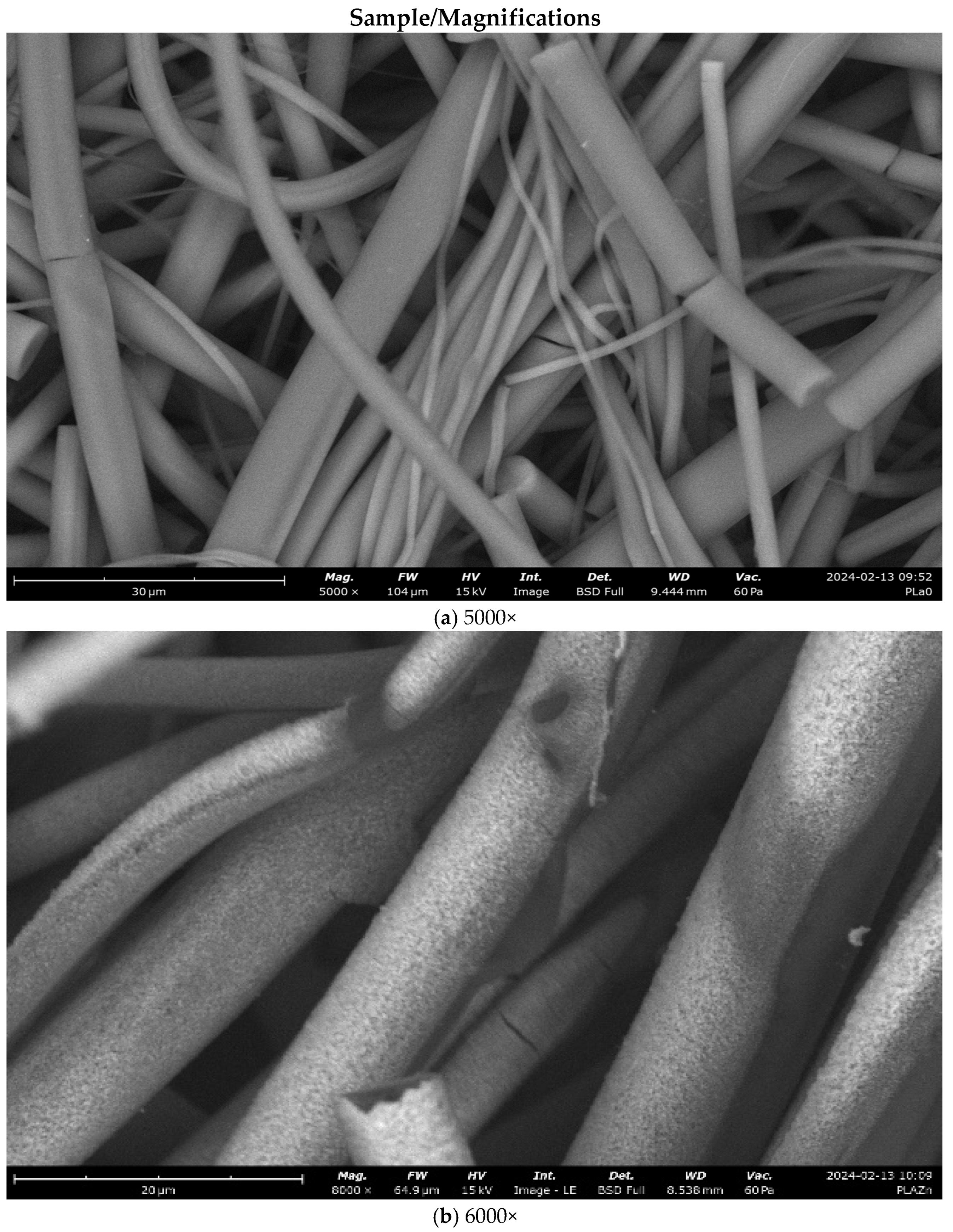
3.1. Magnetron Sputtering Modification of Poly(Lactide) Nonwovens
3.2. Physico-Chemical Characterisation
3.2.1. Atomic Absorption Spectrometry with Flame Excitation (FAAS)
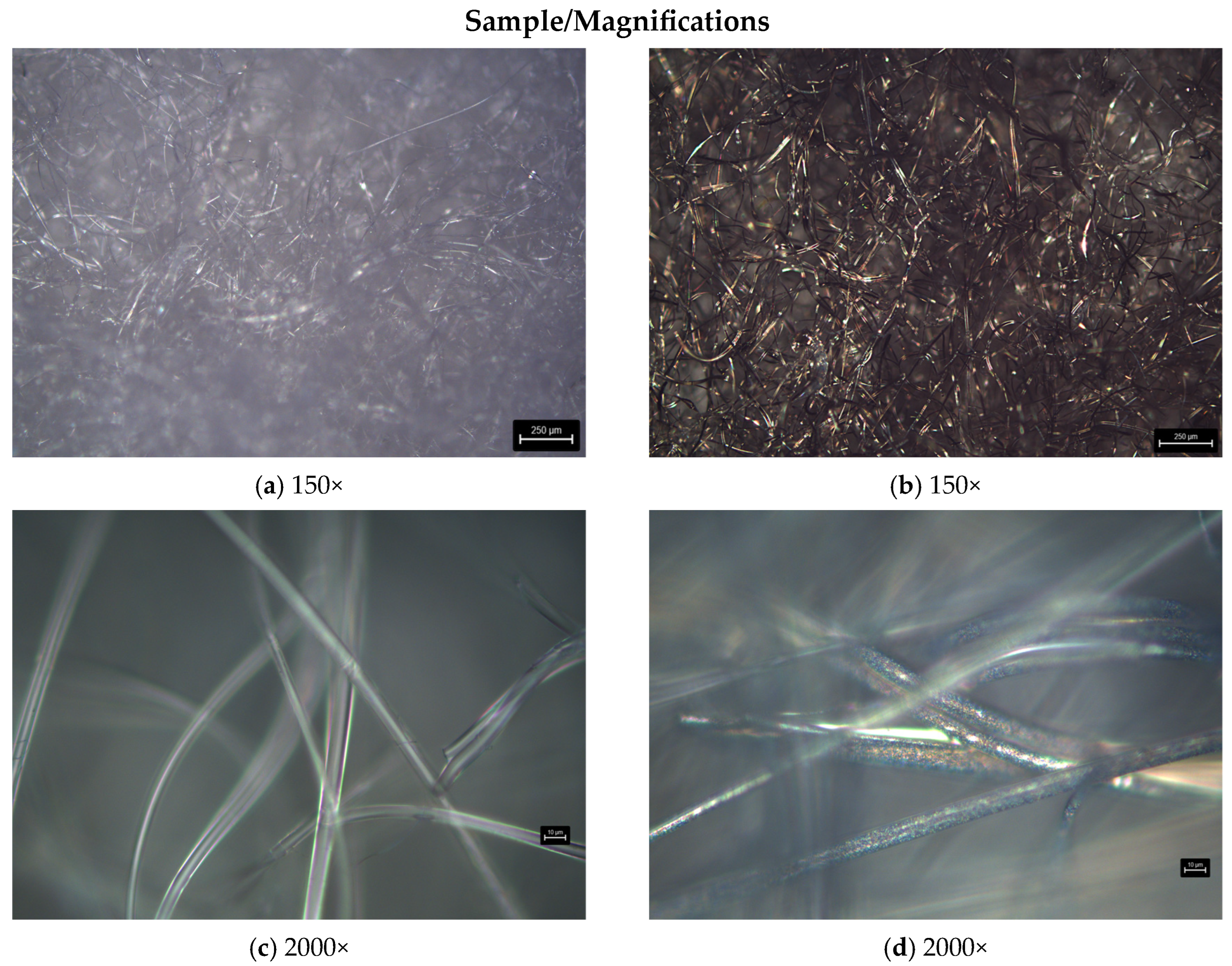
3.2.2. Microscopy Analysis
3.2.3. Specific Surface Area and Total Pore Volume Analysis
3.3. PLA–Zn Composite Biochemical Characterization
3.3.1. Activated Partial Thromboplastin Time (aPTT), Prothrombin Time (PT)
3.3.2. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhivya, S.; Padma, V.V.; Santhin, E. Wound dressings—A review. BioMedicine 2015, 5, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing—A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.I.; Ahmed, N.; Ur-Rehman, A.; Khan, G.M. Polylactide: The polymer revolutionizing the biomedical field. Mater. Biomed. Eng. Thermoset Thermoplast. Polym. 2019, 11, 381–415. [Google Scholar] [CrossRef]
- Ghomi, E.R.; Khalili, S.; Khorasani, S.N.; Neisiany, R.E.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Dong, R.; Liang, Y.; Li, M. Haemostatic materials for wound healing applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, M.; Liu, Q.; Liu, G.; Wang, S.; Li, J. Recent advances in the medical applications of hemostatic materials. Theranostics 2023, 13, 161–196. [Google Scholar] [CrossRef] [PubMed]
- Ijaola, A.O.; Akamo, D.O.; Damiri, F.; Akisin, C.J.; Bamidele, E.A.; Ajiboye, E.G.; Berrada, M.; Onyenokwe, V.O.; Yang, S.-Y.; Asmatulu, E. Polymeric biomaterials for wound healing applications: A comprehensive review. J. Biomater. Sci. Polym. Ed. 2022, 33, 1998–2050. [Google Scholar] [CrossRef] [PubMed]
- Mirhaj, M.; Labbaf, S.; Tavakoli, M.; Seifalian, A. An overview on the recent advances in the treatment of infected wounds: Antibacterial wound dressings. Macromol. Biosci. 2022, 22, 2200014. [Google Scholar] [CrossRef]
- Zwawi, M. Recent advances in bio-medical implants; mechanical properties, surface modifications and applications. Eng. Res. Express 2022, 4, 032003. [Google Scholar] [CrossRef]
- Dong, Q.; Chow, L.C.; Wang, T.; Frukhtbeyn, S.A.; Wang, F.; Yang, M.; Mitchell, J.W. A new bioactive polylactide-based composite with high mechanical strength. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J.S.; Hayman, D. An overview of mechanical properties and material modeling of polylactide (PLA) for medical applications. Ann. Biomed. Eng. 2016, 44, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar]
- Wuisman, P.I.J.M.; Smit, T.H. Bioresorbable polymers: Heading for a new generation of spinal cages. Eur. Spine J. 2006, 15, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G.A. Perspective on polylactic acid-based polymers use for nanoparticles synthesis and applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, K.; Rybak, Z.; Wątrobiński, M.; Struszczyk, M.H.; Filipiak, J.; Szymonowicz, M. Bioresorbable polymeric materials—Current state of knowledge. Polimery 2021, 66, 3–10. [Google Scholar] [CrossRef]
- Kour, K.; Kumar, R.; Singh, G.; Singh, S.; Singh, S.; Sandhu, K. Additive manufacturing of polylactic acid-based nanofibers composites for innovative scaffolding applications. Int. J. Interact. Des. Manuf. 2023. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering—A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Kim, K.-H.; Park, J.W.; Deep, A. Review Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sust. Energ. Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Ghalia, M.A.; Dahman, Y. Review Biodegradable poly(lactic acid)-based scaffolds: Synthesis and biomedical applications. J. Polym. Res. 2017, 24, 74. [Google Scholar] [CrossRef]
- Kliem, S.; Kreutz Bruck, M.; Bonten, C. Review on the biological degradation of polymers in various environments. Materials 2020, 13, 4586. [Google Scholar] [CrossRef] [PubMed]
