A Study on the Corrosion Behavior of RGO/Cu/Fe-Based Amorphous Composite Coatings in High-Temperature Seawater
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Preparation of the Coating
2.2. Experimental Route
2.3. Coating Performance Testing Method
3. Results
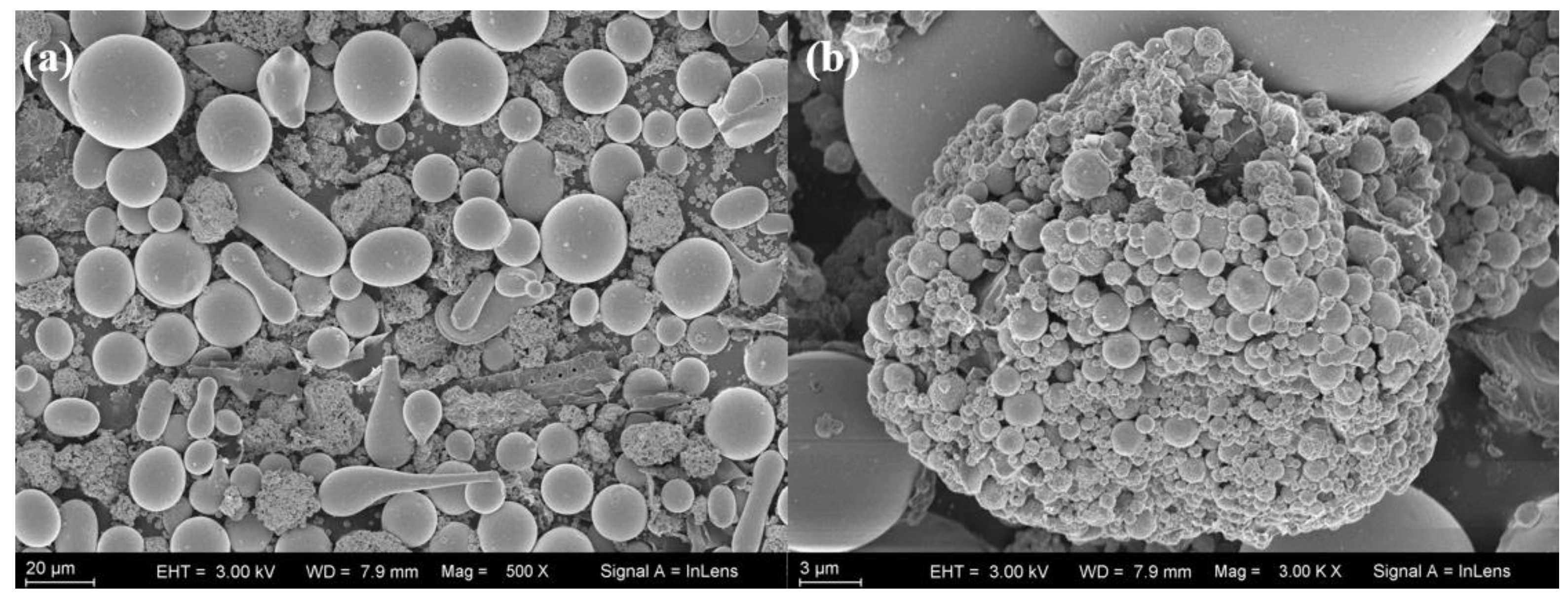
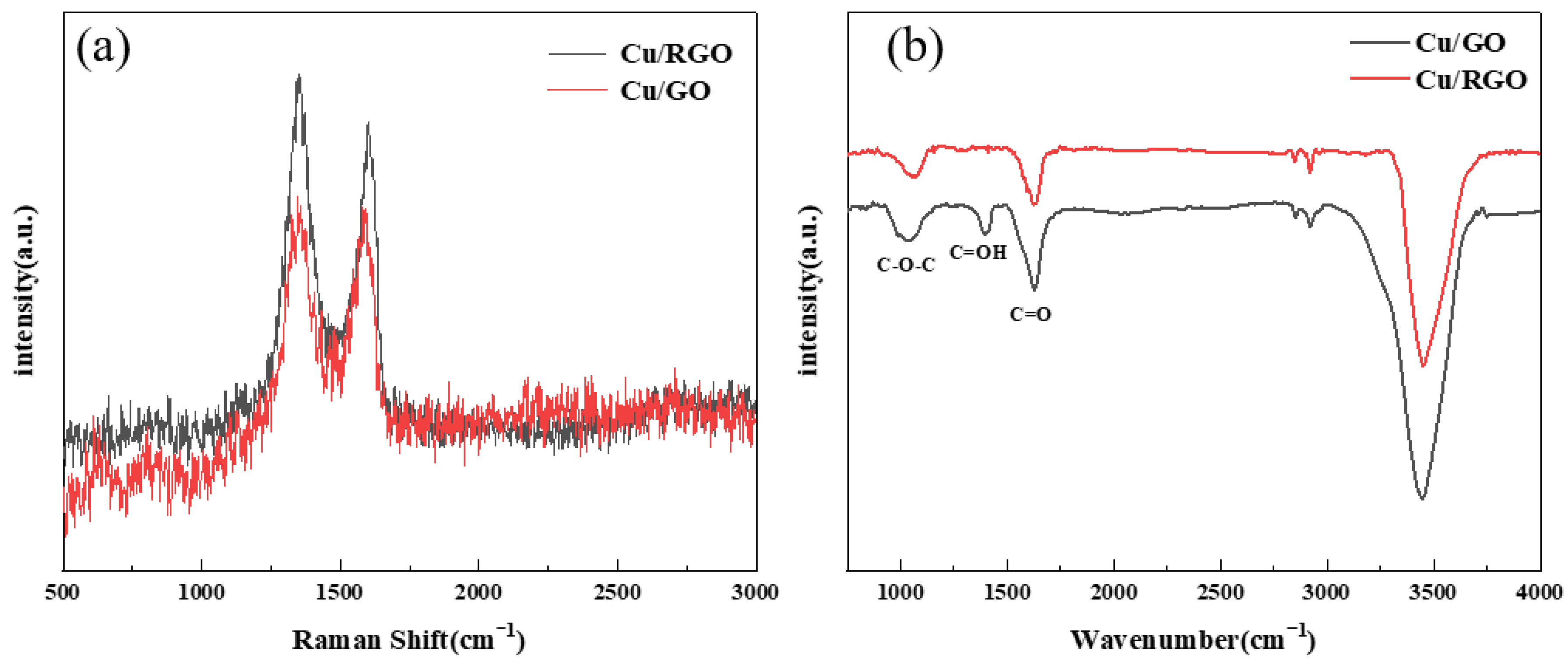
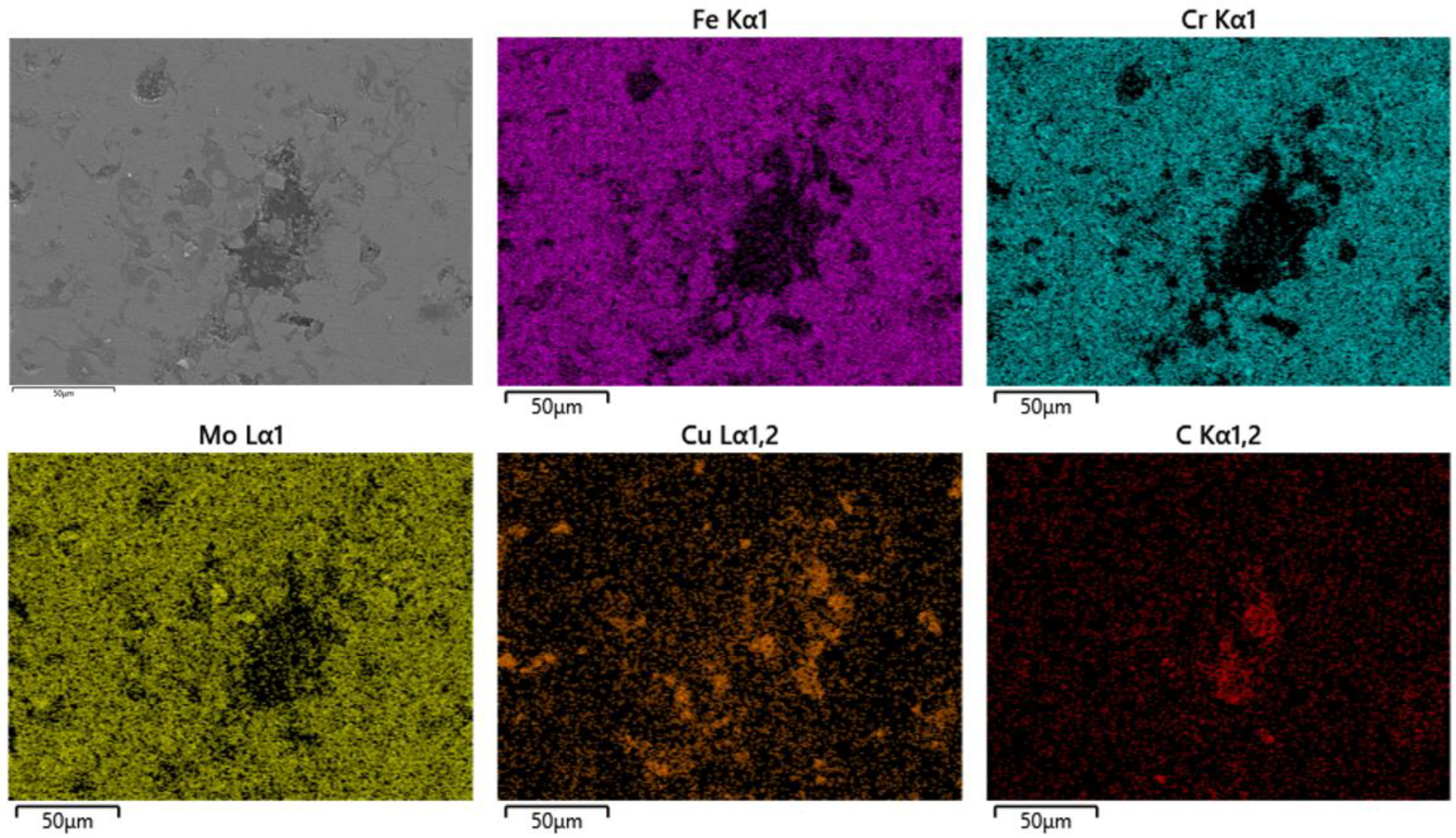
3.1. Characterization of RGO/Cu/Fe-Based Amorphous Composite Coating
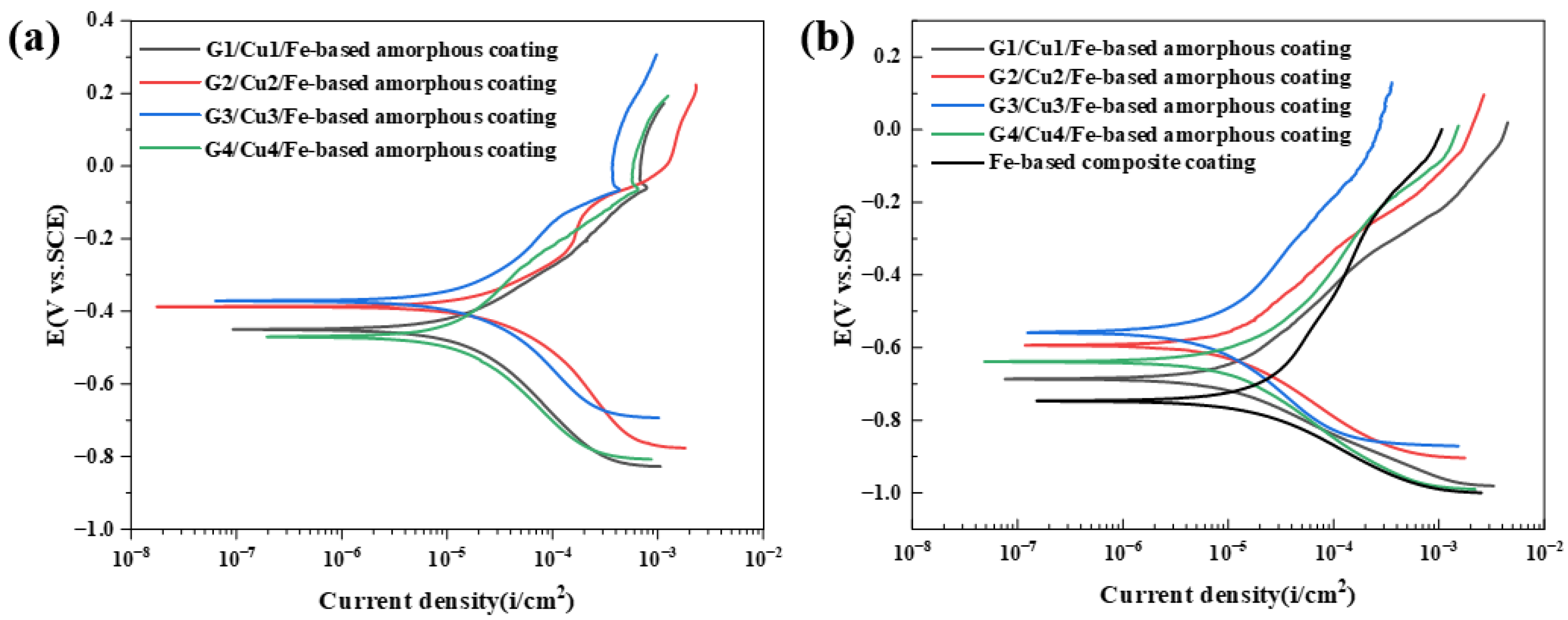
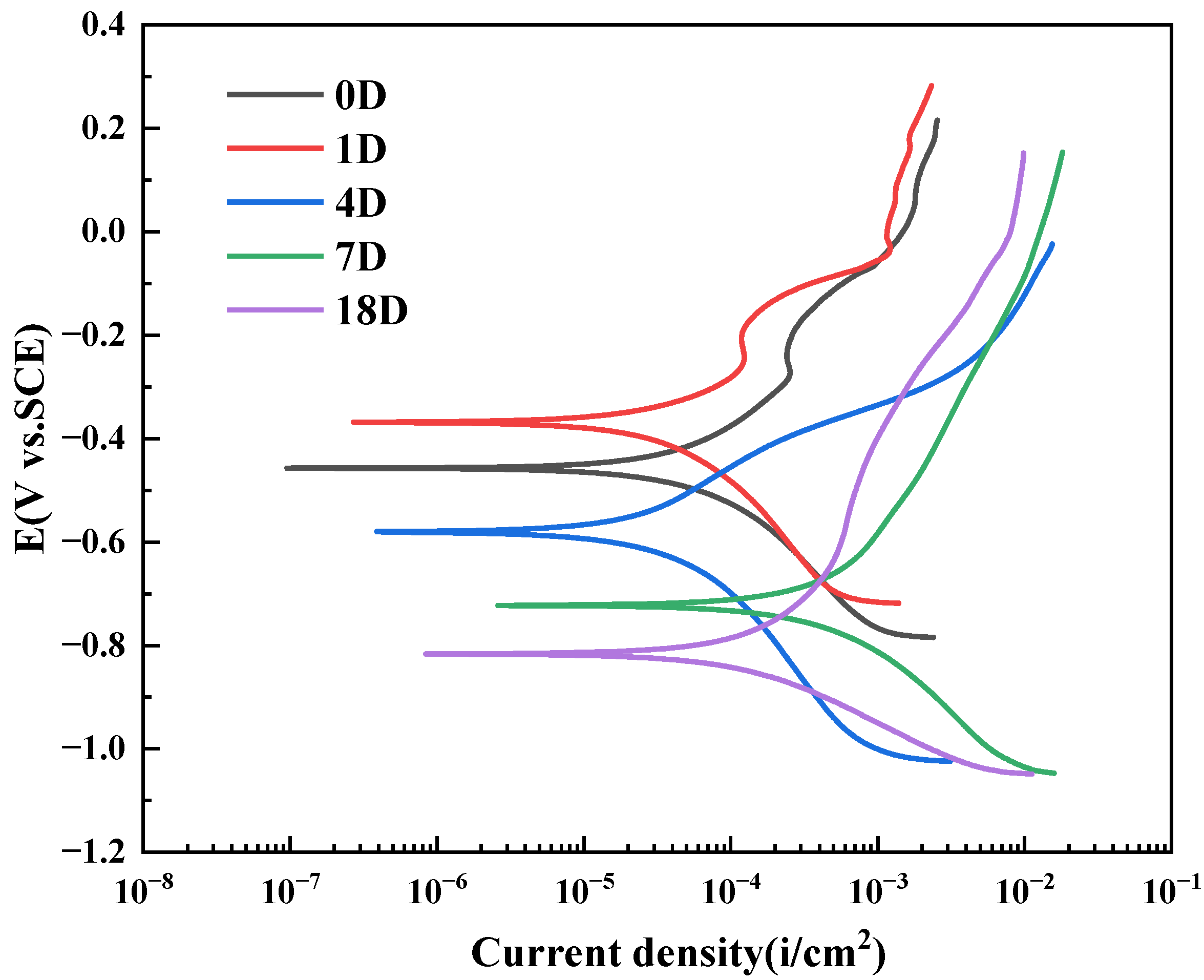
3.2. Analysis of Potentiodynamic Polarization Curve
3.3. Electrochemical Impedance Spectroscopy Analysis
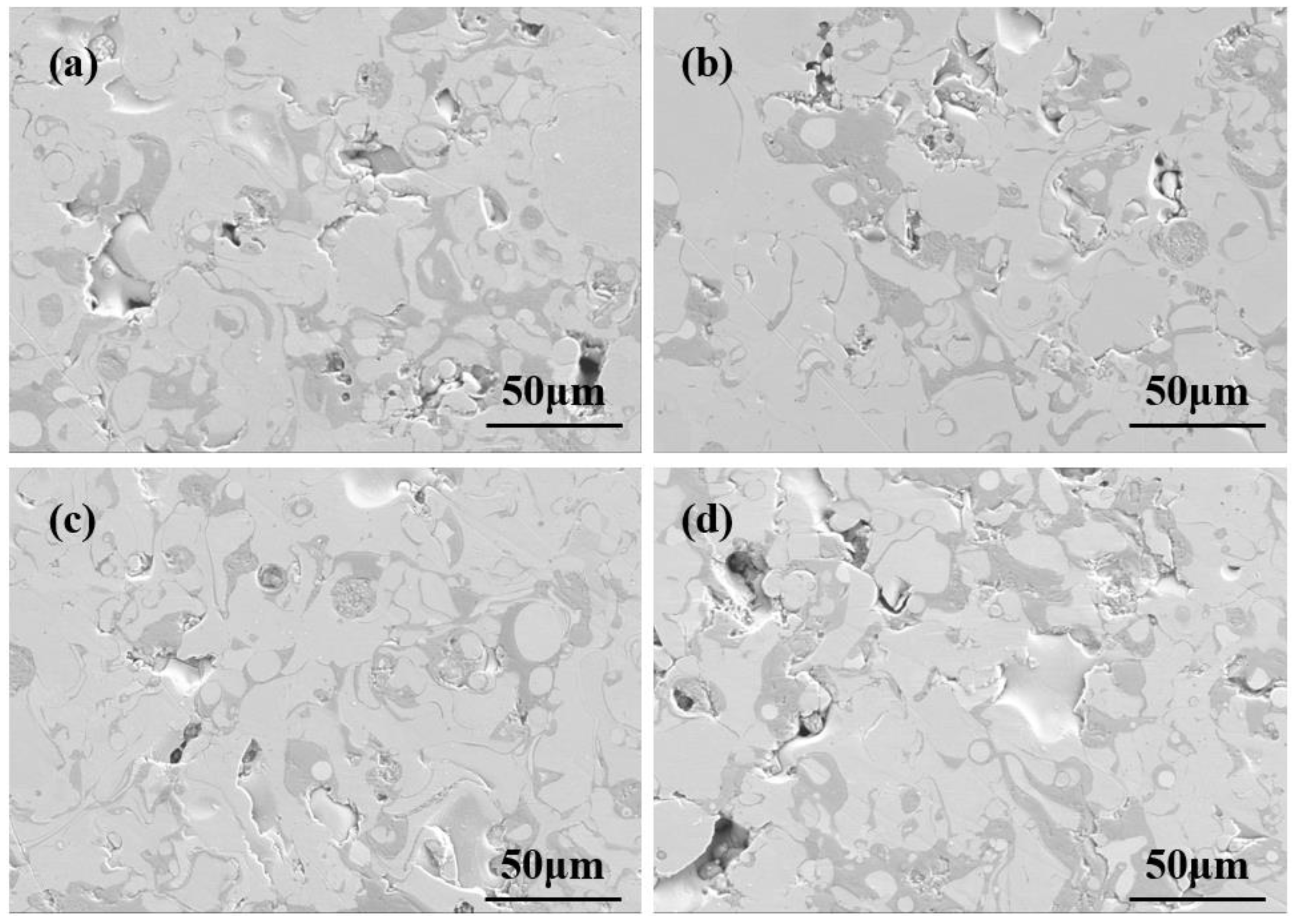
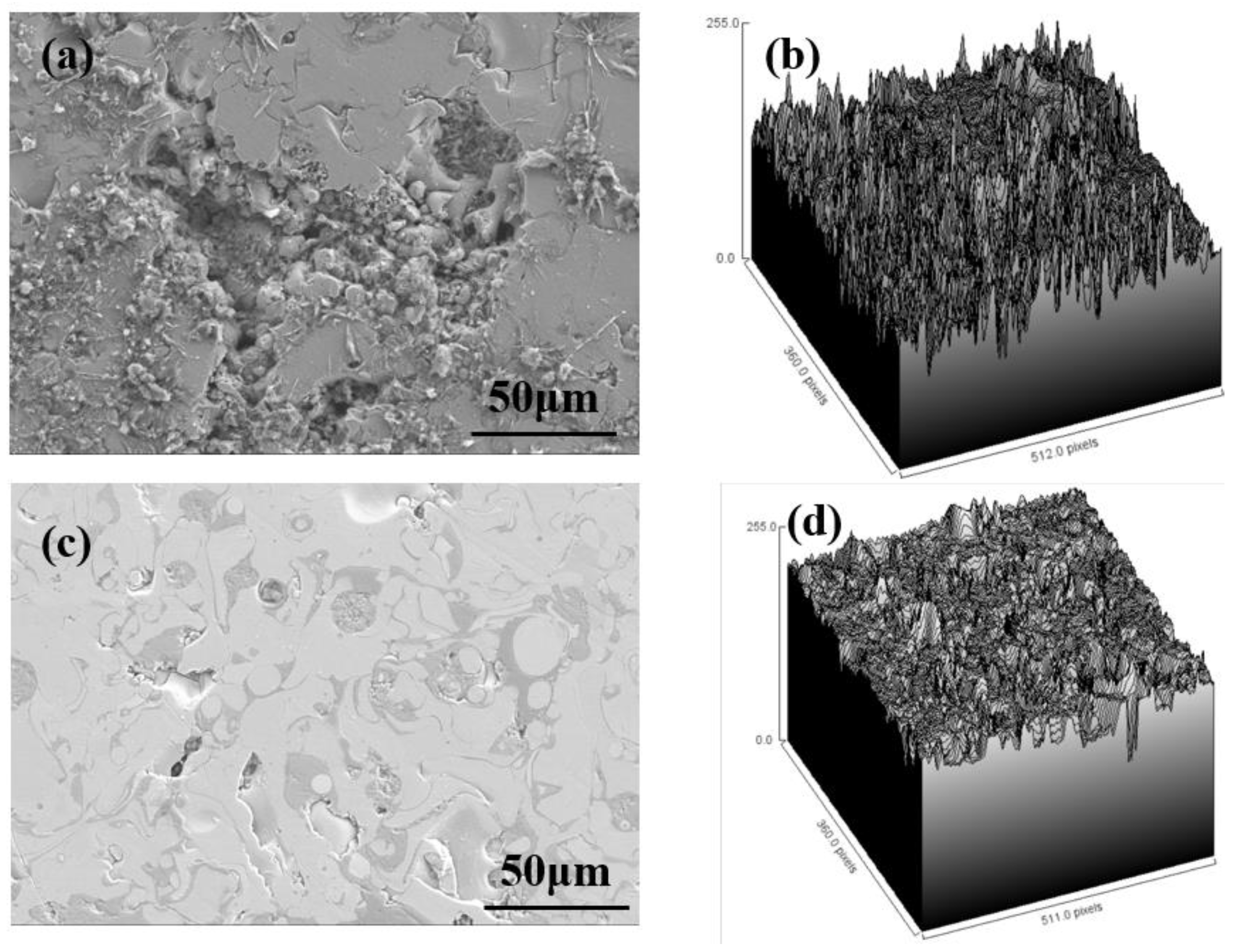
3.4. Surface Morphology Analysis
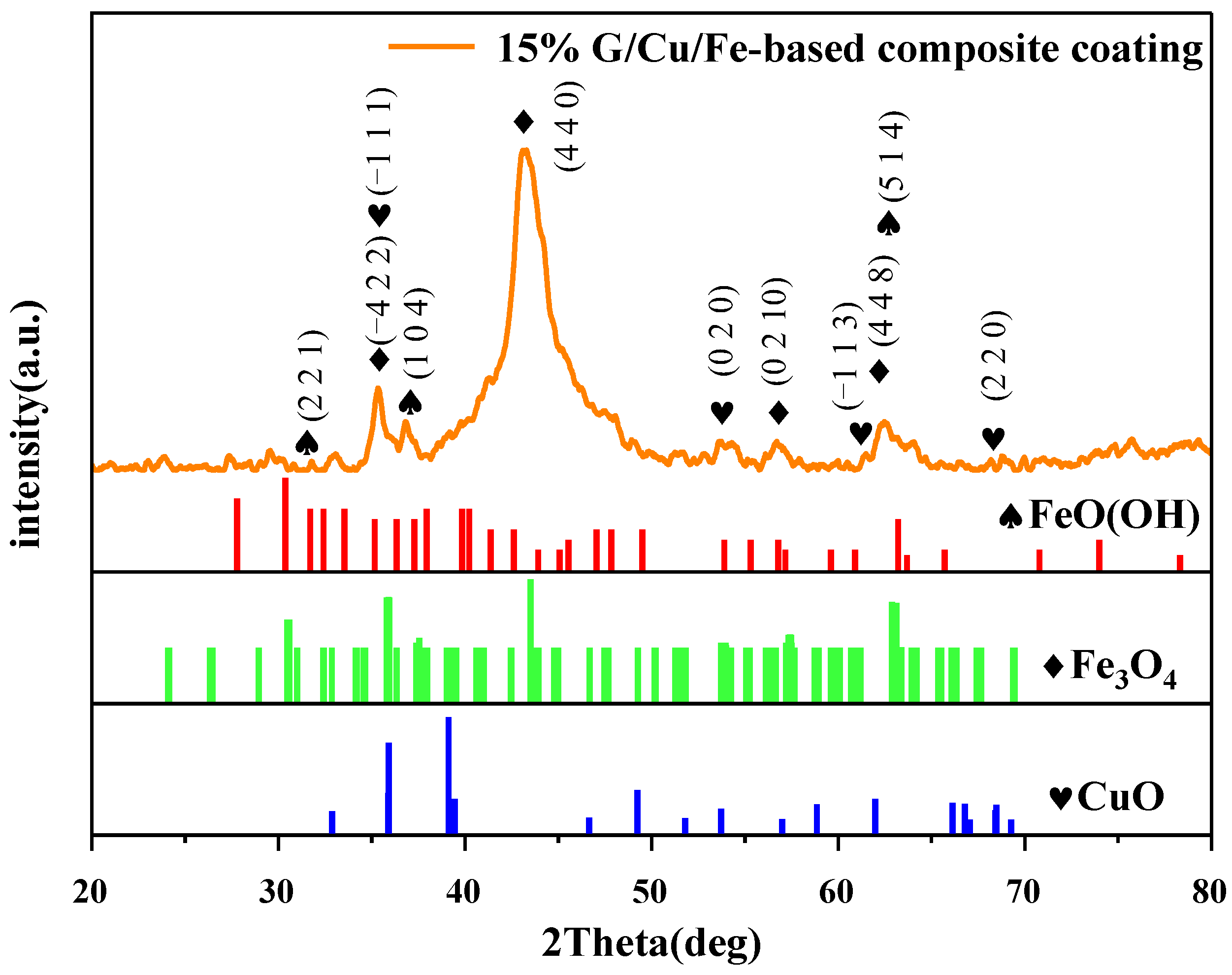
3.5. Phase Analysis of Corrosion Products
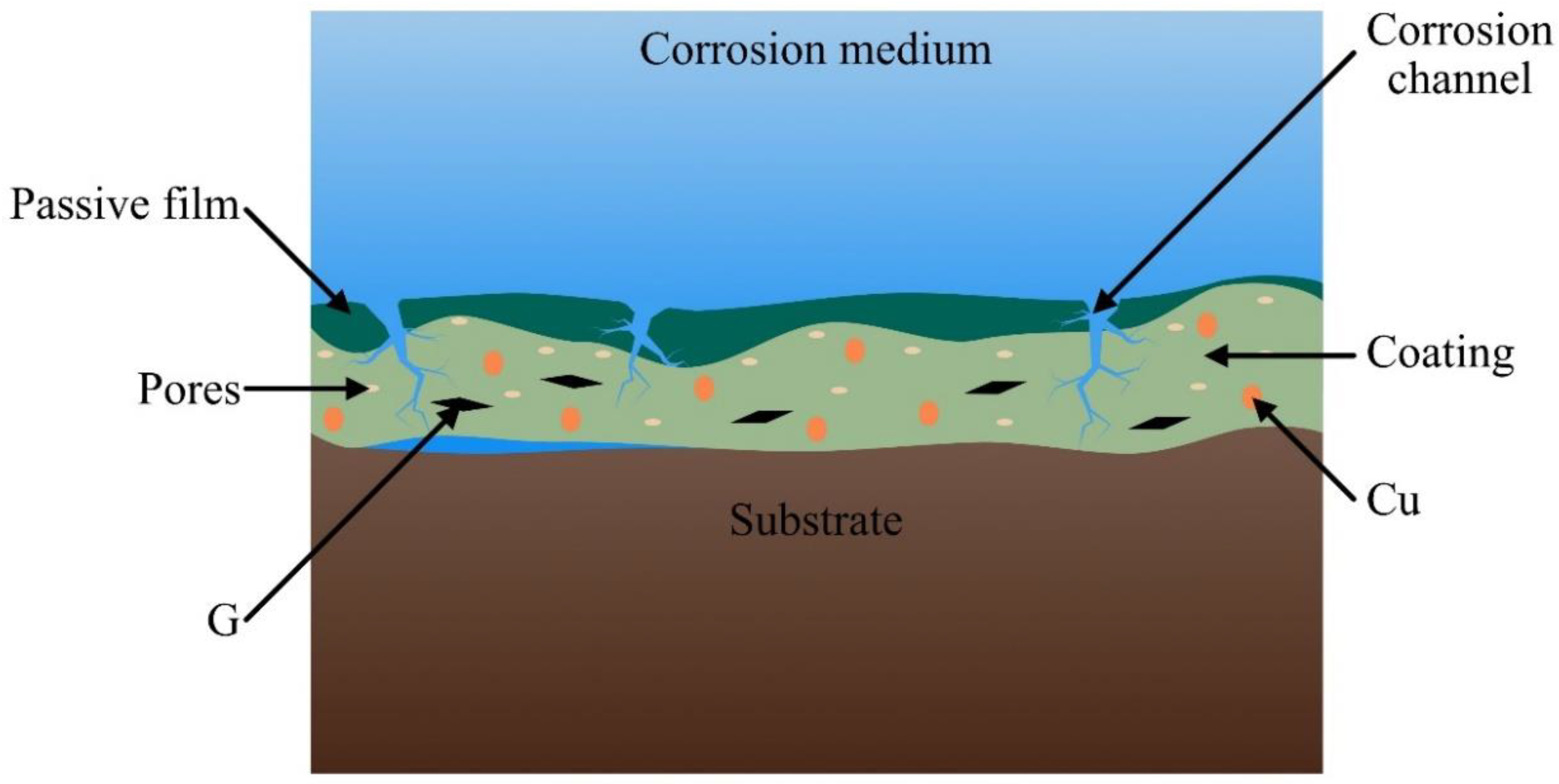
4. Discussion
5. Conclusions
- (1)
- With the increase in the proportion of RGO/Cu introduced, the corrosion resistance of the coating increases firstly and then decreases, with the best result occurring at a doping ratio of 15 wt.%.
- (2)
- The main failure modes of Fe-based amorphous composite coatings under high-temperature seawater are coating cracking and peeling, with limited pitting corrosion.
- (3)
- The introduction of RGO can effectively increase the toughness of the coating, improve its mechanical properties, and help to suppress the propagation of cracks at high temperatures.
- (4)
