Cinnamon Bark Oil as an Effective Fungicide in Protecting the Surface of Wood-Based Softboards against the Development of Mold Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Research Material
2.2. Wood Treatment
2.3. Assessment of the Effectiveness of Treatment against Molds
2.4. GCMS Analysis
2.5. Statistical Analysis
3. Results
3.1. Assessment of Biocidal Effectiveness against Mold Fungi
3.2. Identification of Biocide Volatile Components in SBs
3.3. Graphical Identification of Research Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 Concerning the Making Available on the Market and use of Biocidal Products. Available online: https://echa.europa.eu/pl/information-on-chemicals/biocidal-products (accessed on 20 February 2024).
- ESD for PT 8: Revised Emission Scenario Document for Wood Preservatives (OECD Series No. 2, 2013). Available online: https://echa.europa.eu/pl/guidance-documents/guidance-on-biocides-legislation/emission-scenario-documents (accessed on 20 February 2024).
- Pánek, M.; Reinprecht, L.; Hulla, M. Ten essential oils for beech wood protection—Efficacy against wood-destroying fungi and moulds, and effect on wood discoloration. BioResources 2014, 9, 5588–5603. [Google Scholar] [CrossRef]
- Mazela, B.; Bartkowiak, M.; Ratajczak, I. Animal protein impact on fungicidal properties of treatment formulations. Wood Res. 2007, 52, 13–22. [Google Scholar]
- González-Búrquez, M.d.J.; González-Díaz, F.R.; García-Tovar, C.G.; Carrillo-Miranda, L.; Soto-Zárate, C.I.; Canales-Martínez, M.M.; Penieres-Carrillo, J.G.; Crúz-Sánchez, T.A.; Fonseca-Coronado, S. Comparison between In Vitro Antiviral Effect of Mexican Propolis and Three Commercial Flavonoids against Canine Distemper Virus. Evid. Based Complement. Alternat. Med. 2018, 2018, 7092416. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Huh, N.; Hong, H.J.; Kim, B.S.; Kim, G.H.; Kim, J.J. The antagonistic properties of Trichoderma spp. inhabiting woods for potential biological control of wood-damaging fungi. Holzforschung 2012, 66, 883–887. [Google Scholar] [CrossRef]
- Pop, D.M.; Timar, M.C.; Beldean, E.C.; Varodi, A.M. Combined Testing Approach to Evaluate the Antifungal Efficiency of clove (Eugenia caryophyllata) Essential Oil for Potential Application in Wood Conservation. BioResources 2020, 15, 9474–9489. [Google Scholar] [CrossRef]
- Sparacello, S.; Gallo, G.; Faddetta, T.; Megna, B.; Nicotra, G.; Bruno, B.; Giambra, B.; Palla, F. Thymus vulgaris Essential Oil and Hydro-Alcoholic Solutions to Counteract Wooden Artwork Microbial Colonization. Appl. Sci. 2021, 11, 8704. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Z.; Huang, Q.; Zhang, D. Antifungal Activity of Several Essential Oils and Major Components against Wood-Rot Fungi. Ind. Crops Prod. 2017, 108, 278–285. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, T.; Mi, N.; Wang, Y.; Li, G.; Wang, L.; Xie, Y. Antifungal Activity of Monoterpenes against Wood White-Rot Fungi. Int. Biodeterior. Biodegrad. 2016, 106, 157–160. [Google Scholar] [CrossRef]
- Goktas, O.; Mammadov, R.; Duru, M.E.; Ozen, E.; Colak, A.M. Application of extracts from the poisonous plant, Nerium Oleander L., as a wood preservative. Afr. J. Biotechnol. 2007, 6, 2000–2003. [Google Scholar]
- Ozen, E. A Study about Poisonous Plant (Geophytes) Extracts as a Wood Preservative to Wood Decay Fungi. Ph.D. Thesis, Institute of Natural Science, Mugla University, Mugla, Turkey, 2005. [Google Scholar]
- Yildiz, Ü.C.; Kiliç, C.; Gürgen, A.; Yildiz, S. Possibility of using lichen and mistletoe extracts as potential natural wood preservative. Maderas-Cienc. Tecnol. 2020, 22, 179–188. [Google Scholar] [CrossRef]
- Tascioglu, C.; Yalcin, M.; Sen, S.; Akcay, C. Antifungal properties of some plant extracts used as wood preservatives. Int. Biodeter. Biodegr. 2013, 85, 23–28. [Google Scholar] [CrossRef]
- Hussain, A.; Shrivastav, A.; Jain, S.K. Antifungal Activity of Essential Oils against Local Wood Degrading Cellulolytic Filamentous Fungi. Adv. Biores. 2013, 4, 161–167. [Google Scholar]