- Vaid, R.; Yildirim, E.; Pasquinelli, M.A.; King, M.W. Hydrolytic degradation of polylactic acid fibers as a function of pH and exposure time. Molecules 2021, 26, 7554. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Chen, P.; Shi, X.; Zhang, G.; Wang, C. Preparation of open-porous stereocomplex PLA/PBAT scaffolds and correlation between their morphology, mechanical behavior, and cell compatibility. RSC Adv. 2018, 8, 12933–12943. [Google Scholar] [CrossRef] [PubMed]
- Nobs, L.; Buchegger, F.; Gurny, R.; Allémann, E. Poly(lactic acid) nanoparticles labeled with biologically active Neutravidin™ for active targeting. Eur. J. Pharm. Biopharm. 2004, 58, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, J. Controlled release of a model protein lysozyme from phase sensitive smart polymer systems. Int. J. Pharm. 2004, 271, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.B.; Whitaker, M.J.; Sebald, W.; Clarke, N.; Howdle, S.M.; Shakesheff, K.M.; Oreffo, R.O.C. Human osteoprogenitor bone formation using encapsulated bone morphogenetic protein 2 in porous polymer scaffolds. Tissue Eng. 2004, 10, 1037–1045. [Google Scholar] [CrossRef]
- Stoyanova, N.; Paneva, D.; Mincheva, R.; Toncheva, A.; Manolova, N.; Dubois, P.; Rashkov, I. Poly(l-lactide) and poly(butylene succinate) immiscible blends: From electrospinning to biologically active materials. Mater. Sci. Eng. C 2014, 41, 119–126. [Google Scholar] [CrossRef]
- Tara, S.; Kurobe, H.; Rocco, K.A.; Maxfield, M.W.; Best, C.A.; Yi, T.; Naito, Y.; Breuer, C.K.; Shinoka, T. Well-organized neointima of large-pore poly(l-lactic acid) vascular graft coated with poly(l-lactic-co-ε-caprolactone) prevents calcific deposition compared to small-pore electrospun poly(l-lactic acid) graft in a mouse aortic implantation model. Atherosclerosis 2014, 237, 684–691. [Google Scholar] [CrossRef]
- Gomillion, C.T.; Lakhman, R.K.; Kasi, R.M.; Weiss, R.A.; Kuhn, L.T.; Goldberg, A.J. Lithium-end-capped polylactide thin films influence osteoblast progenitor cell differentiation and mineralization. J. Biomed. Mater. Res. A 2015, 103, 500–510. [Google Scholar] [CrossRef]
- Khavpachev, M.A.; Trofimchuk, E.S.; Nikonorova, N.I.; Garina, E.S.; Moskvina, M.A.; Efimov, A.V.; Demina, V.A.; Bakirov, A.V.; Sedush, N.G.; Potseleev, V.V.; et al. Bioactive polylactide fibrous materials prepared by crazing mechanism. Macromol. Mater. Eng. 2020, 305, 2000163. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Pogorielov, M.; Hapchenko, A.; Deineka, V.; Rogulska, L.; Oleshko, O.; Vodsedalkova, K.; Berezkinova, L.; Vyslouzilova, L.; Klapst’ova, A.; Erben, J. In vitro degradation and in vivo toxicity of NanoMatrix3DVR polycaprolactone and poly(lactic acid) nanofibrous scaffolds. J. Biomed. Mater. Res. Part A 2018, 106A, 2200–2212. [Google Scholar] [CrossRef]
- Spasova, M.; Manolova, N.; Paneva, D.; Mincheva, R.; Dubois, P.; Rashkov, I.; Maximova, V.; Danchev, D. Polylactide stereocomplex-based electrospun materials possessing surface with antibacterial and hemostatic properties. Biomacromolecules 2010, 11, 151–159. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, Z.; Wang, C.; Zhang, Z.; Xu, S.; Han, C.C. A biodegradable trilayered barrier membrane composed of sponge and electrospun layers: Hemostasis and antiadhesion. Biomacromolecules 2015, 16, 3083–3092. [Google Scholar] [CrossRef]
- Birajdar, M.S.; Halake, K.S.; Lee, J. Blood-clotting mimetic behavior of biocompatible microgels. J. Ind. Eng. Chem. 2018, 63, 117–123. [Google Scholar] [CrossRef]
- Wyrwa, R.; Otto, K.; Voigt, S.; Enkelmann, A.; Schnabelrauch, M.; Neubert, T.; Schneider, G. Electrospun mucosal wound dressings containing styptics for bleeding control. Mater. Sci. Eng. C 2018, 93, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Yagi, H.; Tajima, K.; Kuroda, K.; Shinoda, M.; Kitago, M.; Abe, Y.; Oshima, G.; Hirukawa, K.; Itano, O.; et al. Efficacy of new polylactic acid nonwoven fabric as a hemostatic agent in a rat liver resection model. Surg. Innov. 2019, 26, 312–320. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Cheng, N.; Liu, Z.; Wu, Y.B.; Zhou, Q.Q.; Li, C.Y.; Yu, M.; Ramakrishna, S.; Wang, R.; et al. A non-surgical suturing strategy for rapid cardiac hemostasis. Nano Res. 2023, 16, 810–821. [Google Scholar] [CrossRef]