- In high-temperature seawater, the corrosion products on the coating will block cracks and pores, and the corrosion rate will decrease after reaching a maximum value, as a result of the balance between the corrosion and the blocking effect of the corrosion products.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- An, Y.; Hou, G.; Chen, J.; Zhao, X.; Liu, G.; Zhou, H.; Chen, J. Microstructure and tribological properties of iron-based metallic glass coatings prepared by atmospheric plasma spraying. Vacuum 2014, 107, 132–140. [Google Scholar] [CrossRef]
- Cui, S.; Zhai, H.; Tong, W.; Li, W.; Li, X.; Fan, X.; Xiong, D. Effect of hydration reaction on the wear behavior of Fe-based amorphous coating in different wear conditions. J. Non-Cryst. Solids 2023, 602, 122093. [Google Scholar] [CrossRef]
- Li, X.; Zhai, H.; Li, W.; Cui, S.; Ning, W.; Qiu, X. Dry sliding wear behaviors of Fe-based amorphous metallic coating synthesized by d-gun spray. J. Non-Cryst. Solids 2020, 537, 120018. [Google Scholar] [CrossRef]
- Al-Abboodi, H.; Fan, H.; Mhmood, I.A.; Al-Bahrani, M. The dry sliding wear rate of a Fe-based amorphous coating prepared on mild steel by HVOF thermal spraying. J. Mater. Res. Technol. 2022, 18, 1682–1691. [Google Scholar] [CrossRef]
- Mahade, S.; Awe, S.A.; Björklund, S.; Lukáč, F.; Mušálek, R.; Joshi, S. Sliding wear behavior of a sustainable Fe-based coating and its damage mechanisms. Wear 2022, 500–501, 204375. [Google Scholar] [CrossRef]
- Wang, Y.; Li, K.; Scenini, F.; Jiao, J.; Qu, S.; Luo, Q.; Shen, J. The effect of residual stress on the electrochemical corrosion behavior of Fe-based amorphous coatings in chloride-containing solutions. Surf. Coat. Technol. 2016, 302, 27–38. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Sun, L.; Zhang, X.; Shen, J. Environmentally assisted fracture behavior of Fe-based amorphous coatings in chloride-containing solutions. J. Alloys Compd. 2018, 738, 37–48. [Google Scholar] [CrossRef]