- Missio, A.L.; Mattos, B.D.; Ferreira, D.d.F.; Magalhães, W.L.E.; Bertuol, D.A.; Gatto, D.A.; Petutschnigg, A.; Tondi, G. Nanocellulose-tannin films: From trees to sustainable active packaging. J. Clean. Prod. 2018, 184, 143–151. [Google Scholar] [CrossRef]
- Laks, P.E.; McKaig, P.A.; Hemingway, R.W. Flavonoid biocides: Wood preservatives based on condensed tannins. Holzforsch. Int. J. Biol. Chem. Phys. Technol. Wood 1988, 42, 299–306. [Google Scholar] [CrossRef]
- Anouhe, J.-B.S.; Niamké, F.B.; Faustin, M.; Virieux, D.; Pirat, J.-L.; Adima, A.A.; Kati-Coulibaly, S.; Amusant, N. The role of extractives in the natural durability of the heartwood of Dicorynia guianensis Amsh: New insights in antioxydant and antifungal properties. Ann. For. Sci. 2018, 75, 15. [Google Scholar] [CrossRef]
- Eller, F.J.; Hay, W.T.; Kirker, G.T.; Mankowski, M.E.; Sellling, G.W. Hexadecyl ammonium chloride amylose inclusion complex to emulsify cedarwood oil and treat wood against termites and wood-decay fungi. Int. Biodeterior. Biodegrad. 2018, 129, 95–101. [Google Scholar] [CrossRef]
- Kirker, G.T.; Blodgett, A.B.; Arango, R.A.; Lebow, P.K.; Clausen, C.K. The role of extractives in naturally durable wood species. Int. Biodeterior. Biodegrad. 2013, 82, 53–58. [Google Scholar] [CrossRef]
- Broda, M. Natural Compounds for Wood Protection against Fungi—A Review. Molecules 2020, 25, 3538. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Cheng, S.-S.; Wu, C.-L.; Chang, S.-T. Contact and fumigant actions of trans-cinnamaldehyde against wood-decay fungi evaluated by using solid-phase microextraction. Wood Sci. Technol. 2020, 54, 237–247. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.-X.; Lin, J.-G.; Liu, J.; Jiang, M.-S.; Chu, L.-X. Chemical composition and antifungal activity of extracts from the xylem of Cinnamomum camphora. BioResources 2014, 9, 2560–2571. [Google Scholar] [CrossRef]
- Stefanović, M.; Todorović, N.; Milić, G.; Lovrić, A. Antifungal protection of beech wood using plant extracts. IOP Conf. Ser. Mater. Sci. Eng. 2023, 1298, 012009. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Chen, P.-F.; Chang, S.-T. Antifungal activities of essential oils and their constituents from indigenous cinnamon (Cinnamomum osmophloeum) leaves against wood decay fungi. Bioresour. Technol. 2005, 96, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Chittenden, C.; Singh, T. Antifungal activity of essential oils against wood degrading fungi and their applications as wood preservatives. Int. Wood Prod. J. 2011, 2, 44–48. [Google Scholar] [CrossRef]
- Matan, N.; Matan, N. Antifungal activities of anise oil, lime oil, and tangerine oil against moulds on rubberwood (Hevea brasiliensis). Int. Biodeterior. Biodegrad. 2008, 62, 75–78. [Google Scholar] [CrossRef]
- Hu, L.; Qin, L.; Xie, J.; Xu, H.; Yang, Z. Application of plant essential oils in controlling wood mold and stain fungi. BioResources 2021, 16, 1325–1334. [Google Scholar] [CrossRef]
- Thoroski, J.; Blank, G.; Biliaderis, C. Eugenol induced inhibition of extracellular enzyme production by Bacillus subtilis. J. Food Prot. 1989, 52, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Thobunluepop, P.; Pawelzik, E.; Jatisatienr, C.; Vearasilp, S. In vitro screening of the antifungal activity of plant extracts as fungicides against rice seed borne fungi. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science: Leuven, Belgium, 2009; Volume 837, pp. 223–228. [Google Scholar]
- Kamperidou, V. The Biological Durability of Thermally- and Chemically-Modified Black Pine and Poplar Wood Against Basidiomycetes and Mold Action. Forests 2019, 10, 1111. [Google Scholar] [CrossRef]
- Cheng, S.-S.; Liu, J.-Y.; Chang, E.-H.; Chang, S.-T. Antifungal activity of cinnamaldehyde and eugenol congeners against wood-rot fungi. Bioresour. Technol. 2008, 99, 5145–5514. [Google Scholar] [CrossRef]
- EN 310; Wood-Based Panels-Determination of Modulus of Elasticity in Bending and of Bending Strength. European Committee for Standardisation: Brussels, Belgium, 1994.