- Campoccia, D.; Visai, L.; Renò, F.; Cangini, I.; Rizzi, M.; Poggi, A.; Montanaro, L.; Rimondini, L.; Arciola, C.R. Bacterial adhesion to poly-(D,L)lactic acid blended with vitamin E: Toward gentle anti-infective biomaterials. J. Biomed. Mater. Res. A 2015, 103, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yang, C.; Shan, M.; Jia, H.; Zhang, S.; Chen, X.; Liu, W.; Liu, X.; Chen, J.; Wang, X. Synergistic Poly(lactic acid) antibacterial surface combining superhydrophobicity for antiadhesion and chlorophyll for photodynamic therapy. Langmuir 2022, 38, 8987–8998. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Mehadi Hassan, M.; He, X.; Hu, G.; Ren, Y.; Kim, H.; Mirkhani, S.A.; Hu, J.; Sen, A.; Wang, J.; et al. Thymol-loaded polylactic acid electrospun fibrous membranes with synergistic biocidal and anti-bacterial adhesion properties via morphology control. Colloids Surf. A: Physicochem. Eng. Asp. 2023, 677, 132360. [Google Scholar] [CrossRef]
- Tham, C.Y.; Hamid, Z.A.A.; Ahmad, Z.; Ismail, H. Surface engineered poly (lactic acid) (PLA) microspheres by chemical treatment for drug delivery system. Key Eng. Mater. 2014, 594–595, 214–218. [Google Scholar] [CrossRef]
- Davachi, S.M.; Kaffashi, B. Polylactic acid in medicine. Polym. Plast. Technol. Eng. 2015, 54, 944–967. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and medical applications of polylactic acid: A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly(lactic acid) nanofibrous scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Kaczmarek, A.; Mrozińska, Z.; Olczyk, J. Deposition of copper on polyester knitwear fibers by a magnetron sputtering system. Physical properties and evaluation of antimicrobial response of new multi-functional composite materials. Appl. Sci. 2020, 10, 6990. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Mrozińska, Z.; Kaczmarek, A.; Lisiak-Kucińska, A. Deposition of copper on poly(lactide) non-woven fabrics by magnetron sputtering–fabrication of new multi-functional, antimicrobial composite. Materials 2020, 13, 3971. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Boguń, M.; Mrozińska, Z.; Kaczmarek, A. Physical properties, chemical analysis, and evaluation of antimicrobial response of new polylactide/alginate/copper composite materials. Mar. Drugs. 2020, 18, 660. [Google Scholar] [CrossRef] [PubMed]
- Kudzin, M.H.; Giełdowska, M.; Mrozińska, Z.; Boguń, M. Poly(lactic acid)/Zinc/Alginate complex material: Preparation and antimicrobial properties. Antibiotics 2021, 10, 1327. [Google Scholar] [CrossRef] [PubMed]
- Mrozińska, Z.; Ponczek, M.; Kaczmarek, A.; Boguń, M.; Sulak, E.; Kudzin, M.H. Blood coagulation activities of Cotton–Alginate–Copper composites. Mar. Drugs 2023, 21, 625. [Google Scholar] [CrossRef]
- Mrozińska, Z.; Kudzin, M.H.; Ponczek, M.B.; Kaczmarek, A.; Król, P.; Lisiak-Kucińska, A.; Żyłła, R.; Walawska, A. Biochemical Approach to Poly(Lactide)–Copper Composite—Impact on Blood Coagulation Processes. Materials 2024, 17, 608. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Xi, Y.; Weng, Y. Recent advances in PLA-based antibacterial food packaging and its applications. Molecules 2022, 27, 5953. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.C.A. A review on enhancing the antibacterial activity of ZnO: Mechanisms and microscopic investigation. Nanoscale Res. Lett. 2020, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Nokhodchi, A. An overview on antimicrobial and wound healing properties of ZnO nanobiofilms, hydrogels, and bionanocomposites based on cellulose, chitosan, and alginate polymers. Carbohydr. Polym. 2020, 227, 115349. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A mini review of antibacterial properties of ZnO nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Chong, W.J.; Shen, S.; Li, Y.; Trinchi, A.; Pejak, D.; Kyratzis, I.L.; Sola, A.; Wen, C. Additive manufacturing of antibacterial PLA-ZnO nanocomposites: Benefits, limitations and open challenges. J. Mater. Sci. Technol. 2022, 111, 120–151. [Google Scholar] [CrossRef]