- Widjajanto, T.; Darmadi, D.B.; Irawan, Y.S.; Gapsari, F. Comparative microstructure characteristics and properties of arc-sprayed Fe-based and HVOF-sprayed Ni-based coatings on ASME SA 210 C steel tube. Results Eng. 2023, 17, 100985. [Google Scholar] [CrossRef]
- Nayak, S.K.; Kumar, A.; Sarkar, K.; Banerjee, A.; Laha, T. Mechanistic insight into the role of amorphicity and porosity on determining the corrosion mitigation behavior of Fe-based amorphous/nanocrystalline coating. J. Alloys Compd. 2020, 849, 156624. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, W.; Wang, S.; Gu, X.; Wang, J. Characterisation of three-dimensional porosity in an Fe-based amorphous coating and its correlation with corrosion behaviour. Corros. Sci. 2015, 93, 211–221. [Google Scholar] [CrossRef]
- Ye, X.; Shin, Y.C. Synthesis and characterization of Fe-based amorphous composite by laser direct deposition. Surf. Coat. Technol. 2014, 239, 34–40. [Google Scholar] [CrossRef]
- Huang, C.; Wang, J.; Yan, J.; Liao, L. Evaluation of corrosion and wear performance of Fe-based coating and Hastelloy C276 in H2S environment on 304 stainless steel substrate. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131871. [Google Scholar] [CrossRef]
- Lin, T.-J.; Sheu, H.-H.; Lee, C.-Y.; Lee, H.-B. The study of mechanical properties and corrosion behavior of the Fe-based amorphous alloy coatings using high velocity oxygen fuel spraying. J. Alloys Compd. 2021, 867, 159132. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, Y.; Wan, Y.; Jeyaprakash, N.; Wang, Y.; Ma, K.; Yang, J. Influence of scanning speed on microstructure and corrosion resistance of Fe-based amorphous coatings by high-speed laser cladding. Surf. Coat. Technol. 2024, 479, 13044. [Google Scholar] [CrossRef]