- EN 317; Particleboards and Fibreboards-Determination of Swelling in Thickness after Immersion in Water. European Committee for Standardisation: Brussels, Belgium, 1999.
- EN 323; Wood-Based Panels-Determination of Density. European Committee for Standardisation: Brussels, Belgium, 1999.
- Salerno-Kochan, R.; Czarnecki, W.; Szakiel, J.; Jankowski, P. No. 72886; Laboratory Device for Spraying Modification of Leather and Textile Products with Essential Oils. Patent Office of the Republic of Poland: Warszawa, Poland, 2023.
- Betlej, I.; Andres, B.; Krajewski, K.; Kiełtyka-Dadasiewicz, A.; Szadkowska, D.; Zawadzki, J. Effect of Various Mentha sp. Extracts on the Growth of Trichoderma viride and Chaetomium globosum on Agar Medium and Pine Wood. Diversity 2023, 15, 152. [Google Scholar] [CrossRef]
- Bi, Z.; Morrell, J.J.; Lei, Y.; Yan, L.; Ji, M. Eco-friendly and mildly modification of wood cell walls with heat treated wood extracts to improve wood decay resistance. Ind. Crops Prod. 2022, 184, 115079. [Google Scholar] [CrossRef]
- Vovchuk, C.S.; González Garello, T.; Careaga, V.P.; Fazio, A.T. Promising Antifungal Activity of Cedrela fissilis Wood Extractives as Natural Biocides against Xylophagous Fungi for Wood Artwork of Cultural Heritage. Coatings 2024, 14, 237. [Google Scholar] [CrossRef]
- Ventorino, V.; La Storia, A.; Robertiello, A.; Corsi, S.; Romano, I.; Sannino, L.; Pepe, O. Fungal Biodeterioration and Preservation of Miniature Artworks. J. Fungi 2023, 9, 1054. [Google Scholar] [CrossRef] [PubMed]
- Ioan, M.; Anghel, D.F.; Gifu, I.C.; Alexandrescu, E.; Petcu, C.; Diţu, L.M.; Sanda, G.A.; Bala, D.; Cinteza, L.O. Novel Microemulsions with Essential Oils for Environmentally Friendly Cleaning of Copper Cultural Heritage Artifacts. Nanomaterials 2023, 13, 2430. [Google Scholar] [CrossRef]