- Islam, F.; Shohag, S.; Uddin, M.J.; Islam, R.; Nafady, M.H.; Akter, A.; Mitra, S.; Roy, A.; Bin Emran, T.; Cavalu, S. Exploring the journey of zinc oxide nanoparticles (ZnO-NPs) toward biomedical applications. Materials 2022, 15, 2160. [Google Scholar] [CrossRef] [PubMed]
- Pino, P.; Bosco, F.; Mollea, C.; Onida, B. Antimicrobial nano-zinc oxide biocomposites for wound healing applications: A review. Pharmaceutics 2023, 15, 970. [Google Scholar] [CrossRef]
- Rathore, A.; Shah, D.; Kaur, H. Recent advances in metal oxide/polylactic acid nanocomposites and their applications. Polym.-Plast. Tech. Mater. 2023, 62, 231–245. [Google Scholar] [CrossRef]
- Fujihara, J.; Nishimoto, N. Review of zinc oxide nanoparticles: Toxicokinetics, tissue distribution for various exposure routes, toxicological effects, toxicity mechanism in mammals, and an approach for toxicity reduction. Biol. Trace Elem. Res. 2024, 202, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Li, M.; Sun, Y.; Shang, X.; Ma, Y. Biological mechanism of ZnO nanomaterials. J. Appl. Toxicol. 2024, 44, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Korant, B.D.; Kauer, J.C.; Butterworth, B.E. Zinc ions inhibit replication of rhinoviruses. Nature 1974, 248, 588–590. [Google Scholar] [CrossRef]
- Hulisz, D. Efficacy of zinc against common cold viruses: An overview. J. Am. Pharm. Assoc. 2004, 44, 594–603. [Google Scholar]
- Alavi, M.; Kamarasu, P.; McClements, D.J.; Moore, M.D. Metal and metal oxide-based antiviral nanoparticles: Properties, mechanisms of action, and applications. Adv. Colloid Interface Sci. 2022, 306, 102726. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Loconsole, D.; Sallustio, A.; Picca, R.A.; Felici, R.; Chironna, M.; Cioffi, N. On the efficacy of ZnO nanostructures against SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 3040. [Google Scholar] [CrossRef]
- Wolfgruber, S.; Rieger, J.; Cardozo, O.; Punz, B.; Himly, M.; Stingl, A.; Farias, P.M.A.; Abuja, P.M.; Zatloukal, K. Antiviral activity of zinc oxide nanoparticles against SARS-CoV-2. Int. J. Mol. Sci. 2023, 24, 8425. [Google Scholar] [CrossRef]
- Kumar, S.; Ansari, S.; Narayanan, S.; Ranjith-Kumar, C.T.; Surjit, M. Antiviral activity of zinc against hepatitis viruses: Current status and future prospects. Front. Microbiol. 2023, 14, 1218654. [Google Scholar] [CrossRef] [PubMed]
- Minaeian, S.; Khales, P.; Hosseini-Hosseinabad, S.M.; Farahmand, M.; Poortahmasebi, V.; Habib, Z.; Tavakoli, A. Evaluation of activity of zinc oxide nanoparticles on human rotavirus and multi-drug resistant Acinetobacter baumannii. Pharm. Nanotechnol. 2023, 11, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.-L.; Rausalu, K.; Savest, N.; Žusinaite, E.; Vasiliev, G.; Viirsalu, M.; Plamus, T.; Krumme, A.; Merits, A.; Bondarenko, O. Antibacterial and antiviral effects of Ag, Cu and Zn metals, respective nanoparticles and filter materials thereof against coronavirus SARS-CoV-2 and influenza A Virus. Pharmaceutics 2022, 14, 2549. [Google Scholar] [CrossRef] [PubMed]