- Chu, Z.; Wei, F.; Zheng, X.; Zhang, C.; Yang, Y. Microstructure and properties of TiN/Fe-based amorphous composite coatings fabricated by reactive plasma spraying. J. Alloys Compd. 2019, 785, 206–213. [Google Scholar] [CrossRef]
- Chu, Z.; Zheng, X.; Zhang, C.; Xu, J.; Gao, L. Study the effect of AT13 addition on the properties of AT13/Fe-based amorphous composite coatings. Surf. Coat. Technol. 2019, 379, 125053. [Google Scholar] [CrossRef]
- Yang, Z.; Che, J.; Zhang, Z.; Yu, L.; Hu, M.; Sun, W.; Gao, W.; Fan, J.; Wang, L.; Liu, G. High-efficiency graphene/epoxy composite coatings with outstanding thermal conductive and anti-corrosion performance. Compos. Part A Appl. Sci. Manuf. 2024, 181, 108152. [Google Scholar] [CrossRef]
- Li, L.; Nian, F.; Zhang, S.; Xu, Y.; Ma, S.; Li, Y. Hydrophobic and anti-fouling novel anti-corrosion coatings of graphene quantum dots in situ doped with polyphenylene sulfide. Surf. Coat. Technol. 2024, 479, 130527. [Google Scholar] [CrossRef]
- Liu, C.; Qiu, S.; Du, P.; Zhao, H.; Wang, L. An ionic liquid–graphene oxide hybrid nanomaterial: Synthesis and anticorrosive applications. Nanoscale 2018, 10, 8115–8124. [Google Scholar] [CrossRef]
- Wen, J.-G.; Geng, W.; Geng, H.-Z.; Zhao, H.; Jing, L.-C.; Yuan, X.-T.; Tian, Y.; Wang, T.; Ning, Y.-J.; Wu, L. Improvement of corrosion resistance of waterborne polyurethane coatings by covalent and noncovalent grafted graphene oxide nanosheets. ACS Omega 2019, 4, 20265–20274. [Google Scholar] [CrossRef]