- Kowalska, J.; Tyburski, J.; Krzymińska, J.; Jakubowska, M. Cinnamon powder: An in vitro and in vivo evaluation of antifungal and plant growth promoting activity. Eur. J. Plant Pathol. 2020, 156, 237–243. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Gędas, A.; Simões, M. The Effects of Eugenol, Trans-Cinnamaldehyde, Citronellol, and Terpineol on Escherichia coli Biofilm Control as Assessed by Culture-Dependent and -Independent Methods. Molecules 2020, 25, 2641. [Google Scholar] [CrossRef] [PubMed]
- Kartal, S.; Hwang, W.J.; Imamura, Y.; Sekine, Y. Effect of essential oil compounds and plant extracts on decay and termite resistance of wood. Holz. Roh. Werkst. 2006, 64, 455–461. [Google Scholar] [CrossRef]
- Khademibami, L.; Bobadilha, G.S. Recent Developments Studies on Wood Protection Research in Academia: A Review. Front. For. Glob. Change 2022, 5, 793177. [Google Scholar] [CrossRef]
- Maoz, M.; Freitag, C.; Morrell, J.J. Potential Synergy between Natural Product Extracts for Limiting Fungal Decay; Document no. IRG/WP 09-30495. 09-05-24/28 Beijing, China; International Research Group on Wood Protection: Stockholm, Sweden, 2009. [Google Scholar]
- Antonelli, F.; Bartolini, M.; Plissonnier, M.-L.; Esposito, A.; Galotta, G.; Ricci, S.; Petriaggi, B.D.; Pedone, C.; Di Giovanni, A.; Piazza, S.; et al. Essential Oils as Alternative Biocides for the Preservation of Waterlogged Archaeological Wood. Microorganisms 2020, 8, 2015. [Google Scholar] [CrossRef]
- Ipek, E.; Zeytinoglu, H.; Okay, S.; Tuylu, B.A.; Kurkcuoglu, M.; Baser, K.H.C. Genotoxicity and antigenotoxicity of Origanum oil and carvacrol evaluated by Ames Salmonella/microsomal test. Food Chem. 2005, 93, 551–556. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.L.; Yen, T.B.; Chang, H.T.; Chang, S.T. Antifungal Activity and Synergistic Effect of Cinnamaldehyde Combined with Antioxidants Against Wood Decay Fungi; Document no. IRG/WP 07-30445; The International Research Group on Wood Protection: Stockholm, Sweden, 2007. [Google Scholar]