- Shehabeldine, A.M.; Hashem, A.H.; Wassel, A.R.; Hasanin, M. Antimicrobial and antiviral activities of durable cotton fabrics treated with nanocomposite based on zinc oxide nanoparticles, acyclovir, nanochitosan, and clove oil. Appl. Biochem. Biotechnol. 2022, 194, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dong, H.; He, R.; Wang, N.; Zhao, Q.; Yang, L.; Qu, Z.; Sun, L.; Chen, S.; Ma, J.; et al. Hydro electroactive Cu/Zn coated cotton fiber nonwovens for antibacterial and antiviral applications. Int. J. Biol. Macromol. 2022, 207, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Asmat-Campos, D.; Rojas-Jaimes, J.; de Oca-Vásquez, G.M.; Nazario-Naveda, R.; Delfín-Narciso, D.; Juárez-Cortijo, L.; Bayona, D.E.; Diringer, B.; Pereira, R.; Menezes, D.B. Biogenic production of silver, zinc oxide, and cuprous oxide nanoparticles, and their impregnation into textiles with antiviral activity against SARS-CoV-2. Sci. Rep. 2023, 13, 9772. [Google Scholar] [CrossRef] [PubMed]
- Cano-Vicent, A.; Tuñón-Molina, A.; Bakshi, H.; Serra, R.; Iman, M.; Alfagih, I.M.; Tambuwala, M.M.; Serrano-Aroca, A. Biocompatible alginate film crosslinked with Ca2+ and Zn2+ possesses antibacterial, antiviral, and anticancer activities. ACS Omega 2023, 8, 24396–24405. [Google Scholar] [CrossRef]
- Gerwien, F.; Skrahina, V.; Kasper, L.; Hube, B.; Brunke, S. Metals in fungal virulence. FEMS Microbiol. Rev. 2018, 42, fux050. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Le, T. Zinc oxide nanoparticle as a novel class of antifungal agents: Current advances and future perspectives. J. Agric. Food Chem. 2018, 66, 11209–11220. [Google Scholar] [CrossRef]
- Mohammed, A.K.; Salh, K.K.; Ali, F.A. ZnO, TiO2 and Ag nanoparticles impact against some species of pathogenic bacteria and yeast. Cell Mol. Biol. 2021, 67, 24–34. [Google Scholar] [CrossRef]
- Zhai, P.; Chai, Y.; Lu, L. Fungal zinc homeostasis and its potential as an antifungal target: A focus on the human pathogen Aspergillus fumigatus. Microorganisms 2022, 10, 2469. [Google Scholar] [CrossRef] [PubMed]
- Daniel, A.I.; Keyster, M.; Klein, A. Biogenic zinc oxide nanoparticles: A viable agricultural tool to control plant pathogenic fungi and its potential effects on soil and plants. Sci. Total Environ. 2023, 897, 165483. [Google Scholar] [CrossRef] [PubMed]
- Guleria, G.; Kumar, S.; Thakur, S.; Sharma, D.K.; Thakur, S.; Kalia, S.; Shandilya, M. Biomedical potential of hydrothermally synthesized zinc oxide nanoparticles for antifungal evaluation and cytotoxicity analysis. Appl. Organomet. Chem. 2023, 37, e7270. [Google Scholar] [CrossRef]
- Huang, T.; Li, X.; Maier, M.; O’Brien-Simpson, N.M.; Heath, D.E.; O’Connor, A.J. Using inorganic nanoparticles to fight fungal infections in the antimicrobial resistant era. Acta Biomater. 2023, 158, 56–79. [Google Scholar] [CrossRef] [PubMed]
- Mahamuni-Badiger, P.; Ghare, V.; Nikam, C.; Patil, N. The fungal infections and their inhibition by Zinc oxide nanoparticles: An alternative approach to encounter drug resistance. Nucleus 2023. [Google Scholar] [CrossRef]
- Nan, R.; Liu, S.; Zhai, M.; Zhu, M.; Sun, X.; Chen, Y.; Pang, Q.; Zhang, J. Facile synthesis of Cu-doped ZnO nanoparticles for the enhanced photocatalytic disinfection of bacteria and fungi. Molecules 2023, 28, 7232. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Sharma, M.V.; Haque, M.A.; Simal-Gandara, J. Antifungal action and targeted mechanism of Bio fabricated zinc oxide (ZnO) nanoparticles against Ascochytafabae. Heliyon 2023, 9, e19179. [Google Scholar] [CrossRef] [PubMed]
- Mohs, F.E.; Sevringhaus, E.L.; Schmidt, E.R. Conservative amputation of gangrenous parts by chemosurgery. Ann. Surg. 1941, 114, 274–282. [Google Scholar] [CrossRef]
- Carr, M.E.; Powers, P.L. Differential effects of divalent cations on fibrin structure. Blood Coagul. Fibrin. 1991, 2, 741–747. [Google Scholar] [CrossRef]
- Belozerskaya, G.G.; Makarov, V.A.; Zhidkov, E.A.; Malykhina, L.S.; Sergeeva, O.A.; Ter-Arutyunyants, A.A.; Makarova, L.V. Local hemostatics (A review). Pharm. Chem. J. 2006, 40, 353–359. [Google Scholar] [CrossRef]