- Bai, T.; Lv, L.; Du, W.; Fang, W.; Wang, Y. Improving the tribological and anticorrosion performance of waterborne polyurethane coating by the synergistic effect between modified graphene oxide and polytetrafluoroethylene. Nanomaterials 2020, 10, 137. [Google Scholar] [CrossRef]
- Mourya, P.; Goswami, R.N.; Saini, R.; Ray, A.; Khatri, O.P. Epoxy coating reinforced with graphene-PANI nanocomposites for enhancement of corrosion-resistance performance of mild steel in saline water. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133500. [Google Scholar] [CrossRef]
- Pandey, U.; Sharma, C. Comparative study of graphene oxide-multifunctional oxide doping on corrosion resistance of electrodeposited nickel coatings in saline environments. Int. J. Hydrog. Energy 2024, 60, 165–179. [Google Scholar] [CrossRef]
- Pang, W.; Jiang, H.; Wang, S.; He, T.; Chen, H.; Yan, T.; Cheng, M.; Sun, S.; Li, C. Graphene oxides enhanced polyurethane based composite coating with long term corrosion resistance and self-healing property. Eur. Polym. J. 2024, 207, 112825. [Google Scholar] [CrossRef]
- Taravel-Condat, C.; Desamais, N. Qualification of High Strength Carbon Steel Wires for Use in Specific Annulus Environment of Flexible Pipes Containing CO2 and H2S. In Proceedings of the 25th International Conference on Offshore Mechanics and Arctic Engineering, Hamburg, Germany, 4–9 June 2006; pp. 585–591. [Google Scholar]
- Wu, L.; Zhou, Z.; Zhang, K.; Zhang, X.; Wang, G. Electrochemical and passive film evaluation on the corrosion resistance variation of Fe-based amorphous coating affected by high temperature. J. Non-Cryst. Solids 2022, 597, 121892. [Google Scholar] [CrossRef]