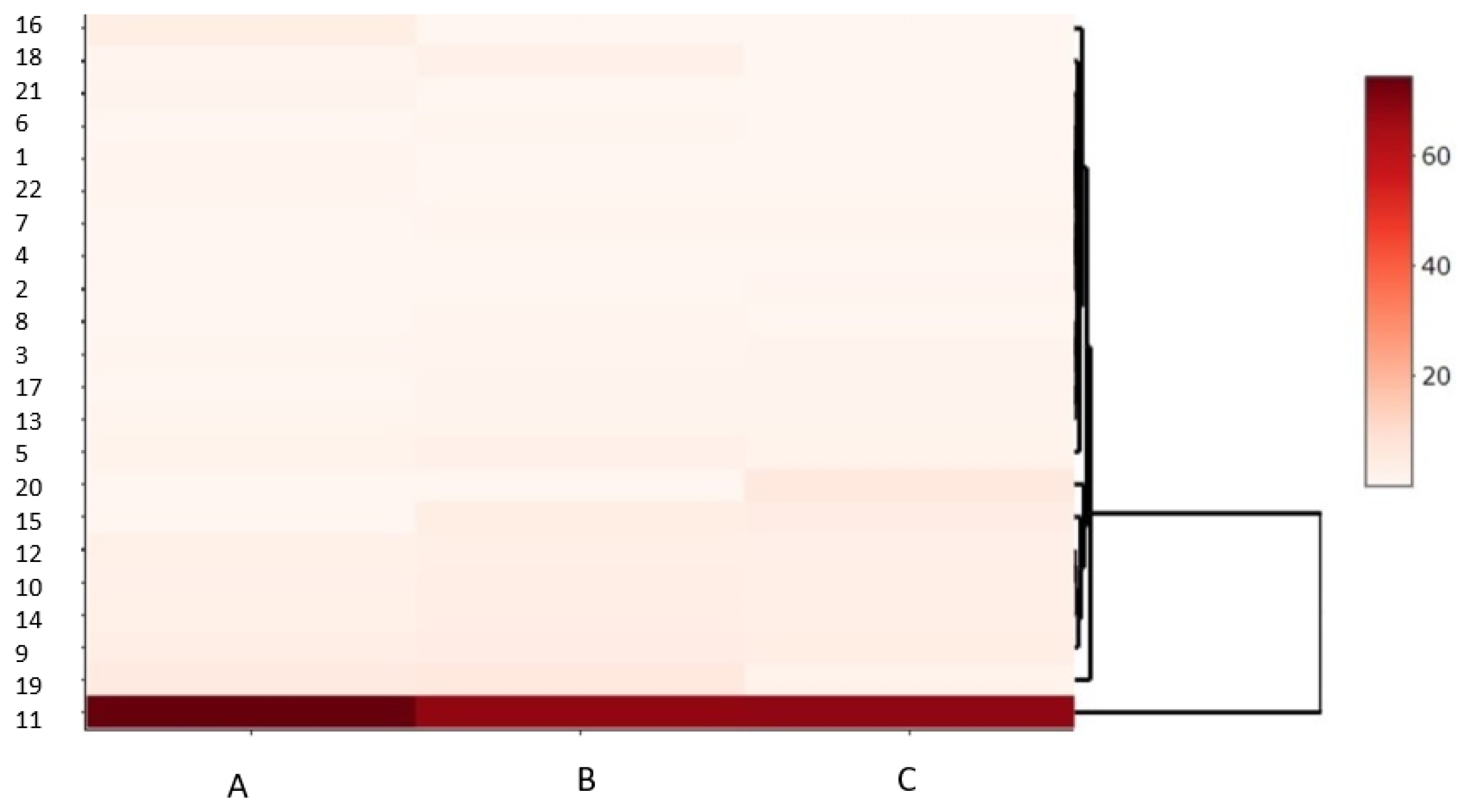
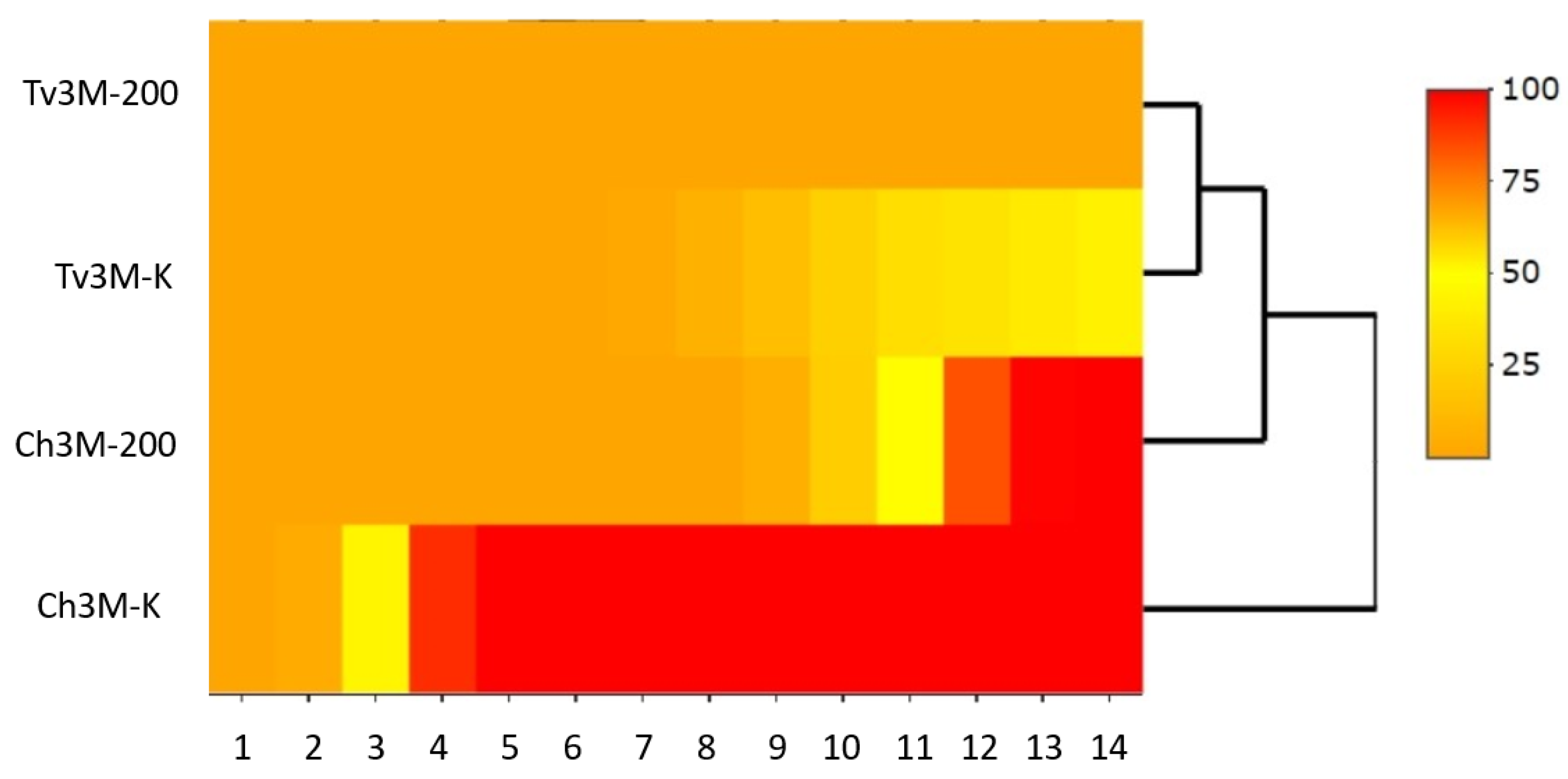
| Source of Variation | Sum of Squares SS | Mean Sum of Squares MS | Fisher’s F-Test F | Significance Level p | Percentage of Contribution P [%] |
|---|---|---|---|---|---|
| Fungi | 20,758 | 20,758 | 110.343 | 0.000000 | 1.0 |
| Concentration | 534,996 | 178,332 | 947.933 | 0.000000 | 25.2 |
| Time since treatment | 28,462 | 14,231 | 75.645 | 0.000000 | 1.3 |
| Test day | 257,004 | 18,357 | 97.580 | 0.000000 | 12.1 |
| Fungi × Concentration | 39,944 | 13,315 | 70.776 | 0.000000 | 1.9 |
| Fungi × Time since treatment | 460,021 | 230,011 | 1222.634 | 0.000000 | 21.7 |
| Concentration*Time since treatment | 66,936 | 11,156 | 59.300 | 0.000000 | 3.1 |
| Fungi × Test day | 20,980 | 1499 | 7.966 | 0.000000 | 1.0 |
| Concentration × Test day | 84,238 | 2006 | 10.661 | 0.000000 | 4.0 |
| Time since treatment × Test day | 30,111 | 1075 | 5.716 | 0.000000 | 1.4 |
| Fungi × Concentration × Time since treatment | 152,131 | 25,355 | 134.777 | 0.000000 | 7.2 |
| Fungi × Concentration × Test day | 39,125 | 932 | 4.952 | 0.000000 | 1.8 |
| Fungi × Time since treatment × Test day | 92,354 | 3298 | 17.533 | 0.000000 | 4.3 |
| Concentration × Time since treatment × Test day | 44,398 | 529 | 2.810 | 0.000000 | 2.1 |
| Fungi × Concentration × Time since treatment × Test day | 116,032 | 1381 | 7.343 | 0.000000 | 5.5 |
| Error | 135,075 | 188 | - | - | 6.4 |
| Source of Variation | Sum of Squares SS | Mean Sum of Squares MS | Fisher’s F-Test F | Significance Level p | Percentage of Contribution P [%] |
|---|---|---|---|---|---|
| Concentration | 368,996.9 | 122,999.0 | 430.839 | 0.000000 | 31.8 |
| Time since treatment | 291,613.5 | 145,806.8 | 510.729 | 0.000000 | 25.2 |
| Test day | 92,436.1 | 6602.6 | 23.127 | 0.000000 | 8.0 |
| Concentration × Time since treatment | 156,571.2 | 26,095.2 | 91.406 | 0.000000 | 13.5 |
| Concentration × Test day | 60,303.9 | 1435.8 | 5.029 | 0.000000 | 5.2 |