- Mammadova-Bach, E.; Braun, A. Zinc homeostasis in platelet-related diseases. Int. J. Mol. Sci. 2019, 20, 5258. [Google Scholar] [CrossRef]
- van Rensburg, M.J.; van Rooy, M.; Bester, M.J.; Serem, J.C.; Venter, C.; Oberholzer, H.M. Oxidative and haemostatic effects of copper, manganese and mercury, alone and in combination at physiologically relevant levels: An ex vivo study. Hum. Exp. Toxicol. 2019, 38, 419–433. [Google Scholar] [CrossRef]
- Ahmed, N.S.; Lopes-Pires, M.; Pugh, N. Zinc: An endogenous and exogenous regulator of platelet function during hemostasis and thrombosis. Platelets 2021, 32, 880–887. [Google Scholar] [CrossRef]
- Szewc, M.; Markiewicz-Gospodarek, A.; Górska, A.; Chilimoniuk, Z.; Rahnama, M.; Radzikowska-Buchner, E.; Strzelec-Pawelczak, K.; Bakiera, J.; Maciejewski, R. The role of zinc and copper in platelet activation and pathophysiological thrombus formation in patients with pulmonary embolism in the course of SARS-CoV-2 infection. Biology 2022, 11, 752. [Google Scholar] [CrossRef]
- Chen, J.; He, J.; Yang, Y.; Qiao, L.; Hu, J.; Zhang, J.; Guo, B. Antibacterial adhesive self-healing hydrogels to promote diabetic wound healing. Acta Biomater. 2022, 146, 119–130. [Google Scholar] [CrossRef]
- Ndlovu, S.P.; Fonkui, T.Y.; Kumar, P.; Choonara, Y.E.; Ndinteh, D.T.; Aderibigbe, B.A. Dissolvable zinc oxide nanoparticle-loaded wound dressing with preferential exudate absorption and hemostatic features. Polym. Bull. 2023, 80, 7491–7518. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Z.; Gan, L.; Zhang, L.; Yang, L.; Wu, P. Application of biomedical microspheres in wound healing. Int. J. Mol. Sci. 2023, 24, 7319. [Google Scholar] [CrossRef]
- Barbucci, R.; Magnani, A. Metal-ion complexes in the angiogenetic effect. Macromol. Symp. 2000, 156, 239–252. [Google Scholar] [CrossRef]
- D’Andrea, L.D.; Romanelli, A.; Di Stasia, R.; Pedone, C. Bioinorganic aspects of angiogenesis. Dalton Trans. 2010, 39, 7625–7636. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Asatourian, A.; Orangi, J.; Sorenson, C.M.; Sheibani, N. Functional role of inorganic trace elements in angiogenesis-Part II: Cr, Si, Zn, Cu, and S. Crit. Rev. Oncol. Hematol. 2015, 96, 143–155. [Google Scholar] [CrossRef]
- Ahtzaz, S.; Nasir, M.; Shahzadi, L.; Amir, W.; Anjum, A.; Arshad, R.; Iqbal, F.; Chaudhry, A.A.; Yar, M.; ur Rehman, I. A study on the effect of zinc oxide and zinc peroxide nanoparticles to enhance angiogenesis-pro-angiogenic grafts for tissue regeneration applications. Mater. Des. 2017, 132, 409–418. [Google Scholar] [CrossRef]
- Hassan, A.; Elebeedy, D.; Matar, E.R.; Fahmy Mohamed Elsayed, A.; Abd El Maksoud, A.I. Investigation of angiogenesis and wound healing potential mechanisms of zinc oxide nanorods. Front. Pharmacol. 2021, 12, 661217. [Google Scholar] [CrossRef]
- Nosrati, H.; Aramideh Khouy, R.; Nosrati, A.; Khodaei, M.; Banitalebi-Dehkordi, M.; Ashrafi-Dehkordi, K.; Sanami, S.; Alizadeh, Z. Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis. J. Nanobiotechnol. 2021, 19, 1. [Google Scholar] [CrossRef]
- Šalandová, M.; van Hengel, I.A.J.; Apachitei, I.; Zadpoor, A.A.; van der Eerden, B.C.J.; Fratila-Apachitei, L.E. Inorganic agents for enhanced angiogenesis of orthopedic biomaterials. Adv. Healthc. Mater. 2021, 10, 2002254. [Google Scholar] [CrossRef]
- Dürig, J.; Calcagni, M.; Buschmann, J. Transition metals in angiogenesis—A narrative review. Mater. Today Bio 2023, 22, 100757. [Google Scholar] [CrossRef]
- Hamed, S.H.; Azooz, E.A.; Al-Mulla, E.A.J. Nanoparticles-assisted Wound Healing: A Review. Nano Biomed. Eng. 2023, 15, 425–435. [Google Scholar] [CrossRef]
- Han, Z.; Deng, L.; Chen, S.; Wang, H.; Huang, Y. Zn2+-Loaded adhesive bacterial cellulose hydrogel with angiogenic and antibacterial abilities for accelerating wound healing. Burns & Trauma 2023, 11, tkac048. [Google Scholar] [CrossRef]