- Escrivà-Cerdán, C.; Blasco-Tamarit, E.; García-García, D.; García-Antón, J.; Akid, R.; Walton, J. Effect of temperature on passive film formation of UNS N08031 Cr–Ni alloy in phosphoric acid contaminated with different aggressive anions. Electrochim. Acta 2013, 111, 552–561. [Google Scholar] [CrossRef]
- Carranza, R.; Alvarez, M. The effect of temperature on the passive film properties and pitting behaviour of a FeCrNi alloy. Corros. Sci. 1996, 38, 909–925. [Google Scholar] [CrossRef]
- Zhang, C.; Chu, Z.; Wei, F.; Qin, W.; Yang, Y.; Dong, Y.; Huang, D.; Wang, L. Optimizing process and the properties of the sprayed Fe-based metallic glassy coating by plasma spraying. Surf. Coat. Technol. 2017, 319, 1–5. [Google Scholar] [CrossRef]
- Wu, C.H.; Liu, C.; Su, D.; Xin, H.L.; Fang, H.-T.; Eren, B.; Zhang, S.; Murray, C.B.; Salmeron, M.B. Bimetallic synergy in cobalt–palladium nanocatalysts for CO oxidation. Nat. Catal. 2019, 2, 78–85. [Google Scholar] [CrossRef]
- Song, Y.; Kim, D.; Hong, S.; Kim, T.-S.; Kim, K.-J.; Park, J.Y. Bimetallic synergy from a reaction-driven metal oxide–metal interface of pt–co bimetallic nanoparticles. ACS Catal. 2023, 13, 13777–13785. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, L.; Huang, L.; He, W.; Li, H.; Wang, S.; Long, Z.; Sun, Z. Identification of active sites in pt–co bimetallic catalysts for co oxidation. ACS Appl. Energy Mater. 2021, 4, 11151–11161. [Google Scholar] [CrossRef]
- Kim, T.-S.; Choi, H.; Kim, D.; Song, H.C.; Oh, Y.; Jeong, B.; Lee, J.; Kim, K.-J.; Shin, J.W.; Byon, H.R.; et al. Catalytic boosting on AuCu bimetallic nanoparticles by oxygen-induced atomic restructuring. Appl. Catal. B Environ. 2023, 331, 122704. [Google Scholar] [CrossRef]