| Time since treatment × Test day | 37,236.4 | 1329.9 | 4.658 | 0.000000 | 3.2 |
| Concentration × Time since treatment × Test day | 49,956.1 | 594.7 | 2.083 | 0.000002 | 4.3 |
| Error | 102,204.5 | 285.5 | - | - | 8.8 |
| Source of Variation | Sum of Squares SS | Mean Sum of Squares MS | Fisher’s F-Test F | Significance Level p | Percentage of Contribution P [%] |
|---|---|---|---|---|---|
| Concentration | 205,963.9 | 68,654.6 | 751.902 | 0.00 | 21.9 |
| Time since treatment | 196,416.1 | 98,208.1 | 1075.570 | 0.00 | 20.8 |
| Test day | 186,252.0 | 13,303.7 | 145.702 | 0.00 | 19.8 |
| Concentration × Time since treatment | 62,606.0 | 10,434.3 | 114.276 | 0.00 | 6.6 |
| Concentration × Test day | 62,723.6 | 1493.4 | 16.356 | 0.00 | 6.7 |
| Time since treatment × Test day | 85,219.1 | 3043.5 | 33.333 | 0.00 | 9.0 |
| Concentration × Time since treatment × Test day | 110,473.1 | 1315.2 | 14.404 | 0.00 | 11.7 |
| Error | 32,870.8 | 91.3 | - | - | 3.5 |
| Factor | Value | Homogeneous Groups Regarding the Covered Area | |
|---|---|---|---|
| Trichoderma viride | Chaetomium globosum | ||
| Concentration | Control | a, b | A |
| 75 | b | B | |
| 120 | c | C | |
| 200 | d | C | |
| Time since treatment | 24 | a | A |
| 2T | a | B | |
| 3M | b | C | |
| Systematic Substance Name | Common Name | No. CAS | RT [min] | Retention of the Preparation in the Sample [g/m2] | ||
|---|---|---|---|---|---|---|
| 200 | 120 | 75 | ||||
| Peak Share in the Chromatogram [%] | ||||||
| Benzaldehyde | - | 100-52-7 | 8.28 | 0.26 | 0.20 | 0.31 |
| Tert-butylobenzen | - | 98-06-6 | 9.70 | trace | 0.56 | 0.40 |
| Isopropenyl-1-methyl-1-cyclohexene | D-Limonen | 5989-27-5 | 9.80 | trace | 0.31 | - |
| 1,3,3-Trimethyl-2-oxabicyclo[2.2.2]octane | Eucalyptol | 470-82-6 | 9.85 | 0.22 | 0.97 | 0.57 |
| p-mentha-1,4-diene | ƴ-Terpinen | 99-85-4 | 10.39 | trace | 0.29 | - |