- Shabestarian, H.; Tabrizi, M.H.; Movahedi, M.; Neamati, A.; Sharifnia, F. Green synthesis of Ag-NPs as a metal nanoparticle and ZnO-NPs as a metal oxide nanoparticle: Evaluation of the in vitro cytotoxicity, anti-oxidant, anti-angiogenic activities. Nanomed. J. 2023, 10, 245–258. [Google Scholar]
- Yoshida, Y.G.; Yan, S.; Xu, H.; Yang, J. Novel metal nanomaterials to promote angiogenesis in tissue regeneration. Eng. Regen. 2023, 4, 265–276. [Google Scholar] [CrossRef]
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic acid/zinc oxide biocomposite films for food packaging application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Liu, Y.; Yang, Z.; Ma, L.; Zhuang, H.; Wang, E.; Wu, C.; Huan, Z.; Guo, F.; et al. Design of a biofluid-absorbing bioactive sandwich-structured Zn–Si bioceramic composite wound dressing for hair follicle regeneration and skin burn wound healing. Bioact. Mater. 2021, 6, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Pusnik-Cresnar, P.K.; Aulova, A.; Bikiaris, D.N.; Lambropoulou, D.; Kuzmic, K.; Zemljic, L.F. Incorporation of metal-based nanoadditives into the PLA matrix: Effect of surface properties on antibacterial activity and mechanical performance of PLA nanoadditive films. Molecules 2021, 26, 4161. [Google Scholar] [CrossRef]
- Higuchi, J.; Klimek, K.; Wojnarowicz, J.; Opalińska, A.; Chodara, A.; Szałaj, U.; Dąbrowska, S.; Fudala, D.; Ginalska, G. Electrospun membrane surface modification by sonocoating with HA and ZnO:Ag nanoparticles—Characterization and evaluation of osteoblasts and bacterial cell behavior in vitro. Cells 2022, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Garg, R.; Kashif, M.; Eddy, N.O. Nano-scale innovations in packaging: Properties, types, and applications of nanomaterials for the future. Food Chem. Adv. 2023, 3, 100560. [Google Scholar] [CrossRef]
- Buzarovska, A.; Selaru, A.; Serban, M.; Pircalabioru, G.G.; Costache, M.; Cocca, M.; Gentile, G.; Averous, L.; Dinescu, S. Biobased multiphase foams with ZnO for wound dressing applications. J. Mater. Sci. 2023, 58, 17594–17609. [Google Scholar] [CrossRef]
- Canales, D.A.; Pinones, N.; Saavedra, M.; Loyo, C.; Palza, H.; Peponi, L.; Leones, A.; Baier, R.V.; Boccaccin, A.R.; Grünelwald, A.; et al. Fabrication and assessment of bifunctional electrospun poly(L-lactic acid) scaffolds with bioglass and zinc oxide nanoparticles for bone tissue engineering. Int. J. Biol. Macromol. 2023, 228, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Chruściel, J.J.; Olczyk, J.; Kudzin, M.H.; Kaczmarek, P.; Król, P.; Tarzyńska, N. Antibacterial and antifungal properties of polyester, polylactide, and cotton nonwovens and fabrics, by means of stable aqueous dispersions containing copper silicate and some metal oxides. Materials 2023, 16, 5647. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, M.F.; Marongiu, F.; Barcellona, D. Performance and Interpretation of Clot Waveform Analysis. In Hemostasis and Thrombosis; Methods in Molecular Biology; Favaloro, E.J., Gosselin, R.C., Eds.; Humana: New York, NY, USA, 2023; Volume 2663. [Google Scholar] [CrossRef]
- Analytical Methods for Atomic Absorption Spectroscopy. The Perkin-Elmer Corporation. 1996. Available online: www.lasalle.edu/ (accessed on 3 July 2020).
- EN ISO 20645:2006; Textile Fabrics—Determination of Antibacterial Activity—Agar Diffusion Plate Test. International Organization for Standardization: Geneva, Switzerland, 2006.
- Scopus Base. 5897 Document Results on Zinc Reactivity. Available online: https://www-1scopus-1com-1000014gw0004.han.p.lodz.pl/results/results.uri?sort=plf-f&src=s&st1=ZINC+REACTIVITY&sid=db823368cc0731ddd5c45db4310e3aef&sot=b&sdt=b&sl=30&s=TITLE-ABS-KEY%28ZINC+REACTIVITY%29&origin=searchbasic&editSaveSearch=&yearFrom=Before+1960&yearTo=Present&sessionSearchId=db823368cc0731ddd5c45db4310e3aef&limit=10 (accessed on 8 February 2024).