- Qian, H.; Xu, Z.; Chen, S.; Liu, Y.; Yan, D. Silicon carbide/enamel composite coatings for steel corrosion protection: Microstructure, thermal expansion behavior, and anti-corrosion performance. Surf. Coat. Technol. 2022, 434, 128172. [Google Scholar] [CrossRef]
- Singh, P.; Satish, C.; Islam, A.; Prasad, S.; Pandit, N.; Keshri, S.; Keshri, A.K. Multifunctional hybrid composite: Plasma sprayed Al and graphene reinforced alumina coating. J. Alloys Compd. 2024, 984, 173984. [Google Scholar] [CrossRef]







| Process Parameters | RGO/Cu/Fe-Based Amorphous Composite Coating |
|---|---|
| Arc voltage (V) | 70 |
| Arc current (A) | 500 |
| Gun distance (mm) | 100 |
| Movement speed of spray gun (m/min) | 5–7 |
| Argon flow rate (dm3/min) | 30 |
| Nitrogen flow rate (dm3/min) | 120 |
| Coating thickness (μm) | 300 |
| NaCl | MgCl2 | Na2SO4 | CaCl2 | KCl | SrCl2 | NaHCO3 | KBr | H3BO3 | NaF |
|---|---|---|---|---|---|---|---|---|---|
| 24.530 | 5.200 | 4.090 | 1.160 | 0.695 | 0.025 | 0.201 | 0.101 | 0.027 | 0.003 |
| Samples | Ecorr (mV) | icorr (μA·cm−2) | CorrRate (mpy) |
|---|---|---|---|
| G1/Cu1/Fe-based amorphous coating | −449.9 | 5.22 | 1.95 |
| G2/Cu2/Fe-based amorphous coating | −387.5 | 2.23 | 0.83 |
| G3/Cu3/Fe-based amorphous coating | −371.3 | 2.22 | 0.83 |
| G4/Cu4/Fe-based amorphous coating | −469.9 | 8.36 | 3.13 |
| Samples | Ecorr (mV) | icorr (μA·cm−2) | CorrRate (mpy) |
| G1/Cu1/Fe-based amorphous coating | −686.7 | 18.95 | 8.66 |
| G2/Cu2/Fe-based amorphous coating | −593.8 | 16.00 | 7.30 |
| G3/Cu3/Fe-based amorphous coating | −557.8 | 12.04 | 5.50 |
| G4/Cu4/Fe-based amorphous coating | −638.2 | 15.33 | 7.00 |
| Fe-based amorphous coating | −746.5 | 25.58 | 11.69 |
| Samples | Ecorr (mV) | icorr (μA·cm−2) | CorrRate (mpy) |
|---|---|---|---|
| 0D | −457.2 | 7.65 | 3.50 |