| 4-methylidene-1-propan-2-ylbicyclo[3.1.0]hexane | Sabinene | 3387-41-5 | 10.94 | trace | 0.33 | 0.31 |
| 3,7-Dimethyl-1,6-octadien-3-yl acetate | Linalyl acetate | 115-95-7 | 11.14 | 1.30 | 1.63 | 1.81 |
| 2-Phenylethanol | - | 60-12-8 | 11.37 | 0.43 | 0.32 | 0.23 |
| exo-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ol | Isoborneol | 12-76-5 | 12.31 | trace | 0.24 | 0.22 |
| 4-Carvomenthenol | Terpinen 4-ol | 562-74-3 | 12.49 | 0.42 | 0.50 | 0.57 |
| 3-Cyclohexene-1-methanol | alfa-Terpineol | 98-55-5 | 12.71 | 3.16 | 4.05 | 3.90 |
| Phenethyl acetate | - | 103-45-7 | 13.68 | 2.35 | 2.69 | 2.99 |
| trans-3-Phenyl-2-propenal | trans-Cinnamaldehyde | 14371-10-9 | 14.05 | 74.45 | 68.38 | 67.84 |
| 1-methoxy-4-(1-propenyl)benzene | anethol | 104-46-1 | 14.16 | 2.37 | 2.62 | 2.82 |
| Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, 2-acetate | Isobornyl acetate | 125-12-2 | 14.21 | 0.70 | 1.02 | 0.99 |
| p-menth-1-en-8-yl acetate | Terpinyl Acetate | 80-26-2 | 15.02 | 2.40 | 2.89 | 3.3 |
| 2-Methoxy-4-(2-propenyl)phenol | Eugenol | 97-53-0 | 15.13 | 3.80 | 3.89 | 3.64 |
| 4-hexen-1-ol, 5-methyl-2-(1-methylethenyl)-, acetate | Lavandulyl acetate | 20777-39-3 | 15.40 | 0.40 | 0.42 | 0.46 |
| 1,3-dimethyl-8-(1-methyl ethyl) tricyclo(4.4.0.0.02,7-)dec-3-ene | copaene | 3856-25-5 | 15.45 | 0.42 | 0.98 | 0.98 |
| 4-Allyl-1,2-dimethoxybenzene, | Methyl eugenol | 93-15-2 | 15.70 | trace | trace | 0.25 |
| Bicyclo[7.2.0]undec-4-ene, 4,11,11-trimethyl-8-methylene- | β-Caryophyllene | 87-44-5 | 16.06 | 0.67 | 1.61 | 1.75 |
| 3-phenyl-2-propen-1-yl acetate | cinnamyl acetate | 103-54-8 | 16.26 | 4.72 | 5.37 | 5.73 |
| 3-phenyl-2-propenoic acid ethyl ester | ethyl (Z)-cinnamate | 4610-69-9 | 16.53 | 0.33 | trace | 0.39 |
| -Methylene-4,12,12-trimethyl-5-oxatricyclo[8.2.0.04,6]dodecane | - | 1139-30-6 | 18.10 | 0.85 | trace | 0.48 |
| Benzyl benzoate | - | 120-51-4 | 20.04 | 0.26 | trace | 0.33 |