- Durrant, P.J.; Durrant, B. Introduction to Advanced Inorganic Chemistry; Longmans, Green&Co., Ltd.: London, UK, 1962. [Google Scholar]
- Harikumar, K.R.; Rao, C.N.R. Role of oxygen transients in the facile scission of C–O bonds of alcohols on Zn surfaces. Chem. Commun. 1999, 4, 341–342. [Google Scholar] [CrossRef]
- Harikumar, K.R.; Vinod, C.P.; Kulkarni, G.U.; Rao, C.N.R. Facile C-O bond scission in alcohols on Zn surfaces. J. Phys. Chem. B 1999, 103, 2445–2452. [Google Scholar] [CrossRef]
- Arora, R.; Paul, S.; Gupta, R. A mild and efficient procedure for the conversion of aromatic carboxylic esters to secondary amides. Can. J. Chem. 2005, 83, 1137–1140. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Sadavarte, V.S.; Uppalla, L.S. Zn mediated transesterification of β-ketoesters. J. Chem. Res. 2001, 1, 16–17. [Google Scholar] [CrossRef]
- Liu, S.; Ji, D.; Yang, Y.; Zhen, X.; Tian, X.; Han, J. A practical procedure for efficient synthesis of α-amino acids. Lett. Org. Chem. 2009, 6, 156–158. [Google Scholar] [CrossRef]
- Ryu, I.; Kuriyama, H.; Miyazato, H.; Minakata, S.; Komatsu, M.; Yoon, J.-Y.; Kim, S. Zinc-induced deoximation of α,α′-dioxo-type oximes and oxime ethers leading to α,β-diketo esters. Bull. Chem. Soc. Jap. 2004, 77, 1407–1408. [Google Scholar] [CrossRef]
- Fang, P.-K.; Wu, Y.-C.; Lin, Y.-C. Investigate the effect of zinc on depolymerized PET. Proc. Int. J. Ind. Eng. Oper. Manag. 2021, 6740–6750. [Google Scholar]
- Airaksinen, S. Role of Excipients in Moisture Sorption and Physical Stability of Solid Pharmaceutical Formulations. Ph.D. Thesis, Faculty of Pharmacy University of Helsinki, Helsinki, Finland, 2005. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar]
- Qi, L.; Tang, X.; Wang, Z.; Peng, X. Pore characterization of different types of coal from coal and gas outburst disaster sites using low temperature nitrogen adsorption approach. Int. J. Mining Sci. Technol. 2017, 27, 371–377. [Google Scholar] [CrossRef]
- Mutch, N.J.; Waters, E.K.; Morrissey, J.H. Immobilized transition metals stimulate contact activation and drive factor XII-mediated coagulation. J. Thromb. Haemost. 2012, 10, 2108–2115. [Google Scholar] [CrossRef]


| Sample | Sputt. Dep. Time [min.] | Zn Concentration | Sample Abbrev. PLA-Zn(M) | ||
|---|---|---|---|---|---|
| g/kg | mc [Mol/kg] /a | % [g/100 g] | |||
| PLA | - | - | PLA | ||
| PLA-Zn(5) | 5 | 21.09 | 0.32 | 2.11 | PLA-Zn(5)(0.3) |
| PLA-Zn(10) | 10 | 43.60 | 0.67 | 4.36 | PLA-Zn(10)(0.7) |
| PLA-Zn(15) | 15 | 105.00 | 1.61 | 10.50 | PLA-Zn(15)(1.6) |
| Sample Name | Zinc Concentration | This Work | Literature Data | |||||
|---|---|---|---|---|---|---|---|---|
| g/kg | mc [Mol/kg] /a | SSA [m2/g] | TPV [cm3/g] | SSA [m2/g] | TPV [cm3/g] | |||
| PLA | - | 0.9842 | 3.836 × 10−3 | 0.9721 [52] 0.221 [51,52] | 3.858 × 10−3 [52] 9.1 × 10−4 [51,52] | |||
| PLA-Zn(t)(mc) | PLA-Zn(5)(0.3) | 21.09 | 0.32 | 0.9405 | 3.235 × 10−3 | |||
| PLA-Zn(10)(0.7) | 43.60 | 0.67 | 0.9056 | 2.683 × 10−3 | ||||
| PLA-Zn(15)(1.6) | 105.00 | 1.61 | 0.7300 | 2.511 × 10−3 | ||||
| Sample Name | Bacterial Average Inhibition Zone (mm) | ||||
|---|---|---|---|---|---|
| E. coli | S. aureus | ||||
| Lit. Data | This Work | Lit. Data | This Work | ||
| PLA | 0 [55] | 0 | 0 [55] | 0 | |
| PLA-Zn(t)(mc) | PLA-Zn(5)(0.3) | - | 2 | 1 | |
| PLA-Zn(10)(0.7) | - | 2 | 2 | ||
| PLA-Zn(15)(1.6) | 2 | 2 | |||
| Concentration of inoculum: E. coli: CFU/mL = 1.6 × 108; S. aureus: CFU/mL = 1.2 × 108 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrozińska, Z.; Ponczek, M.B.; Kaczmarek, A.; Świerczyńska, M.; Kudzin, M.H. Activity in the Field of Blood Coagulation Processes of Poly(Lactide)-Zinc Fiber Composite Material Obtained by Magnetron Sputtering. Coatings 2024, 14, 666. https://doi.org/10.3390/coatings14060666
Mrozińska Z, Ponczek MB, Kaczmarek A, Świerczyńska M, Kudzin MH. Activity in the Field of Blood Coagulation Processes of Poly(Lactide)-Zinc Fiber Composite Material Obtained by Magnetron Sputtering. Coatings. 2024; 14(6):666. https://doi.org/10.3390/coatings14060666
Chicago/Turabian StyleMrozińska, Zdzisława, Michał B. Ponczek, Anna Kaczmarek, Małgorzata Świerczyńska, and Marcin H. Kudzin. 2024. "Activity in the Field of Blood Coagulation Processes of Poly(Lactide)-Zinc Fiber Composite Material Obtained by Magnetron Sputtering" Coatings 14, no. 6: 666. https://doi.org/10.3390/coatings14060666
APA StyleMrozińska, Z., Ponczek, M. B., Kaczmarek, A., Świerczyńska, M., & Kudzin, M. H. (2024). Activity in the Field of Blood Coagulation Processes of Poly(Lactide)-Zinc Fiber Composite Material Obtained by Magnetron Sputtering. Coatings, 14(6), 666. https://doi.org/10.3390/coatings14060666