| 1D | −368.2 | 7.41 | 3.39 |
| 4D | −579.8 | 10.19 | 4.66 |
| 7D | −772.6 | 21.64 | 9.89 |
| 18D | −816.7 | 20.12 | 9.19 |
| Immersion Time | Rs (Ω·cm2) | CPEf (s-secn) | Rf (Ω·cm2) | CPEdl (s-secn) | Rct(Ω·cm2) | Chi-Squared |
|---|---|---|---|---|---|---|
| 0D | 6.18 × 100 | 1.46 × 10−3 | 6.41 × 101 | 2.36 × 10−3 | 6.36 × 102 | 3.35 × 10−4 |
| 1D | 7.25 × 100 | 1.48 × 10−3 | 8.48 × 101 | 3.95 × 10−3 | 8.04 × 102 | 6.10 × 10−5 |
| 4D | 8.50 × 100 | 3.44 × 10−3 | 1.81 × 101 | 3.80 × 10−3 | 3.68 × 102 | 8.18 × 10−4 |
| 7D | 1.07 × 100 | 2.56 × 10−4 | 8.53 × 100 | 4.10 × 10−3 | 1.18 × 102 | 4.19 × 10−4 |
| 18D | 7.87 × 100 | 3.25 × 10−3 | 3.50 × 100 | 2.86 × 10−3 | 1.02 × 102 | 2.87 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Z.; Zhang, Y.; Tang, W.; Xu, Y.; Xu, J. A Study on the Corrosion Behavior of RGO/Cu/Fe-Based Amorphous Composite Coatings in High-Temperature Seawater. Coatings 2024, 14, 556. https://doi.org/10.3390/coatings14050556
Chu Z, Zhang Y, Tang W, Xu Y, Xu J. A Study on the Corrosion Behavior of RGO/Cu/Fe-Based Amorphous Composite Coatings in High-Temperature Seawater. Coatings. 2024; 14(5):556. https://doi.org/10.3390/coatings14050556
Chicago/Turabian StyleChu, Zhenhua, Yunzheng Zhang, Wan Tang, Yuchen Xu, and Jingxiang Xu. 2024. "A Study on the Corrosion Behavior of RGO/Cu/Fe-Based Amorphous Composite Coatings in High-Temperature Seawater" Coatings 14, no. 5: 556. https://doi.org/10.3390/coatings14050556
APA StyleChu, Z., Zhang, Y., Tang, W., Xu, Y., & Xu, J. (2024). A Study on the Corrosion Behavior of RGO/Cu/Fe-Based Amorphous Composite Coatings in High-Temperature Seawater. Coatings, 14(5), 556. https://doi.org/10.3390/coatings14050556