| Octahydro-3,6,8,8-tetramethyl-1H-3a,7-methanoazulen-6-ol-6-acetate | Cedryl acetate | 77-54-3 | 20.13 | 0.42 | - | - |
| Systematic Substance Name | Ordinary Substance Name | No. CAS | RT [min] | Peak Share in the Chromatogram [%] |
|---|---|---|---|---|
| 2-isopropyl-5-methylphenol | Tymol | 89-83-8 | 14.30 | 9.29 |
| hexyl hexanoate | - | 6378-65-0 | 15.44 | 12.46 |
| 3,7,11-trimethyldodeca-1,3,6,10-tetraene | Farnesene | 502-61-4 | 17.02 | 77.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betlej, I.; Andres, B.; Krajewski, K.; Borysiuk, P.; Szakiel, J.; Kowalski, M.; Salerno-Kochan, R.; Balawejder, M.; Cebulak, T.; Auriga, R.; et al. Cinnamon Bark Oil as an Effective Fungicide in Protecting the Surface of Wood-Based Softboards against the Development of Mold Fungi. Coatings 2024, 14, 433. https://doi.org/10.3390/coatings14040433
Betlej I, Andres B, Krajewski K, Borysiuk P, Szakiel J, Kowalski M, Salerno-Kochan R, Balawejder M, Cebulak T, Auriga R, et al. Cinnamon Bark Oil as an Effective Fungicide in Protecting the Surface of Wood-Based Softboards against the Development of Mold Fungi. Coatings. 2024; 14(4):433. https://doi.org/10.3390/coatings14040433
Chicago/Turabian StyleBetlej, Izabela, Bogusław Andres, Krzysztof Krajewski, Piotr Borysiuk, Jerzy Szakiel, Mateusz Kowalski, Renata Salerno-Kochan, Maciej Balawejder, Tomasz Cebulak, Radosław Auriga, and et al. 2024. "Cinnamon Bark Oil as an Effective Fungicide in Protecting the Surface of Wood-Based Softboards against the Development of Mold Fungi" Coatings 14, no. 4: 433. https://doi.org/10.3390/coatings14040433
APA StyleBetlej, I., Andres, B., Krajewski, K., Borysiuk, P., Szakiel, J., Kowalski, M., Salerno-Kochan, R., Balawejder, M., Cebulak, T., Auriga, R., & Rybak, K. (2024). Cinnamon Bark Oil as an Effective Fungicide in Protecting the Surface of Wood-Based Softboards against the Development of Mold Fungi. Coatings, 14(4), 433. https://doi.org/10.3390/coatings14040433









